Abstract
All eukaryotes require mitochondria for survival and growth. The origin of mitochondria can be traced down to a single endosymbiotic event between two probably prokaryotic organisms. Subsequent evolution has left mitochondria a collection of heterogeneous organelle variants. Most of these variants have retained their own genome and translation system. In hydrogenosomes and mitosomes, however, the entire genome was lost. All types of mitochondria import most of their proteome from the cytosol, irrespective of whether they have a genome or not. Moreover, in most eukaryotes, a variable number of tRNAs that are required for mitochondrial translation are also imported. Thus, import of macromolecules, both proteins and tRNA, is essential for mitochondrial biogenesis. Here, we review what is known about the evolutionary history of the two processes using a recently revised eukaryotic phylogeny as a framework. We discuss how the processes of protein import and tRNA import relate to each other in an evolutionary context.
Keywords: mitochondria, protein import, tRNA import, mitosomes, hydrogenosomes
1. Introduction
Mitochondria or mitochondria-like organelles are part of the essential inventory of eukaryotic cells. Their origin can be traced down to an endosymbiotic event between what were probably two prokaryotic organisms: a host cell of uncertain lineage and an intracellular symbiont with the characteristic features of an α proteobacterium (Lang et al. 1999; Andersson et al. 2003; Dyall et al. 2004).
Comparative genomics suggest that mitochondria and the mitochondria-like organelles—hydrogenosomes and mitosomes—share a monophyletic evolutionary origin, with the original endosymbiotic event dated to at least 1.5 Gyr ago (vanderGiezen & Tovar 2005; vanderGiezen et al. 2005). It has long been known that most proteins of mitochondria are nucleus-encoded and imported from the cytosol (Dolezal et al. 2006; Neupert & Herrmann 2007; Bolender et al. 2008; Hildenbeutel et al. 2008; Mokranjac & Neupert 2009). Less well known is that most mitochondria also import tRNAs (Salinas et al. 2008). Import of these macromolecules, both proteins and tRNAs, therefore represents a key aspect of mitochondrial biogenesis. In this review, we will outline what is known about the two processes. Moreover, we discuss how the two processes relate to each other in an evolutionary context.
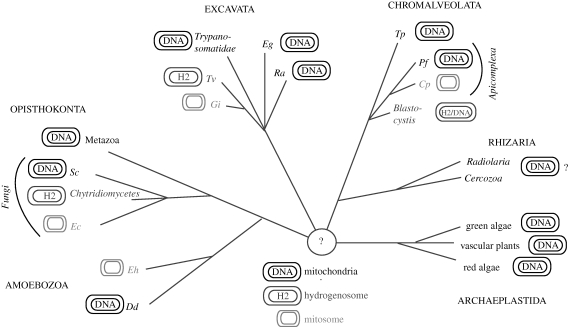
The shared features of mitochondria and mitochondria-like organelles are a double membrane and the fact that most if not all of their proteome is acquired by import of nucleus-encoded proteins from the cytosol. The protein translocation machineries in the outer and the inner membrane consist, with some interesting variations that will be discussed here, of a set of conserved core components. However, in considering organelle morphology and metabolic capabilities, there are breath-taking differences between mitochondria and mitochondria-like organelles from different organisms. This should perhaps not be surprising, given that these organelles and their corresponding host cells have coevolved through a very long time (maybe up to 1.5 Gyr). The net result is a collection of morphologically, genetically and functionally heterogeneous organelle variants that can be divided into three main groups: ‘classic’ mitochondria, hydrogenosomes and mitosomes (vanderGiezen & Tovar 2005; vanderGiezen et al. 2005) (figure 1).
Figure 1.
Occurrence of mitochondria, hydrogenosomes and mitosomes within the six eukaryotic supergroups. Branching order reflects the phylogenetic relationship of taxons but branch length is not to scale. All mitochondria-like organelles derive from the same endosymbiotic event. Hydrogenosomes and mitosomes evolved at least three and four times independently. The mitochondrial-like organelle in Blastocystis shows feature of mitochondria and hydrogenosomes and therefore blurs the distinction between these two organelles. Acronyms for species: Sc, Sacharomyces cerevisiae; Ec, Encephalitozoon cuniculi; Eh, Entamoeba histolytica; Dd, Dictyostelium discoideum; Tv, Trichomonas vaginalis; Gi, Giardia intestinalis; Eg, Euglena gracilis; Ra, Reclinomonas americana; Tp, Tetrahymena pyriformis; Pf, Plasmodium falciparum; Cp, Cryptosporidium parvum.
(a). Mitochondria, hydrogenosomes and mitosomes
Classic mitochondria are found in all aerobic eukaryotes. These organelles oxidize pyruvate and house the citric acid cycle. Their major metabolic activity is to produce ATP by oxidative phosphorylation. However, while mitochondria devote much of their proteome either directly or indirectly to oxidative phosphorylation, this is not the mitochondrial function that is essential for life under all conditions. There are micro-organisms such as Saccharomyces cerevisiae or the bloodstream form of Trypanosoma brucei that can live without oxidative phosphorylation—these organisms nevertheless require mitochondria. This is due to the fact that mitochondria are the only site for the biogenesis of FeS clusters, essential cofactors in mitochondrial and cytosolic proteins (Lill & Mühlenhoff 2008). Mitochondria also play key roles in haem biosynthesis, Ca2+ homeostasis and, in metazoans, in accentuating apoptosis. Perhaps the most striking feature of classic mitochondria is that they all house a vestige of the genome that belonged to the endosymbiont ancestor (Burger et al. 2003).
Hydrogenosomes too produce ATP. They ferment pyruvate to acetate, CO2 and molecular hydrogen (hence the name) and produce ATP by substrate level phosphorylation. Thus, hydrogenosomes can be considered as the anaerobic equivalent of mitochondria (Müller 1993; Hackstein et al. 1999). That said, it is important to emphasize that while hydrogenosomes decarboxylate pyruvate, they do this by pyruvate : ferredoxin oxidoreductase, an enzyme that is not related to the pyruvate dehydrogenase complex found in mitochondria. Moreover, hydrogenosomes do not have cytochromes and most importantly—with very notable exceptions (Boxma et al. 2005)—they do not contain a genome or a translation machinery. Hydrogenosomes are also the site of FeS-cluster biogenesis. Hydrogenosomes are found in a number of phylogenetically unrelated microbial eukaryotes that inhabit anaerobic environments such as rumen-dwelling ciliates and fungi, as well as free living and parasitic flagellates that do not have classical mitochondria (figure 1).
Mitosomes represent the third class of mitochondria-like organelles and, characteristically, mitosomes do not produce ATP and do not have an organellar genome. Mitosomes have been found in anaerobic or micro-aerophilic organisms that do not have classic mitochondria including the archamoeba Entamoeba histolytica (Mai et al. 1999), the diplomonad Giardia intestinalis (Tovar et al. 2003), the microsporidian Encephalitozoon cuniculi (Katinka et al. 2001) and the apicomplexan Cryptosporidium parva (Henriquez et al. 2005) (figure 1). Mitosomes are also expected to be present in many or most of the close relatives of these groups. The only function attributed to mitosomes so far is the synthesis of FeS clusters (Tovar et al. 2003; Goldberg et al. 2008), which therefore appears to be the only function that is shared between all three types of mitochondria-like organelles. The mitosomes of E. histolytica and Mastigamoeba balamuthi may represent an exception to this rule, as in these amoebae the FeS-cluster biogenesis pathway appears to localize outside of the mitosomes. The function of the E. histolytica mitosome therefore remains unknown (Gill et al. 2007; Aguilera et al. 2008).
The grouping of mitochondria-like organelles into classic mitochondria, hydrogenosomes and mitosomes is useful to appreciate the metabolic diversity of these organelles. Given the numerous times that these metabolic specializations have occurred, it is perhaps unsurprising that there are some mitochondria-like organelles that cannot easily be categorized. For example, the recent characterization of mitochondria-like organelles in the anaerobic ciliate Nyctotherus ovalis and the stramenopile Blastocystis revealed features of both classic mitochondria and hydrogenosomes (Boxma et al. 2005; Pérez-Brocal & Clark 2008; Stechmann et al. 2008; Wawrzyniak et al. 2008). In many respects, an acceptance that mitochondria are found in all eukaryotes, allowing for extreme variation in metabolic differences and specializations, would be a more reasonable and useful definition for this organelle than the tripartite categorization in common use.
(b). Eukaryotic phylogeny
In order to understand the evolutionary history of mitochondrial protein and tRNA import, it is important to understand the evolutionary relationships between the different organisms that are studied. This is not a trivial task; the more we know, the more we realize what we do not know about the evolutionary relationship of microbial eukaryotes. Previous models for the ‘tree of life’ that are still very popular have placed many micro-organisms that contain hydrogenosomes or mitosomes (e.g. Trichomonas, Giardia and Microsporidia) as well as the mitochondria-containing trypanosomatids at the base of the eukaryotic evolutionary tree (Sogin 1991). In this model, these select groups of organisms diverged very early from the rest of the eukaryotes which only much later radiated into the so-called crown group. The early diverging eukaryotes were considered to be primitive and hence expected to have retained ancestral traits. Accumulation of much more molecular data and their integration with morphological data led to a paradigm shift in the relationships (Simpson & Roger 2004; Adl et al. 2005): six major supergroups Amoebozoa, Opisthokonta, Excavata, Chromalveolata, Rhizaria and Archaeplastida are now recognized which arose more or less simultaneously from an already complex last universal common ancestor at an unknown time (figure 1). The root of this new phylogeny and how the six supergroups are related to each other is unknown. In this new phylogeny, protozoa such as trypanosomatids, Giardia and Trichomonas are not more early diverging, nor more primitive, than other organisms (Dacks et al. 2008).
It is fair to say that a debate continues on whether or not the new more ‘egalitarian’ view of eukaryotic phylogeny is more appropriate than the old ‘ladder-type model’. In this review, we decided to adopt the new concept of eukaryotic phylogeny as it offers a fresh view on the evolution of mitochondrial protein and tRNA import that has not been discussed before.
Mitochondria are found in species of all six supergroups. Hydrogenosomes are found in at least three of the supergroups and mitosomes are found in four of the supergroups (figure 1). This illustrates that while all mitochondria-like organelles derive from a single endosymbiotic event, what we describe as hydrogenosomes and mitosomes evolved independently more than once in different branches of the eukaryotic evolutionary tree.
There are two important consequences of this new phylogeny. First, it means that the absence of specific traits in any of the previously defined ‘early diverging’ eukaryotes is in most cases better explained by secondary loss of traits rather than reflecting the ancestral state (Dacks et al. 2008). Second, it is important to realize that yeast and metazoans, which include most of the popular model systems in biology such as Drosophila, yeasts, mouse, etc., belong to the same supergroup of the Opisthokonta. Thus, studies in these organisms only reflect a small part of eukaryotic diversity.
(c). Gradual loss of endosymbiont genes
There is little doubt that acquiring a bacterial endosymbiont and its subsequent transformation into a mitochondrion was a key event in the early evolutionary history of eukaryotes. How natural selection has favoured the maintenance of the symbiosis and the conversion of the endosymbiont to an organelle is hotly debated, and a number of scenarios that differ by the suggested metabolic nature of the endosymbiosis are being discussed (Embley & Martin 2006).
A major driving force of this transition was the gradual loss of genes from the genome of the endosymbiont (Adams & Palmer 2003). Interestingly, however, in the case of classic mitochondria, this loss never went to completion and all have retained a genome. The coding content of these genomes varies between five genes in apicomplexans (Feagin 2000) to 97 genes in the protozoan Reclinomonas americana (Lang et al. 1997). With few exceptions, proteins encoded on mitochondrial genomes are components of the respiratory chain or factors of the mitochondrial translation system that produces them. Having a mitochondrial genome is costly as it needs to be maintained, replicated and expressed. Mitochondrial translation alone requires well over 100 proteins (e.g. approx. 80 ribosomal proteins, 20 aminoacyl-tRNA synthetases and translation factors) as well as at least 22–24 different structural RNAs (rRNAs and tRNAs). Given the fact that only few proteins—in the case of some apicomplexans, only three—are actually produced inside the organelle, the continued existence of a mitochondrial genome appears as an incredible waste of resources (Feagin 2000). However, for reasons that we still do not fully understand, it seems that it is not possible to sustain oxidative phosphorylation without producing at least a few proteins inside the organelle. In order to explain this surprising fact, two main hypotheses, the ‘hydrophobicity’ theory (vonHeijne 1986) and the ‘co-location for redox regulation theory’ (Allen & Raven 1996), have been proposed.
Most of the genes that were lost from the endosymbiont genome were essential for the survival of the symbiont and their absence had to be compensated for. This was achieved by two strategies. The most important one was that many mitochondrial protein genes did not disappear for good but were functionally transferred to the nucleus. In order for the organelle to make use of the transferred genes, they needed to acquire regulatory sequences that allowed their transcription in the nucleus as well as their translation in the cytosol. Furthermore and most importantly, the resulting proteins needed to be imported into mitochondria.
A relatively small group of essential protein genes of the endosymbiont did indeed disappear, which was made possible because their role was taken over by pre-existing nuclear-encoded proteins with equivalent functions. In contrast to proteins encoded by genes transferred to the nucleus, most of these were only in part imported into mitochondria and their dual localization allowed them to simultaneously perform their newly acquired mitochondrial as well as their traditional cytosolic function. How many proteins are imported into mitochondria is revealed by comprehensive mitochondrial proteomic studies in various organisms which have identified up to 1000 or more different proteins (Sickmann et al. 2003; DaCruz et al. 2005; Millar et al. 2005; Panigrahi et al. 2009). Most of these are nuclear-encoded, synthesized in the cytosol and therefore need to be imported into mitochondria.
Not only protein but also tRNA genes were lost during mitochondrial evolution. This loss was very variable: some mitochondria have kept the total set of mitochondrial tRNA genes, many have lost a subset and in a few cases the process went to completion and the entire set was lost (Schneider & Marechal-Drouard 2000; Salinas et al. 2008).
2. Mitochondrial protein import
(a). Occurrence of protein import
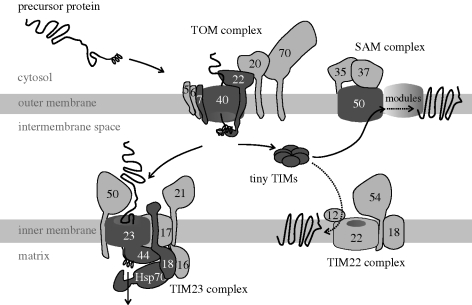
After 30 years of research into mitochondrial protein import, much of it focused on the model yeast S. cerevisiae, a clear picture is emerging of how mitochondrial proteins are recognized and imported by the organelle. As detailed in the following sections, a series of molecular machines serve as protein translocases to import nuclear-encoded mitochondrial proteins from their site of synthesis in the cytosol to the internal compartments of mitochondria. Translocation across the outer membrane is mediated by the TOM complex (translocase of the outer membrane of mitochondria), and translocation across the inner membrane is mediated by a TIM complex (translocase of the inner membrane of mitochondria). Another ubiquitous complex, the sorting and assembly machinery (SAM) complex, receives imported proteins destined for the outer membrane from the TOM complex, and catalyses integration and assembly of this select set of mitochondrial proteins (figure 2).
Figure 2.
The range in complexity of mitochondrial protein transport machines. The protein import machinery from yeast (Saccharomyces cerevisiae) is depicted. Protein substrates (black) translated on cytoplasmic ribosomes contain N-terminal, helical targeting segments that enable their binding to the TOM complex on the mitochondrial surface. There are seven components of the TOM complex, with the Tom40, Tom7 and Tom22 subunits (dark grey) being found generally in eukaryotes, while the other subunits (light grey) are found only in opisthokont. Similarly, the Sam50 subunit of the SAM complex has a common occurrence in eukaryotes, while the other subunits of this complex are more restricted. Four subunits of the TIM23 complex (dark grey) are common to the eukaryotic kingdoms, while the subunits shown in light grey are restricted in their occurrence and probably evolved later. The TIM22 complex might be considered a later addition to the protein import pathway (Schneider et al. 2008). Protein substrates pass from the TOM complex to either the SAM, TIM22 or TIM23 complexes for their transport to the outer membrane, inner membrane or matrix, respectively.
Analysis of genome sequence data shows that representative organisms, from across the major supergroups, all have a SAM complex. The core subunit of the SAM complex is a protein called Sam50 (Kozjak et al. 2003; Paschen et al. 2003; Gentle et al. 2004), which is related to the bacterial protein BamA (Omp85) and was inherited from the α-proteobacterial ancestor to mitochondria. The beta-barrel assembly machinery (BAM) complex, containing BamA, assembles bacterial proteins into the outer membrane (Misra 2007; Gatsos et al. 2008; Knowles et al. 2009), just as the SAM complex assembles mitochondrial proteins into the outer membrane. Whereas the evolutionary progenitor to the SAM complex is clear, there are no obvious bacterial protein translocases that could have given rise to the TOM and TIM complexes. Bacteria are not known to import proteins across their outer or inner membranes. There are hints that evolution may have cobbled together these complexes from various bacterial protein parts, using proteins with no previous function in protein transport. Taken together, the best model suggests that the TOM and TIM complexes were purpose-built to respond to new pressures arising from developments in the endosymbiotic relationship. Perhaps the strongest evidence to date that the hydrogenosomes, mitosomes and mitochondria have all arisen from a common organelle comes from the observations that they all share this common protein import machinery, composed of the TOM and TIM complexes, which evolved specifically to import nuclear-encoded proteins into mitochondria (Dolezal et al. 2006). While this evidence is largely derived from ‘in silico’ studies, components of the TIM complex are found in mitosomes and hydrogenosomes (Dolezal et al. 2005), and a recent study on the TOM complex in Giardia shows that it localized to mitosomes and related to the core TOM complex from the mitochondria of yeast (Dagley et al. 2009).
(b). Targeting of proteins to mitochondria
Work done in yeast and other organisms shows that targeting sequences can be found at the N-terminus, C-terminus or internally in proteins destined for mitochondria. Many membrane proteins, for example, do not have the otherwise typical N-terminal targeting sequence that has been well studied and found to occur on almost all soluble proteins and on many membrane proteins too. It remains, however, a useful generalization to posit that mitochondrial proteins are designated by an N-terminal, positively charged, amphipathic sequence that can form an α helix, thereby displaying surface features that are recognized by the TOM and TIM complexes. While the nature of mitochondrial targeting sequences is more complex, with as many exceptions as there are cases following the rule, a simple N-terminal, basic, amphipathic, helical sequence will direct even a non-mitochondrial protein passenger along the protein import pathway and into the mitochondrial matrix (Lemire et al. 1989). The hydrophobic surface of the amphipathic helix is recognized by Tom20 and a positively charged surface recognized by Tom22 (Brix et al. 1999; Moberg et al. 2004; Saitoh et al. 2007; Yamano et al. 2008). These same segments of the protein that adopt an α-helical conformation in contact with Tom20 (Saitoh et al. 2007) adopt an extended conformation when bound by the matrix-located processing peptidase (MPP).
How mitochondrial targeting sequences evolved has been a difficult question to address. Detailed analyses using comparative genomics made clear the later stages of mitochondrial evolution, e.g. how escape of genes (either the DNA or an RNA copy of the gene) from the endosymbiont could enable transfer to the nucleus and integration in the genome, how adaptive rearrangements could allow gene expression and how exon shuffling and other recombination events could create a mitochondrial targeting sequence (Brennicke et al. 1993; Kadowaki et al. 1996; Kurland & Andersson 2000; Gray et al. 2001). A major impetus for the development of the TOM complex, however, was likely to have relied on pre-adaptive features, literally basic amphipathic N-terminal segments, in many bacterial proteins that would serve as mitochondrial targeting information (Lucattini et al. 2004).
The structural aspects in mitochondrial targeting sequences are conserved across all six supergroups of eukaryotes: mitochondrial proteins from animals or fungi are targeted to mitochondria in organisms from other kingdoms, and vice versa (Bowler et al. 1989; Häusler et al. 1997; Alvarez-Fortes et al. 1998; Bhaduri-McIntosh & Vaidya 1998; Murcha et al. 2003; Dolezal et al. 2005; Burri et al. 2006; Uboldi et al. 2006). In all cases, the targeting sequences studied feature positively charged residues in a context that could adopt an N-terminal, amphipathic helix. In many cases, the targeting sequence also includes features that might assist its folding to a helical structure, and extensions that would allow for processing by the highly conserved inner membrane protease (IMP) or mitochondrial processing protease (MPP) peptidases in the intermembrane space and matrix, respectively. These proteases evolved from bacterial processing peptidases: (i) IMP is related to the signal sequence processing leader peptidase and (ii) MPP is related to single subunit peptidases from α proteobacteria (Gakh et al. 2002; Burri et al. 2005; Brown et al. 2007; Smíd et al. 2008).
The same targeting sequences can serve to target proteins between all three classes of mitochondria-like organelles: hydrogenosomal sequences target proteins into mitosomes or mitochondria, and mitosomal sequences target proteins to mitochondria and hydrogenosomes (Häusler et al. 1997; Dolezal et al. 2005; Burri et al. 2006; Uboldi et al. 2006). In all three cases, the core targeting sequence can be a short segment corresponding to just two to three turns of an α helix. Work done in Leishmania major showed that a simple sequence motif of only eight residues, which described a basic amphipathic sequence, was sufficient to predict up to 100 of the proteins targeted to mitochondria from genome sequence alone (Uboldi et al. 2006). A tailored bioinformatic approach based on the recognition site for MPP processing (RXF/[ILFSAGQ] or R[FNESG]/[ILFSAGQ]) detected 147 proteins from the proteome of T. vaginalis that might be targeted into hydrogenosomes, with the majority of these putative targeting sequences being 10 residues or less (Smíd et al. 2008). Using random DNA sequences, Lemire et al. (1989) showed that a large collection of synthetic sequences of 10–14 residues is necessary and sufficient to target passenger proteins to mitochondria. The summary picture then is that while the extension to be proteolytically cleaved from a mitochondrial protein can in some cases be very long, the information needed for mitochondrial targeting is housed within a portion of the sequence, in just two to three turns of an α-helical segment.
(c). Membrane translocation of proteins
Mitochondrial targeting sequences are recognized sequentially by a series of protein translocases (Neupert & Brunner 2002; Rehling et al. 2003; Koehler 2004; Dolezal et al. 2006; Bolender et al. 2008) (figure 2). Translocation across the outer membrane is mediated by the TOM complex, a molecular machine composed of several integral membrane protein components. The receptors Tom70 and Tom20 (Endo & Kohda 2002; Perry & Lithgow 2005) recognize mitochondrial proteins and then release them into a translocation channel provided by the beta-barrel protein Tom40. In yeast, there are four additional subunits docked onto Tom40 to form the ‘core’ TOM complex: Tom5, Tom6, Tom7 and Tom22 (Meisinger et al. 2001). Structural studies using NMR and electron microscopy, as well as electrophysiology and a range of biochemical assays, have been used to investigate and define the steps of protein import at the level of the outer membrane (Verschoor & Lithgow 1999; Herrmann & Neupert 2000; Endo & Kohda 2002; Neupert & Brunner 2002; Koehler 2004; Rehling et al. 2004; Perry & Lithgow 2005; Bolender et al. 2008).
Studies done in yeast have shown that after passing through the TOM complex, imported proteins can interact with one of the two distinct machines in the inner membrane (figure 2). One of these, the TIM23 complex, is built around a channel formed from Tim23, with this channel allowing for substrate entry to the mitochondrial matrix. Translocation through the TIM23 complex is driven by a motor complex built around a mitochondrial Hsp70. The molecular chaperone Hsp70 is anchored to the membrane by J proteins, Tim16/Pam16 and Tim14/Pam18 (Koehler 2004; Rehling et al. 2004) and the peripheral inner membrane protein Tim44: this tethering of Hsp70 to the translocation channel harnesses its activity to drive substrate proteins into the matrix.
The second TIM complex is built around the Tim22 subunit, and this TIM22 complex functions in the insertion of multi-topic inner membrane proteins. Interestingly, Tim22 and Tim23 appear to have come about through gene duplication events in many organisms: but some organisms, including ‘excavates’ such as T. brucei (Schneider et al. 2008) and opisthokonts like E. cuniculi (Waller et al. 2009), have only a single ‘TimX’ protein and probably therefore a single TIM complex to function both for translocation through the inner membrane and for assembly into the inner membrane. Even in yeast, the TIM23 complex is known to sort some proteins into the inner membrane by a ‘stop-transfer’ mechanism (Neupert & Brunner 2002; Koehler 2004; Kutik et al. 2007), demonstrating the versatility of this machine.
A small TIM complex operates in the mitochondrial intermembrane space to deliver imported membrane proteins from the TOM complex to the SAM complex (in the case of proteins destined for the outer membrane) or the TIM22 complex (in the case of proteins destined for the inner membrane) (Koehler 2004). The small TIM proteins function in partnerships, forming hetero-oligomeric hexamers that serve as chaperones for the membrane proteins en route through the intermembrane space. When catalogued across the major eukaryotic kingdoms, there are four small TIM families: Tim8, Tim9, Tim10 and Tim13 (Gentle et al. 2007). In yeast and in humans, the Tim8 and Tim13 subunits form a hetero-hexameric complex (i.e. Tim83 : Tim133) and the Tim9 and Tim10 subunits form a distinct hetero-hexameric complex (i.e. Tim93 : Tim103) (Webb et al. 2006; Baker et al. 2007). A fifth small TIM, Tim12, was derived recently through a gene duplication (from Tim10 in yeast, and from Tim9 in animals), and an equivalent scenario of ‘four-plus-one’ small TIMs is found in plants and in apicomplexans suggesting that the four TIM families arose early in the evolution of eukaryotes, with the fifth small TIM having been derived independently in many lineages. However, more simple sets of small TIMs can be seen with the mitosomes of the apicomplexan Cryptosporidium having a single small TIM protein (Gentle et al. 2007). Whether this orphan protein forms a homo-hexamer or functions as a smaller chaperone is not clear.
A set of highly conserved subunits in the SAM, TOM and TIM complexes are found in enough representative supergroups that they can be considered ubiquitous in eukaryotes (figure 3). It has been proposed, therefore, that one of the early events in the evolution of the first eukaryotic cells was to establish a protein import pathway, using relatively simple protein translocases (Lucattini et al. 2004; Dolezal et al. 2006). Through the course of evolution, lineage-specific components have been added on to these primary machines, with the best case in point being the Tom20 subunit of the TOM complex. Tom20 is shaded grey in figure 2, because hidden Markov models based on the biochemically defined receptor characterized in fungi (Söllner et al. 1989; Ramage et al. 1993) find homologues only in fungi and animals (Likić et al. 2005). The simplest interpretation is that this protein was developed as an import receptor in the early stage of divergence of the opisthokont lineage. This yeast/animal Tom20 is not an essential component of a viable TOM complex, with yeast mutants lacking Tom20 growing only slightly less well than wild-type yeast (Lithgow et al. 1994). In addition, it is now clear that microsporidians have lost the gene encoding Tom20 secondarily (Burri et al. 2006; Waller et al. 2009). Evidence that other lineages of eukaryotes independently acquired a Tom20-like receptor to improve protein import efficiency comes from analysis of the 20 kDa subunit of the TOM complex in plants (Werhahn et al. 2001): this protein functions as an import receptor, but structural analysis shows that the protein is coded in reverse with respect to the yeast Tom20, clearly demonstrating that this distinct import receptor arose by convergent evolution (Perry et al. 2006).
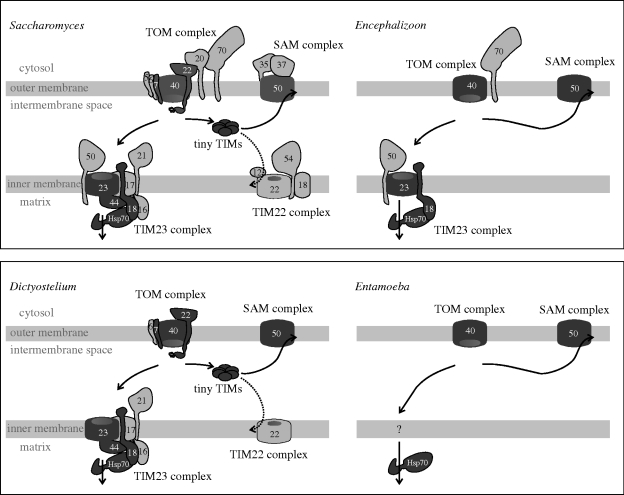
Figure 3.
Protein transport machines in ‘classic’ mitochondria and mitosomes: a tale from two kingdoms. The opisthokont kingdom includes fungi and microsporidia. Protein sequence similarity between species of fungi and microsporidia is high, enabling confident predictions of mitochondrial protein import components in microsporidia (Burri et al. 2006; Waller et al. 2009), and leading to the suggestion that microsporidia have managed a secondary loss of numerous modules from the protein import machinery. At least in the context of the small proteome likely for the mitosomes, small TIM chaperones and the TIM22 complex can be dispensed with and the TOM, SAM and TIM23 complexes can be highly simplified. In the case of Amoebozoa, we might anticipate that further components of the protein import machinery, specific to this lineage, might be found. In Dictyostelium discoideum, the common ‘core’ components in the TOM, TIM23 and TIM22 complexes and the small TIM chaperones are recognizable (Dolezal et al. 2006). However, much of this machinery appears to have been dispensed with secondarily in the amoeba E. histolytica.
More extreme cases of gene loss are starting to become apparent in select, unrelated, parasites. Figure 3 shows what on the surface appears to be a radical gene loss for components of the import apparati found in the microsporidian Encephalizoon cuniculi and the amoeba E. histolytica. As with any sequence-based analysis, one must be aware of the potential for confounding negative results that might simply be explained by extreme sequence divergence. But the analyses that figure 3 summarizes made use of sensitive hidden Markov models, and very close sequence relationships exist for the other components of the protein import machinery in these organisms. Furthermore, microsporidians are opisthokonts, and the pairwise sequence similarities are very high between the components of the import machinery from the yeast Saccharomyces and the microsporidian—yet none of the other components of the machinery were identified even with the most sensitive hidden Markov model searches (Waller et al. 2009). Dictyostelium discoideum is a well-studied model organism and the genome of this amoeba encodes all the expected core subunits of the TOM, SAM and TIM complexes (Barth et al. 2007). This would argue that sequence divergence between the amoebozoa and opisthokonts is not a barrier to detection of components of the mitochondrial import pathway. However, while the related amoeba E. histolytica has mitosomes with clear matrix-located chaperones and Tom40 and Sam50 proteins to serve in a TOM and SAM complex, further import machinery was not detectable (Tovar et al. 1999; Bakatselou et al. 2003; Tovar et al. 2007; Likić et al. in press).
3. Mitochondrial rna import
Just as the loss of protein-coding genes to the nucleus instigated a need for protein import, loss of tRNA-coding genes is coupled with tRNA import into mitochondria. Translation of the few proteins encoded in the mitochondrial genome requires, depending on the genetic code and the wobble rules, at least 20–22 different tRNAs. Experimental analysis in a number of different species confirmed that this has been achieved with an import pathway for tRNAs (Schneider & Marechal-Drouard 2000; Entelis et al. 2001b; Tarassov et al. 2007; Salinas et al. 2008). While it would be possible in principle, there is no evidence in any system for a functional transfer of mitochondrial tRNA genes to the nucleus; instead, imported tRNAs derive from cytosolic tRNAs that are also essential for cytosolic translation. The import of cytosolic tRNAs therefore must have preceded the loss of mitochondrial tRNA genes.
(a). Occurrence of mitochondrial tRNA import
Complete mitochondrial genome sequences are available for more than a 1000 different species of eukaryotes (O'Brien et al. 2009). Bioinformatic analysis of these sequences can predict the number of mitochondrial tRNA genes and match these to the codons that are used by the corresponding mitochondrial translation systems. The conclusion of such an analysis is that most eukaryotes lack some of the essential mitochondrial tRNA genes in their mitochondrial genomes (figure 4). Experimental analysis in a number of these systems has shown that this lack is compensated for by import of the corresponding cytosolic tRNAs. Thus, imported tRNAs always represent a small fraction of the cytosolic tRNA pool. Exclusive mitochondrial localization of a nucleus-encoded tRNA has so far not been found, though a tantalizing possibility exists in the recently described Chlamydomonas reinhardtii tRNALysUUU (Vinogradova et al. 2009).
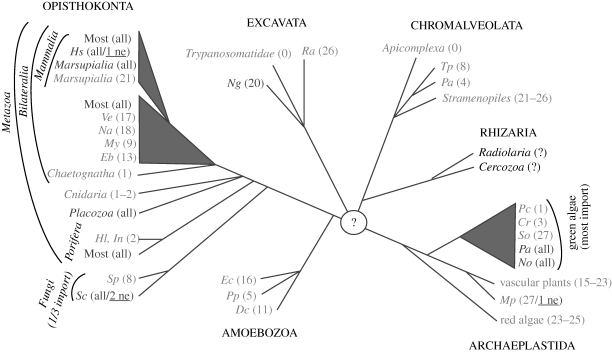
Figure 4.
Occurrence of mitochondrial tRNA import. Unrooted phylogenetic tree of the six eukaryotic supergroups (indicated in capitals). Branching order reflects the phylogenetic relationship of taxons but branch length is not to scale. Bioinformatic analysis of complete mitochondrial genome sequences allows to predict whether the encoded tRNAs are sufficient to read all codons that are used by the corresponding mitochondrial translation systems. Based on this analysis, eukaryotes were divided into two groups: the ones having a complete set of mitochondrial tRNA genes (shown in dark grey) and the ones that lack a variable number of apparently essential mitochondrial tRNA genes (shown in light grey). Taxons shown were chosen to represent organisms having the most complete as well as the most reduced mitochondrial tRNA gene contents, respectively. The numbers of tRNA genes encoded in the different mitochondrial genomes are indicated. ‘All’ indicates that the mitochondrial-encoded tRNA gene set is complete and ‘0’ indicates complete absence of mitochondrial tRNA genes. If organisms retain a single mitochondrial tRNA gene only it is always the tRNAMet. The minimal number of tRNAs required for mitochondrial translation is, depending on the wobble rules and the genetic code variations, between 20–22. Thus, all organisms having 20 or less mitochondrial tRNA genes must import at least some tRNAs from the cytosol. However, even in organisms having more than 22 mitochondrial tRNA genes, the set of mitochondrial-encoded tRNA is often not complete and import of cytosolic tRNAs is required. In most of these cases, it is the tRNAThr that is imported. In a few systems, import of a cytosolic tRNA that has the same decoding capacity as a still existing mitochondrial-encoded tRNA gene has been shown experimentally (shown underlined). Import of these tRNAs is expected to be redundant. Acronyms for species: Hs, Homo sapiens; Ve, Vanhornia eucnemidarum; Na, Neomaskellia andropogonis; My, Mizuhopecten yessoensis; Eb, Epiperipatus biolleyi; Hl, Hypospongia lachne; In, Igornella notabilis; Sp, Spizellomyces punctatus; Sc, Saccharomyces cerevisiae; Ng, Naegleria gruberi; Ra, Reclinomonas americana; Tp, Tetrahymena pyriformis; Pa, Paramecium aurelia; Pc, Polytomella capuana; Cr, Chlamydomonas reinhardtii; Sc, Scenedesmus obliquus; Pa, Pseudendoclonium akinetum; No, Nephroselmis olivacea; Mp, Marchantia polymorpha; Ec, Entamoeba castelanii; Pp, Physarum polycephalum; Dc, Dictyostelium citrinum.
The phylogenetic distribution of mitochondrial tRNA, as predicted by bioinformatics, shows that contrary to popular belief the process is very widespread. At least some tRNAs are imported in the vast majority of species in probably all six eukaryotic supergroups (mitochondrial genome sequences for Rhizaria are still missing) (figure 4). It is organisms with a complete set of mitochondrial tRNA genes that are exceptional rather than the ones lacking them! Essentially, all these exceptions are opisthokont species and yet even within the opisthokonts, we find taxons such as the Cnidaria and the Chaetognatha (arrow worms) that have lost all but one or two mitochondrial tRNA genes. In addition, within the Fungi and the Bilateralia, there are many examples of individual species that have lost at least some mitochondrial tRNA genes whereas their close relatives have kept the whole set (figure 4).
It is likely that the original endosymbiont had a complete set of tRNA genes, and loss of mitochondrial tRNA genes is expected to be irreversible. Thus, having a complete set of mitochondrial tRNAs genes represents the ancestral situation. It follows that the loss of mitochondrial tRNA genes and import of the corresponding cytosolic tRNAs are derived traits. Based on this, we conclude that mitochondrial tRNA import has a polyphyletic evolutionary origin: it was invented many times in different branches of the eukaryotic evolutionary tree.
(b). Limits of bioinformatic analysis
One of the caveats on bioinformatic analyses aimed at the occurrence of mitochondrial tRNA import is that modifications of the wobble nucleotide, which cannot be predicted, can change the decoding capacity of a tRNA, making it difficult to match it to a specific codon set. Some mitochondrial tRNAs have unconventional structures (Wolstenholme et al. 1987) or undergo RNA editing (Bullerwell & Gray 2005) and are therefore hard to recognize. Moreover, the existence of a mitochondrial tRNA gene does not preclude mitochondrial import of a cytosolic tRNA that is able to read the same codons. In fact, a scenario like this may even have been an obligatory evolutionary intermediate that subsequently allowed the loss of the corresponding mitochondrial tRNA genes.
The limitations to predict scenarios for tRNA import can best be illustrated in the yeast S. cerevisiae. The yeast mitochondrial genome encodes an apparently complete set of mitochondrial tRNAs. Nevertheless, yeast mitochondria import a small fraction of one of two cytosolic tRNALys isoacceptors (Tarassov et al. 1995b). Import of this tRNA is redundant under standard growth conditions but becomes essential when cells are grown at elevated temperature (Kamenski et al. 2007). Import of the tRNALys represents the best studied case of mitochondrial tRNA import in any species (discussed below). A recent study suggests that besides the tRNALys also a small fraction of the cytosolic tRNAGln is imported into yeast mitochondria (Rinehart et al. 2005). The function of the imported tRNAGln is presently unknown. However, surprisingly, it seems to be imported by a different pathway than the tRNALys.
Moreover, a complex situation is also found in humans. Just as in yeast, the human mitochondrial genome encodes a complete set of mitochondrial tRNAs. Recent evidence, however, suggests that a fraction of the cytosolic tRNAGln is imported into mitochondria (Rubio et al. 2008). In addition to tRNA import, import of the RNA subunits of RNase P (Puranam & Attardi 2001) and RNase MRP (Chang & Clayton 1989) as well as the 5S rRNA (Magalhaes et al. 1998) has been suggested. In the case of RNase P, these claims are highly controversial as mammalian mitochondrial RNase P has recently been shown to lack an RNA subunit (Holzmann et al. 2008). Import of 5S rRNA is also surprising as no 5S rRNA has been found in mitochondrial ribosomes of mammals (Sharma et al. 2003). However, import of 5S rRNA has been analysed in some detail using an in vitro system and it has been proposed that the RNA might be co-imported with an as-yet-unknown protein (Entelis et al. 2001a).
While it is clear that the described cases are of great interest and need to be further investigated, they are exceptions and, at least in the case of tRNAs, bioinformatics remains a valid tool to analyse the distribution of mitochondrial import on a global scale.
(c). Non-random loss of mitochondrial tRNA genes
The extent of tRNA gene loss from the mitochondrial genome is variable (figure 4). The most extreme situations are found in some poriferan species, Cnidaria, Chaetognatha and the green algae Pseudendoclonium akinetum, which have retained only one or two mitochondrial tRNA genes, and in the trypanosomatids and apicomplexans that lack mitochondrial tRNA genes altogether (O'Brien et al. 2009).
Even though the loss of specific mitochondrial tRNA genes does not show a defined phylogenetic pattern, it follows a distinct order when analysed across all taxa. This can best be explained by the fact that lost mitochondrial tRNA genes of bacterial genetic origin are compensated for by import of a fraction of cytosolic tRNAs that are of eukaryotic origin. The evolutionary origin of tRNAs can formally be demonstrated only for few species that show domain specific features. However, circumstantial evidence such as the fact that nucleus-encoded tRNAs are transcribed by RNA polymerase III suggests that all imported tRNAs are of eukaryotic descent.
Compensation can only be successful if the imported tRNA can be functionally integrated into the bacterial-type translation system of mitochondria. For the initiator tRNAMet, this is difficult. Translation initiation is very different in eukaryotes and in systems of bacterial origin, the latter requiring a specific initiator tRNAsMet that carries a formylated methionine (Mayer et al. 2001). This means that even if a cytosolic eukaryotic initiator tRNAMet would be imported into mitochondria, it would not be functional as it could not be formylated. This explains why the single tRNA gene that has been retained in mitochondria of many Cnidaria, Chaetognatha and P. akinetum is the tRNAMet.
Many mitochondria show variations from the classic genetic code, the most frequent one being a reassignment of the stop codon UGA to tryptophane (Knight et al. 2001). Decoding of the reassigned codon requires an anticodon change in the mitochondrial tRNATrp which for that reason cannot simply be replaced by its cytosolic counterpart that lacks this change. Thus, most mitochondrial genomes have kept the gene for the tRNATrp.
Can the concept that the overall frequency of mitochondrial tRNA gene loss is determined by the efficiency of functional integration of an imported tRNA into the mitochondrial translation system be extended to all tRNAs? It has been suggested that overall the loss of mitochondrial tRNA genes follows a specific order which could be explained by the differential capabilities of mitochondrial aminoacyl-tRNA synthetases to recognize imported eukaryotic-type tRNAs (Schneider 2001a). In such a scenario, the loss of a mitochondrial tRNA gene would essentially be driven by how good its imported counterpart can be aminoacylated in the mitochondrion. The model is supported by the observation that the frequency of the loss of a specific mitochondrial tRNA gene is positively correlated with the similarities of the corresponding bacterial-type and eukaryotic-type aminoacyl-tRNA synthetases as well as the similarities of their corresponding identity elements on the tRNA (Schneider 2001a).
Thus, the difficulty to functionally integrate specific imported tRNAs into the mitochondrial translation system represents a barrier for mitochondrial tRNA gene loss. Translation initiation and mitochondrial codon reassignments appear to be especially strong handicaps for imported tRNAs. Surprisingly, however, two taxonomic groups, trypanosomatids (Schneider 2001b) and apicomplexans (Feagin 2000; Crausaz-Esseiva et al. 2004b), have lost all mitochondrial tRNA genes, indicating that these barriers are not absolute (see below).
(d). Mitochondrial targeting of tRNAs
The mechanisms of mitochondrial tRNA import in the various systems have recently been expertly reviewed (Salinas et al. 2008). Here, we provide a condensed discussion of this subject with the emphasis on aspects we consider important in the evolutionary context. To discuss how tRNAs are imported into mitochondria, it is helpful to subdivide the process into three temporally and spatially ordered steps: (i) targeting of the tRNA to the mitochondrion, (ii) membrane translocation, and (iii) integration of the imported tRNA into the mitochondrial translation system.
The number of imported tRNAs ranges from one only to the whole set and is species-specific. Interestingly, in some taxons such as plants, the import specificity can differ even in closely related species. However, in all species, at least a few tRNAs still exist that are exclusively cytosolic indicating the need of the cell to select a subset of tRNAs for mitochondrial import. This selection depends on targeting signals on the tRNAs and is mediated by proteins. There are only three systems where the signals that are both necessary and sufficient for mitochondrial tRNA import have been characterized in detail.
(i) The first case concerns import of a fraction of one of two tRNALys isoacceptors into yeast mitochondria (Martin et al. 1979). Mitochondrial targeting of the tRNALys requires specific binding to Eno2p, an isoform of the glycolytic enzyme enolase (Entelis et al. 2006). Enolase delivers the tRNA to the surface of mitochondria. There, the tRNA is released and can now bind to the precursor of mitochondrial lysyl-tRNA synthetase to which it has a higher affinity than to enolase. The precursor of mitochondrial lysyl-tRNA synthetase is translated in the vicinity of mitochondria and acts as a carrier for import (see below). The specific binding of the two proteins to imported tRNALys is primarily determined by acceptor stem and anticodon nucleotides that differ between the two tRNALys isoacceptors (Entelis et al. 1998).
(ii) The second example is the trypanosomatid T. brucei in which all mitochondrial tRNAs are derived from cytosolic ones (Hancock & Hajduk 1990; Tan et al. 2002b). The initiator tRNAMet and the tRNASec, however, are not imported (Bouzaidi-Tiali et al. 2007). An in vivo analysis has shown that the single T-stem nucleotide pair at position 51 : 63 is both necessary and sufficient to determine the localization of trypanosomal tRNAs (Crausaz-Esseiva et al. 2004a). Thus, a U : A nucleotide pair at this position, found in the initiator tRNAMet, specifies a cytosolic localization, whereas any other base pair indicates a mitochondrial localization. Interestingly, the U51 : A63 nucleotide pair in the cytosolic initiator tRNAMet has previously been characterized as an anti-determinant for elongation factor 1a (eEF1a) binding (Drabkin et al. 1998). In line with this, it was shown that mitochondrial targeting of trypanosomal tRNAs requires interaction with eEF1a. This also explains the cytosolic localization of the tRNASec which lacks the U51 : A63 cytosolic localization signal but nevertheless does not bind to eEF1a as it has its own specialized elongation factor. Thus, in T. brucei, eEF1a besides its housekeeping function in translation elongation has a second function in selecting a subpopulation of cytosolic tRNAs for mitochondrial import. Moreover, in vivo analysis has shown that eEF1a-mediated targeting of tRNAs to the mitochondria is an obligatory step for membrane translocation of tRNA to occur.
(iii) The third example is Tetrahymena which contains three very similar tRNAGln isoacceptors. Two of them with the anticodons UUA and CUA are cytosol-specific and recognize the stop codon UCA which has been reassigned to glutamine in the nucleus of Tetrahymena. The third tRNAGln with the anticodon UUG recognizes the standard glutamine codons and is in part imported into mitochondria (Rusconi & Cech 1996a). In an in vivo analysis, it was shown that the anticodon UUG of the imported tRNAGln is both necessary and sufficient to induce import of any of the three tRNAGln molecules (Rusconi & Cech 1996b). However, no protein interacting with the import signal has been identified yet.
These three examples illustrate that the targeting signals on the tRNA and the targeting factors are not conserved between the different systems. This is no surprise because it reflects the very different specificities of mitochondrial tRNA import in the three systems. Moreover, finding different targeting signals and mechanisms is in line with the presumed polyphyletic origin of mitochondrial tRNA import. However, despite these differences, there are also some striking similarities between yeast and trypanosomatids. Targeting of the tRNAs to the mitochondria is in both cases essential for subsequent membrane translocation of the tRNA. Moreover, targeting is mediated by cytosolic housekeeping proteins that perform a second function. Further, research is needed to show whether this common principle of mitochondrial tRNA targeting can be extended to even more organisms.
There are a number of other in vivo studies that have attempted to identify the cis-elements on the tRNAs that induce their import into mitochondria (Dietrich et al. 1996; Lima & Simpson 1996; Delage et al. 2003). However, these studies are generally less complete and thus their interpretation is difficult. Moreover, targeting has also been analysed by in vitro import assays (Mahapatra et al. 1998; Rubio et al. 2000; Bhattacharyya et al. 2002). These assays were in most cases done in the absence of cytosol and therefore in this respect may not reflect the in vivo situation. Overall, the studies mentioned above identified all major tRNA domains as being important for mitochondrial tRNA targeting in one or the other system and therefore further illustrate the non-conserved nature of the tRNA targeting signals.
(e). Extent of mitochondrial tRNA localization
Studies in trypanosomatids (Tan et al. 2002b) and Chlamydomonas (Vinogradova et al. 2009), which import all or nearly all of their mitochondrial tRNAs, revealed large variations between the extent of mitochondrial localization of individual tRNAs. In both organisms, these variations were not correlated with the cytosolic concentration of the tRNAs. This raises two questions: why is the extent of mitochondrial localization of different tRNAs so variable and how is it regulated?
(i) The steady-state levels of Chlamydomonas cytosolic tRNAs correlate with the codon usage of nuclear genes as has been shown in other organisms as well. However, in Chlamydomonas, the same is true in mitochondria and the frequency of specific codons in mitochondrial genes appears to correlate with the levels of imported tRNAs that read them (Vinogradova et al. 2009). As the nuclear and the mitochondrial codon usage are different, this requires differential mitochondrial localization of tRNAs. Interestingly, in T. brucei, a similar study failed to reveal such a correlation (Tan et al. 2002b). However, as in this study only a subset of tRNAs was analysed, the question may need to be reinvestigated. Thus, at least in Chlamydomonas, the extent of mitochondrial localization may serve to adapt tRNA abundance to the mitochondrial codon usage.
(ii) What is responsible for the differential localization of imported tRNAs is not known in any system. Different mitochondrial steady-state levels of tRNAs could in principle be achieved by different import efficiencies or by regulating tRNA stability after import. A study in Leishmania suggests that it might be the former. Cytosolic leishmanial tRNAGlu and tRNAGln have a thiomodified uridine at the wobble position whereas their imported counterparts are lacking this modification (Kaneko et al. 2003). Thus, it was proposed that the thiomodified uridine acts as an antideterminant that prevents mitochondrial tRNA import. However, in vivo evidence for this attractive proposal is yet to emerge.
(f). Membrane translocation of tRNAs
tRNAs destined to be imported into mitochondria must be translocated across the outer and the inner mitochondrial membranes. From in vitro import experiments, we know that this process requires ATP and in most but not all cases the membrane potential. Moreover, protease treatment of mitochondria prevents tRNA import indicating that it is mediated by proteins. Information on the nature of these protein factors is available in three systems: S. cerevisiae, Leishmania and plants.
(i). Saccharomyces cerevisiae
After targeting to the mitochondrial surface, the tRNALys is released from enolase and binds to the precursor of mitochondrial lysyl-tRNA synthetase (pre-MSK) (Tarassov et al. 1995b). Subsequently, the folded tRNA is co-imported together with pre-MSK across the protein import channel (Entelis et al. 1998). This may seem surprising as it is difficult to see how the interaction between pre-MSK and the imported tRNALys can be maintained during mitochondrial protein import which requires unfolding of the transported protein. However, the evidence for the co-import model is very convincing. In vivo and in vitro import of the tRNALys strictly depends on the precursor of pre-MSK. The involvement of Tom20 and Tim44, two components of the protein import machinery, has directly been shown in vivo and in vitro (Tarassov et al. 1995a). Thus, in S. cerevisiae, pre-MSK has two functions, it aminoacylates the mitochondria-encoded tRNALys and it is responsible for import of the cytosolic tRNALys. Recent experiments have shown that the two functions can be separated and are associated with distinct regions of the pre-MSK molecule (Kamenski et al. 2007). The imported tRNALys cannot be aminoacylated inside mitochondria. Yet, as it can only bind to pre-MSK when aminoacylated, it is imported as a functional tRNA that can take part in protein synthesis, even though recycling is not possible. Interestingly, the other tRNA that is imported into mitochondria of S. cerevisiae, the tRNAGln, is not co-imported with protein but by an as-yet-unknown mechanism (Rinehart et al. 2005).
(ii). Leishmania
The tRNA import pathway in Leishmania tropica has been elucidated in great detail by the group of S. Adhya. It can only be summarized here, for more information refer to Bhattacharyya & Adhya (2004), Mirande (2007) and Adhya (2008). The inner membrane tRNA import machinery of L. tropica appears to consist of an unconventional protein complex of approximately 580 kDa, termed tRNA import complex (RIC), that was initially characterized by affinity chromatography using an RNA oligonucleotide consisting of an in vitro-defined tRNA-import signal (Goswami et al. 2006). Mass spectrometry analysis revealed that RIC consists of 11 major subunits, eight of which are nucleus-encoded and three that are mitochondria-encoded (Mukherjee et al. 2007). Six of the former are essential for tRNA import and four are identical to subunits of different respiratory complexes (iron sulphur protein and subunit 6b of complex III, cytochrome oxidase subunit 6 of complex IV and F1α subunit of complex V, the ATP synthase complex). Ablation of either of the six essential subunits by an unusual conditional antisense knockdown strategy reduced the level of mitochondrial tRNAs to zero within 24 h. Moreover, the functional RIC complex could be reconstituted into liposomes with recombinant subunits expressed in Escherichia coli. Omission of any of the six essential factors abolished ATP-dependent import of tRNAs into liposomes. How tRNAs are transported across the outer membrane has not been addressed.
Characterization of the tRNA import machinery of the Leishmania mitochondrial inner membrane is a truly amazing feat. However, a closer analysis of the published data raises a number of questions. Conditional ablation of specific leishmanial mRNAs has been tried by many groups using various strategies without success. Yet, in the L. tropica strain used by the Adhya group, the antisense strategy seems to work extremely efficiently. Moreover, reconstitution of the RIC complex—consisting of six different proteins—into liposomes was done starting from denatured proteins that were eluted from sodium dodecyl sulphate (SDS) gels and refolded (Mukherjee et al. 2007). While this might not be impossible, we are not aware of any precedent where such an approach has worked. Finally, L. tropica and T. brucei are closely related. We therefore would expect to find the same tRNA import machinery in both species. However, while the tRNA import machinery has not been identified in T. brucei, at least three of the trypanosomal RIC orthologues are not expressed in the bloodstream stage of the parasite (Panigrahi et al. 2009), even though it does import tRNAs. Thus, there is much left to be sorted out regarding the tRNA import machinery of trypanosomatids.
(iii). Plants
Recently, it was shown that antibodies against the voltage-dependent anion channel (VDAC), the metabolite transporter of the outer mitochondrial membrane, inhibited import of tRNAs into isolated plant mitochondria (Salinas et al. 2006). Consistent with these results, recombinantly expressed VDAC was able to bind tRNAs. In vitro import of tRNAs was also inhibited using antisera against Tom20 and Tom40, two conserved components of the mitochondrial outer membrane protein translocation machinery. However, the fact that in vitro import does not require a mitochondrial precursor protein together with tRNA import competition studies showed that, unlike in yeast, tRNAs and proteins are not co-imported. Based on these results, it was suggested that VDAC may be a major component of the tRNA import channel whereas Tom20 and Tom40 may function as import receptors (Salinas et al. 2006).
In summary, it appears clear that, in agreement with the postulated polyphyletic origin of mitochondrial tRNA import, the membrane translocation mechanism of tRNAs is not conserved between the different organisms. Moreover, there is no evidence for a dedicated tRNA import machinery in any system. Instead, its components, just as the ones required for tRNA targeting, appear to be housekeeping components performing a second function.
(g). Functional integration of imported tRNAs
Imported tRNAs are always of the eukaryotic type, whereas the mitochondrial translation system is of bacterial descent. Many tRNAs might in principle be functionally interchangeable between the cytosol and the mitochondria. However, for the initiator tRNAMet and for tRNAs that read codons that differ from the standard genetic code, this is not the case. Trypanosomatids and apicomplexans did not retain any mitochondrial tRNA genes. Their mitochondrial translation system therefore depends exclusively on imported eukaryotic-type tRNAs and as a consequence requires unique evolutionary adaptations. Some of these adaptations have been characterized in T. brucei and are discussed below.
Even though there is no bacterial-type initiator tRNAMet in trypanosome mitochondria, translation initiation requires a formylated tRNAMet. The tRNA that becomes formylated is a fraction of the imported eukaryotic-type elongator tRNAMet. Formylation is catalysed by an unusual tRNAMet-formyl-transferase that selectively formylates elongator-type tRNAMet and therefore has a substrate specificity diametrically opposed to conventional formyl-transferases (Tan et al. 2002a). Thus, in T. brucei, the elongator tRNAMet has three distinct functions that depend on its localization. In the cytosol, it functions as a conventional eukaryotic elongator tRNAMet, whereas after import in the bacterial-type translation system of mitochondria it is used as both initiator and elongator tRNAMet, depending on whether it is formylated or not (Martin 2002). The formylated methionine on the imported elongator tRNAMet, but no specific feature of the tRNA itself, is the main determinant that is recognized by an apparently conventional bacterial-type initiation factor 2 (Charrière et al. 2005). Thus, the unusual formyl-transferase seems to be the only required adaptation allowing the use of elongator tRNAMet in translation initiation.
In mitochondria of trypanosomatids, the stop codon UGA has been reassigned to tryptophane. The organellar tRNATrp therefore has to decode UGA in addition to the normal tryptophane codon UGG. It is not obvious how this can be achieved by an imported cytosolic tRNATrp that does not recognize the UGA stop codon.
Trypanosomatids solve this problem by a mitochondria-specific RNA editing event that converts the CCA anticodon of the imported tRNATrp to UCA (Alfonzo et al. 1999; Charrière et al. 2006). This allows the tRNA to decode both UGG and UGA codons. However, the CCA anticodon is an identity determinant for the eukaryotic tryptophanyl-tRNA synthetase. Thus, unlike most other imported tRNAs of trypanosomes, the edited tRNATrp in mitochondria cannot be charged by an aminoacyl-tRNA synthetase that is dually targeted to the cytosol and the mitochondrion. Instead, trypanosomatids evolved a highly diverged eukaryotic-type tryptophanyl-tRNA synthetase that is specific for mitochondria and that, unlike its cytosolic counterpart, can aminoacylate both edited and unedited tRNATrp (Charrière et al. 2006). For trypanosomatids loosing the mitochondrial tRNATrp gene was therefore very costly as it required the evolution of a specific enzyme that edits the tRNATrp as well as of a novel type of eukaryotic tryptophanyl-tRNA synthetase.
Many more adaptations of the mitochondrial translation system to imported tRNAs are likely to exist. Studies of how imported tRNAs are functionally integrated into the mitochondrial translation system have so far been restricted to trypanosomatids. It would be interesting to extend them to apicomplexans which are faced with the same problems. Apicomplexans belong to a different eukaryotic supergroup than trypansomatids and therefore may have found different solutions. We believe that investigating the consequences tRNA import imposes on mitochondrial translation is of great interest. Most trypanosomatids and apicomplexans are clinically important pathogens. Thus, the parasite-specific adapations of the mitochondrial translation system may offer novel drug targets. Moreover, exploring the limits of adaptation of a bacterial-type translation systems to eukaryotic components will help to reveal the fundamental requirements of translation.
4. Conclusions
Mitochondrial protein and tRNA import are key processes required for mitochondrial biogenesis. The problems faced in establishing each transport system are similar: most proteins and all tRNAs have to be translocated across the two mitochondrial membranes without disrupting the inner membrane potential. Import requires ATP, is protein-mediated and shows specificity: only a subset of the total cellular complement of proteins, or of tRNAs, are imported. There are of course some differences too: mitochondria in many, perhaps most, organisms import an estimated 500–1000 proteins with varying chemical properties. The number of imported tRNA molecules ranges from 1 to 30 depending on the species, and tRNAs have uniform structures and similar molecular weights (approx. 25 kDa). Also, the final localization of all imported tRNAs is the matrix, whereas proteins need to be sorted to the various mitochondrial subcompartments. It is clear that the evolution of mitochondrial import of macromolecules, both protein and tRNAs, was tightly linked to the gradual loss of genes from the genome of the endosymbiont (Adams & Palmer 2003). However, whereas the loss of most mitochondrial protein genes was shaped by their functional transfer to the nucleus, the disappearance of mitochondrial tRNA genes was largely governed by import of cytosolic tRNAs and their functional integration into the mitochondrial translation system. Moreover, whereas dual targeting to the cytosol and mitochondria is exceptional for proteins, it is the rule for imported tRNAs.
Mitochondrial import and sorting of proteins have been studied for many years. They require one or more of four distinct hetero-oligomeric membrane protein complexes, depending on the nature and the intramitochondrial destination of the transported substrates (Dolezal et al. 2006; Neupert & Herrmann 2007; Bolender et al. 2008; Hildenbeutel et al. 2008; Mokranjac & Neupert 2009). These protein complexes contain a set of core components that are conserved in mitochondria, hydrogenosomes and mitosomes of all species indicating the monophyletic origin of mitochondrial protein import. While our knowledge is at an early stage, it does now appear that a universal pathway handles proteins imported into mitochondria and mitochondria-like organelles, one which already existed in the last common ancestor of all eukaryotes (Dolezal et al. 2006).
Compared with mitochondrial protein import, we know very little about mitochondrial tRNA import. Its phylogenetic distribution suggests that, in contrast to mitochondrial protein import, tRNA import was probably not present in the last common ancestor of all eukaryotes. Instead, it evolved multiple times in most branches of at least four of the supergroups of eukaryotes. Consistent with this idea, the mechanisms for tRNA import vary in the different lineages of eukaryotes (figure 5). Yet, despite these variations, there is a common theme: tRNAs appear to be transported by hitch hiking. They make use of distinct housekeeping proteins in order to be targeted to and imported into mitochondria. The lack of a dedicated tRNA import machinery is in line with the recent evolutionary origins of the process. Moreover, the fact that there are several ways to import tRNAs into mitochondria is good news for people who would like to use mitochondrial tRNA import as a tool to treat diseases caused by mitochondrial tRNA mutations. And it may explain why attempts to transplant tRNA import systems from one organism to another to complement mutated mitochondrial tRNAs have been amazingly successful at least in cell cultures (Entelis et al. 2001b, 2002; Salinas et al. 2008).
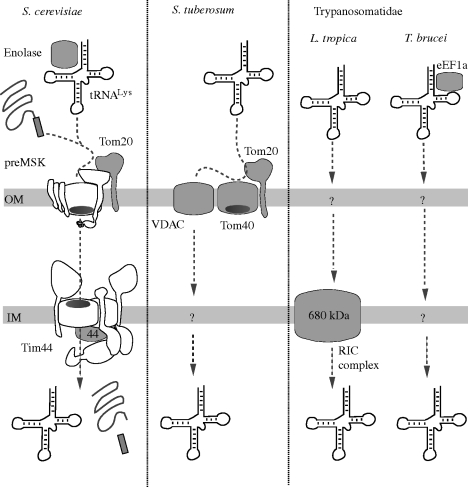
Figure 5.
Current state of knowledge of mitochondrial tRNA import machineries. Components required for targeting and/or membrane translocation of tRNAs have been identified in yeast (S. cerevisiae), potato (Solanum tuberosum) and the Trypanosomatidae (L. tropica and T. brucei). Outer (OM) and inner mitochondrial membranes (IM) are indicated. Factors whose involvement in tRNA import has directly been shown are shaded in grey. Saccharomyces cerevisiae: the tRNALys is targeted to mitochondria by enolase. Subsequently, the tRNA is co-imported with the precursor of mitochondrial lysyl-tRNA synthetase (pre-MSK). Pre-sequence of pre-MSK is shown as a box. Import of the tRNALys is coupled to import of pre-MSK indicating that the entire protein import machinery is required for tRNA import. Saccharomyces tuberosum: the three outer membrane proteins VDAC, Tom40 and Tom20 are required to translocate tRNAs across the outer membrane. Unlike in yeast, tRNAs are not co-imported with proteins. Tom20 and Tom40 appear to function as receptors, whereas the VDAC may build the actual import channel. Trypanosomatidae: mitochondrial tRNA import has been analysed in detail in L. tropica. Inner membrane translocation of tRNAs appears to require a large protein complex termed RIC complex, consisting of six essential proteins (FeS-protein and subunit 6b of complex III, cytochrome oxidase subunit 6 of complex IV, F1a subunit of complex V and two unknown trypanosomatid specific proteins) and five non-essential proteins. In T. brucei, it was shown that eukaryotic elongation factor 1a (eEF1a) is essential for in vivo targeting of tRNAs to the mitochondria.
It should be remembered that the information on the phylogenetic distribution of mitochondrial tRNA import is incomplete. Having a complete set of mitochondrial tRNA genes is essentially restricted to opisthokont species (figure 4), and yet even some of these organisms (including ourselves) are known to import at least some tRNAs. This redundant tRNA import cannot be predicted bioinformatically but only be discovered experimentally. Thus, we cannot yet exclude that the import of redundant tRNAs occurs in many or even all mitochondria that have a complete set of mitochondria-encoded tRNA genes.
Our knowledge of tRNA import machineries is fragmentary: the characterization of the tRNA import machinery in plants is in its initial stages and, while import of the yeast tRNALys is well characterized, it is not known how the yeast tRNAGln is imported. Moreover, in trypanosomatids, the tRNA translocation machinery of the outer membrane has not been identified. It is therefore possible that when a more complete inventory of the tRNA import machineries is available, some conserved core proteins are identified. It is both striking and exciting that in plants and yeast, two of the three systems that have been studied, the tRNA import machinery includes components of the mitochondrial protein import system. Moreover, in yeast tRNA and protein import have even converged to a common macromolecule import pathway. It is of great interest that though both yeast tRNALys and plant tRNAs require the TOM complex for import, the actual tRNA import mechanism is different and only the yeast tRNALys is co-imported with proteins. Future studies on tRNA import promise better understanding of the mechanisms and capabilities of the mitochondrial protein import machinery too.
The suggestion that tRNAs might be imported into mitochondria was initially greeted with great skepticism. Later, it was thought to be restricted to only a few taxa of eukaryotes. Today, we know that it is a quasi-universal process. Should it in future turn out that mitochondrial tRNA import is a universal process and should further experimental work reinforce the connection between mitochondrial tRNA and protein import in different systems, one would be forced to consider that both mitochondrial macromolecular import pathways may have a monophyletic and common evolutionary origin.
Acknowledgements
This work was supported by grants 31003A_121937 (to A.S.) from the Swiss National Foundation and by a grant from the Australian Research Council (to T.L.).
Footnotes
One contribution of 12 to a Theme Issue ‘Evolution of organellar metabolism in unicellular eukaryotes’.
References
- Adams K. L., Palmer J. D.2003Evolution of mitochondrial gene content: gene loss and transfer to the nucleus. Mol. Phylogenet. Evol. 29, 380–395 (doi:10.1016/S1055-7903(03)00194-5) [DOI] [PubMed] [Google Scholar]
- Adhya S.2008Leishmania mitochondrial tRNA importers. Int. J. Biochem. Cell. Biol. 12, 2681–2685 (doi:10.1016/j.biocel.2007.10.025) [DOI] [PubMed] [Google Scholar]
- Adl S. M., et al. 2005The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J. Eukaryot. Microbiol. 52, 399–451 (doi:10.1111/j.1550-7408.2005.00053.x) [DOI] [PubMed] [Google Scholar]
- Aguilera P., Barry T., Tovar J.2008Entamoeba histolytica mitosomes: organelles in search of a function. Exp. Parasitol. 118, 10–16 (doi:10.1016/j.exppara.2007.08.004) [DOI] [PubMed] [Google Scholar]
- Alfonzo J. D., Blanc V., Estevez A. M., Rubio M. A. T., Simpson L.1999C to U editing of anticodon of imported mitochondrial tRNATrp allows decoding of UGA stop codon in Leishmania. EMBO J. 18, 7056–7062 (doi:10.1093/emboj/18.24.7056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. F., Raven J. A.1996Free-radical-induced mutation vs redox regulation: costs and benefits of genes in organelles. J. Mol. Evol. 42, 482–492 (doi:10.1007/BF02352278) [DOI] [PubMed] [Google Scholar]
- Alvarez-Fortes E., Ruiz-Pérez L. M., Bouillaud F., Rial E., Rivas L.1998Expression and regulation of mitochondrial uncoupling protein 1 from brown adipose tissue in Leishmania major promastigotes. Mol. Biochem. Parasitol. 93, 191–202 (doi:10.1016/S0166-6851(98)00029-2) [DOI] [PubMed] [Google Scholar]
- Andersson S. G., Karlberg O., Canbäck B., Kurland C. G.2003On the origin of mitochondria: a genomics perspective. Phil. Trans. R. Soc. Lond. B 358, 165–177 (doi:10.1098/rstb.2002.1193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakatselou C., Beste D., Kadri A. O., Somanath S., Clark C. G.2003Analysis of genes of mitochondrial origin in the genus Entamoeba. J. Eukaryot. Microbiol. 50, 210–214 (doi:10.1111/j.1550-7408.2003.tb00119.x) [DOI] [PubMed] [Google Scholar]
- Baker M. J., Frazier A. E., Gulbis J. M., Ryan M. T.2007Mitochondrial protein-import machinery: correlating structure with function. Trends Cell Biol. 17, 456–464 (doi:10.1016/j.tcb.2007.07.010) [DOI] [PubMed] [Google Scholar]
- Barth C., Le P., Fisher P. R.2007Mitochondrial biology and disease in Dictyostelium. Int. Rev. Cytol. 263, 207–252 (doi:10.1016/S0074-7696(07)63005-8) [DOI] [PubMed] [Google Scholar]
- Bhaduri-McIntosh S., Vaidya A. B.1998Plasmodium falciparum: import of a phosphate carrier protein into heterologous mitochondria. Exp. Parasitol. 88, 252–254 (doi:10.1006/expr.1998.4242) [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S. N., Adhya S.2004The complexity of mitochondrial tRNA import. RNA Biol. 1, 84–88 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S. N., Chatterjee S., Adhya S.2002Mitochondrial RNA import in Leishmania tropica: aptamers homologous to multiple tRNA domains that interact cooperatively or antagonistically at the inner membrane. Mol. Cell. Biol. 22, 4372–4382 (doi:10.1128/MCB.22.12.4372-4382.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolender N., Sickmann A., Wagner R., Meisinger C., Pfanner N.2008Multiple pathways for sorting mitochondrial precursor proteins. EMBO Rep. 9, 42–49 (doi:10.1038/sj.embor.7401126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzaidi-Tiali N., Aeby E., Charrière F., Pusnik M., Schneider A.2007Elongation factor 1a mediates the specificity of mitochondrial tRNA import in T. brucei. EMBO J. 26, 4302–4312 (doi:10.1038/sj.emboj.7601857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C., Alliotte T., Vandenbulcke M., Bauw G., Vandekerckhove J., Vanmontagu M., Inzé D.1989A plant manganese superoxide dismutase is efficiently imported and correctly processed by yeast mitochondria. Proc. Natl Acad. Sci. USA 86, 3237–3241 (doi:10.1073/pnas.86.9.3237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxma B., et al. 2005An anaerobic mitochondrion that produces hydrogen. Nature 434, 74–79 (doi:10.1038/nature03343) [DOI] [PubMed] [Google Scholar]
- Brennicke A., Grohmann L., Hiesel R., Knoop V., Schuster W.1993The mitochondrial genome on its way to the nucleus: different stages of gene transfer in higher plants. FEBS Lett. 325, 140–145 (doi:10.1016/0014-5793(93)81430-8) [DOI] [PubMed] [Google Scholar]
- Brix J., Rudiger S., Bukau B., Schneider-Mergener J., Pfanner N.1999Distribution of binding sequences for the mitochondrial import receptors Tom20, Tom22, and Tom70 in a presequence-carrying preprotein and a non-cleavable preprotein. J. Biol. Chem. 274, 16 522–16 530 (doi:10.1074/jbc.274.23.16522) [DOI] [PubMed] [Google Scholar]
- Brown M. T., Goldstone H. M., Bastida-Corcuera F., Delgadillo-Correa M. G., Mcarthur A. G., Johnson P. J.2007A functionally divergent hydrogenosomal peptidase with protomitochondrial ancestry. Mol. Microbiol. 64, 1154–1163 (doi:10.1111/j.1365-2958.2007.05719.x) [DOI] [PubMed] [Google Scholar]
- Bullerwell C. E., Gray M. W.2005In vitro characterization of a tRNA editing activity in the mitochondria of Spizellomyces punctatus, a Chytridiomycete fungus. J. Biol. Chem. 280, 2463–2470 (doi:10.1074/jbc.M411273200) [DOI] [PubMed] [Google Scholar]
- Burger G., Gray M. W., Lang B. F.2003Mitochondrial genomes: anything goes. Trends Genet. 19, 709–716 (doi:10.1016/j.tig.2003.10.012) [DOI] [PubMed] [Google Scholar]
- Burri L., Strahm Y., Hawkins C. J., Gentle I. E., Puryer M. A., Verhagen A., Callus B., Vaux D., Lithgow T.2005Mature DIABLO/Smac is produced by the IMP protease complex on the mitochondrial inner membrane. Mol. Biol. Cell 16, 2926–2933 (doi:10.1091/mbc.E04-12-1086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri L., Williams B. A., Bursac D., Lithgow T., Keeling P. J.2006Microsporidian mitosomes retain elements of the general mitochondrial targeting system. Proc. Natl Acad. Sci. USA 103, 15 916–15 920 (doi:10.1073/pnas.0604109103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A.1989Mouse RNAase MRP RNA is encoded by a nuclear gene and contains a decamer sequence complementary to a conserved region of mitochondria RNA substrate. Cell 56, 131–139 (doi:10.1016/0092-8674(89)90991-4) [DOI] [PubMed] [Google Scholar]
- Charrière F., Tan T. H., Schneider A.2005Mitochondrial initiation factor 2 of Trypanosoma brucei binds imported formylated elongator-type methionyl-tRNA. J. Biol. Chem. 280, 15 659–15 665 (doi:10.1074/jbc.M411581200) [DOI] [PubMed] [Google Scholar]
- Charrière F., Helgadóttir S., Horn E. K., Söll D., Schneider A.2006Dual targeting of a single tRNATrp requires two different tryptophanyl-tRNA synthetases in Trypanosoma brucei. Proc. Natl Acad Sci. USA 103, 6847–6852 (doi:10.1073/pnas.0602362103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crausaz-Esseiva A., Marechal-Drouard L., Cosset A., Schneider A.2004aThe T-stem determines the cytosolic or mitochondrial localization of trypanosomal methionyl-tRNAs. Mol. Biol. Cell. 15, 2750–2757 (doi:10.1091/mbc.E03-11-0821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crausaz-Esseiva A., Naguleswaran A., Hemphill A., Schneider A.2004bMitochondrial tRNA import in Toxoplasma gondii. J. Biol. Chem. 279, 42 363–42 368 (doi:10.1074/jbc.M404519200) [DOI] [PubMed] [Google Scholar]
- Dacks J. B., Walker G., Field M. C.2008Implications of the new eukaryotic systematics for parasitologists. Parasitol. Int. 57, 97–104 (doi:10.1016/j.parint.2007.11.004) [DOI] [PubMed] [Google Scholar]
- Dacruz S., Parone P. A., Martinou J. C.2005Building the mitochondrial proteome. Expert Rev. Proteomics 2, 541–551 (doi:10.1586/14789450.2.4.541) [DOI] [PubMed] [Google Scholar]
- Dagley M. J., Dolezal P., Likić V. A., Smid O., Purcell A. W., Buchanan S. K., Tachezy J., Lithgow T.2009The protein import channel in the outer mitosomal membrane of Giardia intestinalis. Mol. Biol. Evol. 26, 1941–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delage L., Duchene A. M., Zaepfel M., Marechal-Drouard L.2003The anticodon and the D-domain sequences are essential determinants for plant cytosolic valyl-tRNA import into mitochondria. Plant J. 34, 623–633 (doi:10.1046/j.1365-313X.2003.01752.x) [DOI] [PubMed] [Google Scholar]
- Dietrich A., Marechal-Drouard L., Carneiro V., Cosset A., Small I.1996A single base change prevents import of cytosolic analyl-tRNA into mitochondria in transgenic plants. Plant J. 10, 913–918 (doi:10.1046/j.1365-313X.1996.10050913.x) [DOI] [PubMed] [Google Scholar]
- Dolezal P., Smíd O., Rada P., Zubácová Z., Bursać D., Suták R., Nebesárová J., Lithgow T., Tachezy J.2005Giardia mitosomes and trichomonad hydrogenosomes share a common mode of protein targeting. Proc. Natl Acad. Sci. USA 102, 10 924–10 929 (doi:10.1073/pnas.0500349102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezal P., Likić V., Tachezy J., Lithgow T.2006Evolution of the molecular machines for protein import into mitochondria. Science 313, 314–318 (doi:10.1126/science.1127895) [DOI] [PubMed] [Google Scholar]
- Drabkin H. J., Estrella M., Rajbhandary U. L.1998Initiator-elongator discrimination in vertebrate tRNAs for protein synthesis. Mol. Cell. Biol. 18, 1459–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall S. D., Brown M. T., Johnson P. J.2004Ancient invasions: from endosymbionts to organelles. Science 304, 253–257 (doi:10.1126/science.1094884) [DOI] [PubMed] [Google Scholar]
- Embley T. M., Martin W.2006Eukaryotic evolution, changes and challenges. Nature 440, 623–630 (doi:10.1038/nature04546) [DOI] [PubMed] [Google Scholar]
- Endo T., Kohda D.2002Functions of outer membrane receptors in mitochondrial protein import. Biochim. Biophys. Acta 1592, 3–14 (doi:10.1016/S0167-4889(02)00259-8) [DOI] [PubMed] [Google Scholar]
- Entelis N. S., Kiefer S., Kolesnikova O. A., Martin R. P., Tarassov I. A.1998Structural requirements of lysyl-tRNA for its import into yeast mitochondria. Proc. Natl Acad. Sci. USA 95, 2838–2843 (doi:10.1073/pnas.95.6.2838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entelis N. S., Kolesnikova O. A., Dogan S., Martin R. P., Tarassov I. A.2001a5 S rRNA and tRNA import into human mitochondria. J. Biol. Chem. 276, 45 642–45 653 (doi:10.1074/jbc.M103906200) [DOI] [PubMed] [Google Scholar]
- Entelis N. S., Kolesnikova O. A., Martin R. P., Tarassov I. A.2001bRNA delivery into mitochondria. Adv. Drug Deliv. Rev. 49, 199–215 (doi:10.1016/S0169-409X(01)00135-1) [DOI] [PubMed] [Google Scholar]
- Entelis N., Kolesnikova O., Kazakova H., Brandina I., Kamenski P., Martin R. P., Tarassov I.2002Import of nuclear encoded RNAs into yeast and human mitochondria: experimental approaches and possible biomedical applications. Genet. Eng. (N. Y.) 24, 191–213 [DOI] [PubMed] [Google Scholar]
- Entelis N., Brandina I., Kamenski P., Krasheninnikov I. A., Martin R. P., Tarassov I.2006A glycolytic enzyme, enolase, is recruited as a cofactor of tRNA targeting toward mitochondria in Saccharomyces cerevisiae. Genes Dev. 20, 1609–1620 (doi:10.1101/gad.385706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagin J. E.2000Mitochondrial genome diversity in parasites. Int. J. Parasitol. 30, 371–390 (doi:10.1016/S0020-7519(99)00190-3) [DOI] [PubMed] [Google Scholar]
- Gakh O., Cavadini P., Isaya G.2002Mitochondrial processing peptidases. Biochim. Biophys. Acta 1592, 63–77 (doi:10.1016/S0167-4889(02)00265-3) [DOI] [PubMed] [Google Scholar]
- Gatsos X., Perry A. J., Anwari K., Dolezal P., Wolynec P. P., Likić V. A., Purcell A. W., Buchanan S. K., Lithgow T.2008Protein secretion and outer membrane assembly in Alphaproteobacteria. FEMS Microbiol. Rev. 32, 995–1009 (doi:10.1111/j.1574-6976.2008.00130.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentle I., Gabriel K., Beech P., Waller R., Lithgow T.2004The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. J. Cell Biol. 164, 19–24 (doi:10.1083/jcb.200310092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentle I. E., et al. 2007Conserved motifs reveal details of ancestry and structure in the small TIM chaperones of the mitochondrial intermembrane space. Mol. Biol. Evol. 24, 1149–1160 (doi:10.1093/molbev/msm031) [DOI] [PubMed] [Google Scholar]
- Gill E. E., Diaz-Triviño S., Barberà M. J., Silberman J. D., Stechmann A., Gaston D., Tamas I., Roger A. J.2007Novel mitochondrion-related organelles in the anaerobic amoeba Mastigamoeba balamuthi. Mol. Microbiol. 66, 1306–1320 [DOI] [PubMed] [Google Scholar]
- Goldberg A. V., et al. 2008Localization and functionality of microsporidian iron-sulphur cluster assembly proteins. Nature 452, 624–628 (doi:10.1038/nature06606) [DOI] [PubMed] [Google Scholar]
- Goswami S., Dhar G., Mukherjee S., Mahata B., Chatterjee S., Home P., Adhya S.2006A bifunctional tRNA import receptor from Leishmania mitochondria. Proc. Natl Acad. Sci. USA 103, 8354–8359 (doi:10.1073/pnas.0510869103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. W., Burger G., Lang B. F.2001The origin and early evolution of mitochondria. Genome Biol. 2, 1018.1–1018.5 (doi:10.1186/gb-2001-2-6-reviews1018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstein J. H., Akhmanova A., Boxma B., Harhangi H. R., Voncken F. G.1999Hydrogenosomes: eukaryotic adaptations to anaerobic environments. Trends Microbiol. 7, 441–447 (doi:10.1016/S0966-842X(99)01613-3) [DOI] [PubMed] [Google Scholar]
- Hancock K., Hajduk S. L.1990The mitochondrial tRNAs of Trypanosoma brucei are nuclear encoded. J. Biol. Chem. 265, 19 208–19 215 [PubMed] [Google Scholar]
- Häusler T., Stierhof Y.-D., Blattner J., Clayton C.1997Conservation of mitochondrial targeting sequence function in mitochondrial and hydrogenosomal proteins from the early-branching eukaryotes Crithidia, Trypanosoma and Trichomonas. Eur. J. Cell Biol. 73, 240–251 [PubMed] [Google Scholar]
- Henriquez F. L., Richards T. A., Roberts F., Mcleod R., Roberts C. W.2005The unusual mitochondrial compartment of Cryptosporidium parvum. Trends Parasitol. 21, 68–74 (doi:10.1016/j.pt.2004.11.010) [DOI] [PubMed] [Google Scholar]
- Herrmann J. M., Neupert W.2000Protein transport into mitochondria. Curr. Opin. Microbiol. 3, 210–214 (doi:10.1016/S1369-5274(00)00077-1) [DOI] [PubMed] [Google Scholar]
- Hildenbeutel M., Habib S. J., Herrmann J. M., Rapaport D.2008New insights into the mechanism of precursor protein insertion into the mitochondrial membranes. Int. Rev. Cell. Mol. Biol. 268, 147–190 (doi:10.1016/S1937-6448(08)00805-8) [DOI] [PubMed] [Google Scholar]
- Holzmann J., Frank P., Löffler E., Bennett K. L., Gerner C., Rossmanith W.2008RNase P without RNA: identification and functional reconstitution of the human mitochondrial tRNA processing enzyme. Cell 135, 462–474 (doi:10.1016/j.cell.2008.09.013) [DOI] [PubMed] [Google Scholar]
- Kadowaki K., Kubo N., Ozawa K., Hirai A.1996Targeting presequence acquisition after mitochondrial gene transfer to the nucleus occurs by duplication of existing targeting signals. EMBO J. 15, 6652–6152 [PMC free article] [PubMed] [Google Scholar]
- Kamenski P., Kolesnikova O., Jubenot V., Entelis N., Krasheninnikov I. A., Martin R. P., Tarassov I.2007Evidence for an adaptation mechanism of mitochondrial translation via tRNA import from the cytosol. Mol. Cell 26, 625–637 (doi:10.1016/j.molcel.2007.04.019) [DOI] [PubMed] [Google Scholar]
- Kaneko T., Suzuki T., Kapushoc S. T., Rubio M. A., Ghazvini J., Watanabe K., Simpson L., Suzuki T.2003Wobble modification differences and subcellular localization of tRNAs in Leishmania tarentolae: implication for tRNA sorting mechanism. EMBO J. 22, 657–667 (doi:10.1093/emboj/cdg066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katinka M. D., et al. 2001Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature 414, 450–453 (doi:10.1038/35106579) [DOI] [PubMed] [Google Scholar]
- Knight R. D., Freeland S. J., Landweber L. F.2001Rewriting the keyboard: evolvability of the genetic code. Nature Rev. Genet. 2, 49–58 (doi:10.1038/35047500) [DOI] [PubMed] [Google Scholar]
- Knowles T. J., Scott-Tucker A., Overduin M., Henderson I. R.2009Membrane protein architects: the role of the BAM complex in outer membrane protein assembly. Nat. Rev. Microbiol. 7, 206–214 (doi:10.1038/nrmicro2069) [DOI] [PubMed] [Google Scholar]
- Koehler C. M.2004New developments in mitochondrial assembly. Annu. Rev. Cell Dev. Biol. 20, 309–335 (doi:10.1146/annurev.cellbio.20.010403.105057) [DOI] [PubMed] [Google Scholar]
- Kozjak V., Wiedemann N., Milenkovic D., Lohaus C., Meyer H. E., Guiard B., Meisinger C., Pfanner N.2003An essential role of Sam50 in the protein sorting and assembly machinery of the mitochondrial outer membrane. J. Biol. Chem. 278, 48 520–48 523 (doi:10.1074/jbc.C300442200) [DOI] [PubMed] [Google Scholar]
- Kurland C. G., Andersson S. G.2000Origin and evolution of the mitochondrial proteome. Microbiol. Mol. Biol. Rev. 64, 786–820 (doi:10.1128/MMBR.64.4.786-820.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutik S., Guiard B., Meyer H. E., Wiedemann N., Pfanner N.2007Cooperation of translocase complexes in mitochondrial protein import. J. Cell. Biol. 179, 585–591 (doi:10.1083/jcb.200708199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang B. F., Burger G., O'Kelly C. J., Cedergren R., Golding G. B., Lemieux C., Sankoff D., Turmel M., Gray M. W.1997An ancestral mitochondrial DNA resembling a eubacterial genome in miniature. Nature 387, 493–497 (doi:10.1038/387493a0) [DOI] [PubMed] [Google Scholar]
- Lang B. F., Gray M. W., Burger G.1999Mitochondrial genome evolution and the origin of eukaryotes. Annu. Rev. Genet. 33, 351–397 (doi:10.1146/annurev.genet.33.1.351) [DOI] [PubMed] [Google Scholar]
- Lemire B. D., Fankhauser C., Baker A., Schatz G.1989The mitochondrial targeting function of randomly generated peptide sequences correlates with predicted helical amphiphilicity. J. Biol. Chem. 264, 20 206–20 215 [PubMed] [Google Scholar]
- Likić V. A., Dolezal P., Celik N., Dagley M., Lithgow T.In press Using hidden Markov models to discover new protein transport machines. Meth. Mol. Biol. [DOI] [PubMed] [Google Scholar]
- Likić V. A., et al. 2005Patterns that define the four domains conserved in known and novel isoforms of the protein import receptor Tom20. J. Mol. Biol. 347, 81–93 (doi:10.1016/j.jmb.2004.12.057) [DOI] [PubMed] [Google Scholar]
- Lill R., Mühlenhoff U.2008Maturation of iron–sulfur proteins in eukaryotes: mechanisms, connected processes, and diseases. Annu. Rev. Biochem. 77, 669–700 (doi:10.1146/annurev.biochem.76.052705.162653) [DOI] [PubMed] [Google Scholar]
- Lima B. D., Simpson L.1996Sequence-dependent in vivo import of tRNAs into the mitochondrion of Leishmania tarentolae. RNA 2, 429–440 [PMC free article] [PubMed] [Google Scholar]
- Lithgow T., Junne T., Wachter C., Schatz G.1994Yeast mitochondria lacking the two import receptors Mas20p and Mas70p can efficiently and specifically import precursor proteins. J. Biol. Chem. 269, 15 325–15 330 [PubMed] [Google Scholar]
- Lucattini R., Likic V. A., Lithgow T.2004Bacterial proteins predisposed for targeting to mitochondria. Mol. Biol. Evol. 2004, 652–658 (doi:10.1093/molbev/msh058) [DOI] [PubMed] [Google Scholar]
- Magalhaes P. J., Andreu A. L., Schon E. A.1998Importation of 5S rRNA into human mitochondria in vivo. Mol. Biol. Cell 9, 2375–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahapatra S., Ghosh S., Bera S. K., Ghosh T., Das A., Adhya S.1998The D arm of tyrosine tRNA is necessary and sufficient for import into Leishmania mitochondria in vitro. Nucl. Acids Res. 26, 2037–2041 (doi:10.1093/nar/26.9.2037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai Z., Ghosh S., Frisardi M., Rosenthal B., Rogers R., Samuelson J.1999Hsp60 is targeted to a cryptic mitochondrion-derived organelle (‘crypton’) in the microaerophilic protozoan parasite Entamoeba histolytica. Mol. Cell. Biol. 19, 2198–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N. C.2002Location alters tRNA identity: Trypanosoma brucei's cytosolic elongator methionyl tRNA is both the initiator and elongator in mitochondria. Proc. Natl Acad. Sci. USA 99, 1110–1112 (doi:10.1073/pnas.042011199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. P., Schneller J. M., Stahl A. J. C., Dirheimer G.1979Import of nuclear desoxyribonucleic acid coded lysine-accepting transfer ribonucleic acid (anticodon C-U-U) into yeast mitochondria. Biochemistry 18, 4600–4605 (doi:10.1021/bi00588a021) [DOI] [PubMed] [Google Scholar]
- Mayer C., Stortchevoi A., Kohrer C., Varshney U., Rajbhandary U. L.2001Initiator tRNA and its role in initiation of protein synthesis. Cold Spring Harb. Symp. Quant. Biol. 66, 195–206 (doi:10.1101/sqb.2001.66.195) [DOI] [PubMed] [Google Scholar]
- Meisinger C., et al. 2001Protein import channel of the outer mitochondrial membrane: a highly stable Tom40-Tom22 core structure differentially interacts with preproteins, small tom proteins, and import receptors. Mol. Cell Biol. 21, 2337–2348 (doi:10.1128/MCB.21.7.2337-2348.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar A. H., Heazlewood J. L., Kristensen B. K., Braun H. P., Møller I. M.2005The plant mitochondrial proteome. Trends Plant Sci. 10, 36–43 (doi:10.1016/j.tplants.2004.12.002) [DOI] [PubMed] [Google Scholar]
- Mirande M.2007The ins and outs of tRNA transport. EMBO Rep. 8, 547–549 (doi:10.1038/sj.embor.7400989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra R.2007First glimpse of the crystal structure of YaeT's POTRA domains. ACS Chem. Biol. 2, 649–651 (doi:10.1021/cb700212p) [DOI] [PubMed] [Google Scholar]
- Moberg P., Nilsson S., Ståhl A., Eriksson A. C., Glaser E., Mäler L.2004NMR solution structure of the mitochondrial F1beta presequence from Nicotiana plumbaginifolia. J. Mol. Biol. 336, 1129–1140 (doi:10.1016/j.jmb.2004.01.006) [DOI] [PubMed] [Google Scholar]
- Mokranjac D., Neupert W.2009Thirty years of protein translocation into mitochondria: unexpectedly complex and still puzzling. Biochim. Biophys. Acta. 1793, 33–41 (doi:10.1016/j.bbamcr.2008.06.021) [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Basu S., Home P., Dhar G., Adhya S.2007Necessary and sufficient factors for the import of transfer RNA into the kinetoplast mitochondrion. EMBO Rep. 8, 589–595 (doi:10.1038/sj.embor.7400979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M.1993The hydrogenosome. J. Gen. Microbiol. 139, 2879–2889 [DOI] [PubMed] [Google Scholar]
- Murcha M. W., Lister R., Ho A. Y., Whelan J.2003Identification, expression, and import of components 17 and 23 of the inner mitochondrial membrane translocase from Arabidopsis. Plant Physiol. 131, 1737–1747 (doi:10.1104/pp.102.016808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert W., Brunner M.2002The protein import motor of mitochondria. Nat. Rev. Mol. Cell Biol. 3, 555–565 (doi:10.1038/nrm878) [DOI] [PubMed] [Google Scholar]
- Neupert W., Herrmann J. M.2007Translocation of proteins into mitochondria. Annu. Rev. Biochem. 76, 723–749 (doi:10.1146/annurev.biochem.76.052705.163409) [DOI] [PubMed] [Google Scholar]
- O'Brien E. A., Zhang Y., Wang E., Marie V., Badejoko W., Lang B. F., Burger G.2009GOBASE: an organelle genome database. Nucl. Acids Res. 37, D946–D950 (doi:10.1093/nar/gkn819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahi A. K., Ogata Y., Zíková A., Anupama A., Dalley R. A., Acestor N., Myler P. J., Stuart K. D.2009A comprehensive analysis of Trypanosoma brucei mitochondrial proteome. Proteomics 9, 434–450 (doi:10.1002/pmic.200800477) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen S. A., Waizenegger T., Stan T., Preuss M., Cyrklaff M., Hell K., Rapaport D., Neupert W.2003Evolutionary conservation of biogenesis of beta-barrel membrane proteins. Nature 426, 862–866 (doi:10.1038/nature02208) [DOI] [PubMed] [Google Scholar]
- Pérez-Brocal V., Clark C. G.2008Analysis of two genomes from the mitochondrion-like organelle of the intestinal parasite Blastocystis: complete sequences, gene content, and genome organization. Mol. Biol. Evol. 25, 2475–2482 (doi:10.1093/molbev/msn193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A. J., Lithgow T.2005Protein targeting: entropy, energetics and modular machines. Curr. Biol. 15, R423–R425 (doi:10.1016/j.cub.2005.05.031) [DOI] [PubMed] [Google Scholar]
- Perry A. J., Hulett J. M., Likić V. A., Lithgow T., Gooley P. R.2006Convergent evolution of receptors for protein import into mitochondria. Curr. Biol. 16, 221–229 (doi:10.1016/j.cub.2005.12.034) [DOI] [PubMed] [Google Scholar]
- Puranam R. S., Attardi G.2001The RNase P associated with HeLa cell mitochondria contains an essential RNA component identical in sequence to that of the nuclear RNase P. Mol. Cell. Biol. 21, 548–561 (doi:10.1128/MCB.21.2.548-561.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage L., Junne T., Hahne K., Lithgow T., Schatz G.1993Functional cooperation of mitochondrial protein import receptors. EMBO J. 12, 4115–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehling P., et al. 2003Protein insertion into the mitochondrial inner membrane by a twin-pore translocase. Science 299, 1747–1751 (doi:10.1126/science.1080945) [DOI] [PubMed] [Google Scholar]
- Rehling P., Brandner K., Pfanner N.2004Mitochondrial import and the twin-pore translocase. Nat. Rev. Mol. Cell Biol. 5, 519–530 (doi:10.1038/nrm1426) [DOI] [PubMed] [Google Scholar]
- Rinehart J., Krett B., Rubio M. A., Alfonzo J. D., Soll D.2005Saccharomyces cerevisiae imports the cytosolic pathway for Gln-tRNA synthesis into the mitochondrion. Genes Dev. 19, 583–592 (doi:10.1101/gad.1269305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio M. A., Liu X., Yuzawa H., Alfonzo J. D., Simpson L.2000Selective importation of RNA into isolated mitochondria from Leishmania tarentolae RNA. RNA 6, 988–1003 (doi:10.1017/S1355838200991519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio M. A., Rinehart J. J., Krett B., Duvezin-Caubet S., Reichert A. S., Söll D., Alfonzo J. D.2008Mammalian mitochondria have the innate ability to import tRNAs by a mechanism distinct from protein import. Proc. Natl Acad. Sci. USA 105, 9186–9191 (doi:10.1073/pnas.0804283105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusconi C. P., Cech T. R.1996aMitochondrial import of only one of three nuclear-encoded glutamine tRNAs in Tetrahymena thermophila. EMBO J. 15, 3286–3295 [PMC free article] [PubMed] [Google Scholar]
- Rusconi C. P., Cech T. R.1996bThe anticodon is the signal sequence for mitochondrial import of glutamine tRNA in Tetrahymena. Genes Dev. 10, 2870–2880 (doi:10.1101/gad.10.22.2870) [DOI] [PubMed] [Google Scholar]
- Saitoh T., Igura M., Obita T., Ose T., Kojima R., Maenaka K., Endo T., Kohda D.2007Tom20 recognizes mitochondrial presequences through dynamic equilibrium among multiple bound states. EMBO J. 26, 4777–4788 (doi:10.1038/sj.emboj.7601888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas T., Duchene A. M., Delage L., Nilsson S., Glaser E., Zaepfel M., Marechal-Drouard L.2006The voltage-dependent anion channel, a major component of the tRNA import machinery in plant mitochondria. Proc. Natl Acad. Sci. USA 103, 18 362–18 367 (doi:10.1073/pnas.0606449103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas T., Duchêne A. M., Maréchal-Drouard L.2008Recent advances in tRNA mitochondrial import. Trends Biochem. Sci. 33, 320–329 (doi:10.1016/j.tibs.2008.04.010) [DOI] [PubMed] [Google Scholar]
- Schneider A.2001aDoes the evolutionary history of aminoacyl-tRNA synthetases explain the loss of mitochondrial tRNA genes? Trends Genet. 17, 557–558 (doi:10.1016/S0168-9525(01)02439-8) [DOI] [PubMed] [Google Scholar]
- Schneider A.2001bUnique aspects of mitochondrial biogenesis in trypanosomatids. Int. J. Parasitol. 31, 1403–1415 (doi:10.1016/S0020-7519(01)00296-X) [DOI] [PubMed] [Google Scholar]
- Schneider A., Marechal-Drouard L.2000Mitochondrial tRNA import: are there distinct mechanisms? Trends Cell Biol. 10, 509–513 (doi:10.1016/S0962-8924(00)01854-7) [DOI] [PubMed] [Google Scholar]
- Schneider A., Bursać D., Lithgow T.2008The direct route: a simplified pathway for protein import into the mitochondrion of trypanosomes. Trends Cell Biol. 18, 12–18 (doi:10.1016/j.tcb.2007.09.009) [DOI] [PubMed] [Google Scholar]
- Sharma M. R., Koc E. C., Datta P. P., Booth T. M., Spremulli L. L., Agrawal R. K.2003Structure of the mammalian mitochondrial ribosome reveals an expanded functional role for its component proteins. Cell 115, 97–108 (doi:10.1016/S0092-8674(03)00762-1) [DOI] [PubMed] [Google Scholar]
- Sickmann A., et al. 2003The proteome of Saccharomyces cerevisiae mitochondria. Proc. Natl Acad. Sci. USA 100, 13 207–13 212 (doi:10.1073/pnas.2135385100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson A. G., Roger A. J.2004The real ‘kingdoms' of eukaryotes. Curr. Biol. 14, R693–R696 (doi:10.1016/j.cub.2004.08.038) [DOI] [PubMed] [Google Scholar]
- Smíd O., et al. 2008Reductive evolution of the mitochondrial processing peptidases of the unicellular parasites Trichomonas vaginalis and Giardia intestinalis. PLoS Pathog. 4, e1000243 (doi:10.1371/journal.ppat.1000243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L.1991Early evolution and the origin of eukaryotes. Curr. Opin. Genet. Dev. 1, 457–463 (doi:10.1016/S0959-437X(05)80192-3) [DOI] [PubMed] [Google Scholar]
- Söllner T., Griffiths G., Pfaller R., Pfanner N., Neupert W.1989MOM19, an import receptor for mitochondrial precursor proteins. Cell 59, 1061–1070 (doi:10.1016/0092-8674(89)90762-9) [DOI] [PubMed] [Google Scholar]
- Stechmann A., Hamblin K., Pérez-Brocal V., Gaston D., Richmond G. S., Vandergiezen M., Clark C. G., Roger A. J.2008Organelles in Blastocystis that blur the distinction between mitochondria and hydrogenosomes. Curr. Biol. 18, 580–585 (doi:10.1016/j.cub.2008.03.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T. H. P., Bochud-Allemannn N., Horn E. K., Schneider A.2002aEukaryotic-type elongator tRNAMet of Trypanosoma brucei becomes formylated after import into mitochondria. Proc. Natl Acad. Sci. USA 99, 1152–1157 (doi:10.1073/pnas.022522299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T. H. P., Pach R., Crausaz A., Ivens A., Schneider A.2002btRNAs in Trypanosoma brucei: genomic organization, expression and mitochondrial import. Mol. Cell. Biol. 22, 3707–3717 (doi:10.1128/MCB.22.11.3707-3716.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarassov I., Entelis N., Martin R. P.1995aAn intact protein translocating machinery is required for mitochondrial import of a yeast cytoplasmic tRNA. J. Mol. Biol. 245, 315–323 (doi:10.1006/jmbi.1994.0026) [DOI] [PubMed] [Google Scholar]
- Tarassov I., Entelis N., Martin R. P.1995bMitochondrial import of a cytoplasmic lysine-tRNA in yeast is mediated by cooperation of cytoplasmic and mitochondrial lysyl-tRNA synthetases. EMBO J. 14, 3461–3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarassov I., Kamenski P., Kolesnikova O., Karicheva O., Martin R. P., Krasheninnikov I. A., Entelis N.2007Import of nuclear DNA-encoded RNAs into mitochondria and mitochondrial translation. Cell Cycle 6, 2473–2477 [DOI] [PubMed] [Google Scholar]
- Tovar J., Fischer A., Clark C. G.1999The mitosome, a novel organelle related to mitochondria in the amitochondrial parasite Entamoeba histolytica. Mol. Microbiol. 32, 1013–1021 (doi:10.1046/j.1365-2958.1999.01414.x) [DOI] [PubMed] [Google Scholar]
- Tovar J., Leon-Avila G., Sanchez L. B., Sutak R.2003Mitochondrial remnant organelles of Giardia function in iron-sulphur protein maturation. Nature 426, 127–128 (doi:10.1038/nature01945) [DOI] [PubMed] [Google Scholar]
- Tovar J., Cox S. S., Vandergiezen M.2007A mitosome purification protocol based on percoll density gradients and its use in validating the mitosomal nature of Entamoeba histolytica mitochondrial Hsp70. Meth. Mol. Biol. 390, 167–177 (doi:10.1007/978-1-59745-466-7_11) [DOI] [PubMed] [Google Scholar]
- Uboldi A. D., et al. 2006A mitochondrial protein affects cell morphology, mitochondrial segregation and virulence in Leishmania. Int. J. Parasitol. 36, 1499–1514 (doi:10.1016/j.ijpara.2006.08.006) [DOI] [PubMed] [Google Scholar]
- Vandergiezen M., Tovar J.2005Degenerate mitochondria. EMBO Rep. 6, 525–530 (doi:10.1038/sj.embor.7400440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandergiezen M., Tovar J., Clark C. G.2005Mitochondrion-derived organelles in protists and fungi. Int. Rev. Cytol. 244, 175–225 (doi:10.1016/S0074-7696(05)44005-X) [DOI] [PubMed] [Google Scholar]
- Verschoor A., Lithgow T. J.1999A first glimpse at the structure of the TOM translocase from the mitochondrial outer membrane. J. Cell Biol. 147, 905–908 (doi:10.1083/jcb.147.5.905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova E., Salinas T., Cognat V., Remacle C., Maréchal-Drouard L.2009Steady-state levels of imported tRNAs in Chlamydomonas mitochondria are correlated with both cytosolic and mitochondrial codon usages. Nucl. Acids Res. 37, 1521–1528 (doi:10.1093/nar/gkn1073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonheijne G.1986Why mitochondria need a genome. FEBS Lett. 198, 1–4 (doi:10.1016/0014-5793(86)81172-3) [DOI] [PubMed] [Google Scholar]
- Waller R. F., Jabbour C., Chan N. C., Celik N., Likic V. A., Mulhern T. D., Lithgow T.2009Evidence of a reduced and modified mitochondrial protein import apparatus in microsporidian mitosomes. Eukaryot. Cell. 8, 19–26 (doi:10.1128/EC.00313-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrzyniak I., et al. 2008Complete circular DNA in the mitochondria-like organelles of Blastocystis hominis. Int. J. Parasitol. 38, 1377–1382 (doi:10.1016/j.ijpara.2008.06.001) [DOI] [PubMed] [Google Scholar]
- Webb C. T., Gorman M. A., Lazarou M., Ryan M. T., Gulbis J. M.2006Crystal structure of the mitochondrial chaperone TIM9-10 reveals a six-bladed α-propeller. Mol. Cell 21, 123–133 (doi:10.1016/j.molcel.2005.11.010) [DOI] [PubMed] [Google Scholar]
- Werhahn W., Niemeyer A., Jänsch L., Kruft V., Schmitz U. K., Braun H.2001Purification and characterization of the preprotein translocase of the outer mitochondrial membrane from Arabidopsis. Identification of multiple forms of TOM20. Plant Physiol. 125, 943–954 (doi:10.1104/pp.125.2.943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Macfarlane J. L., Okimoto R., Clary D. O., Wahleithner J. A.1987Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc. Natl Acad. Sci. 84, 1324–1328 (doi:10.1073/pnas.84.5.1324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano K., Yatsukawa Y., Esaki M., Hobbs A. E., Jensen R. E., Endo T.2008Tom20 and Tom22 share the common signal recognition pathway in mitochondrial protein import. J. Biol. Chem. 283, 3799–3807 (doi:10.1074/jbc.M708339200) [DOI] [PubMed] [Google Scholar]