Abstract
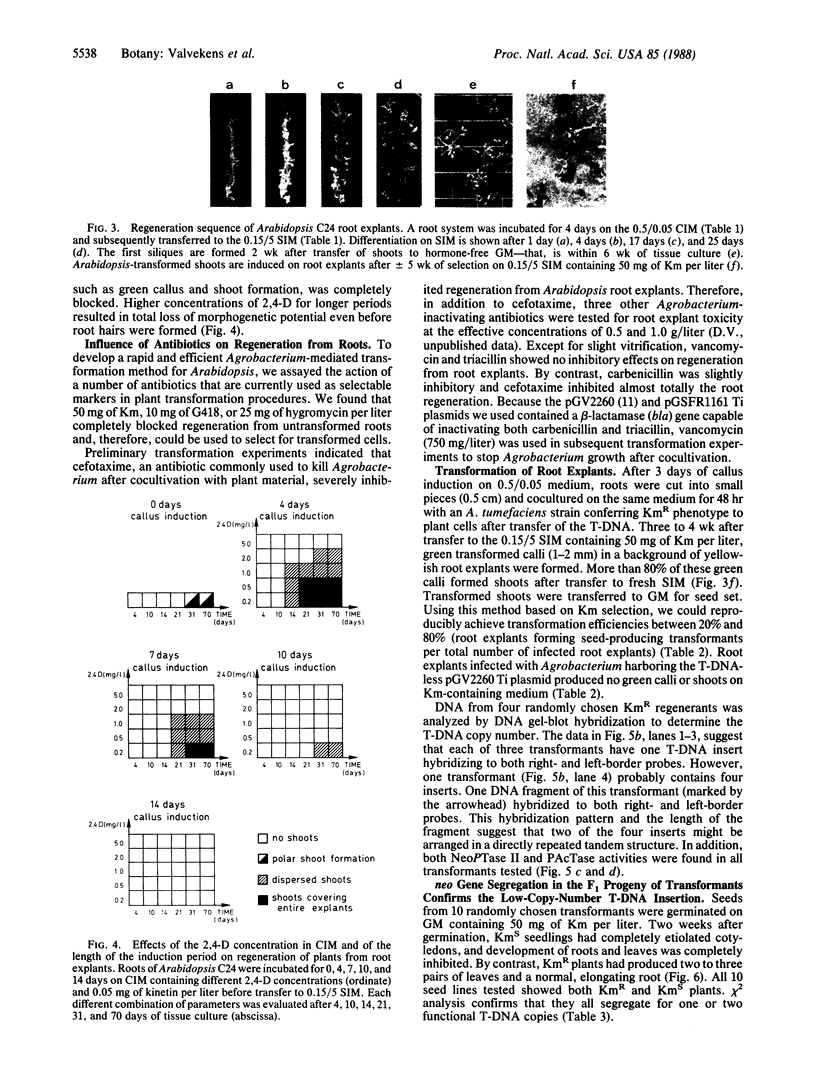
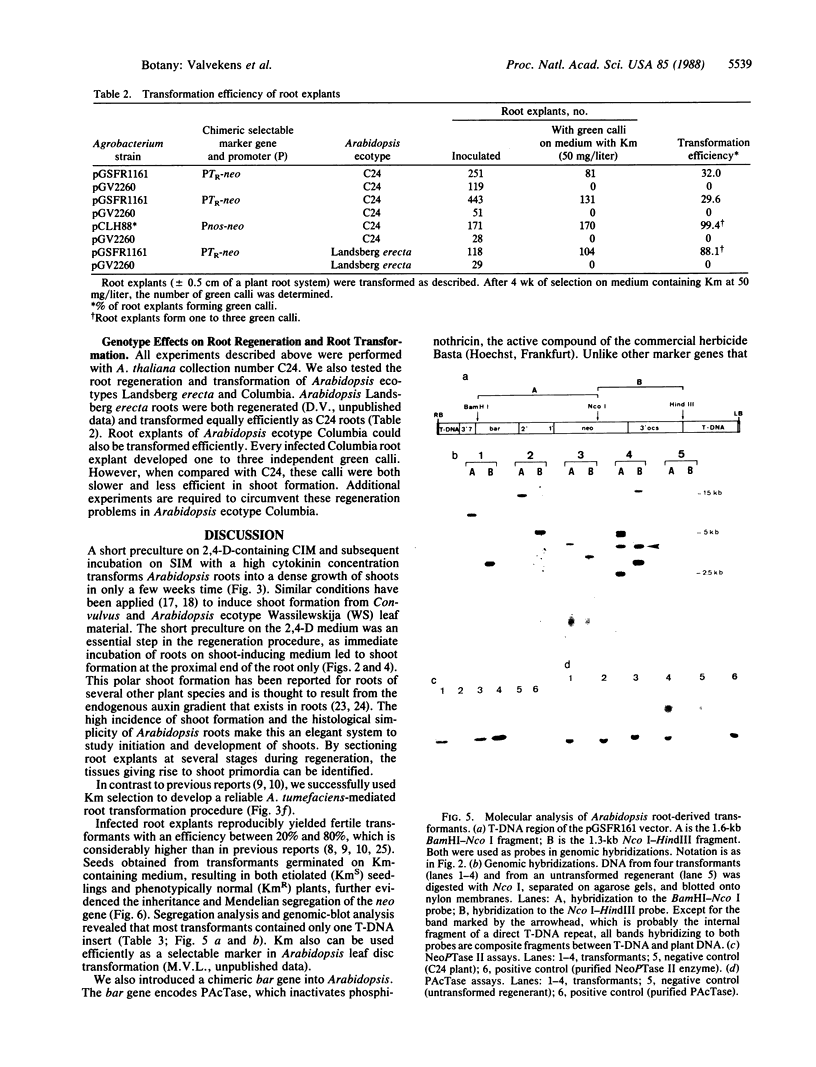
Culture conditions were developed that induce Arabidopsis thaliana (L.) Heynh. root cuttings to regenerate shoots rapidly and at 100% efficiency. The shoots produce viable seeds in vitro or after rooting in soil. A transformation procedure for Arabidopsis root explants based on kanamycin selection was established. By using this regeneration procedure and an Agrobacterium tumor-inducing Ti plasmid carrying a chimeric neomycin phosphotransferase II gene (neo), transformed seed-producing plants were obtained with an efficiency between 20% and 80% within 3 months after gene transfer. F1 seedlings of these transformants showed Mendelian segregation of the kanamycin-resistance trait. The transformation method could be applied to three different Arabidopsis ecotypes. In addition to the neo gene, a chimeric bar gene conferring resistance to the herbicide Basta was introduced into Arabidopsis. The expression of the bar gene was shown by enzymatic assay.
Keywords: herbicide resistance, neomycin phosphotransferase II, plant regeneration, tumor-inducing Ti plasmid, transgenic plants
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Watson B. D., Chiang C. C. Transformation of Tobacco, Tomato, Potato, and Arabidopsis thaliana Using a Binary Ti Vector System. Plant Physiol. 1986 May;81(1):301–305. doi: 10.1104/pp.81.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M. D., Botterman J., Vandewiele M., Dockx J., Thoen C., Gosselé V., Movva N. R., Thompson C., Montagu M. V., Leemans J. Engineering herbicide resistance in plants by expression of a detoxifying enzyme. EMBO J. 1987 Sep;6(9):2513–2518. doi: 10.1002/j.1460-2075.1987.tb02537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson M. L., Warnick D. A. Competence and determination in the process of in vitro shoot organogenesis. Dev Biol. 1983 Feb;95(2):288–293. doi: 10.1016/0012-1606(83)90029-5. [DOI] [PubMed] [Google Scholar]
- Cooley L., Kelley R., Spradling A. Insertional mutagenesis of the Drosophila genome with single P elements. Science. 1988 Mar 4;239(4844):1121–1128. doi: 10.1126/science.2830671. [DOI] [PubMed] [Google Scholar]
- Deblaere R., Bytebier B., De Greve H., Deboeck F., Schell J., Van Montagu M., Leemans J. Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene transfer to plants. Nucleic Acids Res. 1985 Jul 11;13(13):4777–4788. doi: 10.1093/nar/13.13.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Lloyd A. M., Barnason A. R., Rogers S. G., Byrne M. C., Fraley R. T., Horsch R. B. Transformation of Arabidopsis thaliana with Agrobacterium tumefaciens. Science. 1986 Oct 24;234(4775):464–466. doi: 10.1126/science.234.4775.464. [DOI] [PubMed] [Google Scholar]
- Manning J. E., Schmid C. W., Davidson N. Interspersion of repetitive and nonrepetitive DNA sequences in the Drosophila melanogaster genome. Cell. 1975 Feb;4(2):141–155. doi: 10.1016/0092-8674(75)90121-x. [DOI] [PubMed] [Google Scholar]
- Meyerowitz E. M. Arabidopsis thaliana. Annu Rev Genet. 1987;21:93–111. doi: 10.1146/annurev.ge.21.120187.000521. [DOI] [PubMed] [Google Scholar]
- Reiss B., Sprengel R., Will H., Schaller H. A new sensitive method for qualitative and quantitative assay of neomycin phosphotransferase in crude cell extracts. Gene. 1984 Oct;30(1-3):211–217. doi: 10.1016/0378-1119(84)90122-7. [DOI] [PubMed] [Google Scholar]
- Simoens C., Alliotte T., Mendel R., Müller A., Schiemann J., Van Lijsebettens M., Schell J., Van Montagu M., Inzé D. A binary vector for transferring genomic libraries to plants. Nucleic Acids Res. 1986 Oct 24;14(20):8073–8090. doi: 10.1093/nar/14.20.8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J. E., Brenner S. The DNA of Caenorhabditis elegans. Genetics. 1974 May;77(1):95–104. doi: 10.1093/genetics/77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velten J., Schell J. Selection-expression plasmid vectors for use in genetic transformation of higher plants. Nucleic Acids Res. 1985 Oct 11;13(19):6981–6998. doi: 10.1093/nar/13.19.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]