Abstract
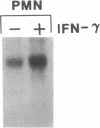
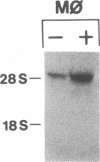
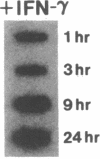
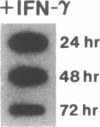
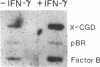
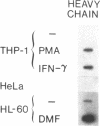
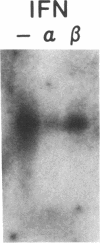
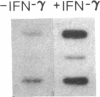
Phagocytic cells, such as macrophages and polymorphonuclear leukocytes, produce a "respiratory burst" in which oxygen is reduced to superoxide and other active oxygen species responsible for many of the microbicidal, tumoricidal, and inflammatory activities of these cells. Interferon gamma has been shown to augment phagocyte superoxide production, but the molecular mechanisms underlying this effect have remained unknown. Recently a key component of the oxidase, phagocyte cytochrome b, has been characterized as a heterodimer of a 91-kDa glycoprotein and a 22-kDa polypeptide. The present studies examined the effects of human recombinant interferon gamma on the expression of the genes for these components of the cytochrome b. In vitro treatment with interferon gamma substantially increases the level of phagocyte cytochrome b heavy chain gene transcripts in normal polymorphonuclear leukocytes, normal monocyte-derived macrophages, and the monocytic leukemia cell line THP-1. Light chain gene transcripts are less affected. In monocyte-derived macrophages and THP-1 cells, the enhanced expression of the heavy chain gene appears in large part attributable to increased rates of transcription. Treatment of monocyte-derived macrophages with human recombinant interferon alpha (a down-regulator of the respiratory burst) decreased the heavy chain transcript levels; interferon beta produced no detectable change. These findings demonstrate the responsiveness of one essential component of the phagocyte oxidase system to activation by interferon gamma and provide a rationale for its use to augment phagocytic function in chronic granulomatous disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984 Nov;64(5):959–966. [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Leukocyte oxidase: defective activity in chronic granulomatous disease. Science. 1967 Feb 17;155(3764):835–836. doi: 10.1126/science.155.3764.835. [DOI] [PubMed] [Google Scholar]
- Berton G., Zeni L., Cassatella M. A., Rossi F. Gamma interferon is able to enhance the oxidative metabolism of human neutrophils. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1276–1282. doi: 10.1016/s0006-291x(86)80421-1. [DOI] [PubMed] [Google Scholar]
- Cassatella M. A., Cappelli R., Della Bianca V., Grzeskowiak M., Dusi S., Berton G. Interferon-gamma activates human neutrophil oxygen metabolism and exocytosis. Immunology. 1988 Mar;63(3):499–506. [PMC free article] [PubMed] [Google Scholar]
- Cassatella M. A., Della Bianca V., Berton G., Rossi F. Activation by gamma interferon of human macrophage capability to produce toxic oxygen molecules is accompanied by decreased Km of the superoxide-generating NADPH oxidase. Biochem Biophys Res Commun. 1985 Nov 15;132(3):908–914. doi: 10.1016/0006-291x(85)91893-5. [DOI] [PubMed] [Google Scholar]
- Chen E., Karr R. W., Frost J. P., Gonwa T. A., Ginder G. D. Gamma interferon and 5-azacytidine cause transcriptional elevation of class I major histocompatibility complex gene expression in K562 leukemia cells in the absence of differentiation. Mol Cell Biol. 1986 May;6(5):1698–1705. doi: 10.1128/mcb.6.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H., Butcher G. A., Cowden W. B. Inhibition of murine malaria (Plasmodium chabaudi) in vivo by recombinant interferon-gamma or tumor necrosis factor, and its enhancement by butylated hydroxyanisole. J Immunol. 1987 Nov 15;139(10):3493–3496. [PubMed] [Google Scholar]
- Cross A. R., Higson F. K., Jones O. T., Harper A. M., Segal A. W. The enzymic reduction and kinetics of oxidation of cytochrome b-245 of neutrophils. Biochem J. 1982 May 15;204(2):479–485. doi: 10.1042/bj2040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinauer M. C., Orkin S. H., Brown R., Jesaitis A. J., Parkos C. A. The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. 1987 Jun 25-Jul 1Nature. 327(6124):717–720. doi: 10.1038/327717a0. [DOI] [PubMed] [Google Scholar]
- Eid N. S., Kravath R. E., Lanks K. W. Heat-shock protein synthesis by human polymorphonuclear cells. J Exp Med. 1987 May 1;165(5):1448–1452. doi: 10.1084/jem.165.5.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Hill M., Gordon S. Interferon alpha/beta selectively antagonises down-regulation of mannosyl-fucosyl receptors on activated macrophages by interferon gamma. Biochem Biophys Res Commun. 1986 Apr 29;136(2):737–744. doi: 10.1016/0006-291x(86)90501-2. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R. A., Orkin S. H., Newburger P. E. Recombinant interferon gamma augments phagocyte superoxide production and X-chronic granulomatous disease gene expression in X-linked variant chronic granulomatous disease. J Clin Invest. 1987 Oct;80(4):1009–1016. doi: 10.1172/JCI113153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Sim R. B., MacPherson G. G., Gordon S. Interaction of human monocytes, macrophages, and polymorphonuclear leukocytes with zymosan in vitro. Role of type 3 complement receptors and macrophage-derived complement. J Clin Invest. 1985 Dec;76(6):2368–2376. doi: 10.1172/JCI112249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984 Oct;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Ginsburg D., Handin R. I., Bonthron D. T., Donlon T. A., Bruns G. A., Latt S. A., Orkin S. H. Human von Willebrand factor (vWF): isolation of complementary DNA (cDNA) clones and chromosomal localization. Science. 1985 Jun 21;228(4706):1401–1406. doi: 10.1126/science.3874428. [DOI] [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett C., Guthrie L. A., Kopaniak M. M., Johnston R. B., Jr, Henson P. M. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985 Apr;119(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Israel A., Kimura A., Fournier A., Fellous M., Kourilsky P. Interferon response sequence potentiates activity of an enhancer in the promoter region of a mouse H-2 gene. Nature. 1986 Aug 21;322(6081):743–746. doi: 10.1038/322743a0. [DOI] [PubMed] [Google Scholar]
- Itami M., Kuroki T., Nose K. Induction of c-fos proto-oncogene by a chemotactic peptide in human peripheral granulocytes. FEBS Lett. 1987 Oct 5;222(2):289–292. doi: 10.1016/0014-5793(87)80388-5. [DOI] [PubMed] [Google Scholar]
- Linial M., Gunderson N., Groudine M. Enhanced transcription of c-myc in bursal lymphoma cells requires continuous protein synthesis. Science. 1985 Dec 6;230(4730):1126–1132. doi: 10.1126/science.2999973. [DOI] [PubMed] [Google Scholar]
- McPhail L. C., Clayton C. C., Snyderman R. The NADPH oxidase of human polymorphonuclear leukocytes. Evidence for regulation by multiple signals. J Biol Chem. 1984 May 10;259(9):5768–5775. [PubMed] [Google Scholar]
- Mehta K., Lopez-Berestein G. Expression of tissue transglutaminase in cultured monocytic leukemia (THP-1) cells during differentiation. Cancer Res. 1986 Mar;46(3):1388–1394. [PubMed] [Google Scholar]
- Mentzer S. J., Guyre P. M., Burakoff S. J., Faller D. V. Spontaneous aggregation as a mechanism for human monocyte purification. Cell Immunol. 1986 Sep;101(2):312–319. doi: 10.1016/0008-8749(86)90144-9. [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Markham A. F., Orkin S. H. Isolation and DNA sequence of a full-length cDNA clone for human X chromosome-encoded phosphoglycerate kinase. Proc Natl Acad Sci U S A. 1983 Jan;80(2):472–476. doi: 10.1073/pnas.80.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Scavuzzo D., Jacobs J. L., Kaplan M. H., Libby D. M., Schindler J., Roberts R. B. In vitro and in vivo activation of human mononuclear phagocytes by interferon-gamma. Studies with normal and AIDS monocytes. J Immunol. 1987 Apr 15;138(8):2457–2462. [PubMed] [Google Scholar]
- Nathan C. F., Horowitz C. R., de la Harpe J., Vadhan-Raj S., Sherwin S. A., Oettgen H. F., Krown S. E. Administration of recombinant interferon gamma to cancer patients enhances monocyte secretion of hydrogen peroxide. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8686–8690. doi: 10.1073/pnas.82.24.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Kaplan G., Levis W. R., Nusrat A., Witmer M. D., Sherwin S. A., Job C. K., Horowitz C. R., Steinman R. M., Cohn Z. A. Local and systemic effects of intradermal recombinant interferon-gamma in patients with lepromatous leprosy. N Engl J Med. 1986 Jul 3;315(1):6–15. doi: 10.1056/NEJM198607033150102. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Tsunawaki S. Secretion of toxic oxygen products by macrophages: regulatory cytokines and their effects on the oxidase. Ciba Found Symp. 1986;118:211–230. doi: 10.1002/9780470720998.ch14. [DOI] [PubMed] [Google Scholar]
- Newburger P. E., Speier C., Borregaard N., Walsh C. E., Whitin J. C., Simons E. R. Development of the superoxide-generating system during differentiation of the HL-60 human promyelocytic leukemia cell line. J Biol Chem. 1984 Mar 25;259(6):3771–3776. [PubMed] [Google Scholar]
- Parkos C. A., Allen R. A., Cochrane C. G., Jesaitis A. J. Purified cytochrome b from human granulocyte plasma membrane is comprised of two polypeptides with relative molecular weights of 91,000 and 22,000. J Clin Invest. 1987 Sep;80(3):732–742. doi: 10.1172/JCI113128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkos C. A., Dinauer M. C., Walker L. E., Allen R. A., Jesaitis A. J., Orkin S. H. Primary structure and unique expression of the 22-kilodalton light chain of human neutrophil cytochrome b. Proc Natl Acad Sci U S A. 1988 May;85(10):3319–3323. doi: 10.1073/pnas.85.10.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer-Pokora B., Kunkel L. M., Monaco A. P., Goff S. C., Newburger P. E., Baehner R. L., Cole F. S., Curnutte J. T., Orkin S. H. Cloning the gene for an inherited human disorder--chronic granulomatous disease--on the basis of its chromosomal location. Nature. 1986 Jul 3;322(6074):32–38. doi: 10.1038/322032a0. [DOI] [PubMed] [Google Scholar]
- Segal A. W. Absence of both cytochrome b-245 subunits from neutrophils in X-linked chronic granulomatous disease. Nature. 1987 Mar 5;326(6108):88–91. doi: 10.1038/326088a0. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Cross A. R., Garcia R. C., Borregaard N., Valerius N. H., Soothill J. F., Jones O. T. Absence of cytochrome b-245 in chronic granulomatous disease. A multicenter European evaluation of its incidence and relevance. N Engl J Med. 1983 Feb 3;308(5):245–251. doi: 10.1056/NEJM198302033080503. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Jones O. T., Webster D., Allison A. C. Absence of a newly described cytochrome b from neutrophils of patients with chronic granulomatous disease. Lancet. 1978 Aug 26;2(8087):446–449. doi: 10.1016/s0140-6736(78)91445-9. [DOI] [PubMed] [Google Scholar]
- Strunk R. C., Cole F. S., Perlmutter D. H., Colten H. R. gamma-Interferon increases expression of class III complement genes C2 and factor B in human monocytes and in murine fibroblasts transfected with human C2 and factor B genes. J Biol Chem. 1985 Dec 5;260(28):15280–15285. [PubMed] [Google Scholar]
- Szuro-Sudol A., Murray H. W., Nathan C. F. Suppression of macrophage antimicrobial activity by a tumor cell product. J Immunol. 1983 Jul;131(1):384–387. [PubMed] [Google Scholar]
- Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980 Aug;26(2):171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- Tsunawaki S., Nathan C. F. Enzymatic basis of macrophage activation. Kinetic analysis of superoxide production in lysates of resident and activated mouse peritoneal macrophages and granulocytes. J Biol Chem. 1984 Apr 10;259(7):4305–4312. [PubMed] [Google Scholar]
- Weil J., Epstein C. J., Epstein L. B., Sedmak J. J., Sabran J. L., Grossberg S. E. A unique set of polypeptides is induced by gamma interferon in addition to those induced in common with alpha and beta interferons. Nature. 1983 Feb 3;301(5899):437–439. doi: 10.1038/301437a0. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Markham A. F., Ricker A. T., Goldberger G., Colten H. R. Isolation of cDNA clones for the human complement protein factor B, a class III major histocompatibility complex gene product. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5661–5665. doi: 10.1073/pnas.79.18.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]