Abstract
Intracellular recordings from mammalian spinal motoneurons in vivo show that the type B gamma-aminobutyric acid receptor agonist, L-(-)-baclofen, when administered systemically to pentobarbital-anesthetized or decerebrate unanesthetized cats decreases the amplitude of monosynaptic group Ia excitatory postsynaptic potentials (EPSPs), markedly increases tetanic and posttetanic potentiation, and reduces or abolishes synaptic depression during high-frequency synaptic activation and in the posttetanic period. These changes occur without detectable alteration in motoneuron input resistance, EPSP shape, or the invasion of action potentials into the intraspinal group Ia terminal arborizations. The baclofen-induced effects are qualitatively similar to those observed in more accessible synaptic systems when presynaptic Ca2+ influx and, concomitantly, transmitter release are reduced. Based on these and other recent findings regarding the mechanism of action of baclofen and the distribution of its receptors in the spinal cord, we suggest that L-(-)-baclofen modifies frequency modulation of Ia synaptic transmission by reducing presynaptic Ca2+ influx and the concomitant level of transmitter release from Ia afferent terminals. The drug appears to be a useful tool in studies of the ionic mechanisms that control the release of transmitter and its frequency modulation at inaccessible mammalian synapses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J. L., Ransom B. R. Amino acid pharmacology of mammalian central neurones grown in tissue culture. J Physiol. 1978 Jul;280:331–354. doi: 10.1113/jphysiol.1978.sp012387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Hudson A. L., Price G. W. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987 Feb;20(2):365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Capek R., Esplin B. Baclofen-induced decrease of excitability of primary afferents and depression of monosynaptic transmission in cat spinal cord. Can J Physiol Pharmacol. 1982 Feb;60(2):160–166. doi: 10.1139/y82-026. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Gynther B. D., Malik R. A pharmacological study of group I muscle afferent terminals and synaptic excitation in the intermediate nucleus and Clarke's column of the cat spinal cord. Exp Brain Res. 1986;64(1):105–113. doi: 10.1007/BF00238205. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Lodge D., Bornstein J. C., Peet M. J. Selective effects of (-)-baclofen on spinal synaptic transmission in the cat. Exp Brain Res. 1981;42(2):158–170. doi: 10.1007/BF00236902. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Lux H. D. gamma-Aminobutyric acid-induced depression of calcium currents of chick sensory neurons. Neurosci Lett. 1985 May 14;56(2):205–210. doi: 10.1016/0304-3940(85)90130-2. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Scott R. H. Inhibition of calcium currents in cultured rat dorsal root ganglion neurones by (-)-baclofen. Br J Pharmacol. 1986 May;88(1):213–220. doi: 10.1111/j.1476-5381.1986.tb09489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol. 1981 Aug;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
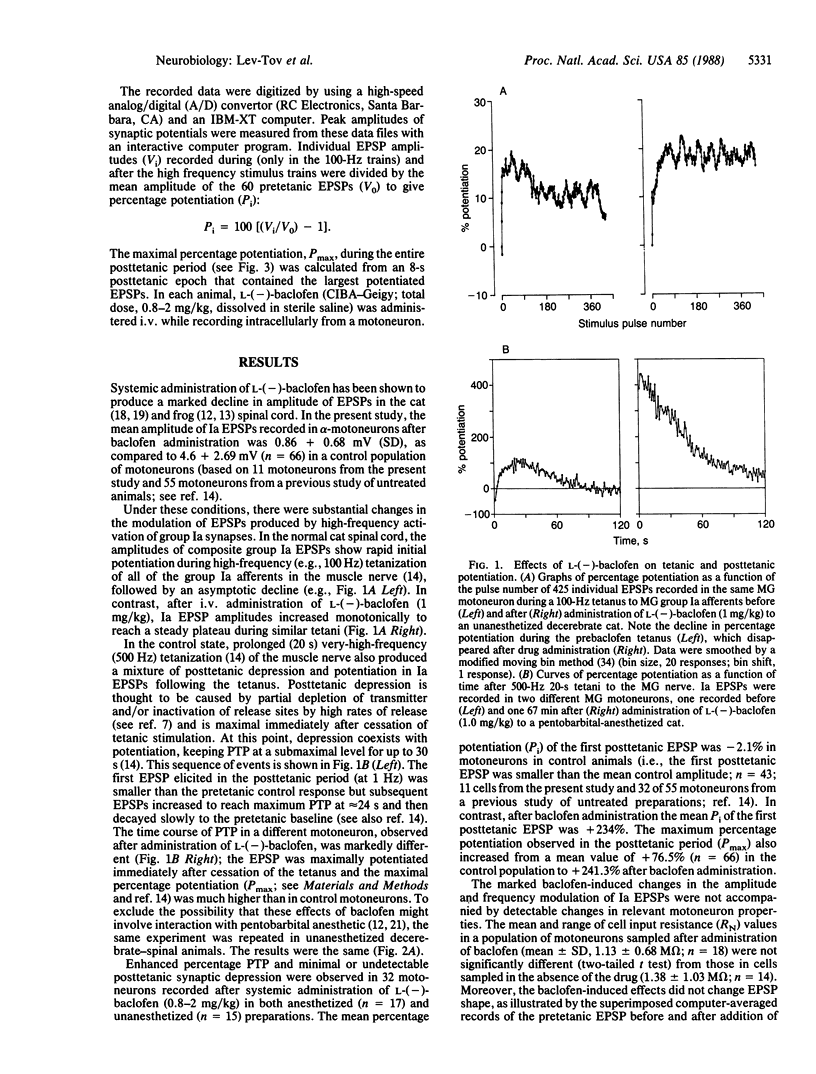
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK K., FUORTES M. G. Stimulation of spinal motoneurones with intracellular electrodes. J Physiol. 1956 Nov 28;134(2):451–470. doi: 10.1113/jphysiol.1956.sp005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S., Krnjević K., Morris M. E., Puil E., Werman R. Action of baclofen on mammalian synaptic transmission. Neuroscience. 1978;3(6):495–515. doi: 10.1016/0306-4522(78)90016-7. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H., Brown D. A. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Tov A., Pinter M. J., Burke R. E. Posttetanic potentiation of group Ia EPSPs: possible mechanisms for differential distribution among medial gastrocnemius motoneurons. J Neurophysiol. 1983 Aug;50(2):379–398. doi: 10.1152/jn.1983.50.2.379. [DOI] [PubMed] [Google Scholar]
- Lev-Tov A., Rahamimoff R. A study of tetanic and post-tetanic potentiation of miniature end-plate potentials at the frog neuromuscular junction. J Physiol. 1980 Dec;309:247–273. doi: 10.1113/jphysiol.1980.sp013507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Henneman E. Composite EPSPs in motoneurons of different sizes before and during PTP: implications for transmission failure and its relief in Ia projections. J Neurophysiol. 1983 Jan;49(1):269–289. doi: 10.1152/jn.1983.49.1.269. [DOI] [PubMed] [Google Scholar]
- Magleby K. L. The effect of repetitive stimulation on facilitation of transmitter release at the frog neuromuscular junction. J Physiol. 1973 Oct;234(2):327–352. doi: 10.1113/jphysiol.1973.sp010348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. Long term changes in augmentation, potentiation, and depression of transmitter release as a function of repeated synaptic activity at the frog neuromuscular junction. J Physiol. 1976 May;257(2):471–494. doi: 10.1113/jphysiol.1976.sp011379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell L. M. Modifiability of spinal synapses. Physiol Rev. 1984 Jan;64(1):260–324. doi: 10.1152/physrev.1984.64.1.260. [DOI] [PubMed] [Google Scholar]
- Munson J. B., Sypert G. W. Properties of single central Ia afferent fibres projecting to motoneurones. J Physiol. 1979 Nov;296:315–327. doi: 10.1113/jphysiol.1979.sp013007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peet M. J., McLennan H. Pre-and postsynaptic actions of baclofen: blockade of the late synaptically-evoked hyperpolarization of CA1 hippocampal neurones. Exp Brain Res. 1986;61(3):567–574. doi: 10.1007/BF00237582. [DOI] [PubMed] [Google Scholar]
- Pierau F. K., Zimmermann P. Action of a GABA-derivative on postsynaptic potentials and membrane properties of cats' spinal motoneurones. Brain Res. 1973 May 17;54:376–380. doi: 10.1016/0006-8993(73)90064-4. [DOI] [PubMed] [Google Scholar]
- Price G. W., Wilkin G. P., Turnbull M. J., Bowery N. G. Are baclofen-sensitive GABAB receptors present on primary afferent terminals of the spinal cord? Nature. 1984 Jan 5;307(5946):71–74. doi: 10.1038/307071a0. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R., Lev-Tov A., Meiri H. Primary and secondary regulation of quantal transmitter release: calcium and sodium. J Exp Biol. 1980 Dec;89:5–18. doi: 10.1242/jeb.89.1.5. [DOI] [PubMed] [Google Scholar]
- Redman S. J., McLachlan E. M., Hirst G. D. Nonuniform passive membrane properties of rat lumbar sympathetic ganglion cells. J Neurophysiol. 1987 Mar;57(3):633–644. doi: 10.1152/jn.1987.57.3.633. [DOI] [PubMed] [Google Scholar]
- Redman S. Junctional mechanisms at group Ia synapses. Prog Neurobiol. 1979;12(1):33–83. doi: 10.1016/0301-0082(79)90010-8. [DOI] [PubMed] [Google Scholar]
- Robertson B., Taylor W. R. Effects of gamma-aminobutyric acid and (-)-baclofen on calcium and potassium currents in cat dorsal root ganglion neurones in vitro. Br J Pharmacol. 1986 Dec;89(4):661–672. doi: 10.1111/j.1476-5381.1986.tb11170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapovalov A. I., Shiriaev B. I. Dual mode of junctional transmission at synapses between single primary afferent fibres and motoneurones in the amphibian. J Physiol. 1980 Sep;306:1–15. doi: 10.1113/jphysiol.1980.sp013381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypert G. W., Munson J. B., Fleshman J. W. Effect of presynaptic inhibition on axonal potentials, terminal potentials, focal synaptic potentials, and EPSPs in cat spinal cord. J Neurophysiol. 1980 Oct;44(4):792–803. doi: 10.1152/jn.1980.44.4.792. [DOI] [PubMed] [Google Scholar]


