Abstract
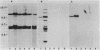
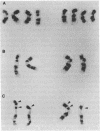
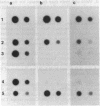
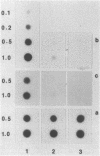
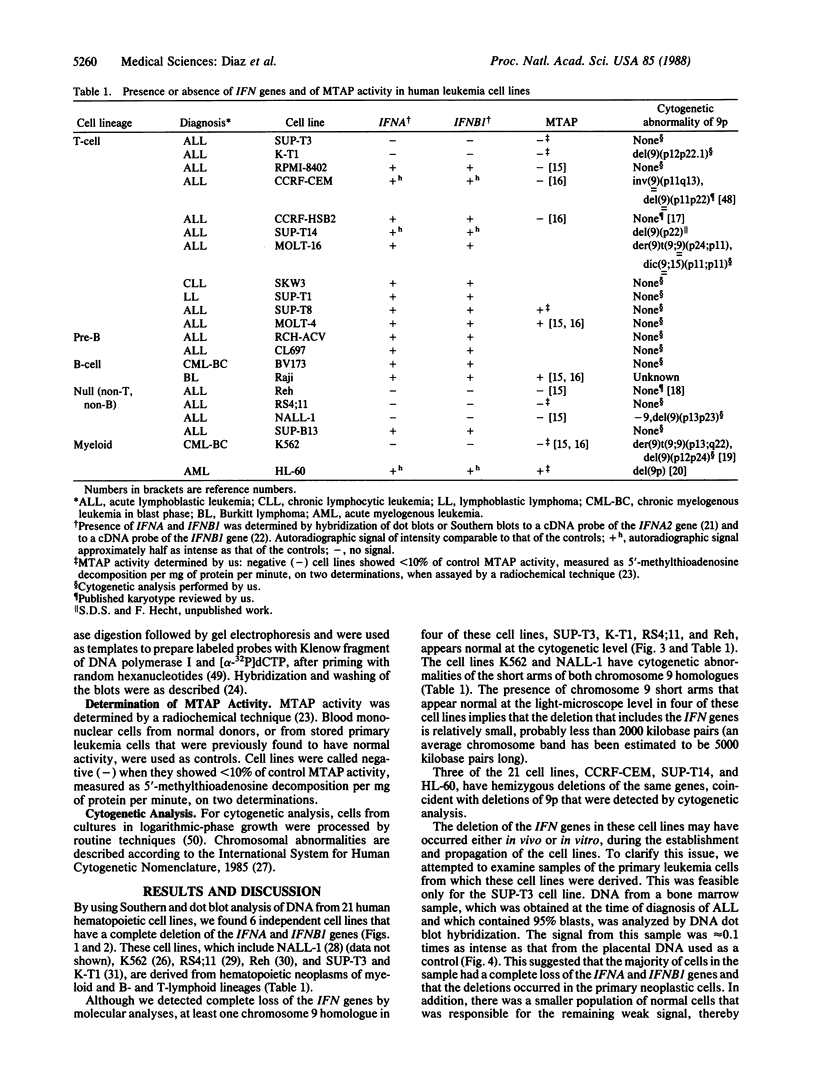
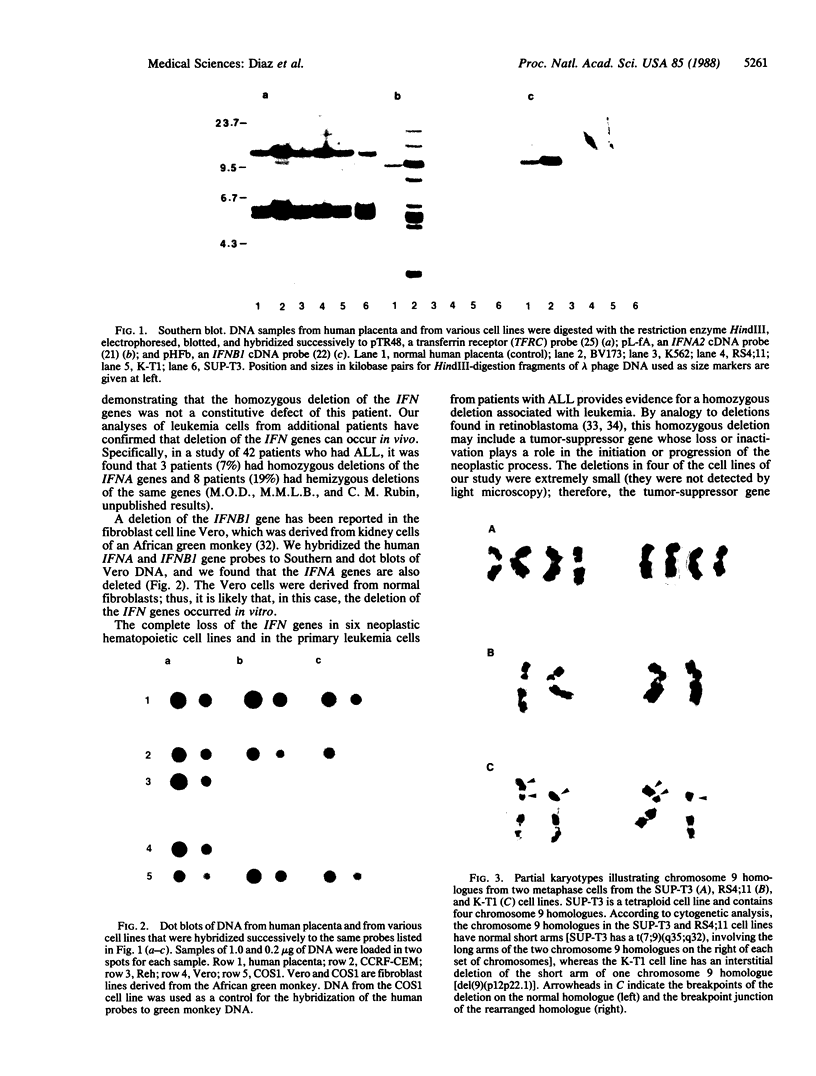
The loss of bands p21-22 from one chromosome 9 homologue as a consequence of a deletion of the short arm [del(9p)], unbalanced translocation, or monosomy 9 is frequently observed in the malignant cells of patients with lymphoid neoplasias, including acute lymphoblastic leukemia and non-Hodgkin lymphoma. The alpha- and beta 1-interferon genes have been assigned to this chromosome region (9p21-22). We now present evidence of the homozygous deletion of the interferon genes in neoplastic hematopoietic cell lines and primary leukemia cells in the presence or absence of chromosomal deletions that are detectable at the level of the light microscope. In these cell lines, the deletion of the interferon genes is accompanied by a deficiency of 5'-methylthioadenosine phosphorylase (EC 2.4.2.28), an enzyme of purine metabolism. These homozygous deletions may be associated with the loss of a tumor-suppressor gene that is involved in the development of these neoplasias. The relevant genes may be either the interferon genes themselves or a gene that has a tumor-suppressor function and is closely linked to them.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedict W. F., Murphree A. L., Banerjee A., Spina C. A., Sparkes M. C., Sparkes R. S. Patient with 13 chromosome deletion: evidence that the retinoblastoma gene is a recessive cancer gene. Science. 1983 Feb 25;219(4587):973–975. doi: 10.1126/science.6336308. [DOI] [PubMed] [Google Scholar]
- Burrone O. R., Milstein C. Control of HLA-A,B,C synthesis and expression in interferon-treated cells. EMBO J. 1982;1(3):345–349. doi: 10.1002/j.1460-2075.1982.tb01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera C. J., Eddy R. L., Shows T. B., Carson D. A. Assignment of the gene for methylthioadenosine phosphorylase to human chromosome 9 by mouse-human somatic cell hybridization. Proc Natl Acad Sci U S A. 1984 May;81(9):2665–2668. doi: 10.1073/pnas.81.9.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A. J., Castleberry R. P., Crist W. M. Lack of association between abnormalities of the chromosome 9 short arm and either "lymphomatous" features or T cell phenotype in childhood acute lymphocytic leukemia. Blood. 1987 Mar;69(3):735–738. [PubMed] [Google Scholar]
- Chen T. R. Modal karyotype of human leukemia cell line, K562 (ATCC CCL 243). Cancer Genet Cytogenet. 1985 May;17(1):55–60. doi: 10.1016/0165-4608(85)90101-3. [DOI] [PubMed] [Google Scholar]
- Chilcote R. R., Brown E., Rowley J. D. Lymphoblastic leukemia with lymphomatous features associated with abnormalities of the short arm of chromosome 9. N Engl J Med. 1985 Aug 1;313(5):286–291. doi: 10.1056/NEJM198508013130503. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., McNurlan M. A. Regulation of cell proliferation and differentiation by interferons. Biochem J. 1985 Mar 1;226(2):345–360. doi: 10.1042/bj2260345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz M. O., Le Beau M. M., Pitha P., Rowley J. D. Interferon and c-ets-1 genes in the translocation (9;11)(p22;q23) in human acute monocytic leukemia. Science. 1986 Jan 17;231(4735):265–267. doi: 10.1126/science.3455787. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fitchen J. H., Riscoe M. K., Dana B. W., Lawrence H. J., Ferro A. J. Methylthioadenosine phosphorylase deficiency in human leukemias and solid tumors. Cancer Res. 1986 Oct;46(10):5409–5412. [PubMed] [Google Scholar]
- Gresser I., Brouty-Boyé D., Thomas M. T., Macieira-Coelho A. Interferon and cell division. I. Inhibition of the multiplication of mouse leukemia L 1210 cells in vitro by interferon preparations. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1052–1058. doi: 10.1073/pnas.66.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henco K., Brosius J., Fujisawa A., Fujisawa J. I., Haynes J. R., Hochstadt J., Kovacic T., Pasek M., Schamböck A., Schmid J. Structural relationship of human interferon alpha genes and pseudogenes. J Mol Biol. 1985 Sep 20;185(2):227–260. doi: 10.1016/0022-2836(85)90401-2. [DOI] [PubMed] [Google Scholar]
- Heron I., Hokland M., Berg K. Enhanced expression of beta2-microglobulin and HLA antigens on human lymphoid cells by interferon. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6215–6219. doi: 10.1073/pnas.75.12.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraki S., Miyoshi I., Kubonishi I., Matsuda Y., Nakayama T., Kishimoto H., Masuji H. Human leukemic "null" cell line (NALL-1). Cancer. 1977 Nov;40(5):2131–2135. doi: 10.1002/1097-0142(197711)40:5<2131::aid-cncr2820400523>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Imanishi J., Hoshino S., Matsuoka H., Kishida T., Minowada J. Antigenic types of various human lymphoblastoid cell interferons. Biken J. 1982 Jun;25(2):97–100. [PubMed] [Google Scholar]
- Kamatani N., Carson D. A. Abnormal regulation of methylthioadenosine and polyamine metabolism in methylthioadenosine phosphorylase-deficient human leukemic cell lines. Cancer Res. 1980 Nov;40(11):4178–4182. [PubMed] [Google Scholar]
- Kamatani N., Nelson-Rees W. A., Carson D. A. Selective killing of human malignant cell lines deficient in methylthioadenosine phosphorylase, a purine metabolic enzyme. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1219–1223. doi: 10.1073/pnas.78.2.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamatani N., Yu A. L., Carson D. A. Deficiency of methylthioadenosine phosphorylase in human leukemic cells in vivo. Blood. 1982 Dec;60(6):1387–1391. [PubMed] [Google Scholar]
- Kowalczyk J., Sandberg A. A. A possible subgroup of ALL with 9p-. Cancer Genet Cytogenet. 1983 Aug;9(4):383–385. doi: 10.1016/0165-4608(83)90086-9. [DOI] [PubMed] [Google Scholar]
- Lewin R. Report urges funds for conservation biology. Science. 1987 Apr 17;236(4799):257–259. doi: 10.1126/science.3563503. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Misawa S., Staal S. P., Testa J. R. Amplification of the c-myc oncogene is associated with an abnormally banded region on chromosome 8 or double minute chromosomes in two HL-60 human leukemia sublines. Cancer Genet Cytogenet. 1987 Sep;28(1):127–135. doi: 10.1016/0165-4608(87)90362-1. [DOI] [PubMed] [Google Scholar]
- Mizoguchi J., Pitha P. M., Raj N. B. Efficient expression in Escherichia coli of two species of human interferon-alpha and their hybrid molecules. DNA. 1985 Jun;4(3):221–232. doi: 10.1089/dna.1985.4.221. [DOI] [PubMed] [Google Scholar]
- Moore D. E., Weise K., Zawydiwski R., Thompson E. B. The karyotype of the glucocorticoid-sensitive, lymphoblastic human T-cell line CCRF-CEM shows a unique deleted and inverted chromosome 9. Cancer Genet Cytogenet. 1985 Jan 1;14(1-2):89–94. doi: 10.1016/0165-4608(85)90219-5. [DOI] [PubMed] [Google Scholar]
- Moore R. N., Larsen H. S., Horohov D. W., Rouse B. T. Endogenous regulation of macrophage proliferative expansion by colony-stimulating factor-induced interferon. Science. 1984 Jan 13;223(4632):178–181. doi: 10.1126/science.6606850. [DOI] [PubMed] [Google Scholar]
- Morris A. G., Morser J., Meager A. Spontaneous production of gamma interferon and induced production of beta interferon by human T-lymphoblastoid cell lines. Infect Immun. 1982 Feb;35(2):533–536. doi: 10.1128/iai.35.2.533-536.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca J. D., Pitha P. M. Transcriptional and posttranscriptional regulation of exogenous human beta interferon gene in simian cells defective in interferon synthesis. Mol Cell Biol. 1986 Jun;6(6):2279–2283. doi: 10.1128/mcb.6.6.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphree A. L., Benedict W. F. Retinoblastoma: clues to human oncogenesis. Science. 1984 Mar 9;223(4640):1028–1033. doi: 10.1126/science.6320372. [DOI] [PubMed] [Google Scholar]
- Naylor S. L., Johnson B. E., Minna J. D., Sakaguchi A. Y. Loss of heterozygosity of chromosome 3p markers in small-cell lung cancer. Nature. 1987 Oct 1;329(6138):451–454. doi: 10.1038/329451a0. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. Phosphate-stimulated breakdown of 5'-methylthioadenosine by rat ventral prostate. Biochem J. 1969 Nov;115(2):241–247. doi: 10.1042/bj1150241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak C., Hagemeijer A. Abnormalities of the short arm of chromosome 9 with partial loss of material in hematological disorders. Leukemia. 1987 Jul;1(7):541–548. [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Analysis of interferon mRNA in human fibroblast cells induced to produce interferon. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7426–7430. doi: 10.1073/pnas.78.12.7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld C., Goutner A., Choquet C., Venuat A. M., Kayibanda B., Pico J. L., Greaves M. F. Phenotypic characterisation of a unique non-T, non-B acute lymphoblastic leukaemia cell line. Nature. 1977 Jun 30;267(5614):841–843. doi: 10.1038/267841a0. [DOI] [PubMed] [Google Scholar]
- Schneider C., Owen M. J., Banville D., Williams J. G. Primary structure of human transferrin receptor deduced from the mRNA sequence. Nature. 1984 Oct 18;311(5987):675–678. doi: 10.1038/311675b0. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., Martuza R. L., Gusella J. F. Loss of genes on chromosome 22 in tumorigenesis of human acoustic neuroma. Nature. 1986 Aug 14;322(6080):644–647. doi: 10.1038/322644a0. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., de la Monte S., Atkins L., Gusella J. F., Martuza R. L. Molecular genetic approach to human meningioma: loss of genes on chromosome 22. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5419–5423. doi: 10.1073/pnas.84.15.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima E. A., Le Beau M. M., McKeithan T. W., Minowada J., Showe L. C., Mak T. W., Minden M. D., Rowley J. D., Diaz M. O. Gene encoding the alpha chain of the T-cell receptor is moved immediately downstream of c-myc in a chromosomal 8;14 translocation in a cell line from a human T-cell leukemia. Proc Natl Acad Sci U S A. 1986 May;83(10):3439–3443. doi: 10.1073/pnas.83.10.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. D., Morgan R., Link M. P., McFall P., Hecht F. Cytogenetic and immunophenotypic analysis of cell lines established from patients with T cell leukemia/lymphoma. Blood. 1986 Mar;67(3):650–656. [PubMed] [Google Scholar]
- Solomon E., Voss R., Hall V., Bodmer W. F., Jass J. R., Jeffreys A. J., Lucibello F. C., Patel I., Rider S. H. Chromosome 5 allele loss in human colorectal carcinomas. Nature. 1987 Aug 13;328(6131):616–619. doi: 10.1038/328616a0. [DOI] [PubMed] [Google Scholar]
- Sparkes R. S., Sparkes M. C., Wilson M. G., Towner J. W., Benedict W., Murphree A. L., Yunis J. J. Regional assignment of genes for human esterase D and retinoblastoma to chromosome band 13q14. Science. 1980 May 30;208(4447):1042–1044. doi: 10.1126/science.7375916. [DOI] [PubMed] [Google Scholar]
- Stong R. C., Korsmeyer S. J., Parkin J. L., Arthur D. C., Kersey J. H. Human acute leukemia cell line with the t(4;11) chromosomal rearrangement exhibits B lineage and monocytic characteristics. Blood. 1985 Jan;65(1):21–31. [PubMed] [Google Scholar]
- Trent J. M., Olson S., Lawn R. M. Chromosomal localization of human leukocyte, fibroblast, and immune interferon genes by means of in situ hybridization. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7809–7813. doi: 10.1073/pnas.79.24.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venuat A. M., Testu M. J., Rosenfeld C. Cytogenetic abnormalities in a human null cell leukemia line (REH). Cancer Genet Cytogenet. 1981 Jun;3(4):327–334. doi: 10.1016/0165-4608(81)90041-8. [DOI] [PubMed] [Google Scholar]
- Weissman B. E., Saxon P. J., Pasquale S. R., Jones G. R., Geiser A. G., Stanbridge E. J. Introduction of a normal human chromosome 11 into a Wilms' tumor cell line controls its tumorigenic expression. Science. 1987 Apr 10;236(4798):175–180. doi: 10.1126/science.3031816. [DOI] [PubMed] [Google Scholar]
- Zbar B., Brauch H., Talmadge C., Linehan M. Loss of alleles of loci on the short arm of chromosome 3 in renal cell carcinoma. 1987 Jun 25-Jul 1Nature. 327(6124):721–724. doi: 10.1038/327721a0. [DOI] [PubMed] [Google Scholar]
- Zullo J. N., Cochran B. H., Huang A. S., Stiles C. D. Platelet-derived growth factor and double-stranded ribonucleic acids stimulate expression of the same genes in 3T3 cells. Cell. 1985 Dec;43(3 Pt 2):793–800. doi: 10.1016/0092-8674(85)90252-1. [DOI] [PubMed] [Google Scholar]