Abstract
Cartilage has a poor intrinsic healing response, and neither the innate healing response nor current clinical treatments can restore its function. Therefore, articular cartilage tissue engineering is a promising approach for the regeneration of damaged tissue. Because cartilage is exposed to mechanical forces during joint loading, many tissue engineering strategies use exogenous stimuli to enhance the biochemical or biomechanical properties of the engineered tissue. Hydrostatic pressure (HP) is emerging as arguably one of the most important mechanical stimuli for cartilage, although no optimal treatment has been established across all culture systems. Therefore, this review evaluates prior studies on articular cartilage involving the use of HP, with a particular emphasis on the treatments that appear promising for use in future studies. Additionally, this review addresses HP bioreactor design, chondroprotective effects of HP, the use of HP for chondrogenic differentiation, the effects of high pressures, and HP mechanotransduction.
Introduction
Injuries to articular cartilage generally result in the formation of mechanically inferior fibrocartilage, which will eventually degrade with use.1 Additionally, current clinical treatments for articular cartilage injuries generally aim to enhance this intrinsic repair response but may often result in the formation of fibrocartilage. Tissue engineering approaches provide tremendous promise for cartilage regeneration. A principal tenet of the cartilage tissue engineering approach is the use of exogenous mechanical stimulation to simulate joint loading and lead to greater chondrocyte metabolic activity and extracellular matrix (ECM) production. Hydrostatic pressure (HP) provides a robust method of chondrocyte stimulation, because it can be applied to chondrocytes in monolayer, three-dimensional (3-D) engineered constructs, as well as explants.
Cartilage is a highly hydrated tissue, comprised of 70% to 80% water per wet weight. The high water content is a result of water being attracted to the negatively charged proteoglycan molecules within the tissue. During joint loading, a uniform normal stress (Fig. 1) is imparted to the chondrocytes. Additionally, as the tissue undergoes a compressive load, the pressurization of the fluid phase initially supports the applied load, because water is trapped within the solid matrix of the tissue because of its low permeability. Although the total stress from pressurization is uniform according to the biphasic theory, the stress may vary throughout the cartilage on a joint surface, because loading is not completely uniform, thus leading to gradients in total stress and pressure, particularly near the joint surface. Eventually, fluid is expelled from the tissue, and the frictional force between the fluid and solid phases of the tissue dissipates energy from the applied load. In the joint, cartilage is typically exposed to stresses between 3 and 10 MPa,2 with stress as high as 18 MPa having been reported in the hip joint.3 These stresses should be translated to HP due to fluid phase pressurization, as described above. Additionally, the human walking cadence generally is up to 1 Hz.4 As such, tissue engineering efforts have generally focused on magnitudes and frequencies within these physiologic ranges. This review addresses bioreactor design for the application of HP, different tissue engineering strategies involving the application of HP, the chondroprotective effects of HP, the use of HP toward chondrogenic differentiation, the effects of high pressures on cartilage, and the mechanotransduction mechanisms that explain the beneficial results from HP application in cartilage tissue engineering studies.
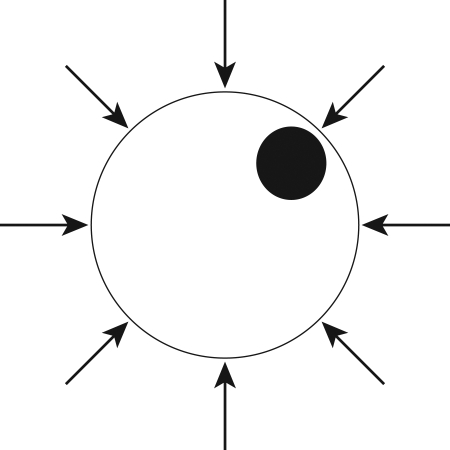
FIG. 1.
Illustration of a chondrocyte exposed to hydrostatic pressure. The cell experiences a uniform normal stress, without any measurable tissue strain.
HP Bioreactors
In general, there are two predominant methods of applying HP to cells, explants and constructs, and they both offer advantages and disadvantages. In the first method, HP is applied by compressing a gas phase that transmits load through the medium to the cells, but this method is limited because pressurizing the gas phase may alter the gas concentration within the culture medium. For instance, Hansen et al.5 observed a 0.36 decrease in the pH of the medium after 10 h of HP application, although the advantage of this approach is that it allows for the controlled alteration of partial pressures within the medium, such as when examining the effects of HP at different oxygen levels.6 Alternatively, a less-complicated approach involves applying HP by compressing only the fluid phase, which limits any changes in gas solubility within the chamber, although because no gas phase alterations are made during pressurization, this method cannot be used when examining the effects of HP at different oxygen levels. This method generally involves connecting a fluid-filled chamber by hose to a piston attached directly to a hydraulic press controlled by a computer (Fig. 2). This is the selected setup in a large number of prior studies, as well as in our own work. Both types of bioreactors also include temperature control, generally by placing the chamber in a water bath, to maintain the culture temperature at 37°C. Furthermore, it is possible to perform experiments with multiple bioreactors to increase the number per group; although this approach is advantageous, because it allows for more-efficient HP application, there may be some variation in the applied pressure conditions between the different bioreactors. Finally, either type of bioreactor may be altered to allow for semicontinuous medium perfusion, as reviewed in detail previously.7
FIG. 2.
Representative hydrostatic pressure (HP) bioreactor design. (a) Computer controls (Instron), which compress piston and generate pressure within chamber. Chamber is placed in water bath to maintain temperature at 37°C. (b) HP chamber, with pressure sensor to verify pressures applied within the chamber. Color images available online at www.liebertonline.com/ten.
Tissue Engineering Strategies with HP
HP has seen extensive use as an agent for increasing the metabolic activity of chondrocytes in tissue engineering studies. In general, these studies have assessed the effects of HP on chondrocytes cultured in monolayer, cartilage explants, and chondrocytes in 3-D culture, with and without a scaffold. In tissue engineering studies involving HP application, it is possible to vary the magnitude, frequency, and duration of application of HP. Additionally, in studies involving 3-D engineered constructs, it is also possible to vary when HP can be applied in construct development. However, little consensus has been reached regarding the ideal levels of each of these parameters, particularly when different culture conditions are used. Table 1 summarizes the major findings of studies that used dynamic HP; the effects of static HP are summarized in Table 2.
Table 1.
Effects of Dynamic Hydrostatic Pressure (HP) on Chondrocytes and Cartilage Tissue Engineering
| Study | Cells | Culture conditions | HP conditions | Main findings |
|---|---|---|---|---|
| Suh et al.8 | 1- to 2-y/o bovine chondrocytes | Monolayer | 0.8 MPa for 5 min on, 30 min off, 10 times | 40% increase in proteoglycan synthesis, enhanced aggrecan mRNA |
| Jortikka et al.9 | 1- to 2-y/o bovine chondrocytes | Monolayer | 5 MPa, 0.5 Hz, for 20 h | Significant increase in sGAG incorporation |
| Smith et al.10 | Adult bovine chondrocytes | Monolayer | 10 MPa HP, 1 Hz for 4 h | Increased aggrecan and collagen II mRNA |
| Smith et al.11 | Adult bovine chondrocytes | Monolayer | 10 MPa, 1 Hz, up to 24 h for 1 day | Increased aggrecan mRNA up to 24 h, maximal increase in collagen II mRNA with 4- and 8-h application |
| Smith et al.11 | Adult bovine chondrocytes | Monolayer | 10 MPa, 1 Hz, for 4 h/d for 4 days | Significant increase in aggrecan and collagen II mRNA |
| Ikenoue et al.12 | Adult human chondrocytes | Monolayer | 1, 5, 10 MPa, 1 Hz, 4 h/d, for 1 or 4 days | Enhanced aggrecan expression with all treatments, enhanced collagen II expression only with 5- and 10-MPa, for 4 days |
| Parkkinen et al.14 | Bovine chondrocytes | Monolayer | 5 MPa, 0.5 Hz, for 1.5 h | Inhibition of sGAG incorporation |
| Parkkinen et al.14 | Adult bovine chondrocytes | Explant | 5 MPa, 0.5 Hz, for 1.5 h | Enhanced sGAG incorporation |
| Carver and Heath15 | Adult P3 equine chondrocytes | PGA mesh | 3.44 or 6.87 MPa, 0.25 Hz, for 20 min every 4 h for 5 weeks | 6.87 MPa increased both GAG and collagen production |
| Carver and Heath15 | 1- to 2-y/o P3 equine chondrocytes | PGA mesh | 3.44 or 6.87 MPa, 0.25 Hz, for 20 min every 4 h for 5 weeks | Both magnitudes increased GAG production; only 6.87-MPa increased collagen production |
| Carver and Heath16 | 1- to 2-y/o P3 equine chondrocytes | PGA mesh | 3.44 MPa, 0.25 Hz, 20 min every 4 h for 5 weeks | Significant increase in GAG production, no effect on collagen production |
| Hu and Athanasiou17 | Immature bovine chondrocytes | Scaffoldless 3-D construct | 10 MPa, 1 Hz, 4 h/day, 5 days/w for up to 8 weeks | Increased collagen production at 4 wks and 8 wks |
| Elder and Athanasiou22 | Immature bovine chondrocytes | Scaffoldless 3-D construct | 1, 5, 10 MPa, 0.1 or 1 Hz, for 1 h/d, from days 10-14 | 10 MPa, 1 Hz significantly increased compressive stiffness and GAG production |
y/o, year-old; mRNA, messenger RNA; sGAG, sulfated glycosaminoglycan; P3, passage 3; PGA, poly(glycolic acid); 3-D, three-dimensional.
Table 2.
Effects of Static Hydrostatic Pressure (HP) on Chondrocytes and Cartilage Tissue Engineering
| Study | Cells | Culture conditions | HP conditions | Main findings |
|---|---|---|---|---|
| Jortikka et al.9 | 1- to 2-y/o bovine chondrocytes | Monolayer | 5 MPa, static, for 20 h | No effect on sGAG incorporation |
| Smith et al.10 | Adult bovine chondrocytes | Monolayer | 10 MPa, static, for 4 h | Decreased collagen mRNA levels |
| Takahashi et al.13 | Chondrosarcoma cell line | Monolayer | 1 and 5 Mpa, static, for 2 h | Significant increase in sGAG incorporation for both magnitudes, increased transforming growth factor beta 1 mRNA with 5 MPa |
| Hall et al.18 | 2-y/o bovine chondrocytes | Explant | 5-15 MPa, static, for 2 h | 5-10 MPa enhanced sGAG incorporation, 5-15 MPa increased proline incorporation |
| Mizuno et al.19 | Immature bovine chondrocytes | 3-D collagen sponges | 2.8 MPa, static, for up to 15 days | Increased GAG production at 5 and 15 days of culture |
| Toyoda et al.20 | Immature bovine chondrocytes | 2% agarose gels | 5 MPa, static, for 4 h | 4 times as much aggrecan mRNA, 50% increase in collagen II mRNA |
| Toyoda et al.21 | Immature bovine chondrocytes | 2% agarose gels | 5 MPa, static, for 4 h | 11% increase in GAG production, 4 times as much aggrecan mRNA |
| Elder and Athanasiou22 | Immature bovine chondrocytes | Scaffoldless 3-D construct | 1, 5, 10 MPa, static, for 1 h/d, from days 10-14 | 5 and 10 MPa significantly increased compressive and tensile stiffness and GAG and collagen production |
| Elder and Athanasiou25 | Immature bovine chondrocytes | Scaffoldless 3-D construct | 10 MPa, static, for 1 h/d, from days 6-10, 10-14, or 14-18 | 10- to 14-day application had greatest effect on compressive and tensile stiffness, and GAG and collagen production |
y/o, year-old; mRNA, messenger RNA; sGAG, sulfated glycosaminoglycan; 3-D, three-dimensional.
In general, studies assessing the effects of HP on normal chondrocytes in monolayer have demonstrated beneficial effects of dynamic HP, whereas static HP has been found to have no effect or a suppressive effect. For instance, Suh et al.8 cultured young bovine chondrocytes in monolayer and exposed them to 0.8 MPa, for 5 min on, 30 min off, 10 times. This treatment resulted in a 40% increase in proteoglycan synthesis and enhanced aggrecan messenger RNA (mRNA), although there was no change in collagen synthesis during pressurization. Also, when using juvenile bovine chondrocytes in monolayer, Jortikka et al.9 demonstrated that HP at 5 MPa, 0.5 Hz, for 20 h significantly increased sulfated glycosaminoglycan (sGAG) incorporation, whereas 5-MPa static HP for the same application time had no effect on sGAG incorporation.
In addition to these studies using chondrocytes from 1- to 2-year-old animals, several studies using adult chondrocytes in monolayer have been performed and have generally demonstrated beneficial effects of dynamic treatment, with no effects or suppressive effects when using static HP. For example, Smith et al.10 exposed adult articular chondrocytes in monolayer to 10-MPa HP, static or 1 Hz, for 4 h. They demonstrated that HP application at 1 Hz increased aggrecan and collagen II mRNA immediately after application, whereas static HP decreased collagen mRNA levels. In a later study, Smith et al.11 cultured normal adult bovine chondrocytes in monolayer and applied HP at 10 MPa, 1 Hz, for up to 24 h for 1 day or for 4 h/d for 4 days. They found that aggrecan mRNA continued to increase up to 24 h of loading, whereas collagen II mRNA expression was increased maximally with 4 and 8 h of HP application. However, they demonstrated the importance of examining multiple loading profiles, because changing to an application of 4 h/d for 4 days led to even greater increases in aggrecan and collagen II mRNA. Additionally, Ikenoue et al.12 again demonstrated the importance of examining multiple application times, culturing normal adult human chondrocytes in monolayer and exposing them to 1-, 5-, or 10-MPa HP, at 1 Hz, for 4 h/d for 1 or 4 days. They demonstrated enhanced collagen II gene expression only for treatment with 5 and 10 MPa, for 4 days; also, although greater aggrecan expression was observed with all treatments, these groups resulted in the greatest enhancement of aggrecan gene expression. This study also indicates that magnitude and frequency have significant effects on chondrocyte metabolism, and it appears that collagen production may be more sensitive than GAG production to the selected HP regimen. On the other hand, a study by Takahashi et al.13 demonstrated beneficial effects when applying static HP to chondrocytes in monolayer, although it must be highlighted that a chondrosarcoma cell line was used rather than primary chondrocytes. They found that 1- and 5-MPa static HP for 2 h resulted in significantly greater sGAG incorporation immediately after HP stimulation and that 5-MPa static HP led to greater expression of transforming growth factor beta 1 (TGF-β1) mRNA. Although these studies generally demonstrate that dynamic HP has beneficial effects on chondrocytes in monolayer, it is difficult to discern an optimal HP regimen because the selected magnitudes, frequencies, and application times are relatively divergent.
Studies assessing the effects of HP on chondrocytes in 3-D culture or in explants have demonstrated different results, and it has been suggested that chondrocytes in monolayer respond differently to HP than tissue. For instance, Parkkinen et al.14 observed enhanced sGAG incorporation in explants exposed to HP at 5 MPa, 0.5 Hz, for 1.5 h, whereas a significant inhibition of sGAG incorporation was found in monolayer cultures exposed to the same regimen. Furthermore, Carver and Heath15 observed that adult and juvenile P3 equine chondrocytes in poly(glycolic acid) (PGA) meshes respond differently to HP at 3.44 or 6.87 MPa, at 0.25 Hz, for 20 min every 4 h for 5 weeks. For adult cells, 6.87-MPa HP was required to increase GAG and collagen production, whereas for juvenile cells, either magnitude increased GAG production, but only 6.87 MPa increased collagen production, suggesting that collagen production may be more sensitive to the applied regimen. In a later study, applying HP at 3.44 MPa, 0.25 Hz, for 20 min every 4 h for 5 weeks to P3 juvenile bovine chondrocytes in PGA meshes resulted in significantly greater GAG production, with no effect on collagen production; however, the results of both studies may stem from the use of passaged chondrocytes.16 Finally, in our own work,17 exposing immature bovine chondrocytes in scaffoldless constructs to HP at 10 MPa, 1 Hz, for 4 h/d, 5 days/week for up to 8 weeks with 10% fetal bovine serym led to greater collagen content than in control at 4 and 8 weeks and prevented the lower GAG per construct observed in the control groups over time.
Additionally, contrary to the majority of studies involving chondrocytes in monolayer, static HP regimens in the physiologic range have generally demonstrated beneficial effects on chondrocytes in 3-D culture or cartilage explants. For example, in explants from 2-year-old bovines, a 2-h application of 5- to 10-MPa static HP enhanced sGAG incorporation, whereas 5- to 15-MPa static HP increased proline incorporation.18 Also, Mizuno et al.19 exposed immature bovine chondrocytes in 3-D collagen sponges to static HP at 2.8 MPa for up to 15 days and observed greater GAG production at 5 and 15 days of culture. Similarly, Toyoda et al.20 found that exposing immature bovine chondrocytes in 2% agarose gels to 5-MPa static HP for 4 h resulted in 4 times as much aggrecan mRNA as well as 50% more collagen II mRNA. In another study using the same constructs and HP regimen, Toyoda et al.21 observed 11% more GAG production and 4 times as much aggrecan mRNA. Also, in our own recent work,22 using scaffoldless articular cartilage constructs as described previously,23,24 a full-factorial comparison was made between three magnitudes (1, 5, and 10 MPa) and three frequencies (static, 0.1, and 1 Hz) of HP for 1 h/d, from days 10 to 14 of construct development. It was determined that static HP at 5 or 10 MPa and cyclic HP at 10 MPa, 1 Hz, resulted in significant greater compressive stiffness and GAG production; however, only static HP at 5 or 10 MPa resulted in significantly greater tensile stiffness and collagen production. An additional exciting finding of the study was the additive and synergistic effects when applying HP and growth factors, with the combination of 10 MPa static HP and TGF-β1 resulting in 164% and 231% greater compressive and tensile stiffness, respectively, as well as 85% and 173% greater GAG and collagen production, respectively. An additional study from our group examined the effects of HP applied at different times during construct development. It was demonstrated that HP applied at 10 MPa for 1 h/d from days 10 to 14 had the greatest effect on construct compressive and tensile properties, as well as GAG and collagen content, when applied from days 10 to 14 of construct development.25 The results of these studies are promising because they indicate that static HP, within physiologic magnitudes, results in greater ECM production and eventually better biomechanical properties. However, different effects result depending on the exact culture conditions selected.
When comparing these 3-D studies, it must be noted that there are important differences between the 3-D culture systems that may alter the response of the cells to HP. For instance, explants provide the normal cell–ECM interactions, and it is likely that the scaffoldless constructs used in our own work resulted in similar cell–ECM interactions by the time HP was applied to the constructs. However, it is possible that materials such as collagen sponges provide initial binding motifs that are not necessarily normal, and cells may therefore spread. On the other hand, scaffolds such as agarose hydrogels are inert and provide no motifs for interaction, although cells can interact with the ECM after it begins to form later in culture.
Although physiologic magnitudes clearly have beneficial effects on chondrocyte gene expression, protein production, and biomechanical properties, based on the varying results observed in these studies regarding effects of HP regimens, magnitude, frequency, and application time must all be optimized for each system. It is possible that a regimen may yield beneficial effects in a 3-D culture system while simultaneously resulting in little effect or a detrimental effect in a monolayer system. Additionally, the cell type used appears to play a significant role in the response to HP application, with immature bovine chondrocytes appearing to have a greater metabolic response to HP stimulation than adult human chondrocytes. Finally, performing HP studies on 3-D constructs leads to additional issues, because the optimal time to begin applying HP during construct development must be determined. Perhaps application of HP early in culture might yield similar results to monolayer studies because of the absence of abundant ECM, whereas studies later in construct development when a significant ECM is present may yield substantially different results.
Chondroprotective Effects of HP
HP also appears to be useful in providing chondroprotective effects to chondrocytes subjected to an inflammatory stimulus. For instance, the application of HP at 10 MPa, 1 Hz, for 12 or 24 h to human osteoarthritic chondrocytes in monolayer resulted in less expression of matrix metalloproteinase (MMP)-2, interleukin (IL)-6, and monocyte chemoattractant protein (MCP)-1.26 Additionally, Lee et al.27 demonstrated chondroprotective effects of HP on human osteoarthritic chondrocytes in monolayer. They found that applying HP at 10 MPa, 1 Hz, for 4 h, after an 18-h treatment with the known inflammatory mediator lipopolysaccharide (LPS) mitigated the damaging effects of LPS, because there were low nitric oxide and nitric oxide synthase levels, which are known to have deleterious effects on ECM production. There were also higher collagen II and aggrecan mRNA levels than in unpressurized cells treated with LPS. A later study by Lee et al.28 identified chondroprotective effects of HP after shear stimulation, because the application of HP at 10 MPa, 1 Hz, to human osteoarthritic chondrocytes after shear stress inhibited nitric oxide release. Additional chondroprotective effects of HP on osteoarthritic chondrocytes were observed in work by Fioravanti et al.,29 because coupling HP with exogenous application of hyaluronic acid resulted in significant chondroprotective effects from the deleterious effects of IL-1β treatment on chondrocyte metabolism; significantly greater GAG production was also noted. Furthermore, Gavenis et al.30 found that applying 40 kPa of HP at 0.0125 Hz to human osteoarthritic chondrocytes resulted in 53.3% greater GAG content by 14 days; however, GAG/dry weight remained only 0.06%. These results indicate that primary osteoarthritic chondrocytes could potentially be used in tissue engineering strategies, which is exciting, because they would of a somewhat readily available autologous cell source. However, it must be mentioned that Islam et al.31 observed an increase in the number of apoptotic cells when applying HP at 5 MPa, 1 Hz, for 4 h to osteoarthritic human chondrocytes in monolayer, indicating that osteoarthritic chondrocytes may be sensitive to the selected HP regimen.
Additionally, because tissue engineered constructs will eventually need to be implanted, it may be that constructs, once implanted, might benefit from HP. As such, chondroprotective effects have also been demonstrated in normal primary chondrocytes. HP at 5 MPa, 0.5 Hz, 3 h/d for 3 days led to upregulation of tissue inhibitor of metalloproteinases-1 and downregulation of MMP-13 and collagen I gene expression in bovine chondrocytes cultured in alginate beads.32 These results appear promising because they indicate that HP could possibly be used after implantation of an engineered construct, as well as to delay the onset of osteoarthritis in already healthy tissue.
HP and Differentiation
In addition to its wide use as an agent for mechanical stimulation in tissue engineering, there has bee tremendous use of HP as a method of differentiating cells toward a chondrogenic phenotype, as summarized in Table 3. For instance, Angele et al.33 cultured adult human bone marrow mesenchymal stem cells (BMSCs) in aggregate culture, and found that HP at 5 MPa, 1 Hz, 4 h/d for 1 day had no effect, whereas 7 days of treatment resulted in a significant increase in collagen and GAG content as early as 7 days after removal of the HP stimulus, with a maximal increase observed 21 days after removal of the HP stimulus. This study suggests that multiple days of HP application are required for an effect, and as seen in our own work with HP,22 the maximum effects of HP may be delayed until several weeks after removal of the stimulus. In a later study, Luo and Seedhom34 seeded ovine BMSCs on polyester scaffolds and demonstrated that after 4 weeks of culture, HP at 0.1 MPa, 0.25 Hz, for 30 min/d for 10 days resulted in increased GAG and collagen content, with shorter timepoints having no effect on collagen content. However, because the constructs were assessed immediately after HP application, it is possible that the longer application time required to see effects on collagen content may be due to the delayed effects of the earlier days of HP application, as discussed above. Additionally, Wagner et al.35 seeded human BMSCs in type I collagen sponges and observed that HP at 1 MPa, 1 Hz, for 4 h/d for 10 days resulted in increased aggrecan, collagen II, and sox9 messenger RNA (mRNA) and increased histological staining for GAGs. However, they also observed an increase in collagen I mRNA; this effect may have been due to the use of an osteochondrogenic medium, because most other studies have used only a chondrogenic medium. The finding of Scherer et al.6 that a chondrogenic medium was required for HP to promote chondrogenesis of bovine BMSCs in high-density monolayer further supports this hypothesis. These results are exciting because they indicate the potential for using dynamic HP to develop a cell source for future cartilage tissue engineering studies, because chondrogenic differentiation of MSCs represents a relatively limitless autologous cell source.
Table 3.
Effects of Hydrostatic Pressure (HP) on Chondrogenic Differentiation
| Study | Cells | Culture conditions | HP conditions | Main findings |
|---|---|---|---|---|
| Angele et al.33 | Human BMSCs | Aggregate culture | 5 MPa, 1 Hz, for 4 h/d, for 1 or 7 days | 7-day treatment increased collagen and GAG content; 1 day treatment had no effect |
| Luo and Seedhom34 | Ovine BMSCs | Polyester scaffolds | 0.1 MPa, 0.25 Hz, 30 min/d, for 10 days | Increased GAG and collagen content |
| Wagner et al.35 | Human BMSCs | Type I collagen sponges | 1 MPa, 1 Hz, 4 h/d, for 10 days | Enhanced aggrecan, collagen II, and sox9 mRNA, but increased collagen I mRNA levels |
| Miyanishi et al.36 | Human BMSCs | Pellet culture with 10 ng/mL TGF-β3 | 10 MPa, 1 Hz, 4 h/d, for up to 14 days | HP and TGF-β3 treatment increased collagen II, aggrecan, and sox9 mRNA levels |
| Miyanishi et al.37 | Human BMSCs | Pellet culture with 10 ng/mL TGF-β3 | 0.1, 1, or 10 MPa, at 1 Hz, 4 h/d, for 3, 7, or 14 days | All magnitudes increased aggrecan and sox9 mRNA, but only 10 MPa increased collagen II mRNA |
| Elder et al.38 | Murine embryonic fibroblasts | Monolayer | 5 MPa, 1 Hz, 7200 cycles/d, for 3 days | 200% increase in GAG production, 225% increase in collagen synthesis |
| Heyland et al.39 | Dedifferentiated porcine chondrocytes | Alginate beads | 0.3 MPa, 1 Hz, for 6 h/d | 25% increase in GAG production, 65% increase in collagen II production |
| Kawanishi et al.40 | Dedifferentiated bovine chondrocytes | Pellet culture | 5 MPa, 0.5 Hz, 4 h/d, for 4 days | 5 times as much aggrecan mRNA and 4 times as much collagen mRNA, no effect on collagen I mRNA levels |
BMSC, bone marrow stromal cell; y/o, year-old; mRNA, messenger RNA; GAG, glycosaminoglycan; TGF-β3, transforming growth factor beta 3.
As described above, in tissue engineering studies, combined treatment with growth factors and HP as agents for chondrogenesis appears promising. For example, Miyanishi et al.36 cultured adult human BMSCs in pellet culture and exposed them to HP at 10 MPa, 1 Hz, 4 h/d for up to 14 days, with and without 10 ng/mL of TGF-β3. The combined treatment with HP and TGF-β3 resulted in a significant increase in collagen II, aggrecan, and sox9 mRNA levels that was greater than the increased levels from either treatment alone. In a follow-up study, Miyanishi et al.37 created pellet cultures of adult human BMSCs and applied HP at 0.1, 1, and 10 MPa, 1 Hz, for 4 h/d for 3, 7, or 14 days, along with 10 ng/mL of TGF-β3. In this study, all magnitudes significantly increased aggrecan and sox9 mRNA, but only 10 MPa significantly increased collagen II mRNA. Furthermore, 10 MPa was the only treatment to significantly increase both GAG and collagen production, with the maximum effect observed after 14 days of HP application. Again, this observation may be due to the delayed effects of HP as discussed above, and it would be interesting to determine whether similar results would be obtained if assessment was delayed until 5 to 10 days after removal of the HP stimulus. These results indicate that growth factor application, specifically using TGF-β3, may substantially enhance the chondrogenic differentiation potential of HP alone.
HP has also been used as a method of chondroinduction of other cell types, such as fibroblasts and dedifferentiated chondrocytes. For instance, Elder et al.38 found that 7200 cycles per day of HP at 5 MPa, 1 Hz, for 3 days applied to murine embryonic fibroblasts in monolayer resulted in an almost 200% increase in GAG production, along with an almost 225% increase in collagen synthesis. Additionally, Heyland et al.39 cultured dedifferentiated porcine chondrocytes in alginate beads and observed a 25% increase in GAG production, as well as a 65% increase in collagen II production after HP application at 0.3 MPa, 1 Hz, for 6 h/d. Finally, Kawanishi et al.40 grew pellet cultures of dedifferentiated bovine chondrocytes (passage 3) and demonstrated that HP application at 5 MPa, 0.5 Hz, for 4 h/d for 4 days led to 5 times as much aggrecan mRNA and 4 times as much collagen II mRNA. However, HP had a negligible effect on collagen I mRNA levels, with control and HP treated pellets having similar decreases in collagen I mRNA. Based on these results, HP appears to be a promising method of differentiating cells to a chondrocytic phenotype, although in the case of dedifferentiated chondrocytes, HP may have a greater effect on enhancing collagen II production than diminishing collagen I production.
Effects of High HPs
As described above, there has been extensive work demonstrating the beneficial effects of physiologic magnitudes of HP on the gene expression and biochemical and biomechanical properties of chondrocytes in monolayer, engineered constructs, as well as explants. However, raising pressures above these physiologic levels has been shown to have limited or even detrimental effects, as summarized in Table 4. Also, although static HP has generally been shown to have beneficial effects when using physiologic magnitudes for 3-D tissue engineering studies, it becomes far more detrimental than dynamic loading at higher pressures. For example, Hall et al.18 examined the effects of 20- to 50-MPa static HP for 20 s, 5 min, or 2 h on bovine explants and found that short-term application times had no effect on GAG and collagen synthesis rates, whereas 2-h application resulted in a significant decrease in GAG and collagen synthesis. In another 3-D study, Nakamura et al.41 seeded normal adult rabbit chondrocytes in alginate beads and found that 50-MPa static HP for 12 or 24 h resulted in a significant increase in the number of apoptotic cells. Additionally, they found that 50-MPa static HP led to a dramatic increase in heat-shock protein 70 (hsp70) mRNA. In another study, Fioravanti et al.42 studied the effects of high HP on normal human chondrocytes in alginate beads and found that 24-MPa static HP applied for 3 h decreased mitochondria and Golgi body number and altered the actin and tubulin of normal chondrocytes such that they more closely resembled osteoarthritic cells in these characteristics. In general, it has been demonstrated that applying HP to 3-D cultures above the physiologic range results in decreased ECM production, as well as a heat shock response.
Table 4.
Effects of High Hydrostatic Pressure (HP)
| Study | Cells | Culture conditions | HP conditions | Main findings |
|---|---|---|---|---|
| Hall et al.18 | Adult bovine chondrocytes | Explant | 20-50 MPa static, for 20 s, 5 min, or 2 h | 2-h application decreased GAG and collagen synthesis, but no effect with short-term HP application |
| Nakamura et al.41 | Adult rabbit chondrocytes | Alginate beads | 50 MPa, static, for 12 or 24 h | 50 MPa increased number of apoptotic cells and increased hsp70 mRNA |
| Fioravanti et al.42 | Adult human chondrocytes | Alginate beads | 24 MPa, static, for 3 h | Decreased mitochondria and Golgi body number; altered actin and tubulin |
| Parkkinen et al.43 | Bovine chondrocytes | Monolayer | 30 MPa, static, for 2 h | Compaction of Golgi body and decreased GAG synthesis |
| Parkinnen et al.44 | Bovine chondrocytes | Monolayer | 30 MPa, static, for 2 h | Complete loss of stress fibers |
| Lammi et al.45 | Bovine chondrocytes | Monolayer | 30 MPa, static | 37% decrease in GAG synthesis, decreased aggrecan mRNA levels |
| Sironen et al.46 | Immortalized human chondrocytes | Monolayer | 30 MPa, static, for up to 24 h | Static HP increased expression of stress response genes |
| Sironen et al.47 | Human chondrosarcoma cell line | Monolayer | 30 MPa, static, for 6 h | Decreased osteonectin, fibronectin, and procollagen gene levels |
| Takahashi et al.48 | Human chondrosarcoma cell line | Monolayer | 50 MPa, static, for 2 h | Increased interleukin-6 and tumor necrosis factor alpha mRNA, and decreased expression of proteoglycan core protein |
| Kaarniranta et al.49 | Human chondrosarcoma cell line | Monolayer | 30 MPa, static, for 12 h | Doubling of hsp70 mRNA |
mRNA, messenger RNA; hsp, heat shock protein; GAG, glycosaminoglycan.
Several studies on chondrocytes in monolayer have shown similar detrimental results. For instance, Parkkinen et al.43 assessed the effects of high pressures on bovine chondrocytes in monolayer and found that a 2-h application of 30-MPa static HP, and to a lesser extent 15-MPa static HP, resulted in a microtubule-dependent compaction of the Golgi apparatus, with a concomitant decrease in GAG synthesis, but 15- and 30-MPa HP at 0.05 or 0.125 Hz had no effect on the Golgi apparatus or GAG synthesis. Similarly, in a later study, Parkinnen et al.44 assessed the effects of 2 h of HP stimulation on bovine chondrocytes in monolayer and found that 30-MPa static HP led to a reversible complete loss of stress fibers, whereas 30-MPa HP at 0.05 or 0.125 Hz just changed the appearance of the stress fibers. It was suggested that the altered stress fibers may be the result of small strains on the cells or microfilaments or of alterations in the intracellular ion concentrations, as described further below. In a similar study using bovine chondrocytes in monolayer, Lammi et al.45 found that 30-MPa static HP resulted in a 37% decrease in GAG synthesis, accompanied by decreased aggrecan mRNA levels; this treatment also resulted in the production of atypically large aggrecan molecules. These results are interesting because the altered aggrecan size demonstrates that HP can affect production of ECM at the level of post-translation, in addition to the aforementioned effects on transcription and translation.
Detrimental effects of high HP have also been observed in other chondrocyte-like cell lines cultured in monolayer. For example, Sironen et al.46 assessed the effects of 30-MPa HP, static or 1 Hz, for up to 24 h on immortalized human chondrocyte cell lines and chondrosarcoma cells cultured in monolayer. They found that static HP resulted in significantly increased hsp70, hsp40, Gadd45, and Gadd153 gene expression, all of which are genes associated with stress responses. Additionally, they demonstrated that static HP had a greater effect on the increased gene expression than HP at 1 Hz. In a separate study, Sironen et al.47 used a complementary DNA array to assess the effects of a 6-h treatment with 30-MPa static HP on human chondrosarcoma cells. This treatment had negative effects on the ECM content, leading to decreased osteonectin, fibronectin, and procollagen levels. Furthermore, Takahashi et al.48 found that, in a human chondrosarcoma cell line, a 2-h application of 50-MPa static HP significantly increased IL-6 and TNF-α mRNA and also led to decreased expression of proteoglycan core protein; these results are indicative of osteoarthritic changes. Finally, Kaarniranta et al.49 observed a doubling of hsp70 mRNA after 12 h of treatment with 30-MPa static HP, whereas treatment with 30-MPa, 0.5 Hz HP did not change the level of hsp70. However, treatment with 4-MPa HP, either static or dynamic, did not alter the expression of hsp70.
Taken together, these results suggest that HP magnitudes outside of the physiologic range may result in a stress response, especially when using static HP, so tissue engineering strategies should focus on more physiologic magnitudes. Although it is unlikely that these higher pressures are useful for cartilage tissue engineering strategies, they indicate that high pressures may play a role in the progression of osteoarthritis, because many osteoarthritic changes can be observed in chondrocytes exposed to these high pressures.
HP Mechanotransduction
Unlike direct compression and shear mechanical stimulation, HP does not result in macroscopic deformation of cartilage. According to the biphasic theory, the solid matrix of cartilage is intrinsically incompressible, and no tissue deformation will be observed under an external hydrostatic load, even though the tissue may be anisotropic. Bachrach et al.50 tested this theoretical prediction on normal bovine cartilage explants and found that static pressures in the physiologic range, up to 12 MPa, did not result in measurable cartilage deformation. Similarly, Tanck et al.51 found that physiologic HP magnitudes on fetal cartilage result only in extremely small deformations of approximately 2 μ-strain, as a result of the relative incompressibility of the solid matrix of articular cartilage. However, even though there is only a minute tissue strain, this, along with the strain imparted by the compressibility of water itself, may be great enough to impart strain on the chondrocytes themselves, although it has previously been demonstrated that the cells are relatively incompressible at these physiologic pressures.52 Therefore, because HP generally produces a state of stress with no or little strain, alternative mechanisms have been proposed to explain the mechanotransduction pathways of HP application.
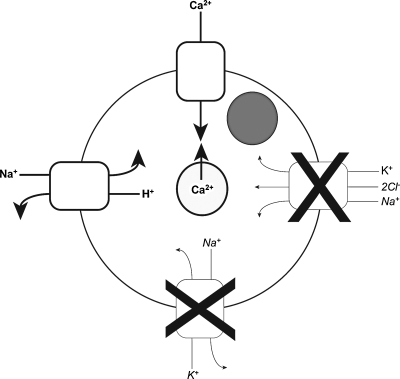
Several studies have indicated that it is likely that HP has direct effects on cell membrane ion channels (Fig. 3). Hall53 examined the effects of static HP on isolated bovine chondrocytes for 20 s or 10 min and found that the sodium–potassium (Na/K) pump was substantially inhibited when going from 2.5 to 5 MPa and that this inhibition increased slightly when pressure was increased up to 50 MPa. For example, 10-MPa static HP for 10 min resulted in 53% less activity of the Na/K pump than in a control. Additionally, the Na/K/chloride (2Cl) transporter was inhibited by increasing pressure up to 50 MPa, and it was found that increasing the magnitude of 10 s of static HP application from 7.5 to 15 MPa resulted in an almost 40% reduction in Na/K/2Cl transporter activity. An additional study by Browning et al.54 examined the effects of static HP, ranging from 2 to 30 MPa for up to 180 s, on juvenile bovine chondrocytes in monolayer. They found that 20- and 30-MPa static HP application resulted in a significant increase in the activity of the Na/hydrogen(H) pump. Furthermore, adding the kinase inhibitor staurosporine prevented the HP-induced stimulation of Na/H exchange, suggesting that direct activation of the transporter is a phosphorylation-dependent process. In a similar study, Mizuno55 assessed the effects of 5 min of static HP at 0.5 MPa on immature bovine articular chondrocytes in monolayer and found that the application of HP to middle zone cells resulted in a doubling of intracellular calcium. It was determined that this increase was dependent upon direct effects of HP on stretch-activated calcium channels, as well as the release of intracellular calcium. Likewise, Browning et al.56 assessed the effects of short-term application of static HP on isolated juvenile bovine articular chondrocytes. They found that 30 s of static HP application at 30 MPa resulted in an approximate 3tripling in intracellular calcium, largely caused by calcium release from intracellular stores. Additionally, they found that this intracellular calcium release was dependent on inositol triphosphate (IP3) mediation and that similar induction of IP3-mediated calcium release occurred at more-physiologic pressure magnitudes, such as 10 MPa.
FIG. 3.
Hydrostatic pressure mechanotransduction. Pressurization inhibits sodium/potassium (Na/K) and Na/K/chloride channels, whereas it activates Na/hydrogen and stretch-activated calcium (Ca) channels, and triggers release of intracellular Ca stores.
As reviewed previously,57 it is likely that the direct effects of HP on transmembrane ion transporter function are due to the pressure's effects on the conformations of the transmembrane proteins. Although HP does not measurably deform cartilage because of the intrinsically incompressible nature of its phases, the transporter proteins themselves have void spaces created by their folding orientation that can be compressed. As these spaces undergo increased strain as pressure rises, the protein will eventually alter its orientation to achieve a lower-energy folding state. Thus, as described above, a pressure-dependent change in intracellular ion concentrations will be observed. It is widely known that alterations in intracellular ion concentrations result in changes in cellular gene expression and protein synthesis.58 Thus, it is likely that specific pressures result in certain ion concentration changes that led to the specific effects on gene expression, protein production, and eventually biomechanical properties in the tissue engineering studies described previously.
Conclusions
Cartilage regeneration has been an extremely difficult problem because of the poor intrinsic healing capacity of the tissue, but mechanical stimulation with HP has provided significant beneficial effects on gene expression and protein production of chondrocytes in monolayer and has led to enhanced biochemical and biomechanical properties in engineered constructs. It is apparent that physiologic magnitudes, particularly between 5 and 10 Mpa, have beneficial effects on cartilage properties, although there are substantial differences in the effects observed between monolayer and 3-D culture, because static HP regimens have little effect or are detrimental to chondrocytes in monolayer, whereas static HP in the physiologic range enhances the functional properties of 3-D engineered constructs. Additionally, work involving HP application to osteoarthritic chondrocytes demonstrates that osteoarthritic chondrocytes may be used in tissue engineering strategies and that HP could potentially be used as a treatment modality to delay osteoarthritic changes. Furthermore, physiologic magnitudes of HP, particularly with intermittent loading frequencies, can be used as a differentiation factor for MSCs, embryonic stem cells, and dedifferentiated chondrocytes. Finally, high HPs, particularly between 30 and 50 MPa but as low as 15 MPa, have detrimental effects on chondrocytes and generally result in a stress response and decreased metabolic activity. These detrimental effects are especially apparent with loading times exceeding 2 h.
Although the work performed up to this point appears promising, additional work must be performed in each system to optimize the magnitude, frequency, duration of application, and application time in construct development. Additionally, based on the additive and synergistic effects of HP and growth factor application, it is likely that, after optimization, HP will need to be used in combination with other exogenous stimuli such as growth factors, as well as with other mechanical stimuli such as direct compression, to yield a construct with biochemical and biomechanical properties approaching those of native tissue.
References
- 1.Buckwalter J.A. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther. 1998;28:192. doi: 10.2519/jospt.1998.28.4.192. [DOI] [PubMed] [Google Scholar]
- 2.Afoke N.Y. Byers P.D. Hutton W.C. Contact pressures in the human hip joint. J Bone Joint Surg Br. 1987;69:536. doi: 10.1302/0301-620X.69B4.3611154. [DOI] [PubMed] [Google Scholar]
- 3.Hodge W.A. Carlson K.L. Fijan R.S. Burgess R.G. Riley P.O. Harris W.H. Mann R.W. Contact pressures from an instrumented hip endoprosthesis. J Bone Joint Surg Am. 1989;71:1378. [PubMed] [Google Scholar]
- 4.Waters R.L. Lunsford B.R. Perry J. Byrd R. Energy-speed relationship of walking: standard tables. J Orthop Res. 1988;6:215. doi: 10.1002/jor.1100060208. [DOI] [PubMed] [Google Scholar]
- 5.Hansen U. Schunke M. Domm C. Ioannidis N. Hassenpflug J. Gehrke T. Kurz B. Combination of reduced oxygen tension and intermittent hydrostatic pressure: a useful tool in articular cartilage tissue engineering. J Biomech. 2001;34:941. doi: 10.1016/s0021-9290(01)00050-1. [DOI] [PubMed] [Google Scholar]
- 6.Scherer K. Schunke M. Sellckau R. Hassenpflug J. Kurz B. The influence of oxygen and hydrostatic pressure on articular chondrocytes and adherent bone marrow cells in vitro. Biorheology. 2004;41:323. [PubMed] [Google Scholar]
- 7.Darling E.M. Athanasiou K.A. Articular cartilage bioreactors and bioprocesses. Tissue Eng. 2003;9:9. doi: 10.1089/107632703762687492. [DOI] [PubMed] [Google Scholar]
- 8.Suh J.K. Baek G.H. Aroen A. Malin C.M. Niyibizi C. Evans C.H. Westerhausen-Larson A. Intermittent sub-ambient interstitial hydrostatic pressure as a potential mechanical stimulator for chondrocyte metabolism. Osteoarthritis Cartilage. 1999;7:71. doi: 10.1053/joca.1998.0163. [DOI] [PubMed] [Google Scholar]
- 9.Jortikka M.O. Parkkinen J.J. Inkinen R.I. Karner J. Jarvelainen H.T. Nelimarkka L.O. Tammi M.I. Lammi M.J. The role of microtubules in the regulation of proteoglycan synthesis in chondrocytes under hydrostatic pressure. Arch Biochem Biophys. 2000;374:172. doi: 10.1006/abbi.1999.1543. [DOI] [PubMed] [Google Scholar]
- 10.Smith R.L. Rusk S.F. Ellison B.E. Wessells P. Tsuchiya K. Carter D.R. Caler W.E. Sandell L.J. Schurman D.J. In vitro stimulation of articular chondrocyte mRNA and extracellular matrix synthesis by hydrostatic pressure. J Orthop Res. 1996;14:53. doi: 10.1002/jor.1100140110. [DOI] [PubMed] [Google Scholar]
- 11.Smith R.L. Lin J. Trindade M.C. Shida J. Kajiyama G. Vu T. Hoffman A.R. van der Meulen M.C. Goodman S.B. Schurman D.J. Carter D.R. Time-dependent effects of intermittent hydrostatic pressure on articular chondrocyte type II collagen and aggrecan mRNA expression. J Rehabil Res Dev. 2000;37:153. [PubMed] [Google Scholar]
- 12.Ikenoue T. Trindade M.C. Lee M.S. Lin E.Y. Schurman D.J. Goodman S.B. Smith R.L. Mechanoregulation of human articular chondrocyte aggrecan and type II collagen expression by intermittent hydrostatic pressure in vitro. J Orthop Res. 2003;21:110. doi: 10.1016/S0736-0266(02)00091-8. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K. Kubo T. Kobayashi K. Imanishi J. Takigawa M. Arai Y. Hirasawa Y. Hydrostatic pressure influences mRNA expression of transforming growth factor-beta 1 and heat shock protein 70 in chondrocyte-like cell line. J Orthop Res. 1997;15:150. doi: 10.1002/jor.1100150122. [DOI] [PubMed] [Google Scholar]
- 14.Parkkinen J.J. Ikonen J. Lammi M.J. Laakkonen J. Tammi M. Helminen H.J. Effects of cyclic hydrostatic pressure on proteoglycan synthesis in cultured chondrocytes and articular cartilage explants. Arch Biochem Biophys. 1993;300:458. doi: 10.1006/abbi.1993.1062. [DOI] [PubMed] [Google Scholar]
- 15.Carver S.E. Heath C.A. Increasing extracellular matrix production in regenerating cartilage with intermittent physiological pressure. Biotechnol Bioeng. 1999;62:166. [PubMed] [Google Scholar]
- 16.Carver S.E. Heath C.A. Semi-continuous perfusion system for delivering intermittent physiological pressure to regenerating cartilage. Tissue Eng. 1999;5:1. doi: 10.1089/ten.1999.5.1. [DOI] [PubMed] [Google Scholar]
- 17.Hu J.C. Athanasiou K.A. The effects of intermittent hydrostatic pressure on self-assembled articular cartilage constructs. Tissue Eng. 2006;12:1337. doi: 10.1089/ten.2006.12.1337. [DOI] [PubMed] [Google Scholar]
- 18.Hall A.C. Urban J.P. Gehl K.A. The effects of hydrostatic pressure on matrix synthesis in articular cartilage. J Orthop Res. 1991;9:1. doi: 10.1002/jor.1100090102. [DOI] [PubMed] [Google Scholar]
- 19.Mizuno S. Tateishi T. Ushida T. Glowacki J. Hydrostatic fluid pressure enhances matrix synthesis and accumulation by bovine chondrocytes in three-dimensional culture. J Cell Physiol. 2002;193:319. doi: 10.1002/jcp.10180. [DOI] [PubMed] [Google Scholar]
- 20.Toyoda T. Seedhom B.B. Kirkham J. Bonass W.A. Upregulation of aggrecan and type II collagen mRNA expression in bovine chondrocytes by the application of hydrostatic pressure. Biorheology. 2003;40:79. [PubMed] [Google Scholar]
- 21.Toyoda T. Seedhom B.B. Yao J.Q. Kirkham J. Brookes S. Bonass W.A. Hydrostatic pressure modulates proteoglycan metabolism in chondrocytes seeded in agarose. Arthritis Rheum. 2003;48:2865. doi: 10.1002/art.11250. [DOI] [PubMed] [Google Scholar]
- 22.Elder B.D. Athanasiou K.A. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS ONE. 2008;3:e2342. doi: 10.1371/journal.pone.0002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elder B.D. Athanasiou K.A. Effects of confinement on the mechanical properties of self-assembled articular cartilage constructs in the direction orthogonal to the confinement surface. J Orthop Res. 2008;26:238. doi: 10.1002/jor.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu J.C. Athanasiou K.A. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;12:969. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 25.Elder B.D. Athanasiou K.A. Temporal effects of hydrostatic pressure on tissue engineered bovine articular cartilage constructs. Tissue Eng Part A 2008 Oct 2. doi: 10.1089/ten.tea.2008.0200. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trindade M.C. Shida J. Ikenoue T. Lee M.S. Lin E.Y. Yaszay B. Yerby S. Goodman S.B. Schurman D.J. Smith R.L. Intermittent hydrostatic pressure inhibits matrix metalloproteinase and pro-inflammatory mediator release from human osteoarthritic chondrocytes in vitro. Osteoarthritis Cartilage. 2004;12:729. doi: 10.1016/j.joca.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Lee M.S. Ikenoue T. Trindade M.C. Wong N. Goodman S.B. Schurman D.J. Smith R.L. Protective effects of intermittent hydrostatic pressure on osteoarthritic chondrocytes activated by bacterial endotoxin in vitro. J Orthop Res. 2003;21:117. doi: 10.1016/S0736-0266(02)00085-2. [DOI] [PubMed] [Google Scholar]
- 28.Lee M.S. Trindade M.C. Ikenoue T. Schurman D.J. Goodman S.B. Smith R.L. Intermittent hydrostatic pressure inhibits shear stress-induced nitric oxide release in human osteoarthritic chondrocytes in vitro. J Rheumatol. 2003;30:326. [PubMed] [Google Scholar]
- 29.Fioravanti A. Cantarini L. Chellini F. Manca D. Paccagnini E. Marcolongo R. Collodel G. Effect of hyaluronic acid (MW 500-730 kDa) on proteoglycan and nitric oxide production in human osteoarthritic chondrocyte cultures exposed to hydrostatic pressure. Osteoarthritis Cartilage. 2005;13:688. doi: 10.1016/j.joca.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Gavenis K. Kremer A. Von Walter M. Hollander D.A. Schneider U. Schmidt-Rohlfing B. Effects of cyclic hydrostatic pressure on the metabolism of human osteoarthritic chondrocytes cultivated in a collagen gel. Artif Organs. 2007;31:91. doi: 10.1111/j.1525-1594.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 31.Islam N. Haqqi T.M. Jepsen K.J. Kraay M. Welter J.F. Goldberg V.M. Malemud C.J. Hydrostatic pressure induces apoptosis in human chondrocytes from osteoarthritic cartilage through up-regulation of tumor necrosis factor-alpha, inducible nitric oxide synthase, p53, c-myc, and bax-alpha, and suppression of bcl-2. J Cell Biochem. 2002;87:266. doi: 10.1002/jcb.10317. [DOI] [PubMed] [Google Scholar]
- 32.Wong M. Siegrist M. Goodwin K. Cyclic tensile strain and cyclic hydrostatic pressure differentially regulate expression of hypertrophic markers in primary chondrocytes. Bone. 2003;33:685. doi: 10.1016/s8756-3282(03)00242-4. [DOI] [PubMed] [Google Scholar]
- 33.Angele P. Yoo J.U. Smith C. Mansour J. Jepsen K.J. Nerlich M. Johnstone B. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. J Orthop Res. 2003;21:451. doi: 10.1016/S0736-0266(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 34.Luo Z.J. Seedhom B.B. Seedhom B.B. Light and low-frequency pulsatile hydrostatic pressure enhances extracellular matrix formation by bone marrow mesenchymal cells: an in-vitro study with special reference to cartilage repair. Proc Inst Mech Eng [H] 2007;221:499. doi: 10.1243/09544119JEIM199. [DOI] [PubMed] [Google Scholar]
- 35.Wagner D.R. Lindsey D.P. Li K.W. Tummala P. Chandran S.E. Smith R.L. Longaker M.T. Carter D.R. Beaupre G.S. Hydrostatic pressure enhances chondrogenic differentiation of human bone marrow stromal cells in osteochondrogenic medium. Ann Biomed Eng. 2008;36:813. doi: 10.1007/s10439-008-9448-5. [DOI] [PubMed] [Google Scholar]
- 36.Miyanishi K. Trindade M.C. Lindsey D.P. Beaupre G.S. Carter D.R. Goodman S.B. Schurman D.J. Smith R.L. Effects of hydrostatic pressure and transforming growth factor-beta 3 on adult human mesenchymal stem cell chondrogenesis in vitro. Tissue Eng. 2006;12:1419. doi: 10.1089/ten.2006.12.1419. [DOI] [PubMed] [Google Scholar]
- 37.Miyanishi K. Trindade M.C. Lindsey D.P. Beaupre G.S. Carter D.R. Goodman S.B. Schurman D.J. Smith R.L. Dose- and time-dependent effects of cyclic hydrostatic pressure on transforming growth factor-beta3-induced chondrogenesis by adult human mesenchymal stem cells in vitro. Tissue Eng. 2006;12:2253. doi: 10.1089/ten.2006.12.2253. [DOI] [PubMed] [Google Scholar]
- 38.Elder S.H. Fulzele K.S. McCulley W.R. Cyclic hydrostatic compression stimulates chondroinduction of C3H/10T1/2 cells. Biomech Model Mechanobiol. 2005;3:141. doi: 10.1007/s10237-004-0058-3. [DOI] [PubMed] [Google Scholar]
- 39.Heyland J. Wiegandt K. Goepfert C. Nagel-Heyer S. Ilinich E. Schumacher U. Portner R. Redifferentiation of chondrocytes and cartilage formation under intermittent hydrostatic pressure. Biotechnol Lett. 2006;28:1641. doi: 10.1007/s10529-006-9144-1. [DOI] [PubMed] [Google Scholar]
- 40.Kawanishi M. Oura A. Furukawa K. Fukubayashi T. Nakamura K. Tateishi T. Ushida T. Redifferentiation of dedifferentiated bovine articular chondrocytes enhanced by cyclic hydrostatic pressure under a gas-controlled system. Tissue Eng. 2007;13:957. doi: 10.1089/ten.2006.0176. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura S. Arai Y. Takahashi K.A. Terauchi R. Ohashi S. Mazda O. Imanishi J. Inoue A. Tonomura H. Kubo T. Hydrostatic pressure induces apoptosis of chondrocytes cultured in alginate beads. J Orthop Res. 2006;24:733. doi: 10.1002/jor.20077. [DOI] [PubMed] [Google Scholar]
- 42.Fioravanti A. Benetti D. Coppola G. Collodel G. Effect of continuous high hydrostatic pressure on the morphology and cytoskeleton of normal and osteoarthritic human chondrocytes cultivated in alginate gels. Clin Exp Rheumatol. 2005;23:847. [PubMed] [Google Scholar]
- 43.Parkkinen J.J. Lammi M.J. Pelttari A. Helminen H.J. Tammi M. Virtanen I. Altered Golgi apparatus in hydrostatically loaded articular cartilage chondrocytes. Ann Rheum Dis. 1993;52:192. doi: 10.1136/ard.52.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parkkinen J.J. Lammi M.J. Inkinen R. Jortikka M. Tammi M. Virtanen I. Helminen H.J. Influence of short-term hydrostatic pressure on organization of stress fibers in cultured chondrocytes. J Orthop Res. 1995;13:495. doi: 10.1002/jor.1100130404. [DOI] [PubMed] [Google Scholar]
- 45.Lammi M.J. Inkinen R. Parkkinen J.J. Hakkinen T. Jortikka M. Nelimarkka L.O. Jarvelainen H.T. Tammi M.I. Expression of reduced amounts of structurally altered aggrecan in articular cartilage chondrocytes exposed to high hydrostatic pressure. Biochem J. 1994;304:723. doi: 10.1042/bj3040723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sironen R. Elo M. Kaarniranta K. Helminen H.J. Lammi M.J. Transcriptional activation in chondrocytes submitted to hydrostatic pressure. Biorheology. 2000;37:85. [PubMed] [Google Scholar]
- 47.Sironen R.K. Karjalainen H.M. Torronen K. Elo M.A. Kaarniranta K. Takigawa M. Helminen H.J. Lammi M.J. High pressure effects on cellular expression profile and mRNA stability. A cDNA array analysis. Biorheology. 2002;39:111. [PubMed] [Google Scholar]
- 48.Takahashi K. Kubo T. Arai Y. Kitajima I. Takigawa M. Imanishi J. Hirasawa Y. Hydrostatic pressure induces expression of interleukin 6 and tumour necrosis factor alpha mRNAs in a chondrocyte-like cell line. Ann Rheum Dis. 1998;57:231. doi: 10.1136/ard.57.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaarniranta K. Elo M. Sironen R. Lammi M.J. Goldring M.B. Eriksson J.E. Sistonen L. Helminen H.J. Hsp70 accumulation in chondrocytic cells exposed to high continuous hydrostatic pressure coincides with mRNA stabilization rather than transcriptional activation. Proc Natl Acad Sci U S A. 1998;95:2319. doi: 10.1073/pnas.95.5.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bachrach N.M. Mow V.C. Guilak F. Incompressibility of the solid matrix of articular cartilage under high hydrostatic pressures. J Biomech. 1998;31:445. doi: 10.1016/s0021-9290(98)00035-9. [DOI] [PubMed] [Google Scholar]
- 51.Tanck E. van Driel W.D. Hagen J.W. Burger E.H. Blankevoort L. Huiskes R. Why does intermittent hydrostatic pressure enhance the mineralization process in fetal cartilage? J Biomech. 1999;32:153. doi: 10.1016/s0021-9290(98)00165-1. [DOI] [PubMed] [Google Scholar]
- 52.Wilkes R.P. Athanasiou K.A. The intrinsic incompressibility of osteoblast-like cells. Tissue Eng. 1996;2:167. doi: 10.1089/ten.1996.2.167. [DOI] [PubMed] [Google Scholar]
- 53.Hall A.C. Differential effects of hydrostatic pressure on cation transport pathways of isolated articular chondrocytes. J Cell Physiol. 1999;178:197. doi: 10.1002/(SICI)1097-4652(199902)178:2<197::AID-JCP9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 54.Browning J.A. Walker R.E. Hall A.C. Wilkins R.J. Modulation of Na + x H + exchange by hydrostatic pressure in isolated bovine articular chondrocytes. Acta Physiol Scand. 1999;166:39. doi: 10.1046/j.1365-201x.1999.00534.x. [DOI] [PubMed] [Google Scholar]
- 55.Mizuno S. A novel method for assessing effects of hydrostatic fluid pressure on intracellular calcium: a study with bovine articular chondrocytes. Am J Physiol Cell Physiol. 2005;288:C329. doi: 10.1152/ajpcell.00131.2004. [DOI] [PubMed] [Google Scholar]
- 56.Browning J.A. Saunders K. Urban J.P. Wilkins R.J. The influence and interactions of hydrostatic and osmotic pressures on the intracellular milieu of chondrocytes. Biorheology. 2004;41:299. [PubMed] [Google Scholar]
- 57.Kornblatt J.A. Kornblatt M.J. The effects of osmotic and hydrostatic pressures on macromolecular systems. Biochim Biophys Acta. 2002;1595:30. doi: 10.1016/s0167-4838(01)00333-8. [DOI] [PubMed] [Google Scholar]
- 58.Horowitz S.B. Lau Y.T. A function that relates protein synthetic rates to potassium activity in vivo. J Cell Physiol. 1988;135:425. doi: 10.1002/jcp.1041350309. [DOI] [PubMed] [Google Scholar]