Abstract
Although understanding large-scale spatial variation in species' distributions is a major goal in macroecology, relatively little attention has been paid to the factors limiting species' ranges. An understanding of these factors may improve predictions of species' movements in response to global change. We present a measure of landscape impermeability, defined as the proportion of resident species whose ranges end in an area. We quantify and map impermeability for Afrotropical birds and use multi-model inference to assess support for a wide suite of hypotheses about its potential environmental correlates. Non-spatial analyses emphasize the importance of broad-scale environmental patterns of energy availability and habitat heterogeneity in limiting species' distributions. Conversely, spatial analyses focus attention on small-scale factors of habitat and topographic complexity. These results hold even when only species from the top quartile of range sizes are assessed. All our analyses highlight that range edges are concentrated in heterogeneous habitats. Global change is expected to alter the nature and distribution of such habitats, necessitating range movement by many resident species. Therefore, impermeability provides a simple measure for identifying regions, where continuing global change and human encroachment are likely to cause profound changes in regional diversity patterns.
Keywords: range edges, species' distributions, birds, climate, habitat heterogeneity, spatial analysis
1. Introduction
The geographic ranges of many species are expected to change in response to ongoing global climate change. When trying to predict future ranges, researchers are often forced to make assumptions, for example that species will not colonize regions outside their present ranges and that there are no obstacles to colonization of newly suitable locations (e.g. Thomas et al. 2004). An understanding of current constraints acting at species' range boundaries may therefore make predictions about the future movements of species more accurate.
Range boundaries have often been studied from a single- or two-species perspective (Holt & Keitt 2005). For example, population genetic models have shown how gene flow from the centre of a species' range may thwart local adaptation at the range edge, which can either promote or disrupt the generation of stable range limits (Kirkpatrick & Barton 1997). Interspecific interactions may also stabilize range limits, even in the absence of strong gradients in environmental variables or dispersal barriers (Case & Taper 2000; Case et al. 2005). While much attention has been given to identifying the patterns and environmental correlates of species richness and range size (e.g. Jetz & Rahbek 2002; Hawkins et al. 2003; Currie et al. 2004), there have been few large-scale analyses devoted to deciphering patterns in the distribution of range boundaries (Svenning & Condit 2008; but see Williams et al. 1999). Furthermore, the use of species distribution modelling (SDM), where a species' climatic envelope is inferred from the climatic variables found within its range, under the assumption that this is an adequate description of a species' realized niche (Guisan & Thuiller 2005), defines edges largely as the location where a variable becomes unsuitable, rather than by modelling the conditions currently constraining range expansion. Here, we identify the areas where high proportions of range boundaries are clustered to provide an ensemble, macroecological perspective on species' limits. We therefore focus on the factors affecting generalized limits of species' distributions and do not consider temporary, or sink, populations or individuals occurring outside this general limit (Gaston 2003; Fortin et al. 2005). We do not incorporate the roles of population dynamics (Kirkpatrick & Barton 1997), genetics (Bridle & Vines 2007) or biotic interactions (Terborgh 1985; Case et al. 2005) in limiting individual species' ranges. We locate regions where the range limits of multiple species coincide, and identify the environmental conditions within these areas.
Every species has a unique set of environmental variables under which it can survive and reproduce. Outside this niche space a species is unable to persist in the long-term. While many abiotic and biotic factors have been proposed to limit species' ranges (reviewed by Gaston 2003), the availability of ambient and productive energy has long been considered the most important factor (Currie et al. 2004). The range edges of many species coincide with climatic thresholds and have been found to change in broad synchrony with changing climate (Gaston 2003). Consequently, SDMs have been used widely to predict the expected new range of a species under one or more climatic change scenarios (Thomas et al. 2004). Recently observed changes in avian community composition suggest, however, that the current pace of climate change may be too rapid, with species' ranges lagging behind their climatic envelopes (Devictor et al. 2008). Furthermore, there is growing evidence that non-climatic factors also limit ranges (e.g. Beale et al. 2008). Habitat heterogeneity has been shown to influence species richness and average range sizes of an area (Rahbek & Graves 2001; Davies et al. 2007). Complex habitats, or steep altitudinal gradients, often harbour high numbers of endemic species, uniquely adapted to one of the array of narrow niches found there (Terborgh 1977). Heterogeneous habitats are expected to contain high densities of range edges, from resident endemics and large-range species unable to cross the varied terrain (Kark et al. 2007).
Evolutionary processes of niche conservatism and niche evolution (Hawkins et al. 2007; Rangel et al. 2007), along with Pleistocene glacial cycles and older climate changes (Davies et al. 2007; Hawkins et al. 2007; Rahbek et al. 2007), have also recently been invoked to explain species' distribution patterns and will probably also impact on the patterning of range boundaries. Indeed, evolutionary explanations of high avian species' richness in the montane tropics include: climatic stability over time; persistence of old species within refugial environments; and the generation of new species through fine-scale niche partitioning along environmental and topographic gradients (Rahbek & Graves 2001). In short, where evolutionary and ecological explanations of high species richness converge, for example in the montane tropics, the density of range edges will also be high. Range edges will also cluster at the margin between tropical and temperate zones if it is true that most species are generated in the tropics and that their ranges expand out of the tropics only rarely (Hawkins et al. 2007; Rangel et al. 2007).
Here we use the birds of the Afrotropics to conduct the first large-scale taxonomic and spatial analysis of the distribution and environmental correlates of range boundaries. To do so, we develop a measure of landscape impermeability (ω), calculated as the proportion of resident species with range boundaries within an area (e.g. 1° grid cell). This measure gives an indication of how readily species' ranges have extended through an area and captures factors beyond hard landscape features (e.g. coastline) that prevent high proportions of species from expanding their ranges. Our measure is similar to beta-diversity measures (Koleff et al. 2003; Gaston et al. 2007), and spatial patterning of ω is expected to be similar to that of beta-diversity. However, ω is simpler and easier to interpret because it has relevance within a focal cell. It is therefore not necessary to define the neighbourhood within which turnover is examined, and yet ω captures compositional changes through space when viewed across grid cells.
We test a range of potential predictors of ω in three categories—habitat type, energy availability and habitat heterogeneity—reflecting the identified roles of both average environment (Gaston et al. 2007) and environmental variability (Buckley & Jetz 2008; Melo et al. 2009) in explaining patterns of global avian turnover. While we expect the signal of ω to be high in areas rich in restricted-range endemics (e.g. the montane tropics), we are also interested in identifying additional patterns and in capturing the range-limiting factors of species of all range sizes (Jetz & Rahbek 2002). Finally, we also look at differences in the spatial patterning of ω between passerines and non-passerines to assess whether characteristics of these major groups influences a species' ability to occupy the landscape. This focus on what limits species, rather than on what determines where they are found, sheds new light on the processes governing patterns in the distribution of species diversity and provides information regarding areas where responses to ongoing global change are expected to be most difficult.
2. Material and methods
(a). Range data
We used data for all 2075 terrestrial Afrotropical bird species taken from a global database of bird ranges (Orme et al. 2005, 2006). We included all endemic Afrotropical species and the Afrotropical range of non-endemic species. All range maps for this region were digitized from expert-drawn distribution maps from a single source (‘The Birds of Africa’, references in the electronic supplementary material). The distribution data in this source provide consistent, detailed range polygons, constructed without recourse to environmental modelling.
The digitized vector maps were converted into a Behrmann equal area grid containing 2569 land cells at a resolution of 96.5 km. This scale, approximately equivalent to a 1° grid, minimizes the overestimation of species occupancy of cells arising from using broad-scale distribution maps (Hurlbert & Jetz 2007), especially for species with ragged range edges. The scale is also not so coarse as to obscure patterns in edge distribution, particularly in relation to restricted-range species (Fortin et al. 2005). Species were scored as present in a grid cell if any part of the breeding range fell within the cell. A grid cell was counted as containing a species' range edge if any part of the perimeter of the species' range, including the boundaries of sections of disjunct range, fell within the cell.
Issues of differences in sampling effort across the realm do exist, for example between the well-studied southern African avifauna (Allan et al. 1997) and the under-studied tropical forests of central Africa. However, The Birds of Africa remains the best available source for our analyses and a recent comparison of gridded survey data (Allan et al. 1997) and these range maps for southern Africa concluded that congruence was adequate using grid cells of 1° (our scale of analysis) and larger (Hurlbert & Jetz 2007).
(b). Calculating impermeability
Impermeability (ω) was calculated as the proportion of resident species that also had a range edge in a cell. As ω is bounded between zero and one, and has non-constant variance and a non-normal error distribution, we used logit-transformed ω [log(ω/1 − ω)] in all models.
(c). Predictor variables
We identified the biome (Olson et al. 2001) occupying the largest proportion of each cell but restricted analyses to the four biomes represented in at least 50 cells. Mean ω for the cells in discarded biomes (listed in the electronic supplementary material) did not differ significantly from those in the remaining four major biomes: tropical and subtropical moist broadleaf forests; tropical and subtropical grasslands, savannas and shrublands; montane grasslands and savannas; and deserts and xeric shrublands (results not shown). In addition, all cells with ω > 0.9 were identified as cells containing ranges with boundaries clipped to lakes or coast and hence where ω was trivially high. In total, 696 cells were discarded from the original dataset, resulting in the removal of 170 species restricted to the omitted cells (163 from coastal cells and seven from minor biomes; see electronic supplementary material). All the omitted species are restricted-range species (mean occurrence: 4.2 cells, range: 1–45 cells) and, since almost all occur in coastal/lakeside cells which are always completely impermeable, their omission is unlikely to obscure additional patterns in ω at this scale of analysis.
Biome type was used as a predictor variable describing broad-scale habitat type; we also used mean elevation (metres) as a second measure to capture habitat type. As measures of habitat heterogeneity within each cell we used: the number of the four major biomes present to indicate large-scale habitat heterogeneity; the number of land cover types (listed in the electronic supplementary material) to represent small-scale landscape heterogeneity; and log10 elevational range, to capture topographic complexity. To investigate the correlation of climatic factors with ω, we used mean annual temperature (°C) as a proxy for ambient energy and mean annual actual evapotranspiration (AET, mm) as a proxy for productive energy (all references in the electronic supplementary material). The data for each of these variables was re-sampled from the original resolutions into the equal-area grid (figure S1 in the electronic supplementary material).
Anthropogenic impacts are expected to be important in determining the boundaries of ranges, particularly given the changes expected in human population and land use within the region (Millennium Ecosystem Assessment 2005). However, the absence of estimates of range change resulting from anthropogenic impact, a temporal disassociation between available measures of impact and the avian range data and the known positive correlation between measures of human impact and biodiversity at coarse spatial scales (Luck 2007), suggests that establishing cause and effect of any relationship between human impact and ω will be difficult. Therefore, we consider only environmental correlates in our reported models. In addition, a correlation test accounting for spatial autocorrelation (Clifford et al. 1989) revealed no correlation between mean human population density and ω (r = −0.00281, n = 2018, effective sample size (ess) = 61.72, t = −0.0216, p = 0.98).
(d). Data analysis
Preliminary analyses were performed to limit the scope of the most complex model considered. We calculated ordinary least squares (OLS) univariate regressions of ω against each predictor across the entire dataset and within each biome (tables S1 and S2 in the electronic supplementary material). Significantly different relationships were often found in the biome-specific analyses, indicating that biome type is a key factor affecting landscape impermeability. We then used regression trees to visualize the structure of the data and to identify potential interaction terms (figure S2 in the electronic supplementary material). Finally, we used a generalized additive model (Wood 2006) to examine the possibility of significant nonlinearity between ω and the predictor variables (figure S3 in the electronic supplementary material). All of the main effects showed broadly linear relationships with ω, except for AET and elevational range which approximated quadratic relationships. Our most complex model therefore includes all main effects and second-order polynomial terms for these two variables. We included first-order interaction terms between each main effect and biome, and also between AET and both temperature and elevational range, resulting in a maximal model containing 19 terms. Variance inflation factors between the main effects were all low (≤4) indicating that there is no strong collinearity among them (table S3 in the electronic supplementary material; Fox 2002).
There has been a growing reaction against stepwise model simplification to a single minimum adequate model, particularly for broad-scale analyses where multiple alternative hypotheses may be relevant (Johnson & Omland 2004; Diniz-Filho et al. 2008). Here, we fitted all 14 752 valid simplifications of our maximal model and follow a multi-model inference approach (Burnham & Anderson 2002; Johnson & Omland 2004). Models were fitted using OLS multiple regression and we obtained AICc, the sample size corrected version of AIC, for each model. We then computed the Akaike weights of each model across the full set of models using ΔAICc values and identified the set of most highly weighted models with a combined Akaike weight of greater than 0.95 (the 95% confidence set; Burnham & Anderson 2002).
(e). Spatial modelling
Cells close to one another in space will tend to have more similar values of both response and explanatory variables than cells located further apart (Legendre 1993). Such spatial autocorrelation can inflate Type I error rates and cause bias in the magnitude of effect of explanatory variables (Davies et al. 2007). Coefficients may also be estimated incorrectly and their variances strongly underestimated. Irrespective of the model selection method used, highly supported non-spatial models are thus expected to be more inclusive than equivalently supported spatial models (Diniz-Filho et al. 2008). However, OLS models may still be useful, despite their higher Type I error rates, in capturing broad-scale drivers of macroecological patterns (Diniz-Filho et al. 2007). Interpretation of both non-spatial and spatial models and explicit consideration of scale and the hierarchical nature of diversity drivers may generate a more complete picture than one or the other mode of analysis alone (Diniz-Filho et al. 2003, 2007). We follow this course here and present the results of both non-spatial and spatially explicit regression analyses following exploration of the spatial structure of our data.
Correlograms of Moran's I confirmed the presence of substantial spatial autocorrelation in explanatory and response variables and in the residuals of models in our OLS best-model subset (figure S4a,b in the electronic supplementary material). Following examination of semi-variograms and calculation of AIC values (Rangel et al. 2006; data not shown) to determine the form of the spatial structure in the data, we refitted all models using a generalized least squares (GLS) approach (Pinheiro & Bates 2000; Beguería & Pueyo 2009) including an exponential spatial correlation structure. GLS incorporates this spatial structure directly into model residuals and correlograms of normalized residuals from fitted models are used to check that spatial autocorrelation had been adequately accounted for (figure S4c in the electronic supplementary material; Pinheiro & Bates 2000).
When fitting a spatial model using GLS, the range (ρ) over which autocorrelation operates is usually optimized for each set of explanatory variables, as the structure of the autocorrelation will vary with the suite of variables chosen. However, changes in the correlation structure will affect the calculation of AICc for a model and so, in order to simplify multi-model inference across models, we used a fixed range (ρ = 39.095 km). This value was obtained as the mean of independently estimated ρ from a random subset of 200 models. Pearson's correlation between the AICc values of these 200 models calculated using both optimized and fixed ρ is almost perfect (r = 0.999, t198 = 29432, p-value < 0.0001), suggesting that this restriction does not unduly affect subsequent model weighting. In addition, visual inspection of Moran's I correlograms indicated that spatial autocorrelation is similarly accounted for in both model sets (data not shown) and that residual autocorrelation was similarly reduced using the fixed or optimized ρ.
(f). Range size and taxonomic influences
Results from studies of species richness and range size (Jetz & Rahbek 2002; Orme et al. 2006) suggest that correlates of ω will differ between large- and small-range species. Following the study of Jetz & Rahbek (2002), the species were divided into range size quartiles based on the number of cells occupied within the truncated dataset and the spatial distribution of ω was calculated for each quartile. The analyses described above were repeated using the species within the top range size quartile to investigate the expected high influence of this quartile on analyses of the dataset as a whole. In addition, we split our dataset into passeriformes and all remaining birds and recalculated ω for each group. We calculated the correlation between the two subsets to investigate the degree to which taxonomic biogeographic structure is reflected in landscape impermeability.
3. Results
(a). Spatial distribution of ω
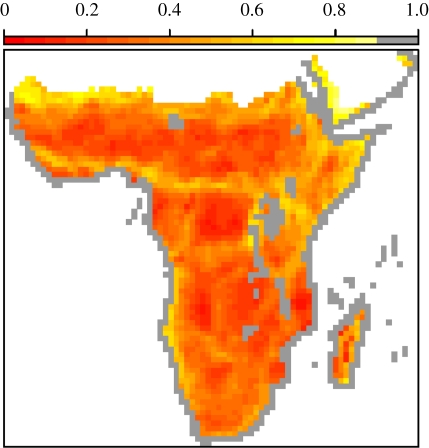
Impermeability (ω) shows strong spatial patterns throughout the Afrotropics (figure 1). Permeable regions include the resource-rich Guinean and Congo basin forests and the savannahs of the Sahel; ω increases markedly at the boundaries of these productive regions. Impermeability is also high in the montane habitats of northeastern Africa and along the edges of the Sahara desert in the north and the Namib desert in the south.
Figure 1.
Landscape impermeability using untransformed ω for Afrotropical birds. Red, high ω; yellow, low ω. Grey cells are omitted from all analyses (see text for further justification).
(b). Non-spatial OLS analyses
Non-spatial modelling produces a 95 per cent confidence set containing 10 models (table 1). As expected, these highly supported models are inclusive (summarized in table 2; see also table S4 in the electronic supplementary material), with the maximal model being the most highly weighted (weight, wi = 0.184) and the remaining nine models including a mean of 16.9 terms. On the basis of F ratios, all models in the top set highlight the importance of available energy (AET, AET2) as well as elevational range, temperature and biome heterogeneity. Interactions with biome typically have lower explanatory power, but are present in models retained in the preferred model set. Although correlograms of the raw residuals from the top OLS model exhibit reduced spatial autocorrelation compared with those of individual variables, there is still evidence (figure S4b in the electronic supplementary material) for substantial short-range autocorrelation (Diniz-Filho et al. 2003).
Table 1.
Akaike weights (wi), AICc and the number of terms in each model for all the non-spatial and spatial models in the 95 per cent confidence sets.
| wi | AICc | terms |
|---|---|---|
| non-spatial | ||
| 0.18 | 2578.1 | 19 |
| 0.17 | 2578.3 | 18 |
| 0.17 | 2578.3 | 16 |
| 0.16 | 2578.4 | 17 |
| 0.09 | 2579.5 | 17 |
| 0.09 | 2579.5 | 18 |
| 0.03 | 2581.9 | 16 |
| 0.03 | 2582.0 | 15 |
| 0.02 | 2582.2 | 18 |
| 0.02 | 2582.6 | 17 |
| spatial | ||
| 0.81 | −47.2 | 3 |
| 0.15 | −43.8 | 3 |
Table 2.
The minimum and maximum F ratio and the number of times retained (n) for each term across the 95 per cent confidence set of 10 non-spatial models (a). F ratios for significant terms in the two models in the 95 per cent confidence set for the spatial models (b). Superscripts show significance at p < 0.001, along with the sign of the coefficient where relevant.
| (a) non-spatial |
(b) spatial |
||||
|---|---|---|---|---|---|
| main effects | min F | max F | n | model 1 | model 2 |
| biome | 61.17* | 61.54* | 10 | ||
| mean elevation | 0.05 | 0.05 | 10 | ||
| mean annual AET | 494.79+ | 497.82+ | 10 | ||
| mean annual temperature | 123.66+ | 124.42+ | 10 | ||
| elevational range | 357.10+ | 359.28+ | 10 | 33.59+ | |
| landscape heterogeneity | 0.29 | 0.30 | 6 | 23.86+ | 28.58+ |
| biome heterogeneity | 203.49+ | 204.74+ | 10 | 50.61+ | 61.74+ |
| mean annual AET2 | 283.59− | 291.32− | 10 | ||
| elevational range2 | 40.51+ | 43.92+ | 10 | 19.47+ | |
| biome interactions | |||||
| mean elevation | 15.17* | 15.48* | 10 | ||
| mean annual AET | 25.98* | 26.41* | 10 | ||
| mean annual temperature | 19.03* | 19.49* | 10 | ||
| elevational range | 41.81* | 42.93* | 10 | ||
| landscape heterogeneity | 6.37* | 6.48* | 4 | ||
| biome heterogeneity | 3.85* | 3.87* | 6 | ||
| mean annual AET2 | 17.72* | 18.81* | 10 | ||
| elevational range2 | 8.25* | 11.34* | 10 | ||
| other interactions | |||||
| mean annual AET: mean annual temperature | 16.48− | 21.93− | 10 | ||
| mean annual AET: elevational range | 1.90+ | 2.38+ | 5 | ||
(c). Spatial GLS analyses
Accounting for spatial autocorrelation using a GLS approach results in a 95 per cent confidence set of two substantially simpler models and better-fitting models (table 1) with little remaining autocorrelation (figure S4c in the electronic supplementary material). Both models include biome heterogeneity as their strongest explanatory variable (table 2), followed by landscape heterogeneity and either elevational range or elevational range2. Highly spatially autocorrelated variables such as AET and temperature (figure S4a in the electronic supplementary material) are dropped from these models.
(d). Effect of range size and taxonomy on ω
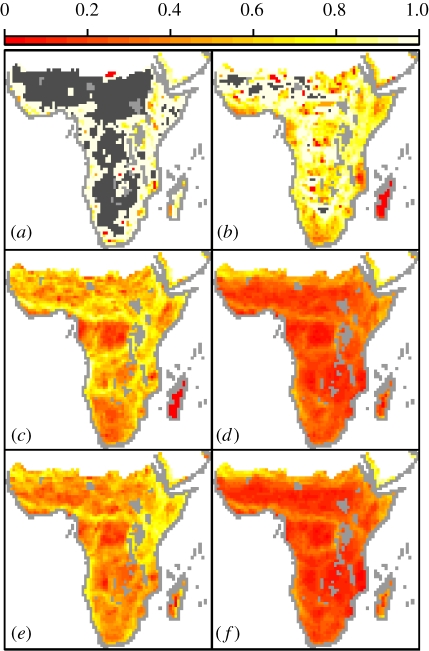
Broad-scale patterns in ω vary considerably within range size quartiles (figure 2a–d) but are dominated by species in the top range size quartile (figure 2d), which inevitably contribute disproportionately to the number of species' presence (73.6%) and edge (54.8%) records in the dataset. Impermeability for this quartile is strongly correlated with overall ω (r = 0.93, n = 2018, ess = 16.30, t = 13.27, p < 0.0001). Both spatial and non-spatial models (table S5 in the electronic supplementary material) for this quartile mirror those for the whole dataset, with OLS models only suggesting a more significant role for temperature and with GLS models supporting the importance of habitat heterogeneity variables (biome heterogeneity, landscape heterogeneity and elevational range). In addition, mean ω for this quartile is significantly higher in cells where restricted-range species are also found (F1,2016 = 28.50, p < 0.0001), highlighting the congruence in highly impermeable areas between small- and large-range species. Finally, the correlation between passerine and non-passerine ω was moderately strong and significantly positive (r = 0.61, n = 2018, ess = 32.94, t = 5.50, p < 0.0001). Given the larger average range size of non-passerines, it is unsurprising that the patterning of ω for this subset (figure 2f) mirrors that of the largest range size quartile (figure 2d) while that for the passerines (figure 2e) is an amalgam of the three smaller quartiles (figure 2a–c).
Figure 2.
Landscape impermeability for subsets of species from the dataset: range size quartiles (a–d) from the narrowest-ranged (a) to the widest-ranged (d), species (e) passerines and (f) non-passerines. Grey cells as in figure 1 with dark grey cells (a,b) showing cells which contain no species from that subset.
4. Discussion
We find strong broad-scale patterns of impermeability across Afrotropical birds, despite the idiosyncrasies of survival, reproduction and immigration that inevitably define individual species' range limits. Of the variables assessed, measures of habitat variability (biome and landscape heterogeneity and elevational range) are the most consistent predictors of impermeability (ω). These variables are significant in both non-spatial and spatial analyses (table 2) and show that transitional or complex habitats act as barriers for a majority of species, even those with the largest ranges (table S5 in the electronic supplementary material). These results support those of van Rensburg et al. (2004) who found greater avian turnover at biome transitions in South Africa, but differ from the early conclusions of Allan et al. (1997) who believed that the botanically defined biomes of the Afrotropics were not ‘entirely relevant to [its] avifauna.’ Whether the clustering of avian range boundaries at biome edges is because of active habitat selection or enforced limits does not detract from the congruence found between avian and vegetation turnover or that high ω areas are those where free range expansion is impeded.
We expected impermeability to be high at the transitions between biomes and in topographically complex regions for two reasons. First, such areas act as barriers to the expansion of mid- and large-range species as they reach the limits of their environmental tolerances. Second, they will be rich in restricted-range endemics adapted to niches uniquely found within the transitional habitat. Range edges for these two groups therefore coincide where habitat heterogeneity is greatest and, indeed, mean ω for the widest-ranging species is significantly higher in cells also occupied by the narrowest-ranging species. Furthermore, only measures of habitat heterogeneity are included in the best-supported spatial models for these wide-ranging species (table S5 in the electronic supplementary material).
Landscape heterogeneity, measured as the number of ecosystem types within a grid cell (references in electronic supplementary material), shows a more complex relationship with ω. Our initial single-predictor analyses show that high landscape heterogeneity in tropical and subtropical grasslands, savannahs and shrublands is associated with lower impermeability (table S2b in the electronic supplementary material). This, however, is driven by the strong signal arising from the species-poor, highly impermeable boundary of this biome with the Sahara (figure 1). In spatial models, which account for the spatial non-independence of this signal, we find a strong positive association between landscape heterogeneity and impermeability across all biomes (table 2; see also Rosenzweig 1995).
Model choice also affects conclusions on the importance of energy availability. In our non-spatial analyses, ω is low where energy availability (AET, temperature and their interaction) is high. However, the strength of these relationships decreases greatly when spatial structure in these variables is accounted for. The fact that climatic variables drop out in the best spatial models indicates that the matching spatial structures of the explanatory and response variables might be driving the strength of these relationships. Additional analyses in other realms are required to determine if there is a genuine effect of climate on ω that is not simply a function of the broad-scale covariance in these variables.
Macroclimatic variables may be true range-limiting factors, but for large-range species only (Jetz & Rahbek 2002; Rahbek et al. 2007). Spatially explicit analyses take account of the same large-range species contributing similar signal in many adjacent cells and change the focus of analysis from long-distance clinal variables, such as temperature and AET, towards predictors acting at finer geographical scales (Diniz-Filho et al. 2003, 2007). Biome heterogeneity, landscape heterogeneity and elevational range are the only predictors remaining in our best spatial models (table 2; table S5 in the electronic supplementary material) suggesting that spatial analyses permit detection of additional explanatory variables acting at scales where the macroclimate is expected to vary only slightly (Hawkins & Diniz-Filho 2006; Diniz-Filho et al. 2007). Interestingly, these measures of habitat heterogeneity are also the only variables remaining in the best spatial models for species in the largest range size quartile. This suggests that additional factors beyond climatic isotherms limit large-range species, and that even species capable of maintaining a large range do not necessarily cross regions of major habitat turnover.
Our results complement analyses of beta-diversity in the Afrotropical avifauna (Williams et al. 1999). These found that, at higher latitudes, turnover was dominated by richness gradients associated with the changing climate (at the edge of the Sahara and Kalahari deserts), while at low latitudes most signal was derived from species' replacements along complex habitats (along the boundary of the humid equatorial forests and to the north and west of Lake Victoria). Different environmental factors therefore operate at different scales in shaping macroecological patterns (Rahbek & Graves 2001), and non-spatial and spatial analyses should together explain the wider hierarchy of factors affecting species of all range sizes (Diniz-Filho et al. 2003, 2007).
We do not assess the scale-dependency of our results, because such an assessment would be confounded by the scale limitations of broad-scale distribution maps (Hurlbert & Jetz 2007). Our finding of the importance of elevational range and habitat heterogeneity is consistent with the observed fine-scale elevational zonation of avian communities within the tropical forest of the Udzungwa Mountains, Tanzania (Romdal & Rahbek 2009) and of the earlier results of Terborgh (1977) for birds along an elevational transect in the Cordillera Vilcabamba, Peru. However, neither scale of analysis directly determines the causal factors limiting species within heterogeneous habitats, and a detailed understanding of such limits will probably require fine-scale mapping of species' abundances in combination with models of the population dynamics at range edges (Case et al. 2005). Environmental models may also incompletely explain variation in ω if range limits are set primarily by historical factors such as the location of refugia (Davies et al. 2007; Rahbek et al. 2007). This would also help explain the high ω found in montane habitats where ecological factors promoting small ranges and refugial environments are coincident (Kark et al. 2007). It is likely that finer-scale analyses would further emphasize some areas of high impermeability associated with excluded coastal cells (figure 1, e.g. in Angola, in Kenya and along the Rift Valley), as these would re-introduce some narrow-ranged species culled from the dataset that are also associated with transitional habitats in these regions.
It is likely that areas of high ω will show early responses to the adverse impacts of global change. We show that the edges of wide-ranging and the entirety of narrow-ranging species' distributions are concentrated in heterogeneous areas. Under global change, it is likely that the nature and location of these habitats will change (e.g. Hannah et al. 2002). Species will respond idiosyncratically to these habitat movements (Davis & Shaw 2001) and changes in community composition in high ω areas are expected to be common (Devictor et al. 2008). The steepness of the elevational gradient in mountainous areas may allow some species to keep pace with their shifting niches (given the high number of, albeit narrow, niches that can be packed into a certain area; Rahbek & Graves 2001; Luoto & Heikkinen 2008). However, certain habitats are projected to have no analogues in the near future (Williams et al. 2007), and only limited adaptation to changing climates is expected (e.g. Gienapp et al. 2008). Şekercioğlu et al. (2008) also highlight the elevated extinction risk of highland birds, with warming temperatures expected to force species uphill, sometimes resulting in complete range extirpation.
Human presence is known to correlate positively with many biodiversity measures at coarse spatial scales (Luck 2007), partly because available energy facilitates both dense human populations and diverse natural assemblages, and partly because human settlements in transitional habitats can probably access more diverse resources (Hugo & van Rensburg 2008). However, in our preliminary analyses, we found no correlation between human population density and ω. While this may reflect the temporal discord between the two datasets, it seems that the areas we identify as particularly vulnerable to disturbance in the face of climate change are not currently facing unusually high human densities.
Previous studies have highlighted transitional habitats as dynamic centres of endemism meriting conservation attention (Kark et al. 2007). We concur with this study, and others, in suggesting that both transition zones and the surrounding areas into which species are likely to ‘want’ to move, alongside montane areas, are important in systematic conservation planning (Luoto & Heikkinen 2008). Our analyses complement others suggesting that climate envelope models do not fully capture species' distributional limits (Beale et al. 2008) and make a start at answering the call for a more inclusive understanding of range-limiting factors (Svenning & Condit 2008; Gaston 2009).
Acknowledgements
We thank Peter Bennett, Tim Blackburn, Kevin Gaston and Ian Owens for access to the bird range data. Lab members kindly commented on earlier drafts; special thanks are extended to Thomas Ezard, Eric Ameca y Juarez, Natalie Cooper, Susanne Fritz, Shai Meiri and Alex Pigot. J. A. F. Diniz-Filho and Robert D. Holt provided insightful comments on earlier versions of this manuscript. For a critical reading by a non-macroecologist, L.M. thanks Nils Bunnefeld. This work was funded by a Grantham studentship to L.M., by a Natural Environment Research Council grant (NE/B503492/1) to A.P. and a Research Excellence Award from Imperial College.
References
- Allan D. G., Harrison J. A., Herremans M., Navarro R. A., Underhill L. G.1997Southern African geography: its relevance to birds. In The atlas of southern African birds (eds Harrison J. A., Allan D. G., Underhill L. G., Herremans M., Tree A. J., Parker V., Brown C. J.). Johannesburg, South Africa: Birdlife South Africa [Google Scholar]
- Beale C. M., Lennon J. J., Gimona A.2008Opening the climate envelope reveals no macroscale associations with climate in European birds. Proc. Natl Acad. Sci. USA 105, 14 908–14 912 (doi:10.1073/pnas.0803506105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beguería S., Pueyo Y.2009A comparison of simultaneous autoregressive and generalized least squares models for dealing with spatial autocorrelation. Global Ecol. Biogeogr. 18, 273–279 (doi:10.1111/j.1466-8238.2009.00446.x) [Google Scholar]
- Bridle J. R., Vines T. H.2007Limits to evolution at range margins: when and why does adaptation fail? Trends Ecol. Evol. 22, 140–147 (doi:10.1016/j.tree.2006.11.002) [DOI] [PubMed] [Google Scholar]
- Buckley L. B., Jetz W.2008Linking global turnover of species and environments. Proc. Natl Acad. Sci. USA 105, 17 836–17 841 (doi:10.1073/pnas.0803524105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham K. P., Anderson D. R.2002Model selection and multi-model inference, a practical information-theoretic approach New York, NY: Springer [Google Scholar]
- Case T. J., Taper M. L.2000Interspecific competition, environmental gradients, gene flow, and the coevolution of species' borders. Am. Nat. 155, 583–605 (doi:10.1086/303351) [DOI] [PubMed] [Google Scholar]
- Case T. J., Holt R. D., McPeek M. A., Keitt T. H.2005The community context of species' borders: ecological and evolutionary perspectives. Oikos 108, 28–46 (doi:10.1111/j.0030-1299.2005.13148.x) [Google Scholar]
- Clifford P., Richardson S., Hemon D.1989Assessing the significance of the correlation between two spatial processes. Biometrics 45, 123–134 (doi:10.2307/2532039) [PubMed] [Google Scholar]
- Currie D. J., et al. 2004Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 7, 1121–1134 (doi:10.1111/j.1461-0248.2004.00671.x) [Google Scholar]
- Davies R. G., et al. 2007Topography, energy and the global distribution of bird species richness. Proc. R. Soc. B 274, 1189–1197 (doi:10.1098/rspb.2006.0061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. B., Shaw R. G.2001Range shifts and adaptive responses to quaternary climate change. Science 292, 673–679 (doi:10.1126/science.292.5517.673) [DOI] [PubMed] [Google Scholar]
- Devictor V., Julliard R., Couvet D., Jiguet F.2008Birds are tracking climate warming, but not fast enough. Proc. R. Soc. B 275, 2743–2748 (doi:10.1098/rspb.2008.0878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz-Filho J. A. F., Bini L. M., Hawkins B. A.2003Spatial autocorrelation and red herrings in geographical ecology. Global Ecol. Biogeogr. 12, 53–64 (doi:10.1046/j.1466-822X.2003.00322.x) [Google Scholar]
- Diniz-Filho J. A. F., Hawkins B. A., Bini L. M., De Marco P., Blackburn T. M.2007Are spatial regression methods a panacea or a Pandora's box? A reply to Beale et al. (2007). Ecography 30, 848–851 [Google Scholar]
- Diniz-Filho J. A. F., Rangel T. F. L. V. B., Bini L. M.2008Model selection and information theory in geographical ecology. Global Ecol. Biogeogr. 17, 479–488 (doi:10.1111/j.1466-8238.2008.00395.x) [Google Scholar]
- Fortin M. J., Keitt T. H., Maurer B. A., Taper M. L., Kaufman D. M., Blackburn T. M.2005Species' geographic ranges and distributional limits: pattern analysis and statistical issues. Oikos 108, 7–17 (doi:10.1111/j.0030-1299.2005.13146.x) [Google Scholar]
- Fox J.2002An R and S-Plus companion to applied regression Thousands Oaks, CA: Sage [Google Scholar]
- Gaston K. J.2003The structure and dynamics of geographical ranges Oxford, UK: Oxford University Press [Google Scholar]
- Gaston K. J.2009Geographic range limits: achieving synthesis. Proc. R. Soc. B 276, 1395–1406 (doi:10.1098/rspb.2008.1480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston K. J., et al. 2007Spatial turnover in the global avifauna. Proc. R. Soc. B 274, 1567–1574 (doi:10.1098/rspb.2007.0236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gienapp P., Teplitsky C., Alho J. S., Mills J. A., Merila J.2008Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 167–178 (doi:10.1111/j.1365-294X.2007.03413.x) [DOI] [PubMed] [Google Scholar]
- Guisan A., Thuiller W.2005Predicting species distribution: offering more than simple habitat models. Ecol. Lett. 8, 993–1009 (doi:10.1111/j.1461-0248.2005.00792.x) [DOI] [PubMed] [Google Scholar]
- Hannah L., Midgley G. F., Lovejoy T., Bond W. J., Bush M., Lovett J. C., Scott D., Woodward F. I.2002Conservation of biodiversity in a changing climate. Conserv. Biol. 16, 264–268 (doi:10.1046/j.1523-1739.2002.00465.x) [DOI] [PubMed] [Google Scholar]
- Hawkins B. A., Diniz-Filho J. A. F.2006Beyond Rapoport's rule: evaluating range size patterns of New World birds in a two-dimensional framework. Global Ecol. Biogeogr. 15, 461–469 [Google Scholar]
- Hawkins B. A., et al. 2003Energy, water, and broad-scale geographic patterns of species richness. Ecology 84, 3105–3117 (doi:10.1890/03-8006) [Google Scholar]
- Hawkins B. A., Diniz-Filho J. A. F., Jaramillo C. A., Soeller S. A.2007Climate, niche conservatism, and the global bird diversity gradient. Am. Nat. 170, S16–S27 (doi:10.1086/519009) [DOI] [PubMed] [Google Scholar]
- Holt R. D., Keitt T. H.2005Species' borders: a unifying theme in ecology. Oikos 108, 3–6 (doi:10.1111/j.0030-1299.2005.13145.x) [Google Scholar]
- Hugo S., van Rensburg B. J.2008The maintenance of a positive spatial correlation between South African bird species richness and human population density. Global Ecol. Biogeogr. 17, 611–621 (doi:10.1111/j.1466-8238.2008.00391.x) [Google Scholar]
- Hurlbert A. H., Jetz W.2007Species richness, hotspots, and the scale dependence of range maps in ecology and conservation. Proc. Natl Acad. Sci. USA 104, 13 384–13 389 (doi:10.1073/pnas.0704469104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetz W., Rahbek C.2002Geographic range size and determinants of avian species richness. Science 297, 1548–1551 (doi:10.1126/science.1072779) [DOI] [PubMed] [Google Scholar]
- Johnson J. B., Omland K. S.2004Model selection in ecology and evolution. Trends Ecol. Evol. 19, 101–108 (doi:10.1016/j.tree.2003.10.013) [DOI] [PubMed] [Google Scholar]
- Kark S., Allnutt T. F., Levin N., Manne L. L., Williams P. H.2007The role of transitional areas as avian biodiversity centres. Global Ecol. Biogeogr. 16, 187–196 (doi:10.1111/j.1466-8238.2006.00274.x) [Google Scholar]
- Kirkpatrick M., Barton N. H.1997Evolution of a species' range. Am. Nat. 150, 1–23 [DOI] [PubMed] [Google Scholar]
- Koleff P., Gaston K. J., Lennon J. J.2003Measuring beta diversity for presence-absence data. J. Anim. Ecol. 72, 367–382 (doi:10.1046/j.1365-2656.2003.00710.x) [Google Scholar]
- Legendre P.1993Spatial autocorrelation - trouble or new paradigm. Ecology 74, 1659–1673 (doi:10.2307/1939924) [Google Scholar]
- Luck G. W.2007A review of the relationships between human population density and biodiversity. Biol. Rev. 82, 607–645 (doi:10.1111/j.1469-185X.2007.00028.x) [DOI] [PubMed] [Google Scholar]
- Luoto M., Heikkinen R. K.2008Disregarding topographical heterogeneity biases species turnover assessments based on bioclimatic models. Global Change Biol. 14, 483–494 (doi:10.1111/j.1365-2486.2007.01527.x) [Google Scholar]
- Melo A. S., Rangel T. F. L. V. B., Diniz-Filho J. A. F.2009Environmental drivers of beta-diversity patterns in New-World birds and mammals. Ecography 32, 226–236 (doi:10.1111/j.1600-0587.2008.05502.x) [Google Scholar]
- Millennium Ecosystem Assessment 2005Ecosystems and human well-being: scenarios Washington, DC: World Resources Institute [Google Scholar]
- Olson D. M., et al. 2001Terrestrial ecoregions of the worlds: a new map of life on Earth. Bioscience 51, 933–938 (doi:10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2) [Google Scholar]
- Orme C. D. L., et al. 2005Global hotspots of species richness are not congruent with endemism or threat. Nature 436, 1016–1019 (doi:10.1038/nature03850) [DOI] [PubMed] [Google Scholar]
- Orme C. D. L., et al. 2006Global patterns of geographic range size in birds. PloS Biol. 4, 1276–1283 (doi:10.1371/journal.pbio.0040208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J. C., Bates D. M.2000Mixed-effects models in S and S-Plus New York, NY: Springer [Google Scholar]
- Rahbek C., Graves G. R.2001Multiscale assessment of patterns of avian species richness. Proc. Natl Acad. Sci. USA 98, 4534–4539 (doi:10.1073/pnas.071034898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbek C., Gotelli N. J., Colwell R. K., Entsminger G. L., Rangel T. F. L. V. B., Graves G. R.2007Predicting continental-scale patterns of bird species richness with spatially explicit models. Proc. R. Soc. B-Biol. Sci. 274, 165–174 (doi:10.1098/rspb.2006.3700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel T. F. L. V. B., Diniz-Filho J. A. F., Bini L. M.2006Towards an integrated computational tool for spatial analysis in macroecology and biogeography. Global Ecol. Biogeogr. 15, 321–327 (doi:10.1111/j.1466-822X.2006.00237.x) [Google Scholar]
- Rangel T. F. L. V. B., Diniz-Filho J. A. F., Colwell R. K.2007Species richness and evolutionary niche dynamics: a spatial pattern-oriented simulation experiment. Am. Nat. 170, 602–616 (doi:10.1086/521315) [DOI] [PubMed] [Google Scholar]
- Romdal T. S., Rahbek C.2009Elevational zonation of afrotropical forest bird communities along a homogeneous forest gradient. J. Biogeogr. 36, 327–336 (doi:10.1111/j.1365-2699.2008.01996.x) [Google Scholar]
- Rosenzweig M. L.1995. In Species diversity in space and time Cambridge, UK: Cambridge University Press [Google Scholar]
- Şekercioğlu C. H., Schneider S. H., Fay J. P., Loarie S. R.2008Climate change, elevational range shifts, and bird extinctions. Conserv. Biol. 22, 140–150 (doi:10.1111/j.1523-1739.2007.00852.x) [DOI] [PubMed] [Google Scholar]
- Svenning J. -C., Condit R.2008Biodiversity in a warmer world. Science 322, 206–207 (doi:10.1126/science.1164542) [DOI] [PubMed] [Google Scholar]
- Terborgh J.1977Bird species-diversity on an Andean elevational gradient. Ecology 58, 1007–1019 (doi:10.2307/1936921) [Google Scholar]
- Terborgh J.1985The role of ecotones in the distribution of Andean birds. Ecology 66, 1237–1246 (doi:10.2307/1939177) [Google Scholar]
- Thomas C. D., et al. 2004Extinction risk from climate change. Nature 427, 145–148 (doi:10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- van Rensburg B. J., Koleff P., Gaston K. J., Chown S. L.2004Spatial congruence of ecological transition at the regional scale in South Africa. J. Biogeogr. 31, 843–854 (doi:10.1046/j.1365-2699.2003.00996.x) [Google Scholar]
- Williams P. H., de Klerk H. M., Crowe T. M.1999Interpreting biogeographical boundaries among Afrotropical birds: spatial patterns in richness gradients and species replacement. J. Biogeogr. 26, 459–474 (doi:10.1046/j.1365-2699.1999.00294.x) [Google Scholar]
- Williams J. W., Jackson S. T., Kutzbacht J. E.2007Projected distributions of novel and disappearing climates by 2100 AD. Proc. Natl Acad. Sci. USA 104, 5738–5742 (doi:10.1073/pnas.0606292104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S.2006Generalized Additive Models: an introduction with R Boca Raton, FL: Chapman & Hall/CRC [Google Scholar]