Abstract
Estrogens attenuate osteoclastogenesis and stimulate osteoclast apoptosis, but the molecular mechanism and contribution of these effects to the overall antiosteoporotic efficacy of estrogens remain controversial. We selectively deleted the estrogen receptor (ER)α from the monocyte/macrophage cell lineage in mice (ERαLysM−/−) and found a 2-fold increase in osteoclast progenitors in the marrow and the number of osteoclasts in cancellous bone, along with a decrease in cancellous bone mass. After loss of estrogens these mice failed to exhibit the expected increase in osteoclast progenitors, the number of osteoclasts in bone, and further loss of cancellous bone. However, they lost cortical bone indistinguishably from their littermate controls. Mature osteoclasts from ERαLysM−/− were resistant to the proapoptotic effect of 17β-estradiol. Nonetheless, the effects of estrogens on osteoclasts were unhindered in mice bearing an ERα knock-in mutation that prevented binding to DNA. Moreover, a polymeric form of estrogen that is not capable of stimulating the nuclear-initiated actions of ERα was as effective as 17β-estradiol in inducing osteoclast apoptosis in cells with the wild-type ERα. We conclude that estrogens attenuate osteoclast generation and life span via cell autonomous effects mediated by DNA-binding-independent actions of ERα. Elimination of these effects is sufficient for loss of bone in the cancellous compartment in which complete perforation of trabeculae by osteoclastic resorption precludes subsequent refilling of the cavities by the bone-forming osteoblasts. However, additional effects of estrogens on osteoblasts, osteocytes, and perhaps other cell types are required for their protective effects on the cortical compartment, which constitutes 80% of the skeleton.
Attenuation of osteoclast apoptosis by a DNA-binding independent action of the ERα accounts for the protective effects of estrogens on cancellous, but not cortical, bone.
Throughout life millions of microscopic quanta of the mammalian skeleton are periodically regenerated by osteoclasts and osteoblasts, two highly specialized cell types that excavate old bone and form new bone, respectively (1). During this regeneration process, the cavities dug by osteoclasts are subsequently refilled with newly formed bone made by osteoblasts. Normally, bone excavation (resorption) and refilling are tightly balanced by the coordinated production of osteoclasts and osteoblasts from their respective hematopoietic and mesenchymal progenitors and by the timing of death of the mature cells by apoptosis. An imbalance between bone resorption and formation is the hallmark of all osteopenic states and can result from a relative increase or decrease in the number of either cell type, resulting from changes in the rate of their generation, life span, or both. All the osteoclasts and the majority of osteoblasts die by apoptosis (2). Some osteoblasts escape this fate and are entombed in the mineralized matrix, as osteocytes. These former osteoblasts represent the most abundant cell type in bone. Osteocytes orchestrate the process of remodeling by signaling the need for the turnover of a particular segment of bone (by sensing tissue microdamage and perhaps hypoxia) and by guiding the recruitment of osteoblasts and osteoclasts to the prespecified site (3,4,5).
Estrogens protect the adult skeleton against bone loss by slowing the rate of bone remodeling and by maintaining a focal balance between bone formation and resorption (1,6). Slowing of bone remodeling is due to the attenuating effects of estrogens on the birth rate of osteoclast and osteoblast progenitors (7,8,9,10,11). Maintenance of a focal balance between formation and resorption, on the other hand, apparently results from opposite effects of estrogens on the life span of osteoclasts and osteoblasts/osteocytes: a proapoptotic effect on osteoclasts and an antiapoptotic effect on osteoblasts and osteocytes (10,12,13,14,15). Conversely, estrogen deficiency leads to an increase in the rate of bone remodeling, resulting from an increase in the generation of both osteoclasts and osteoblasts and a corresponding increase in bone resorption and formation, with resorption exceeding formation. In contrast to classical estrogen actions, which require direct interaction of the estrogen receptor (ER) with DNA, the effects of estrogens on the generation of osteoblasts and the life span of osteoblasts and osteoclasts evidently result from extranuclear actions of the ER and activation of cytoplasmic kinases (9,12,14,16).
Recently, Nakamura et al. (17) reported that female mice in which the ERα was specifically deleted in mature osteoclasts using the cathepsin K promoter (ERαΔOc/ΔOc) exhibited decreased bone volume due to an increase in the number of osteoclasts, resulting from the loss of a cell-autonomous antiapoptotic effect of estrogens on mature osteoclasts. The proapoptotic effect of estrogens on osteoclasts, according to these authors, was mediated by an increase in Fas ligand (FasL) production by osteoclasts. The ERαΔOc/ΔOc mice did not exhibit any changes in osteoclast progenitors under basal conditions and did not undergo any bone loss 2 wk after ovariectomy (OVX). Based on these results, Nakamura and colleagues concluded that the proapoptotic effect of estrogens on mature osteoclasts accounts for their osteoprotective properties. In vitro studies by Krum et al. (18) have confirmed the role of FasL in the effect of estrogens on osteoclast apoptosis. However, in contrast to the findings of Nakamura et al., Krum and colleagues were unable to confirm that estrogens increase the transcription of the FasL gene in osteoclasts. Instead, they showed that estrogens stimulate FasL expression in osteoblasts and concluded that FasL must therefore act in a paracrine fashion to stimulate osteoclasts apoptosis.
In the studies presented here, we have investigated the molecular mechanism of estrogen action in osteoclasts and have dissected the contribution of estrogen effects on this cell type to the overall beneficial effects of estrogens on skeletal homeostasis. For this purpose we used mice in which we deleted the ERα from early osteoclast precursors and their progeny using the promoter of the lysozyme M (LysM) gene, which is expressed in cells of the monocyte/macrophage lineage and neutrophils (19). These mice were designated ERα LysM−/−. In addition, we have used here mice bearing an ERα knock-in mutation that prevents binding to DNA (ERαNERKI/−), which were previously generated by Jakacka et al. (20). ERαNERKI/− mice have an atrophic uterus, but they maintain responsiveness to estrogen-induced activation of ERKs and ERK-mediated phosphorylation of transcription factors (21).
Results
Generation of ERαLysM−/− mice
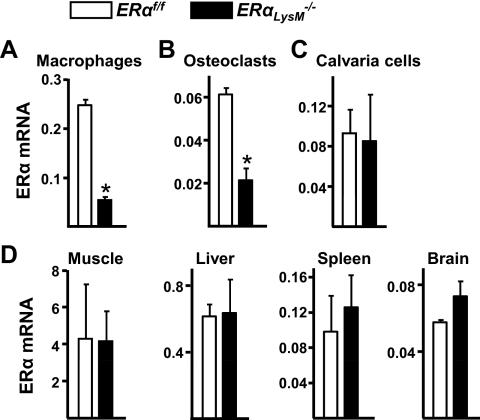
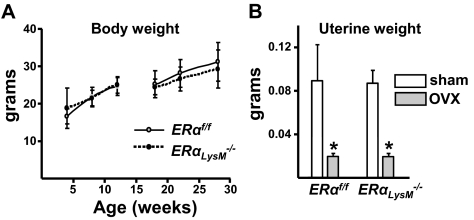
To elucidate cell-autonomous effects of estrogens on osteoclasts, we generated mice with targeted deletion of ERα in osteoclast precursor cells. To this end, mice harboring a floxed ERα allele (ERαf/f) (Fig. 1) were crossed with mice expressing the Cre recombinase under the control of regulatory elements of the LysM gene (19). Excision of the ERα gene was confirmed by quantitative (q)RT-PCR of ERα mRNA levels in ex vivo cultures of bone marrow-derived macrophages and osteoclasts. As shown in Fig. 2, A and B, macrophages and osteoclasts from ERαLysM−/− mice exhibited a 60–80% decrease of ERα mRNA expression. On the other hand, ERα mRNA expression in calvaria-derived osteoblasts, muscle, liver, spleen, or brain was indistinguishable between ERαLysM−/− and the ERαf/f control mice (Fig. 2, C and D). Total body weight of female ERαLysM−/− mice was indistinguishable from the littermate control mice between 4 and 28 wk of age (Fig. 3A). Likewise, the uterine weight determined after the animals were killed at 28 wk of age was indistinguishable between the two genotypes (Fig. 3B).
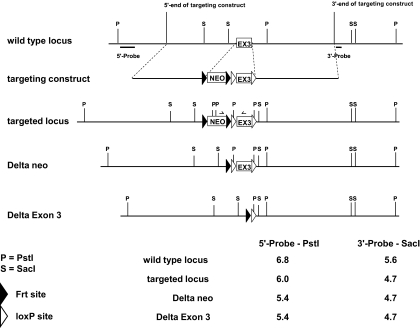
Figure 1.
Generation of conditional ERα mice. A targeting vector was constructed in which ERα exon 3 was flanked by loxP sites. The targeting vector was used to electroporate embryonic stem (ES) cells, and a correctly targeted clone was injected into blastocysts to generate chimeric mice. After germline transmission of the targeted allele, the frt-flanked neomycin cassette was removed by crossing with mice expressing the FLPe recombinase in germ cells.
Figure 2.
ERαLysM −/− mice express lower levels of ERα in macrophages and osteoclasts. A–D, Quantitative RT-PCR analysis of mRNA from: A, macrophages formed from nonadherent bone marrow cells cultured for 4 d in the present of M-CSF. B, Mature osteoclasts generated from bone marrow cells cultured with M-CSF and RANKL for 5 d. C, Osteoblastic cells derived from calvaria of 2- to 3-month-old mice. D, Soft tissues harvested from 12-wk-old mice (n = 5). Bars, Mean ± sd, *, P < 0.05. In panels A and B, the mean was calculated using cells from three individual animals and triplicate determinations per animal. In panel C, the mean was calculated from triplicate determinations obtained from cells pooled from three animals.
Figure 3.
ERαLysM−/− mice exhibit no changes in total body or uterine weight. A, Total body weight of two cohorts of female ERαLysM−/− and ERαf/f littermate mice. No differences were observed between the two genotypes (n = 9–23 mice per group). B, Wet uterine weight of 28-wk-old female mice which were sham-operated or ovariectomized (OVX) 6 wk earlier (n =10–13 mice per group). Bars, Mean ± sd; *, P < 0.05 vs. respective sham-operated mice.
ERαLysM−/− mice exhibit increased osteoclast progenitors in the bone marrow and osteoclasts in cancellous bone
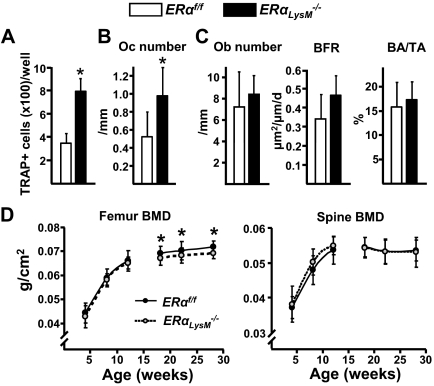
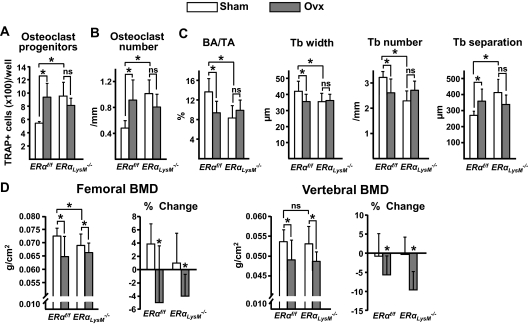
At 12 wk of age the number of osteoclast progenitors in female ERαLysM−/− mice was 2-fold higher when compared with their littermate controls, as determined in ex vivo cultures of bone marrow aspirates (Fig. 4A). Consistent with the increase of the progenitors, the number of osteoclasts in vertebral cancellous bone was also increased by 2-fold in the ERαLysM−/− mice (Fig. 4B). Osteoblast number, bone formation rate, and bone mass, as determined by histology, were not different in the two genotypes (Fig. 4C). Likewise, bone mineral density (BMD) by dual energy x-ray absorptiometry (DEXA) was not different at 4, 8, or 12 wk of age in either the femur or the spine (Fig. 4D). In a serial BMD analysis of a second cohort of female ERαLysM−/− mice, however, a small decrease of femoral BMD was noted at 18, 22, and 28 wk of age as compared with the littermate controls, whereas vertebral BMD remained indistinguishable between the two genotypes at all times (Fig. 4D).
Figure 4.
ERαLysM−/− mice exhibit increased number of osteoclast progenitors and osteoclasts. A, Number of TRAP-positive cells developed from bone marrow cells, extracted from femurs of 12-wk-old mice, and cultured in the presence of M-CSF and RANKL for 5 d. Triplicate cultures were performed from each of seven individual animals. B and C, Histomorphometric analysis of longitudinal undecalcified sections of L1–L3 vertebrae from 12-wk-old female mice (n = 8 mice per group). D, BMDs determined by DEXA in two cohorts (4–12 and 18–28 wk of age) of female ERαLysM−/− and ERαf/f littermate mice (n = 7–23 mice per group). Oc, Osteoclasts; Ob, osteoblasts; BFR, bone formation rate; BA/TA, bone area per tissue area. *, P < 0.05 vs. ERαf/f mice.
Osteoclast progenitor number in the bone marrow obtained from the second cohort at 28 wk revealed the same 2-fold increase in ERαLysM−/− mice that was detected at 12 wk of age in the first cohort (Fig. 5A). In addition, and in spite of the failure to detect reduced bone mass by DEXA in the vertebrae of ERαLysM−/− mice, histomorphometric analysis of vertebral cancellous bone sections revealed a doubling of the number of osteoclasts, similar to what was found in the first cohort at 12 wk of age (Fig. 5B). However, in contrast to the findings at 12 wk, at 28 wk of age ERαLysM−/− mice exhibited lower cancellous bone mass as compared with the controls; and consistent with this there was a decrease in trabecular width and number and an increase in trabecular separation (Fig. 5C).
Figure 5.
ERαLysM−/− mice lose bone mass after OVX. Female mice (22 wk of age) were sham operated or ovariectomized and killed 6 wk later. A, Number of TRAP-positive cells generated from bone marrow cells cultured with M-CSF and RANKL for 5 d. B and C, Histomorphometric analysis of longitudinal undecalcified sections of L1–L3 vertebrae from 28-wk-old female mice (n = 8 mice per group). D, First and third panels depict BMD determinations by DEXA before mice were killed. Second and fourth panels depict the percent change from the initial BMD, which was determined 1 d before surgery (n = 10–13 mice per group). BA/TA, Bone area per tissue area; Tb, trabecular; ns, not significant. *, P < 0.05.
ERαLysM−/− mice lose cortical but not cancellous bone after OVX
After 6 wk of estrogen deficiency, littermate control mice showed the expected bone changes, including an increase in osteoclast progenitors in the bone marrow and the number of osteoclasts in vertebral cancellous bone sections, a decrease in cancellous bone mass, trabecular width, and number, and an increase in trabecular separation (Fig. 5, A–C) (7,13). None of these cellular and microarchitectural changes could be detected in vertebral cancellous bone sections from the estrogen-deficient ERαLysM−/− mice. Most surprisingly, however, ERαLysM−/− mice exhibited a similar loss of both vertebral and femoral BMD by DEXA to that of the littermate controls (Fig. 5D), suggesting that the much larger cortical compartment remained sensitive to estrogens in the ERαLysM−/− mice despite the deletion of the ERα in osteoclast and their precursors.
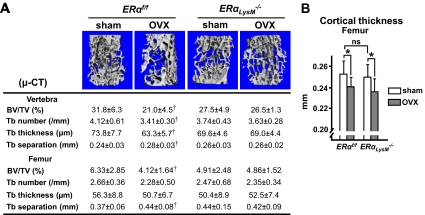
To explore this possibility, we performed micro-computerized tomography (CT) analysis of vertebrae and femurs from sham-operated and OVX ERαLysM−/− mice and the corresponding controls. The difference in vertebral and femoral cancellous bone volume by micro-CT in the estrogen-sufficient state between ERαLysM−/− and controls did not reach statistical significance (Fig. 6A), whereas it did by histology in the vertebrae and by DEXA BMD in the femur (Fig. 5, C and D), most likely reflecting the small size of this change. Nonetheless, micro-CT confirmed the histological finding that OVX caused loss of cancellous bone volume and a decrease in trabecular number and thickness, and an increase in trabecular separation in the littermate controls. None of these changes occurred in the ERαLysM−/− mice. On the other hand, loss of estrogens caused a similar decrease in cortical bone, as indicated by the decrease in femoral cortical width, in ERαLysM−/− and control mice (Fig. 6B).
Figure 6.
Cancellous but not cortical bone is preserved in OVX ERαLysM−/−mice. A and B, Micro-CT measurements were made in bones of the 28-wk-old mice described in Fig. 2 (n = 10–13 mice per group). A, Representative images of vertebral cancellous bone (top) and analysis of vertebral and femoral cancellous bone (bottom). †, P < 0.05 vs. respective sham-operated mice. μ-CT, μ-computed tomography. B, Cortical thickness determined in femurs. BV/TV, bone volume per tissue volume; Tb, trabecular; ns, not significant; *, P < 0.05.
Estrogens induce osteoclast apoptosis via a nonclassical ERα action
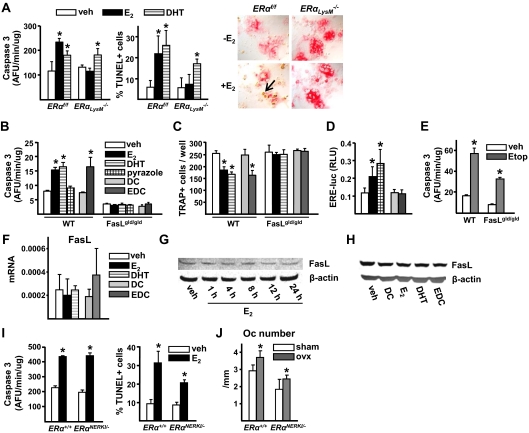
As expected, 17β-estradiol (E2) at 10−8 m increased osteoclast apoptosis in cells from ERαf/f control mice, as determined by either TUNEL (terminal deoxynucleotide transferase-mediated dUTP nick end labeling) or caspase 3 activity assays (Fig. 7A). E2, however, had no effect on the apoptosis of osteoclasts from ERαLysM−/− mice, confirming earlier evidence that the proapoptotic effect of estrogens on mature osteoclasts is indeed cell autonomous (14). In contrast to E2, the nonaromatizable androgen dihydrotestosterone (DHT) promoted apoptosis of osteoclasts from ERαLysM−/− mice and littermate controls, demonstrating that the insensitivity of the ERαLysM−/− cells to estrogens was indeed sex steroid specific.
Figure 7.
Estrogens induce osteoclast apoptosis via a nonclassical ERα action. A, Caspase 3 activity (left panel) or TUNEL assay (middle panel) in mature osteoclasts generated from bone marrow cells from C57BL/6 mice cultured with M-CSF and RANKL for 5 d and treated with vehicle (veh), E2, or DHT (10−8 m) for 12 h. The photomicrographs (×40) show representative areas of the osteoclast culture stained with TUNEL (right panel); the arrow indicates an apoptotic osteoclast. AFU, Arbitrary fluorescent units. B, Caspase 3 activity (right panel) in mature osteoclasts, developed from nonadherent bone marrow cells from FasLgld/gld or wild-type mice, and treated with veh or 10−8 m of each of the indicated compounds for 12 h. C, Number of TRAP-positive cells generated from bone marrow cells from the same mice as in panel C, cultured with M-CSF and RANKL for 5 d in the presence of 10−8 m of the indicated compounds. D, C2C12 cells were transfected with an ERE-luc reporter plasmid and treated with 10−8 m of each of the indicated compounds for 24 h. Luciferase activity is depicted as relative luciferase units (RLU) normalized for Renilla activity. Bars represent the mean ± sd of triplicate determinations, *, P < 0.05 vs. vehicle. E, Caspase 3 activity in mature osteoclasts developed from nonadherent bone marrow cells from wild-type or FasLgld/gld mice and treated with vehicle (veh) or 50 μm etoposide for 12 h. Bars, Mean ± sd of triplicate determinations, *, P < 0.05 vs. respective vehicle. F, mRNA levels of FasL by quantitative RT-PCR in mature osteoclasts generated as in panel A and treated with veh, E2, DHT, dendrimer conjugate, and EDC (10−8 m) for 6 h. G, Western blot analysis of FasL in mature osteoclasts treated with 10−8 m E2 for the indicated times. H, Western blot analysis of FasL in mature osteoclasts treated with 10−8 m concentration of the indicated compounds for 6 h. I, Caspase 3 activity (left panel) or TUNEL assay (right panel) in mature osteoclasts [generated in bone marrow cultures from ERαNERKI/− or wild-type littermates as described in panel A treated for 12 h with vehicle (veh) or E2 (10−8 m]. J, Number of osteoclasts in cancellous bone of undecalcified vertebral sections (L1–L3) from 26-wk-old female ERα+/+ littermate control and ERαNERKI/− mice ovariectomized 6 wk earlier (n = 6 mice per group). Means were calculated from triplicate determination unless indicated otherwise. *, P < 0.05 vs. respective veh or sham-operated controls. DC, dendrimer conjugate; Etop, etoposide; Oc, osteoclast; WT, wild type.
Because of the results of Nakamura et al. and Krum et al. (17,18) suggesting that the proapoptotic effect of E2 on osteoclasts results from stimulation of FasL expression through an estrogen response element (ERE)-mediated mechanism of ERα action, we proceeded to examine the role of FasL on the proapoptotic actions of estrogens on osteoclasts using bone marrow aspirates from wild-type or FasLgld/gld mice that lack FasL, as well as from mice bearing an ERα knock-in mutation that prevents binding to DNA, designated as ERαNERKI/− (20).
Both E2 and DHT increased mature osteoclast apoptosis in wild-type controls of the FasLgld/gld mice (Fig. 7B). 1,3,5-Tris (4-hydroxylphenyl)-4-propylpyrazole-triol designated hereafter as pyrazole, a synthetic high-affinity ERα ligand that is equipotent to E2 in inducing ERE-mediated transcription, as confirmed here (Fig. 7D), but lacks the ability of E2 to activate cytoplasmic kinases (22), however, failed to stimulate osteoclast apoptosis in cells from these mice. On the other hand, a polymeric form of estrogen (estrogen dendrimer conjugate, abbreviated EDC) that is not capable of stimulating the nuclear-initiated actions of ERα, also confirmed here (Fig. 7D), but can activate kinases (23,24), was as effective as E2 in stimulating osteoclast apoptosis (Fig. 7B). E2, DHT, and EDC also decreased the number of osteoclasts in bone marrow cultures from the wild-type controls (Fig. 7C). None of these compounds, however, could stimulate osteoclast apoptosis or decrease osteoclast number in cultures from the FasLgld/gld mice (Fig. 7, B and C), indicating that FasL is required for the proapoptotic effect of all these agents. The topoisomerase inhibitor etoposide did stimulate osteoclast apoptosis in cells from the FasLgld/gld mice, eliminating the possibility that cells from these mice are resistant to all inducers of apoptosis (Fig. 7E).
Strikingly, in spite of the requirement of FasL for the proapoptotic effects of E2, DHT, and EDC, none of these compounds was able to stimulate the expression of FasL mRNA in mature osteoclasts (Fig. 7F), confirming several previous reports that estrogens do not stimulate FasL in osteoclasts (18,25,26,27). Consistent with the lack of an effect at the mRNA level, there was no change in FasL protein levels in mature osteoclasts treated with E2 between 1 and 24 h (Fig. 7G). And, like E2, DHT and EDC had no effect on the FasL protein in mature osteoclasts treated with these compounds for 6 h (Fig. 7H). The lack of an effect of these compounds on FasL mRNA and protein was reproduced in three independent experiments. Parallel experiments were performed in macrophages from wild-type mice, generated by culturing bone marrow cells with macrophage colony-stimulating factor (M-CSF) for 5 d. As in mature osteoclasts, E2, DHT, and EDC had no effect on FasL mRNA or protein in macrophages (data not shown).
In strong support of the rest of the evidence against an ERE-mediated mechanism of estrogen action in osteoclasts, E2 was as effective in promoting osteoclast apoptosis in cells from ERαNERKI/− mice as it was in cells from wild-type (ERα+/+) controls (Fig. 7I). Moreover, female ERαNERKI/− mice exhibited an increase in the number of osteoclasts in vertebral cancellous bone sections after loss of estrogens, similar to that observed in the ERα+/+ littermates (Fig. 7J).
Discussion
In the work described in this paper, we have selectively deleted ERα in cells of the monocyte/macrophage lineage in mice and found that the number of osteoclast progenitors was 2-fold higher as was the number of osteoclasts in cancellous bone. In addition, our ERα LysM−/− mice exhibited a decrease in cancellous bone mass. After OVX these mice did not show the expected increase in osteoclast progenitors, the number of osteoclasts in bone, or further loss of cancellous bone. However, they lost cortical bone indistinguishably from their littermate controls. The proapoptotic effect of estrogens on osteoclasts was completely abrogated in the ERαLysM−/− mice but was unaffected in the ERαNERKI/− mice. Moreover, a polymeric form of estrogen that is not capable of stimulating the nuclear-initiated actions of ERα was as effective as E2 in inducing osteoclast apoptosis in cells with the wild-type ERα. These results demonstrate that estrogens attenuate osteoclast generation and life span via cell-autonomous effects. These effects, however, cannot be exerted by the classical estrogen response element (ERE)-mediated mechanism of estrogen action. More important, our findings strongly suggest that, although elimination of the proapoptotic effect of estrogens on osteoclasts is sufficient for loss of bone in the cancellous compartment in which complete perforation of trabeculae by osteoclastic resorption precludes subsequent refilling of the cavities by the bone-forming osteoblasts, the effects of estrogens on osteoblasts and perhaps other cell types are indispensable for their protective effects on the cortical compartment, which constitutes 80% of the skeleton.
The Flox-Cre mice of the present report exhibited a 60–80% deletion of the targeted gene and had a clear-cut phenotype. This is in agreement with two previous publications that also demonstrated efficient deletion of conditional alleles in osteoclasts using the LysM promoter (28,29). In a third paper by Aliprantis et al. (30) the authors also detected deletion of NFATc1 in osteoclasts, but in that instance it was not sufficient to yield a phenotype. This indicates that different conditional alleles are deleted with varying efficiency using the LysM-Cre gene.
In agreement with our findings in the murine model used here, loss of estrogen at menopause leads to two structurally different forms of bone loss, which differ in cellular mechanisms and rate as well as biomechanical effects (31). Excessive depth of resorption cavities in the thin rods and plates of cancellous bone, evidently caused by an increase in the number and life span of osteoclasts, leads to the perforation of structural elements and discontinuation of the bone structure. This results in rapid bone loss that cannot be compensated by osteoblast refilling of the resorption cavity, simply because the template is no longer there. In cortical bone, osteoclastic resorption causes subendosteal cavitation and transformation of the inner third of the cortex to a cancellous-like structure, which then undergoes the same changes as the cancellous bone. The second form of bone loss results from incomplete refilling of the resorption cavities by osteoblasts and is responsible for slower bone loss. Incomplete refilling by osteoblasts causes simple thinning, as opposed to perforation and complete loss of structural elements, in both cancellous and cortical bone. Based on this knowledge, we submit that cortical bone was unaffected in estrogen-replete ERαLysM−/− mice because unhindered osteoblast life span counteracted the loss of the direct effect on cells of the osteoclastic lineage. Normal osteoblast life span, on the other hand, could not counteract increased osteoclastic resorption in cancellous bone because refilling of the cavities was impossible as a result of the complete perforation of trabeculae and thereby elimination of the necessary template on which osteoblasts perform their bone-forming function.
The demonstration of increased osteoclast progenitors in our study, but not in the one by Nakamura et al., most likely reflects the fact that the ERα in our mice was deleted in osteoclast precursors, whereas in theirs it was deleted only in mature osteoclasts. In our studies, 6 wk of estrogen deprivation caused a significant loss of cortical bone, but no loss of cancellous bone. In contrast, in the Nakamura study there was no bone loss in either the cortical or the cancellous bone compartment after 2 wk of estrogen deprivation. This dichotomy strongly suggests that prolongation of mature osteoclast life span is sufficient for the rapid loss of cancellous bone that ensues after the loss of estrogens, but prolongation of mature osteoclast life span is insufficient for the loss of cortical bone in the estrogen-deficient state. In other words, an increase in osteoclastogenesis combined with the increased osteoclast life span, as well as additional effects on other cell types, are required for the manifestation of bone loss in cortical sites. We therefore conclude that cortical bone loss after OVX was not seen in the Nakamura study most likely because they did not allow sufficient time for estrogen deficiency to produce its adverse effects on this compartment.
Similar to the observations of Nakamura et al. (17), we found that E2 could not stimulate the apoptosis of ERα-deficient mature osteoclasts. Likewise, E2 could not induce the apoptosis of osteoclasts derived from FasL-deficient mice. However, in contrast to the results of Nakamura et al. (17), but in agreement with the findings of Krum et al. (18) as well as several other studies (25,26,27), we were unable to demonstrate a stimulatory effect of E2 on FasL production in primary cultures of murine osteoclasts. FasL-deficient osteoclasts were also resistant to the proapoptotic effect of the nonaromatizable androgen DHT. Strikingly, a polymeric form of estrogen that is not capable of stimulating the nuclear-initiated actions of ERα (23,24) stimulated osteoclast apoptosis in wild-type osteoclasts. Conversely, pyrazole, an ERα-specific ligand that lacks the ability to activate ERKs even though is fully capable of activating ERα-mediated transcription, did not affect osteoclast apoptosis. More to the point, E2 stimulated osteoclast apoptosis in cultures from mice with an ERα mutant that does not bind to DNA, as effectively as in wild-type controls. Furthermore, OVX increased the number of osteoclasts in mice with this mutation. It is very important to note here that the ERαNERKI/− mice lose cancellous bone mass after OVX similar to wild-type littermates (9,32). Collectively, these results contradict the conclusions of Nakamura et al. and Krum et al., that the effects of estrogens on osteoclast apoptosis are mediated by a classic genotropic mechanism of ER action. Instead, our findings strongly suggest that, as in the case of the effects of estrogens on osteoblast generation and life span (9,11,12,14), the effects of estrogens on osteoclast life span are mediated via an extranuclear function of the ERα. In the present study, DHT induced apoptosis of osteoclasts lacking the ERα. Therefore, if DHT were to stimulate osteoclast apoptosis via a transcriptional mechanism similar to that proposed by Nakamura et al. and Krum et al. for estrogens, such effect must be mediated by an androgen response element on the FasL gene promoter.
Mitochondria and reactive oxygen species (ROS) play a crucial role in osteoclast differentiation and function (33,34,35,36,37). Moreover, the protective effects of estrogens on bone, including the attenuation of osteoclastogenesis and osteoclast life span, evidently result from their ability to attenuate oxidative stress (14,38,39). Depending on the cell type, ROS may be required for FasL-induced apoptosis (40), play no role (41), or even act as an antiapoptotic signal in Fas-activated cells (42). Considering all the available evidence together, it is very unlikely that the ability of E2, the EDC, and DHT to promote osteoclast apoptosis in a FasL-dependent fashion results from an ERE-mediated stimulation of FasL expression. Instead, FasL must provide a tonic stimulatory signal for osteoclast apoptosis that is potentiated by estrogens or androgens via one or more nontranscriptional mechanisms: 1) attenuation of the osteoclastogenic and antiapoptotic effect of the receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL), secondary to decreasing ROS production by stimulating glutathione reductase activity (37,38,39); 2) attenuation of the antiapoptotic effect of NF-kB via protein-protein interaction of the ERα with NF-κB (6,43,44,45,46), which for example will attenuate the ability of NF-κB to inhibit TNF receptor I-induced apoptosis via augmentation of the synthesis of cellular Fadd-like IL-1β-converting enzyme-inhibitory protein (47,48); and 3) suppression of the transcriptional activity of c-jun (49). The view that stimulation of FasL in osteoclasts is unlikely to be the mechanism by which estrogens promote osteoclast apoptosis is supported further by evidence that estrogens have the opposite effect, i.e. prevent the apoptosis of osteoblasts (12,13,14), even though murine and human osteoblasts do undergo Fas-mediated apoptosis in response to FasL (50,51,52,53,54). This evidence is particularly incongruent with the contention of Krum and colleagues (18) that osteoblasts are the source of FasL for the apoptosis of osteoclasts.
Deletion of the ERα by means of the LysM promoter in this work led to an increase in the number of macrophages in the bone marrow, strongly suggesting that estrogens acting through the ERα directly control macrophage production and/or life span. Future work will be necessary to elucidate the extent to which these particular effects of estrogens contribute to the antiinflammatory properties of these hormones (55).
In summary, the evidence reported in this paper indicates that estrogens attenuate osteoclast generation and life span via cell-autonomous actions mediated by DNA binding-independent action of ERα. Loss of these actions is sufficient for loss of bone in the cancellous compartment in which complete perforation of trabeculae by osteoclastic resorption precludes subsequent refilling of the cavities by the bone-forming osteoblasts. On the other hand, estrogen actions on other cell types, such as the prolongation of osteoblast life span, inexorably participate in the protective effects of estrogens on the cortical compartment.
Materials and Methods
Generation of conditional ERα mice
To generate a conditional allele for ERα, a targeting vector was constructed in which ERα exon 3 was flanked by loxP sites (Fig. 1). The targeting vector was used to electroporate embryonic stem (ES) cells, and a correctly targeted clone was injected into blastocysts to generate chimeric mice. After germline transmission of the targeted allele, the frt-flanked neomycin cassette was removed by crossing with mice expressing the FLPe recombinase in germ cells (The Jackson Laboratory, Bar Harbor, ME; stock no. 003946). Mice harboring the conditional ERα allele were backcrossed into the C57BL/6 strain for six generations before crossing with any Cre-expressing lines. To disrupt the ERα gene in macrophage/monocyte lineage cells, ERαf/f mice were crossed with mice expressing the Cre recombinase under the control of lysozyme M gene-regulatory elements (LysM-Cre) (19). Experimental mice were generated by breeding ERαf/+ mice heterozygous for the LysM-Cre allele with ERαf/f mice.
Genotyping by Southern blotting and PCR
To detect the targeted locus, genomic DNA was digested with either PstI or SacI, and the fragments were separated on 0.7% agarose gel. Southern blotting was performed as described previously (56). PCR was performed in 20-μl reactions using the REDExtract-N-Amp PCR reagent (Sigma), 400 nm each primer, and 4 μl of the tail genomic DNA. The primers used for detection of the Δneo ERα-loxP allele were as follows: 5′-forward: TCGTTTTGAATTAATTATGAATGTCTG and 3′-reverse: AGATTATCCATAAAATAACAAAA TGCT. These primers produce an amplicon of 83 bp from the wild-type ERα allele or 232 bp from the ERαf allele. The primers used for detection of ERα exon 3 deletion were as follows: 5′-forward: TCGTTTTGAATTAATTATGAATGTCTG and 3′-reverse: TTCATGTGTTGTGCAAATAGC. These primers detect an amplicon of 277 bp after exon 3 deletion. The primers used for LysM-Cre detection were as follows: 5′-forward: GCGGTCTGGCAGTAAAAACTATC and 3′-reverse: GTGAAACAGCATTGCTGTCACTT. The PCR program was as follows: initial denaturation for 3 min at 94 C followed by 35 cycles of 45 sec at 94 C, 45 sec at 55 C, 1 min at 72 C, and a final extension of 10 min at 72 C.
Animal experimentation
ERαNERKI/+ (20) and ERα+/− mice (57) were provided by J. Larry Jameson (Northwestern University, Chicago, IL) and Pierre Chambon (Institute for Genetics and Cellular and Molecular Biology, Strasbourg, France), respectively. ERαNERKI/+ mice were crossed with heterozygous ERα+/− female mice to produce animals carrying only one NERKI allele (ERαNERKI/−). Twenty-two-week-old female ERαf/f mice and their ERαLysM−/− littermates, and ERαNERKI/− mice and their ERα+/+ littermates were sham operated or ovariectomized. Animals were killed 6 wk later, and the tissues were dissected for further analyses. FasLgld/gld mice were purchased from The Jackson Laboratory. All procedures were approved by Institutional Animal Care and Use Committees of the University of Arkansas for Medical Sciences and the Central Arkansas Veterans Healthcare System.
DEXA BMD
BMD measurements were performed by DEXA using a PIXImus-II densitometer (GE Healthcare Lunar, Piscataway, NJ) as previously described (58).
Micro-CT
Micro-CT analysis of the distal end of the femora and the fifth lumbar vertebrae was done after the bones were dissected, cleaned, fixed in 10% Millonig's formalin, and transferred to ethanol, loaded into 12.3-mm diameter scanning tubes and imaged (μCT40; Scanco, Akron, OH). Scans were integrated into three dimensional voxel images (1024 × 1024 pixel matrices for each individual planar stack), and a Gaussian filter (ς = 0.8, support = 1) was used to reduce signal noise. A threshold of 200 was applied to all scans, at medium resolution (E = 55 kVp, I = 145 μA, integration time = 200 msec). The entire vertebral body was scanned with a transverse orientation excluding any bone outside the vertebral body. In the distal femur, 151 transverse slices were taken from the epicondyles and extending toward the proximal end of the femur. The cortical bone and the primary spongiosa were manually excluded from the analysis. All trabecular measurements were made by drawing contours every 10 to 20 slices and using voxel counting for bone volume per tissue volume and sphere-filling distance transformation indices, without preassumptions about the bone shape as a rod or plate for trabecular microarchitecture. Cortical thickness was measured at the femoral mid-diaphysis.
Bone histology
Mice were injected with tetracycline 6 and 2 d before harvesting. Isolated bone samples were processed for histology. The lumbar vertebrae (L1, L2, and L3) were fixed in 10% Millonig's formalin, transferred to 100% ethanol, and embedded undecalcified in methyl methacrylate and the histomorphometric examination was performed in longitudinal sections using the OsteoMeasure Analysis System (OsteoMetrics, Inc., Decatur, GA) as previously described (59,60). Vertebral cancellous bone measurements were restricted to the secondary spongiosa. BFR was defined as the distance between the double labels divided by the interval between the fluorochrome administrations and multiplied by the sum of the double-labeled perimeter plus one half of the single-labeled perimeter.
Transient transfections and luciferase assay
C2C12 cells were plated on a 48-well plate and 16 h later transfected with 0.1 μg of green fluorescent protein, 0.1 μg of and ERE-luciferase reporter plasmid (provided by D. McDonnell, Duke University, Durham, NC), and 0.2 μg of pcDNA using Lipofectamine Plus (Invitrogen, Carlsbad, CA). Cells were treated 24 h later with the different compounds for another 24 h. Luciferase activity was determined using the Dual-LuciferaseReporter assay system (Promega Corp., Madison, WI), according to the manufacturer's instructions. Light intensity was measured with a luminometer, and luciferase activity was divided by the Renilla activity (control reporter) to normalize for transfection efficiency.
Cell culture
To quantify osteoclast progenitor cells, bone marrow was flushed from long bones and plated at different densities: 25,000, 35,000, and 50,000 cells/cm2 in 48-well plates. After 4–5 d of culture in α-MEM medium (Invitrogen), supplemented with 10% fetal bovine serum, 1% penicillin, streptomycin, glutamine, 30 ng/ml M-CSF, and 30 ng/ml sRANKL (R&D Systems, Minneapolis, MN), osteoclasts were fixed with 10% neutral buffered formalin for 15 min and stained for tartrate-resistant acid phosphatase (TRAP). Multinuclear TRAP+ cells were quantified. Macrophages and osteoclasts were developed from bone marrow cells flushed from femurs and tibias of 2- to 3-month-old mice and plated in α-MEM supplemented with 10% fetal bovine serum and 1% penicillin, streptomycin, glutamine. Twenty-four hours later, nonadherent cells were replated and cultured either for 4 d in the presence of 130 ng/ml M-CSF to obtain macrophages, or for 4 d in the presence of 30 ng/ml RANKL and 30 ng/ml M-CSF to obtain osteoclasts.
Apoptosis assays
Osteoclast apoptosis was evaluated 12 h after the addition of vehicle, E2, DHT (Sigma-Aldrich), EDC, or pyrazol (provided by John A. Katzenellenbogen, University of Illinois). The EDC was used within 3 months from its preparation, a time point at which it has been established that there is no disassociation of E2 from the dendrimer. The TUNEL method was performed using the FragEL DNA fragmentation detection kit (EMD Chemicals, San Diego, CA) in cells previously stained for TRAP. Multinuclear TRAP-positive and TUNEL-positive cells were enumerated. Caspase-3 activity was measured by determining the degradation of the fluorometric substrate DEVD (Biomol Research Laboratories, Plymouth Meeting, PA), and protein concentration was measured using a Bio-Rad detergent-compatible kit (Bio-Rad Laboratories, Hercules, CA) as described previously (61).
Quantitative RT-PCR
Total RNA was extracted using Ultraspec (Biotecx Laboratories, Houston, TX) and reverse transcribed using the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. Taqman quantitative RT-PCR was performed as previously described (62). The primers and probes for murine ERα (Mm00433148_mH), FasL (Mm00438864_m1), and rRNA18S (Mm00475528_m1) were manufactured by the TaqMan Gene Expression Assays service (Applied Biosystems). Relative mRNA expression levels were normalized to the housekeeping gene ribosomal protein S2 using the ΔCt method (63).
Western blot analysis
FasL and β-actin levels in cultured osteoclasts were determined using a rabbit polyclonal antibody for FasL and a mouse monoclonal antibody for β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA).
Statistical analysis
All data are reported as the mean ± sd. Group mean values were compared, as appropriate, by Student's unpaired two-tailed t test or one-way ANOVAs with Bonferroni's multiple comparison test. A P value ≤0.05 was considered significant.
Acknowledgments
We thank J. A. Katzenellebongen, Illinois University; J. L. Jameson, Northwestern University; and P. Chambon, Institute for Genetics and Cellular and Molecular Biology, Strasburg, France, for the provision of critical materials and animals. We also thank A. Warren, R. Shelton, K. Vyas, A. DeLoose, X. Qiu, W. Webb, C. Wicker III, and S. Berryhill from the Osteoporosis and Metabolic Bone Diseases Center, University of Arkansas for Medical Sciences for technical assistance; and Beth Bailey for help with the preparation of the manuscript.
Footnotes
This work was supported by the National Institutes of Health (P01 AG13918, R01 AR49794); the Department of Veterans Affairs (Merit Review grants to S.C.M., R.S.W. and R.L.J. and a Veterans Affairs Research Enhancement Award Program); and Tobacco Settlement funds provided by the University of Arkansas for Medical Sciences. M.M.M. is recipient of an award from Marques de Valdecilla Foundation, Santander, Spain. E.A. is supported by a Ph.D. fellowship from the University of Pisa, Italy.
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 6, 2010
Abbreviations: BMD, Bone mineral density; CT, computerized tomography; DEXA, dual energy x-ray absorptiometry; DHT, dihydrotestosterone; E2, 17β-estradiol; EDC, estrogen dendrimer conjugate; ER, estrogen receptor; ERE, estrogen response element; FasL, Fas ligand; LysM, lysozyme M; M-CSF, macrophage colony-stimulating factor; NF-κB, nuclear factor-κB; OVX, ovariectomy; Pyrazole, 1,3,5-Tris(4-hydroxylphenyl)-4-propylpyrazole-triol; RANKL, receptor activator of NF-κB ligand; ROS, reactive oxygen species; TRAP, tartrate-resistant acid phosphatase; TUNEL, terminal deoxynucleotide transferase-mediated dUTP nick end labeling.
References
- Manolagas SC 2000 Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21:115–137 [DOI] [PubMed] [Google Scholar]
- Jilka RL, Bellido T, Almeida M, Plotkin LI, O'Brien CA, Weinstein RS, Manolagas SC 2008 Apoptosis and bone cells. In: Bilezikian JP, Raisz LG, Martin T, eds. Principles of bone biology. San Diego: Academic Press; 235–259 [Google Scholar]
- Manolagas SC 2006 Perspective: choreography from the tomb: an emerging role of dying osteocytes in the purposeful, and perhaps not so purposeful, targeting of bone remodeling. BoneKey-Osteovision 3:5–14; 10.1138/20060193 [Google Scholar]
- Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, Bellido T 2006 Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res 21:605–615 [DOI] [PubMed] [Google Scholar]
- Verborgt O, Gibson GJ, Schaffler MB 2000 Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res 15:60–67 [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Kousteni S, Jilka RL 2002 Sex steroids and bone. Recent Prog Horm Res 57:385–409 [DOI] [PubMed] [Google Scholar]
- Jilka RL, Hangoc G, Girasole G, Passeri G, Williams DC, Abrams JS, Boyce B, Broxmeyer H, Manolagas SC 1992 Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science 257:88–91 [DOI] [PubMed] [Google Scholar]
- Di Gregorio GB, Yamamoto M, Ali AA, Abe E, Roberson P, Manolagas SC, Jilka RL 2001 Attenuation of the self-renewal of transit amplifying osteoblast progenitors in the murine bone marrow by 17β-estradiol. J Clin Invest 107:803–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M, Martin-Millan M, Ambrogini E, Han L, Weinstein RS, Jilka RL, O'Brien CA, Manolagas SC 12 October 2009 Estrogens attenuate oxidative stress, osteoblast differentiation and apoptosis by DNA binding-independent actions of the ERα. J Bone Miner Res 10.1359/jbmr.09107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DE, Dai A, Tiffee JC, Li HH, Mundy GR, Boyce BF 1996 Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-β. Nat Med 2:1132–1136 [DOI] [PubMed] [Google Scholar]
- Usui M, Yoshida Y, Tsuji K, Oikawa K, Miyazono K, Ishikawa I, Yamamoto T, Nifuji A, Noda M 2004 Tob deficiency superenhances osteoblastic activity after ovariectomy to block estrogen deficiency-induced osteoporosis. Proc Natl Acad Sci USA 101:6653–6658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC 2001 Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104:719–730 [PubMed] [Google Scholar]
- Kousteni S, Chen JR, Bellido T, Han L, Ali AA, O'Brien CA, Plotkin L, Fu Q, Mancino AT, Wen Y, Vertino AM, Powers CC, Stewart SA, Ebert R, Parfit AM, Weinstein RS, Jilka RL, Manolagas SC 2002 Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science 298:843–846 [DOI] [PubMed] [Google Scholar]
- Chen JR, Plotkin LI, Aguirre JI, Han L, Jilka RL, Kousteni S, Bellido T, Manolagas SC 2005 Transient versus sustained phosphorylation and nuclear accumulation of ERKs underlie anti-versus pro-apoptotic effects of estrogens. J Biol Chem 280:4632–4638 [DOI] [PubMed] [Google Scholar]
- Kameda T, Mano H, Yuasa T, Mori Y, Miyazawa K, Shiokawa M, Nakamaru Y, Hiroi E, Hiura K, Kameda A, Yang NN, Hakeda Y, Kumegawa M 1997 Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J Exp Med 186:489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousteni S, Han L, Chen JR, Almeida M, Plotkin LI, Bellido T, Manolagas SC 2003 Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest 111:1651–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S 2007 Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell 130:811–823 [DOI] [PubMed] [Google Scholar]
- Krum SA, Miranda-Carboni GA, Hauschka PV, Carroll JS, Lane TF, Freedman LP, Brown M 2008 Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J 27:535–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I 1999 Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 8:265–277 [DOI] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL 2002 An estrogen receptor (ER)α deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol 16:2188–2201 [DOI] [PubMed] [Google Scholar]
- Kousteni S, Almeida M, Han L, Bellido T, Jilka RL, Manolagas SC 2007 Induction of osteoblast differentiation by selective activation of kinase-mediated actions of the estrogen receptor. Mol Cell Biol 27:1516–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA 2000 Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-α-selective agonists. J Med Chem 43:4934–4947 [DOI] [PubMed] [Google Scholar]
- Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, Katzenellenbogen BS 2006 Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol 20:491–502 [DOI] [PubMed] [Google Scholar]
- Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS 2008 Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol 22:2116–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saintier D, Khanine V, Uzan B, Ea HK, de Vernejoul MC, Cohen-Solal ME 2006 Estradiol inhibits adhesion and promotes apoptosis in murine osteoclasts in vitro. J Steroid Biochem Mol Biol 99:165–173 [DOI] [PubMed] [Google Scholar]
- Park H, Jung YK, Park OJ, Lee YJ, Choi JY, Choi Y 2005 Interaction of Fas ligand and Fas expressed on osteoclast precursors increases osteoclastogenesis. J Immunol 175:7193–7201 [DOI] [PubMed] [Google Scholar]
- Kovacić N, Lukić IK, Grcević D, Katavić V, Croucher P, Marusić A 2007 The Fas/Fas ligand system inhibits differentiation of murine osteoblasts but has a limited role in osteoblast and osteoclast apoptosis. J Immunol 178:3379–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenner L, Hoebertz A, Beil T, Keon N, Karreth F, Eferl R, Scheuch H, Szremska A, Amling M, Schorpp-Kistner M, Angel P, Wagner EF 2004 Mice lacking JunB are osteopenic due to cell-autonomous osteoblast and osteoclast defects. J Cell Biol 164:613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Zhao H, Kitaura H, Bhattacharyya S, Brewer JA, Muglia LJ, Ross FP, Teitelbaum SL 2006 Glucocorticoids suppress bone formation via the osteoclast. J Clin Invest 116:2152–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliprantis AO, Ueki Y, Sulyanto R, Park A, Sigrist KS, Sharma SM, Ostrowski MC, Olsen BR, Glimcher LH 2008 NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest 118:3775–3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt AM 1984 Age-related structural changes in trabecular and cortical bone: cellular mechanisms and biomechanical consequences. Calcif Tissue Int 36 (Suppl 1):S123–S128 [DOI] [PubMed] [Google Scholar]
- Syed F, Mödder UI, Fraser DG, Spelsberg TC, Rosen CJ, Krust A, Chambon P, Jameson JL, Khosla S 2005 Skeletal effects of estrogen are mediated by opposing actions of classical and non-classical estrogen receptor pathways. J Bone Miner Res 20:1992–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR 1990 Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest 85:632–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levasseur R, Barrios R, Elefteriou F, Glass II DA, Lieberman MW, Karsenty G 2003 Reversible skeletal abnormalities in γ-glutamyl transpeptidase-deficient mice. Endocrinology 144:2761–2764 [DOI] [PubMed] [Google Scholar]
- Bai XC, Lu D, Liu AL, Zhang ZM, Li XM, Zou ZP, Zeng WS, Cheng BL, Luo SQ 2005 Reactive oxygen species stimulates receptor activator of NF-κB ligand expression in osteoblast. J Biol Chem 280:17497–17506 [DOI] [PubMed] [Google Scholar]
- Lean JM, Jagger CJ, Kirstein B, Fuller K, Chambers TJ 2005 Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology 146:728–735 [DOI] [PubMed] [Google Scholar]
- Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, Kim N, Lee SY 2005 A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 106:852–859 [DOI] [PubMed] [Google Scholar]
- Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC 2007 Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 282:27285–27297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, Urry ZL, Chambers TJ 2003 A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest 112:915–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Azad N, Kongkaneramit L, Chen F, Lu Y, Jiang BH, Rojanasakul Y 2008 The Fas death signaling pathway connecting reactive oxygen species generation and FLICE inhibitory protein down-regulation. J Immunol 180:3072–3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug H, Enari M, Nagata S 1994 No requirement of reactive oxygen intermediates in Fas-mediated apoptosis. FEBS Lett 351:311–313 [DOI] [PubMed] [Google Scholar]
- Aronis A, Melendez JA, Golan O, Shilo S, Dicter N, Tirosh O 2003 Potentiation of Fas-mediated apoptosis by attenuated production of mitochondria-derived reactive oxygen species. Cell Death Differ 10:335–344 [DOI] [PubMed] [Google Scholar]
- Galien R, Evans HF, Garcia T 1996 Involvement of CCAAT/ enhancer-binding protein and nuclear factor-κB binding sites in interleukin-6 promoter inhibition by estrogens. Mol Endocrinol 10:713–722 [DOI] [PubMed] [Google Scholar]
- Galien R, Garcia T 1997 Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-κB site. Nucleic Acids Res 25:2424–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B, Yang MX 1995 Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-κB and C/EBP β. Mol Cell Biol 15:4971–4979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell DP, Norris JD 1997 Analysis of the molecular pharmacology of estrogen receptor agonists and antagonists provides insights into the mechanism of action of estrogen in bone. Osteoporos Int 7(Suppl 1):S29–S34 [DOI] [PubMed] [Google Scholar]
- Temkin V, Karin M 2007 From death receptor to reactive oxygen species and c-Jun N-terminal protein kinase: the receptor-interacting protein 1 odyssey. Immunol Rev 220:8–21 [DOI] [PubMed] [Google Scholar]
- Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, Liu YC, Karin M 2006 The E3 ubiquitin ligase itch couples JNK activation to TNFα-induced cell death by inducing c-FLIP(L) turnover. Cell 124:601–613 [DOI] [PubMed] [Google Scholar]
- Shevde NK, Bendixen AC, Dienger KM, Pike JW 2000 Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci USA 97:7829–7834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC 1998 Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res 13:793–802 [DOI] [PubMed] [Google Scholar]
- Kawakami A, Eguchi K, Matsuoka N, Tsuboi M, Koji T, Urayama S, Fujiyama K, Kiriyama T, Nakashima T, Nakane PK, Nagataki S 1997 Fas and Fas ligand interaction is necessary for human osteoblast apoptosis. J Bone Miner Res 12:1637–1646 [DOI] [PubMed] [Google Scholar]
- Kawakami A, Nakashima T, Tsuboi M, Urayama S, Matsuoka N, Ida H, Kawabe Y, Sakai H, Migita K, Aoyagi T, Nakashima M, Maeda K, Eguchi K 1998 Insulin-like growth factor I stimulates proliferation and Fas-mediated apoptosis of human osteoblasts. Biochem Biophys Res Commun 247:46–51 [DOI] [PubMed] [Google Scholar]
- Kogianni G, Mann V, Ebetino F, Nuttall M, Nijweide P, Simpson H, Noble B 2004 Fas/CD95 is associated with glucocorticoid-induced osteocyte apoptosis. Life Sci 75:2879–2895 [DOI] [PubMed] [Google Scholar]
- Duque G, El Abdaimi K, Henderson JE, Lomri A, Kremer R 2004 Vitamin D inhibits Fas ligand-induced apoptosis in human osteoblasts by regulating components of both the mitochondrial and Fas-related pathways. Bone 35:57–64 [DOI] [PubMed] [Google Scholar]
- Georgiadou P, Sbarouni E 2009 Effect of hormone replacement therapy on inflammatory biomarkers. Adv Clin Chem 47:59–93 [DOI] [PubMed] [Google Scholar]
- Fu Q, Manolagas SC, O'Brien CA 2006 Parathyroid hormone controls receptor activator of NF-κB ligand gene expression via a distant transcriptional enhancer. Mol Cell Biol 26:6453–6468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M 2000 Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 127:4277–4291 [DOI] [PubMed] [Google Scholar]
- O'Brien CA, Jilka RL, Fu Q, Stewart S, Weinstein RS, Manolagas SC 2005 IL-6 is not required for parathyroid hormone stimulation of RANKL expression, osteoclast formation, and bone loss in mice. Am J Physiol Endocrinol Metab 289:E784–E793 [DOI] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Takahashi K, Parfitt AM, Manolagas SC 1996 Linkage of decreased bone mass with impaired osteoblastogenesis in a murine model of accelerated senescence. J Clin Invest 97:1732–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC 1998 Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of their deleterious effects on bone. J Clin Invest 102:274–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T 1999 Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest 104:1363–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S 2005 Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by β-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem 280:41342–41351 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]