Abstract
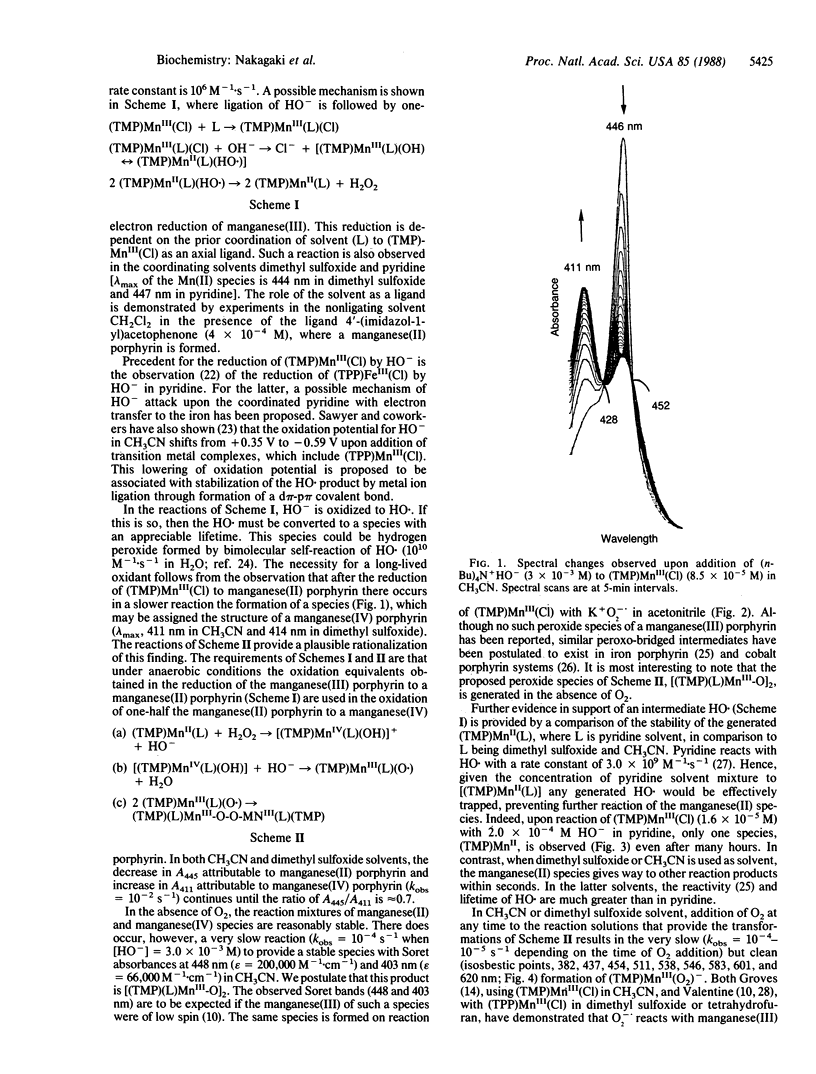
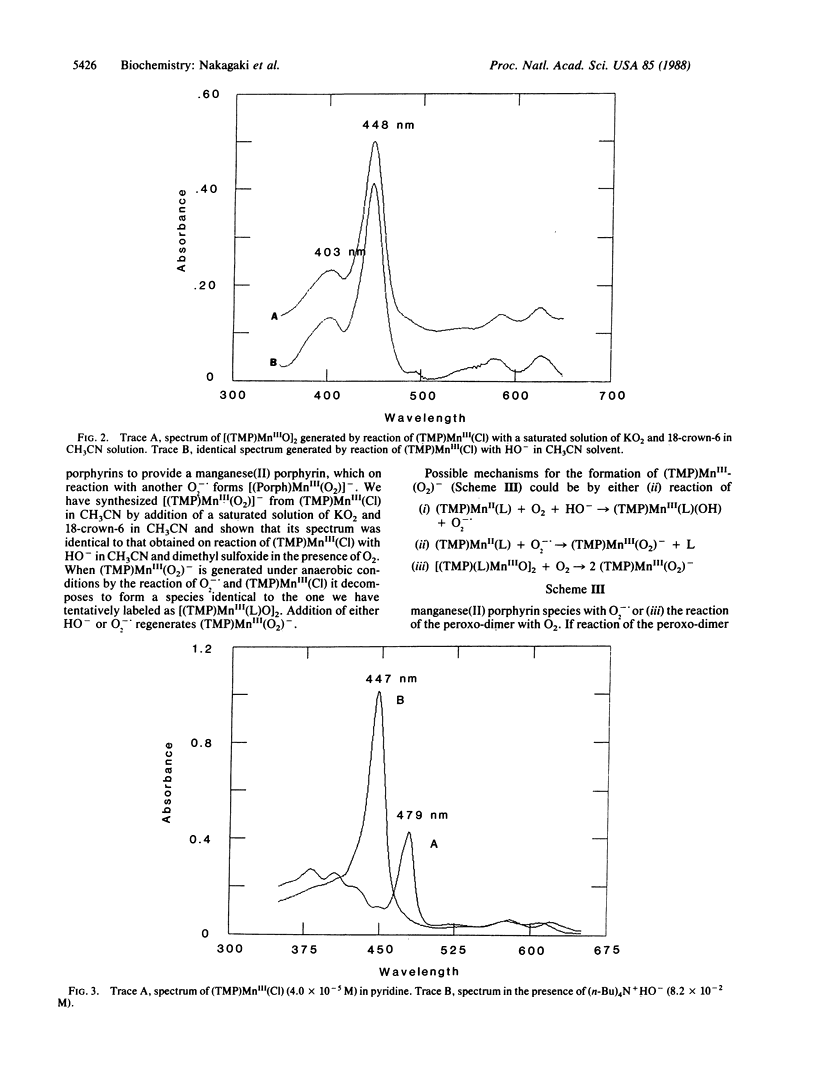
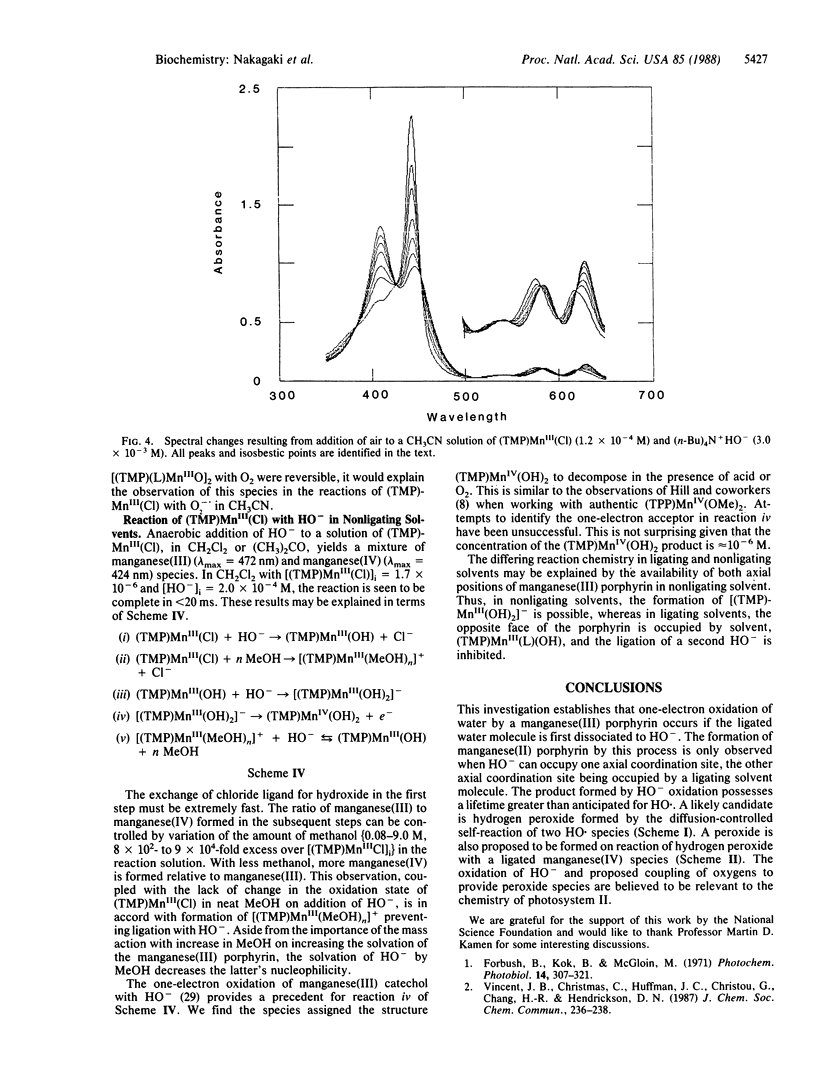
The reaction of HO- with 5,10,15,20-tetrakis(2,4,6-trimethylphenyl)porphinatomanganese(III) chloride [(TMP)MnIII(Cl)] in ligating solvents (CH3CN, dimethyl sulfoxide, pyridine) results in formation of (TMP)MnII (≈106 M-1·s-r), which in a slower reaction is converted to a product whose structure is suggested to be that of a porphyrin manganese(III) peroxo dimer. Admittance of O2 at any time during these reactions leads to formation of the manganese(III) peroxide (TMP)MnIII(O2)-. In nonligating solvents [CH2Cl2, (CH3)2CO], the reaction of HO- with (TMP)MnIII(Cl) yields (TMP)MnIV(OH)2.
Keywords: photosystem II S4 state, water oxidation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Frasch W. D., Mei R. Kinetics of O2 evolution from H2O2 catalyzed by the oxygen-evolving complex: investigation of the S1-dependent reaction. Biochemistry. 1987 Nov 17;26(23):7321–7325. doi: 10.1021/bi00397a019. [DOI] [PubMed] [Google Scholar]
- Hoffman B. M., Weschler C. J., Basolo F. The dioxygen adduct of meso-tetraphenylporphyrinmanganese(II), a synthetic oxygen carrier. J Am Chem Soc. 1976 Sep 1;98(18):5473–5482. doi: 10.1021/ja00434a012. [DOI] [PubMed] [Google Scholar]
- Stynes D. V., Stynes H. C., Ibers J. A., James B. R. Kinetics of the reaction of amine complexes of cobalt(II) protoporphyrin IX dimethyl ester with oxygen. Evidence for hydrogen bonding with coordinated oxygen. J Am Chem Soc. 1973 Feb 21;95(4):1142–1149. doi: 10.1021/ja00785a024. [DOI] [PubMed] [Google Scholar]


