Abstract
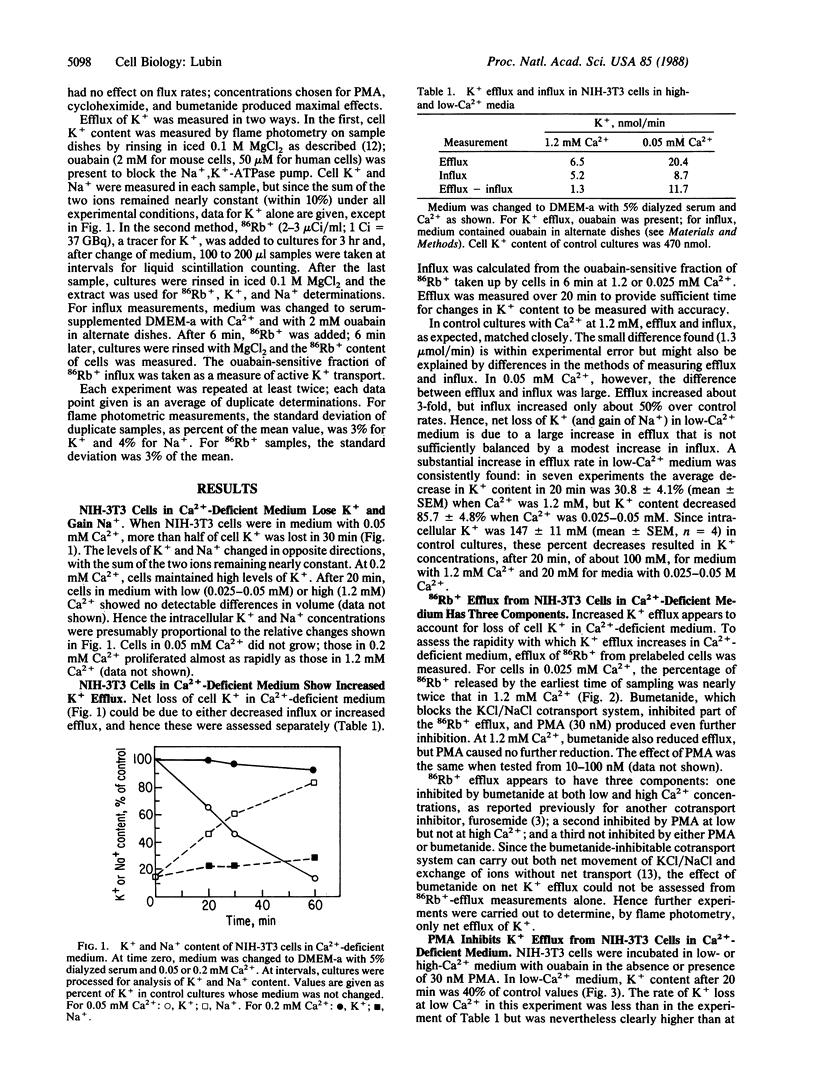
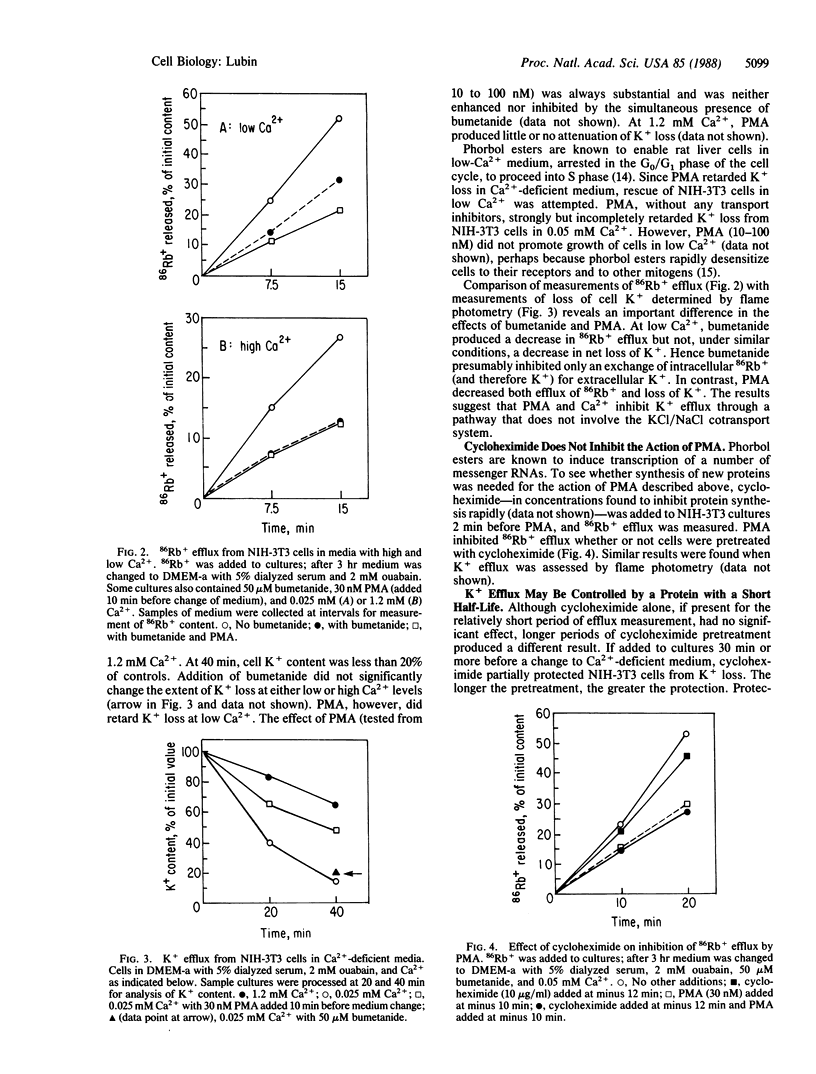
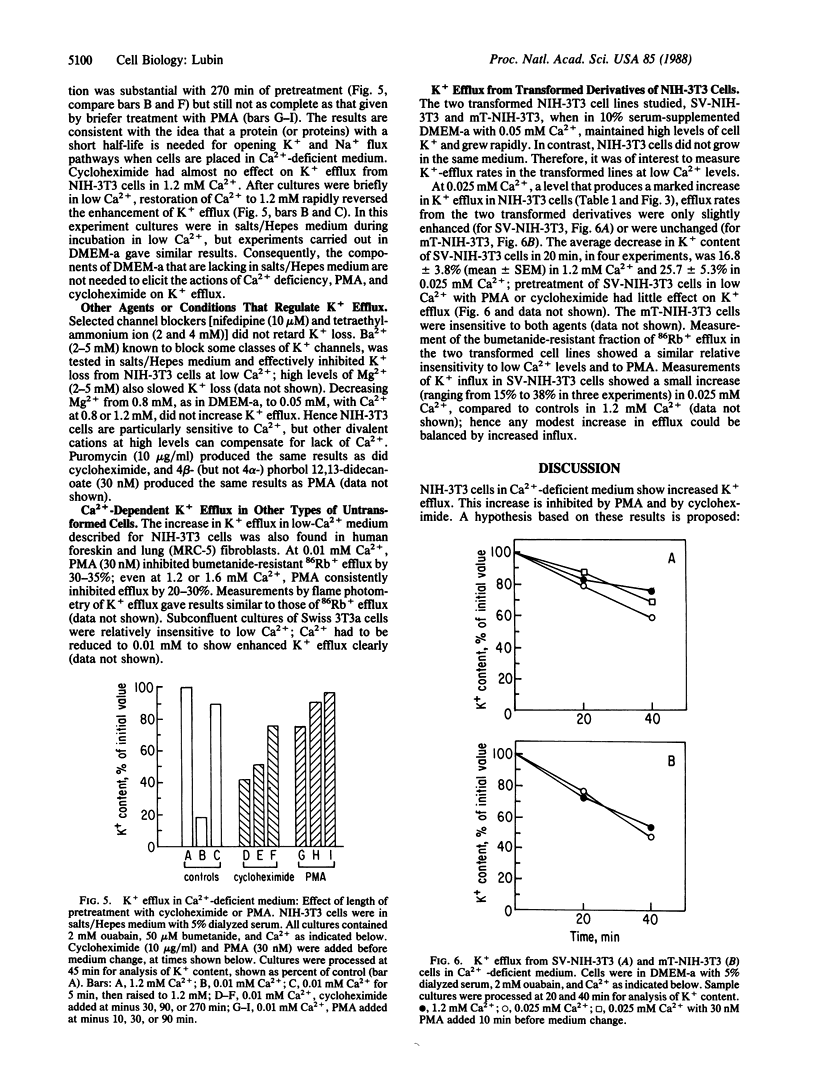
In culture medium deficient in Ca2+, NIH mouse 3T3 cells lose K+, gain Na+, and stop growing. A marked increase in the rate of K+ efflux accounts for this loss; Na+, K+-ATPase pump activity increases but does not fully compensate for enhanced K+ efflux. Phorbol esters and cycloheximide inhibit K+ loss in Ca2+-deficient medium. Phorbol esters inhibit K+ efflux from human fibroblasts as well, even at physiological levels of Ca2+. Two cell lines derived from NIH-3T3, one transformed by a simian virus 40 deletion mutant, the other by the polyoma virus oncogene encoding the middle-sized tumor antigen, retain K+ and can multiply in medium with low Ca2+. Efflux of K+ from these cells is relatively insensitive to reduced Ca2+ concentration, phorbol esters, and cycloheximide. The results suggest the following hypothesis: a channel, nonselective for K+ and Na+, opens when NIH-3T3 cells are in Ca2+-deficient medium; the channel is controlled by the receptor for phorbol ester (protein kinase C) and may also be regulated by a short-lived protein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Lopez-Barneo J. External calcium ions are required for potassium channel gating in squid neurons. Science. 1987 May 8;236(4802):712–714. doi: 10.1126/science.2437654. [DOI] [PubMed] [Google Scholar]
- Balk S. D., Whitfield J. F., Youdale T., Braun A. C. Roles of calcium, serum, plasma, and folic acid in the control of proliferation of normal and Rous sarcoma virus-infected chicken fibroblasts. Proc Natl Acad Sci U S A. 1973 Mar;70(3):675–679. doi: 10.1073/pnas.70.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton A. L., Kleine L. P., Whitfield J. F., Bossi D. Involvement of the Ca2+/phospholipid-dependent protein kinase in the G1 transit of T51B rat liver epithelial cells. Exp Cell Res. 1985 Sep;160(1):197–205. doi: 10.1016/0014-4827(85)90248-4. [DOI] [PubMed] [Google Scholar]
- Cahn F., Lubin M. Inhibition of elongation steps of protein synthesis at reduced potassium concentrations in reticulocytes and reticulocyte lysate. J Biol Chem. 1978 Nov 10;253(21):7798–7803. [PubMed] [Google Scholar]
- Collins M. K., Rozengurt E. Homologous and heterologous mitogenic desensitization of Swiss 3T3 cells to phorbol esters and vasopressin: role of receptor and postreceptor steps. J Cell Physiol. 1984 Feb;118(2):133–142. doi: 10.1002/jcp.1041180205. [DOI] [PubMed] [Google Scholar]
- Ernst M., Adam G. Regulation of passive potassium transport of normal and transformed 3T3 mouse cell cultures by external calcium concentration and temperature. J Membr Biol. 1981;61(3):155–172. doi: 10.1007/BF01870521. [DOI] [PubMed] [Google Scholar]
- GEYER R. P., SHOLTZ K. J., BOWIE E. J. Influence of calcium on potassium concentration in rat liver in vitro. Am J Physiol. 1955 Sep;182(3):487–492. doi: 10.1152/ajplegacy.1955.182.3.487. [DOI] [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol. 1986 Sep;88(3):293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladenson J. H., Bowers G. N., Jr Free calcium in serum. II. Rigor of homeostatic control, correlations with total serum calcium, and review of data on patients with disturbed calcium metabolism. Clin Chem. 1973 Jun;19(6):575–582. [PubMed] [Google Scholar]
- Ledbetter M. L., Lubin M. Control of protein synthesis in human fibroblasts by intracellular potassium. Exp Cell Res. 1977 Mar 15;105(2):223–236. doi: 10.1016/0014-4827(77)90120-3. [DOI] [PubMed] [Google Scholar]
- Lopez-Rivas A., Adelberg E. A., Rozengurt E. Intracellular K+ and the mitogenic response of 3T3 cells to peptide factors in serum-free medium. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6275–6279. doi: 10.1073/pnas.79.20.6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin M. Control of growth by intracellular potassium and sodium concentrations is relaxed in transformed 3T3 cells. Biochem Biophys Res Commun. 1980 Dec 16;97(3):1060–1067. doi: 10.1016/0006-291x(80)91483-7. [DOI] [PubMed] [Google Scholar]
- Lubin M. Intracellular potassium and macromolecular synthesis in mammalian cells. Nature. 1967 Feb 4;213(5075):451–453. doi: 10.1038/213451a0. [DOI] [PubMed] [Google Scholar]
- McCleskey E. W., Almers W. The Ca channel in skeletal muscle is a large pore. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7149–7153. doi: 10.1073/pnas.82.20.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill G. A., Robbins E. The role of calcium in the regulation of the steady-state levels of sodium and potassium in the HeLa cell. J Gen Physiol. 1967 Mar;50(4):781–792. doi: 10.1085/jgp.50.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson N. B., Chen S., Blanck G., Pollack R. SV40 transformation of Swiss 3T3 cells can cause a stable reduction in the calcium requirement for growth. J Cell Biol. 1984 Dec;99(6):2314–2321. doi: 10.1083/jcb.99.6.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet R. Serum-induced net K+ influx performed by the diuretic-sensitive transport system in quiescent NIH 3T3 mouse fibroblasts. Biochim Biophys Acta. 1985 Feb 28;813(1):141–144. doi: 10.1016/0005-2736(85)90355-4. [DOI] [PubMed] [Google Scholar]
- Quastel M. R., Segel G. B., Lichtman M. A. The effect of calcium chelation on lymphocyte monovalent cation permeability, transport and concentration. J Cell Physiol. 1981 May;107(2):165–170. doi: 10.1002/jcp.1041070202. [DOI] [PubMed] [Google Scholar]
- Raptis L., Lamfrom H., Benjamin T. L. Regulation of cellular phenotype and expression of polyomavirus middle T antigen in rat fibroblasts. Mol Cell Biol. 1985 Sep;5(9):2476–2486. doi: 10.1128/mcb.5.9.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. C., Vernon K. D. Correlation of the effect of Ca+2 on Na+ and K+ permeability and membrane potential of Ehrlich ascites tumor cells. J Cell Physiol. 1979 Feb;98(2):359–369. doi: 10.1002/jcp.1040980212. [DOI] [PubMed] [Google Scholar]
- Swierenga S. H., Whitfield J. F., Boynton A. L., MacManus J. P., Rixon R. H., Sikorska M., Tsang B. K., Walker P. R. Regulation of proliferation of normal and neoplastic rat liver cells by calcium and cyclic AMP. Ann N Y Acad Sci. 1980;349:294–311. doi: 10.1111/j.1749-6632.1980.tb29534.x. [DOI] [PubMed] [Google Scholar]
- Van Driessche W., Aelvoet I., Erlij D. Oxytocin and cAMP stimulate monovalent cation movements through a Ca2+-sensitive, amiloride-insensitive channel in the apical membrane of toad urinary bladder. Proc Natl Acad Sci U S A. 1987 Jan;84(1):313–317. doi: 10.1073/pnas.84.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenner C., Hackney J. Inhibition of 86Rb+ and 22Na+ efflux of Ehrlich lettré tumor cells by Ca2+. Arch Biochem Biophys. 1976 Sep;176(1):37–42. doi: 10.1016/0003-9861(76)90138-7. [DOI] [PubMed] [Google Scholar]


