Abstract
Background
Zolpidem is a non-benzodiazepine sedative/hypnotic that acts at GABAA receptors to influence inhibitory neurotransmission throughout the central nervous system. A great deal is known about the behavioral effects of this drug in humans and laboratory animals, but little is known about zolpidem’s specific effects on neurochemistry in vivo.
Objectives
We evaluated how acute administration of zolpidem affected levels of GABA, glutamate, glutamine, and other brain metabolites.
Methods
Proton magnetic resonance spectroscopy (1H MRS) at 4 Tesla was employed to measure the effects of zolpidem on brain chemistry in 19 healthy volunteers. Participants underwent scanning following acute oral administration of a therapeutic dose of zolpidem (10 mg) in a within-subject, single-blind, placebo-controlled, single-visit study. In addition to neurochemical measurements from single voxels within the anterior cingulate (ACC) and thalamus, a series of questionnaires were administered periodically throughout the experimental session to assess subjective mood states.
Results
Zolpidem reduced GABA levels in the thalamus, but not the ACC. There were no treatment effects with respect to other metabolite levels. Self-reported ratings of “dizzy”, “nauseous”, “confused”, and “bad effects” were increased relative to placebo, as were ratings on the sedation/intoxication (PCAG) and psychotomimetic/dysphoria (LSD) scales of the Addiction Research Center Inventory. Moreover, there was a significant correlation between the decrease in GABA and “dizzy”.
Conclusions
Zolpidem engendered primarily dysphoric-like effects and the correlation between reduced thalamic GABA and “dizzy” may be a function of zolpidem’s interaction with α1GABAA receptors in the cerebellum, projecting through the vestibular system to the thalamus.
Keywords: zolpidem, spectroscopy, GABA, glutamate, glutamine, thalamus, anterior cingulate
Introduction
Zolpidem (Ambien®) is one of the most frequently prescribed sleep aids in the United States (Morlock et al. 2006). Although it is structurally distinct from the benzodiazepines, this short-acting hypnotic acts at the benzodiazepine recognition site on the GABAA receptor to enhance inhibition throughout the central nervous system. However, zolpidem interacts preferentially with GABAA receptors containing an α1 subunit (i.e., α1GABAA receptors; Benavides et al. 1988; Hadingham et al. 1993; Sanna et al. 2002), whereas classical benzodiazepines interact indiscriminately with GABAA receptors containing α1, α2, α3, or α5 subunits (they are inactive at α4- and α6-subunit containing GABAA receptors; Pritchett and Seeburg 1990). This receptor subtype selectivity is believed to underlie zolpidem’s superior hypnotic ability (McKernan et al. 2000; Rudolph et al. 1999).
The myriad behavioral effects of zolpidem are well documented in both laboratory animals (e.g., Sanger et al. 1987) and humans (see review by Rush 1998). In addition to its clinical usefulness for the treatment of insomnia, zolpidem’s use also has been associated with a host of untoward effects such as abuse and dependence liability (Evans et al. 1990; Liappas et al. 2003; Rush et al. 1999; Victorri-Vigneau et al. 2007), somnambulism (Harazin and Berigan 1999; Sharma and Dewan 2005; Yang et al. 2005), nightmares (Ganzoni et al. 1995; Hajak and Bandelow 1998; Roger et al. 1993; Toner et al. 1999), hallucinations, delirium, and/or psychosis (Brodeur and Stirling 2001; Huang et al. 2003; Markowitz and Brewerton 1996; Toner et al. 1999; Tsai et al. 2003), and even compulsive behaviors such as sleep-eating (Morgenthaler and Silber 2002; Najjar 2007; Tsai et al. 2007). Effects such as these suggest that it is important to understand the basis of zolpidem’s effects beyond its purported GABAA receptor pharmacology.
Despite its popularity, the specific effects of zolpidem on neurochemistry in vivo are unknown. Previous proton magnetic resonance spectroscopy (1H MRS) studies have provided conflicting reports regarding the effects of clinically relevant doses of benzodiazepines. Brambilla et al. (2002) demonstrated no effects of lorazepam on metabolites such as N-acetylaspartate (NAA), phosphocreatine + creatine (PCr + Cr), trimethylamines (TMA), myo-inositol (mI), glutamate (Glu), or glutamine (Gln) in the dorsolateral prefrontal cortex, while Goddard et al. (2004) observed a clonazepam-induced increase in Glu concomitant with a reduction in GABA levels in the occipital cortex. Preliminary reports from conference proceedings indicated that lorazepam increased choline (Cho), Cr, and mI in the parietal white matter (Davanzo et al. 1997), and intravenous midazolam transiently increased mI and lactate levels in the striatum (Burau et al. 1997). Although similar to the benzodiazepines, zolpidem’s effect on neuronal metabolites has not been addressed empirically using MRS.
The present study employed 1H MRS at 4 Tesla in healthy volunteers to assess the effects of acute oral administration of a therapeutic dose of zolpidem (10 mg) in a single-blind, placebo-controlled study. The scanning procedure utilized standard protocols for measuring GABA (Mescher et al. 1998), as well as protocols developed recently that permitted the simultaneous and reliable measurement of Glu and Gln (Öngür et al. 2008) in two separate voxels of interest within the anterior cingulate (ACC) and thalamus. A series of questionnaires also were administered periodically throughout the experimental session in order to assess subjective mood states associated with drug exposure. Together these measures were aimed at beginning to understand the neurobiological mechanisms that mediate the effects of this popular sedative/hypnotic.
Materials and methods
Prior to beginning the study protocol, we undertook a separate test/re-test study to determine the reliability of our metabolite measurements. Six healthy male (4) and female (2) volunteers (ages 25–36) were scanned twice within one week of another at the same time of day. All scan parameters and data analyses were identical on both days, and are described as follows.
Participants
Nineteen healthy male (7) and female (12) volunteers between the ages of 21–35 (average age 25 ± 0.88 years) and with an average body mass index of 24.26 (± 0.83 kg/m2) completed this study. Participants reported ≤10 lifetime recreational experiences with drugs or abuse other than alcohol (average 4.26 ± 0.90 drinks per week), and they were non-smokers. They had not been diagnosed with a DSM-IV Axis I disorder, they had no history of neurological disorders, they had normal structural MRI scans during the screening visit, and they were not taking psychotropic medications. On the day of the study participants were screened for drug use using QuickTox® urine screen kits (Branan Medical Corporation; Irvine, CA) and breath alcohol level (AlcoSensor, Intoximeter; Saint Louis, MO). Female participants were administered a QuPID® urine pregnancy test (Stanbio Laboratory; Boerne, TX); pregnancy was a contraindication in this study. Any participant who would have been positive on any screen would have been rescheduled and sent home. All participants were transported to and from the laboratory via taxicab. This study was reviewed and approved by the McLean Hospital Institutional Review Board, and therefore was in accord with the Declaration of Helsinki. All volunteers (those enrolled in both the test/re-test pre-study and the zolpidem protocol) provided informed consent and were compensated for their participation.
Procedure and drug treatment
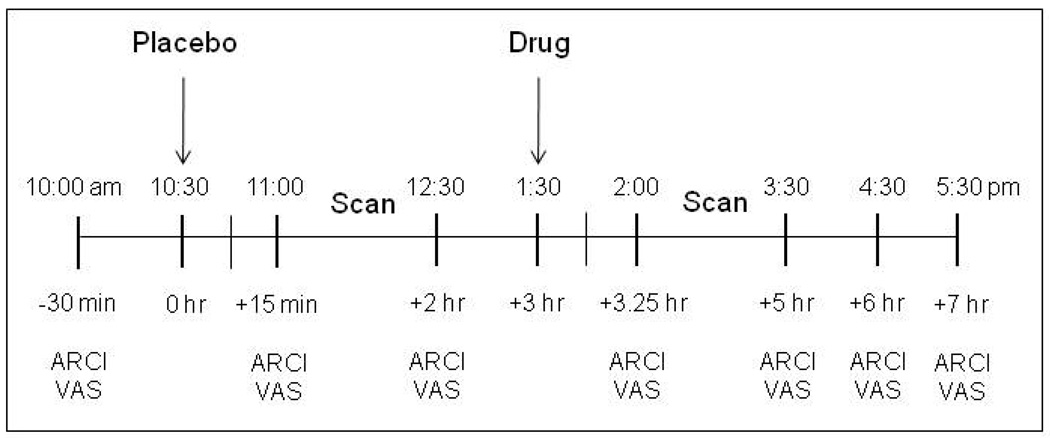
Participants visited the laboratory once for this single-blind, placebo-controlled, double-scan study (see Figure 1 for a detailed outline of the study visit procedure). Participants received a capsule containing placebo 30 min prior to their first 1H MRS scanning session, and an identical capsule containing a therapeutic dose of zolpidem (10 mg; Troy et al. 2000) 30 min prior to the second scanning session. Oral administration of zolpidem has been estimated to result in 70% bioavailability (Hoehns and Perry 1993), and maximum plasma concentrations should be reached within an average of 1.7 hr following ingestion (Langtry and Benfield 1990; Salva and Costa 1995). Throughout the 7.5-hour session participants provided subjective ratings at regular intervals using computerized questionnaires.
Fig. 1.
Procedure for MRS study visit. After a baseline period during which the questionnaires were administered, participants received placebo. The first 90-min scanning session took place 30 min later. At the beginning of the third hour participants received an acute oral dose of zolpidem (10 mg). The second 90-min scanning session took place 30 min later. Throughout the session participants answered questionnaire sets assessing drug-induced subjective effects: Addiction Research Center Inventory (ARCI) and an investigator-constructed visual analog scale (VAS).
Equipment
The computerized questionnaires were administered on Macintosh computers running in-house software (Study Log Master v.79). All magnetic resonance data were collected on a Varian Unity INOVA 4T whole-body MR system running VNMRj 1.1b (Varian Inc; Palo Alto, CA), and using a 16-rung, volumetric birdcage design RF head coil (Robarts Research Institute; London, Canada) operating at 170.3MHz for proton imaging and spectroscopy.
Subjective assessments
Participants answered a series of computerized questionnaires in order to provide information about the drug-induced subjective effects they were experiencing. The Addiction Research Center Inventory (ARCI) is a standardized set of scales intended to evaluate the subjective effects of drugs (Haertzen 1966). A shortened version was used that consists of 49 true/false items that have been derived in order to measure stimulant-like (Benzedrine group: BG), amphetamine-like (AMPH), euphoric (Morphine-Benzedrine group: MBG), sedative- or intoxicating-like (Pentobarbital-Chlorpromazine-Alcohol group: PCAG), and psychotomimetic or dysphoric (Lysergic acid diethylamide: LSD) drug effects (Jasinski 1977). A visual analog scale (VAS) required participants to respond to a list of 15 items by placing a mark on a 100-mm continuum that used “not at all” or “extremely” as anchors (Table 1).
Table 1.
Responses on an investigator-constructed visual analog scale (VAS)
| Response itema | Placebo | Zolpidem | Pb |
|---|---|---|---|
| How anxious do you feel right now? | 6.61 ± 2.07 | 13.21 ± 3.98 | 0.15 |
| How high do you feel right now? | 9.95 ± 3.57 | 20.53 ± 4.88 | 0.09 |
| How sleepy do you feel right now? | 30.50 ± 4.76 | 36.11 ± 5.62 | 0.45 |
| How much are the good effects of the drug you took? |
10.05 ± 3.53 | 19.97 ± 5.55 | 0.14 |
| How dizzy do you feel right now? | 5.53 ± 1.89 | 22.13 ± 4.85 | 0.003* |
| How nauseous do you feel right now? | 2.55 ± 1.18 | 12.82 ± 4.19 | 0.02* |
| How loose do you feel right now? | 37.45 ± 6.28 | 36.68 ± 6.37 | 0.93 |
| How much are the bad effects of the drug you took? |
3.16 ± 1.33 | 13.53 ± 4.31 | 0.03* |
| How confused do you feel right now? | 3.87 ± 1.19 | 15.05 ± 3.72 | 0.007* |
| How carefree do you feel right now? | 30.42 ± 5.55 | 30.74 ± 5.70 | 0.97 |
| How restless do you feel right now? | 11.84 ± 3.14 | 19.37 ± 3.90 | 0.14 |
| How much do you like the drug you took? | 20.87 ± 5.15 | 21.50 ± 5.45 | 0.93 |
| How willing would you be to take it again? | 31.74 ± 6.74 | 24.18 ± 5.34 | 0.39 |
| How willing would you be to pay for it? | 9.66 ± 5.08 | 11.61 ± 4.20 | 0.77 |
| How mentally slow do you feel right now? | 15.61 ± 3.94 | 27.40 ± 5.64 | 0.10 |
Participants were required to respond by placing a mark on a 100-mm continuum that used “not at all” or “extremely” as anchors. Responses are expressed as means ± SEM.
P values with alpha set at 0.05
asterisks represent differences between placebo and zolpidem (10 mg) at that significance level.
Proton imaging
Once participants were positioned inside the bore of the magnet, scout images confirmed optimal positioning at the magnet’s isocenter. The unsuppressed water signal was used to manually shim the global water signal. The transmitter was set back onto the water resonance, and a series of high-contrast T1-weighted anatomical images were taken in the sagittal and axial planes. The parameters for these images were as follows: TE/TR=6.2 s/11.4 ms, field-of-view (FOV)=22×22×8 cm (sagittal) and 22×22×16 cm (axial), readout duration=4 ms, receive bandwidth = ± 32 kHz, in-plane matrix size=128×256×16 (sagittal) and 256×256×64 (axial), in-plane resolution= 0.94 × 1.9 mm (sagittal) and 0.94 × 0.94 mm (axial), readout points=512, slice thickness=2.5 mm, flip-angle= 11°.
Proton MRS
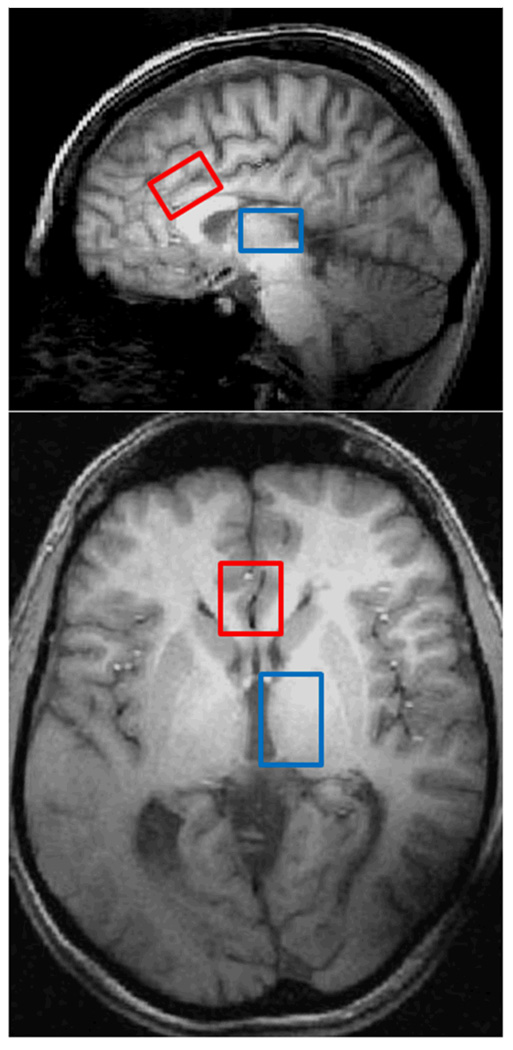
The high-resolution anatomical images were used as a guide to place single voxels in the ACC (3×2×2 cm), and subsequently, the thalamic region (2×2×3 cm) as shown in Figure 2. Proton spectroscopy implemented a 2D-JPRESS approach (Öngür et al. 2008) followed by a MEGAPRESS sequence (Mescher et al. 1998). Manual shimming of the magnetic field within the prescribed voxel was done with global water linewidths ranging from 9–12 Hz. Following the automated optimization of water suppression power and tip angles, the 2D-JPRESS sequence collected 24 TE-stepped spectra (echo-time ranging from 30–490 ms in 20-ms increments), which provided enough J-resolved bandwidth (50 Hz) to resolve Glu from Gln, as well as a range of other metabolites. Additional acquisition parameters were: TR =2 s, f1 acquisition bandwidth =50 Hz, spectral bandwidth =2 kHz, readout duration =512 ms, NEX =16, total scan duration =13 min. Once the first dataset was collected, a second set was collected from the same voxel using a MEGAPRESS sequence to obtain difference-edited GABA spectra. MEGAPRESS acquisition parameters were identical to those for the 2D-JPRESS, except that each individual transient was collected separately for frequency/phase-corrective summation and difference editing offline. Total scan duration =13 min. Once these two procedures (2D-JPRESS followed by MEGAPRESS) were completed for the ACC, they were repeated on a second voxel in the left thalamus. Therefore, two voxels of interest were assessed in each 90-min scanning session.
Fig. 2.
Parasagittal (top) and axial (bottom) views of the brain from T1-weighted images illustrating the ACC (red) and thalamus (blue) voxel placement in one representative participant.
Proton MRS processing
All spectroscopic data processing and analyses were undertaken on a LINUX workstation using reconstruction code written on-site and commercial fitting software (LCModel; Provencher 1993). In order to quantify Glu, Gln, and the other metabolites with the JPRESS data [e.g., NAA, Cr, mI, and Cho], the 24 TE-stepped free-induction decay series (FIDS) were zero-filled out to 64 points, Gaussian-filtered to minimize residual ringing from NAA and Cr signals, and Fourier-Transformed in the TE-dimension. This resulted in a set of 64 J-resolved spectra over 50 Hz. Using GAMMA-simulated J-resolved basis sets, every J-resolved spectral extraction throughout a bandwidth of 50 Hz was fit with its theoretically-correct LCModel template (Smith et al. 1994). The integrated area under the entire 2D surface for each metabolite then was calculated by summing the raw peak areas across all 64 J-resolved extractions for each metabolite. Metabolites with zero amplitude did not contribute to the final integral. The Cr denominator was the sum of the Cr and phosphocreatine integrals. Two spectra from the JPRESS acquisition in the thalamus were excluded from analysis due to low signal-to-noise and/or spectral resolution.
In order to quantify GABA with the MEGAPRESS data, the difference-edited spectra were fitted with LCModel using basis sets acquired at 4T. The individual “ON” and “OFF” averages were frequency- and phase-corrected by using the NAA resonance of the interleaved “OFF” spectra as a navigator. Individual spectra within the time-series first were averaged in groups of 16 to increase signal-to-noise for this purpose. The phase- and frequency-correction factors from the “OFF” NAA resonance for each averaged group were used for both the “ON” and “OFF” datasets. This was necessary since the NAA resonance was saturated in the “ON” spectra due to the frequency-selective Gaussian editing pulse applied at 1.89 ppm. This strategy has proven effective in tracking and correcting for motion artifacts throughout the 13-min acquisition which otherwise would result in imperfect subtraction and contamination of the difference-edited GABA signal with Cr at 3.00ppm. All corrected “ON” and “OFF” spectra then were averaged separately to produce a single 68 ms “ON” and “OFF” spectrum, which subsequently was subtracted to produce the final, optimized, difference-edited GABA spectrum. The appropriate LCModel templates were used to fit the 68 ms “OFF” spectrum and the difference-edited GABA spectrum. The GABA resonance at 3.00 ppm (as well as the co-edited Glu and Gln peaks at 3.75 ppm and ~2.3 ppm, respectively), were normalized to the fitted Cr resonance from the 68 ms sub-spectrum. One spectrum from the MEGAPRESS acquisition in both the ACC and thalamus were excluded from analysis due to low signal-to-noise and/or spectral resolution.
Image segmentation
The high-resolution T1-weighted images were segmented into grey matter (GM), white matter (WM), and cerebral spinal fluid (CSF) compartments using the segmentation tool in the commercial software package FSL 4.1 (FMRIB Software Library; Analysis Group, FMRIB; Oxford, UK). Subsequently, the volumetric tissue contribution for each oblique voxel was determined and relative contributions of GM and WM were calculated using in-house software.
Data analysis
Our primary metabolites of interest were GABA, Gln, and Glu, while NAA, Cr, Cho, and mI were of secondary interest. Measurements were made two times during one study visit in order to assess the effect of zolpidem on metabolites under each condition (placebo vs. zolpidem).
Subjective data were collected repeatedly over the course of the experimental session. Questionnaires were administered at −30 min, +15 min, +2 hr, + 3 hr 15 min, + 5 hr, + 6hr, and + 7hr for a total of 7 time points (see Figure 1 for clarification of study time line). Data were collapsed such that the two time points before drug administration (+15 min and +2 hrs) were considered “placebo”, while the two time points after drug (+3 hr, 15 min and +5 hrs) were considered “zolpidem”.
Metabolite and subjective data were analyzed initially by two-way ANOVAs examining the effects of sex and drug treatment. Since no data were affected by sex they were analyzed subsequently by one-way ANOVAs examining the effect of drug treatment (or brain region as appropriate).
Pearson product moment correlations were used to quantify relationships between zolpidem-induced changes in neurochemistry (metabolite level following placebo administration-metabolite level following zolpidem administration) and changes in subjective effects (subjective rating following placebo-subjective rating following zolpidem).
All data were analyzed using standard statistical software (SigmaStat 3.1; Systat Software, Inc.; San Jose, CA and SPSS 13; SPSS Inc.; Chicago, IL). ANOVA treatment effects were assessed further post hoc using Bonferroni. Alpha was set at 0.05.
Results
Test/re-test reliability
The within-subject coefficients of variation (CV=standard deviation/mean of both scans) for GABA using MEGAPRESS were 14.7% and 9.5% in ACC and thalamus, respectively. Using 2D-JPRESS, the CV in ACC and thalamus were 5.3% and 8.0% for Glu, 14.0% and 17.8% for Gln, 4.4% and 6.4% for NAA, 3.3% and 1.9% for Cho, and 8.4% and 9.6% for mI, respectively. Of all metabolites, Gln appears to have the poorest CV. However, reliability of Gln detection is at the low end of the published range of 16% to 50% (Kassem and Bartha, 2003; Schulte and Boesiger, 2006; Théberge et al. 2005) and in line with the 19% reported when the entire 2D area was fitted (Schulte and Boesiger, 2006).
Tissue segmentation
The relative percentages of GM and WM in ACC and thalamus were unchanged by treatment with zolpidem. In the ACC there was an average of 63 ± 4% GM and 37 ± 4% WM under baseline (placebo) conditions, while there was 62 ± 5% GM and 38 ± 5 % WM under the treatment (zolpidem) condition. In the thalamus, there was an average of 40 ± 9% GM and 60 ± 9% WM following placebo, while there was 41 ± 9% GM and 59 ± 9% WM following zolpidem.
Brain metabolites
Treatment with zolpidem had no effect on Cr in either the ACC [F(1,36)= 0.18, p=0.67] or the thalamus [F(1,30)= 0.07, p=0.80]. Since there were no drug-induced changes in Cr, all reported metabolite levels are expressed as arbitrary units relative to Cr.
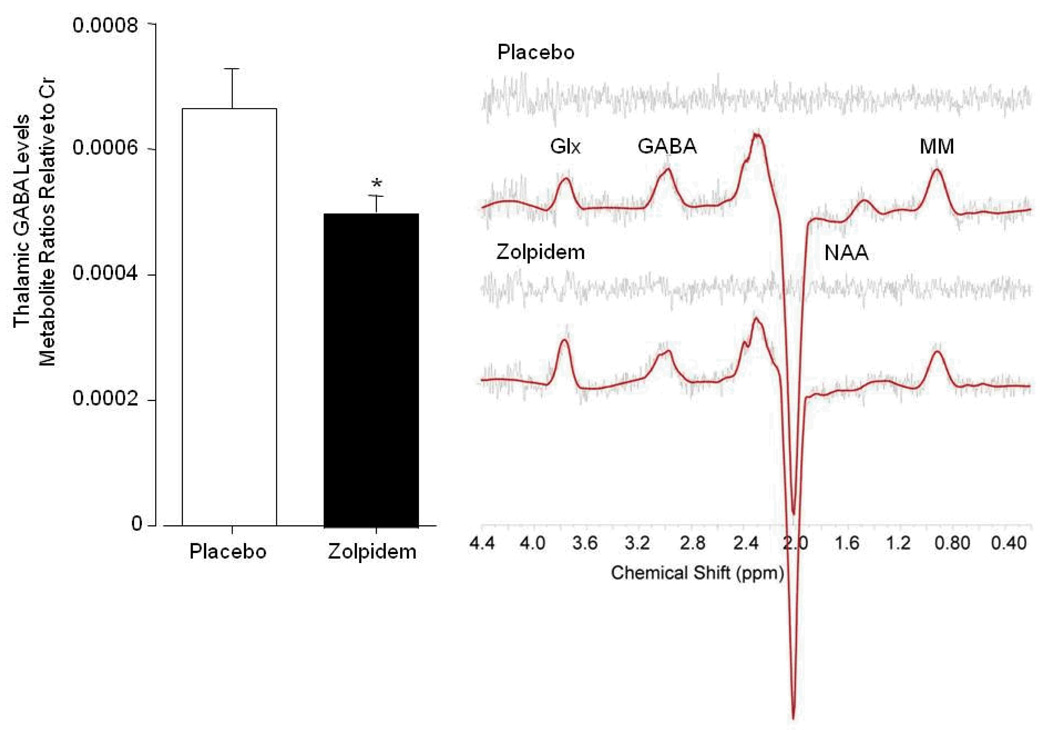
Figure 3 illustrates the main finding that zolpidem decreased GABA by 25% in the thalamus [F(1,34)= 5.82, p= 0.02]. In contrast, zolpidem had no effect on GABA in the ACC [F(1,34)= 0.26, p=0.62]. Importantly, baseline (placebo) levels of GABA within the ACC (0.000350 ± 0.000023) were significantly lower than those within the thalamus (0.000665 ± 0.000063; [F(1,34)= 22.00, p<0.001]).
Fig. 3.
Zolpidem-induced decrease in thalamic GABA/Cr levels (left). Representative MEGAPRESS difference spectra demonstrating the reduction in the 3.00ppm GABA resonance area within a single voxel placed in the left thalamus (right). Spectra are displayed with LCModel fit and residual, and are unfiltered. Glx= glutamate/glutamine, GABA= γ-aminobutryric acid, MM= macromolecules, NAA= N-acetyl aspartate.
Unlike the results obtained for GABA, zolpidem had no effect on Glu [F(1,30)= 0.10, p=0.76] or Gln [F(1,30)= 1.34, p=0.26] in the thalamus. Levels of Glu [F(1,36)= 0.05, p=0.83] and Gln [F(1,36)= 0.74, p=0.40] also were unchanged by zolpidem in the ACC. However, as observed with GABA, baseline levels of Glu were different between the two brain regions. Glu levels were higher in the ACC (0.87 ± 0.03) relative to the thalamus (0.73 ± 0.02; [F(1,33)= 11.22, p= 0.002]). There were no baseline differences in Gln between the two brain regions (ACC: 0.27 ± 0.01 vs. thalamus: 0.31 ± 0.02; [F(1,33)= 3.25, p=0.08]).
Similar analyses revealed no effects of zolpidem on metabolites of secondary interest. NAA (ACC: [F(1,36)= 0.71, p= 0.404]; thalamus: [F(1,30)= 0.70, p= 0.409]), Cho (ACC: [F(1, 36)= 0.07, p= 0.791]; thalamus: [F(1,30)= 0.20, p= 0.657]), and mI (ACC: [F(1,36)= 1.67, p= 0.204]; thalamus: [F(1,30)= 2.12, p= 0.156]) were unchanged in both brain regions. Although baseline levels of NAA were lower in the ACC (1.06 ± 0.02) than in the thalamus (1.26 ± 0.04; [F(1,33)= 21.40, p< 0.001]), Cho was the same in both regions (ACC: 0.24 ± 0.01 vs. thalamus: 0.25 ± 0.01; [F(1, 33)= 3.46, p=0.07]) as was mI (ACC: 0.82 ± 0.01 vs. thalamus: 0.76 ± 0.04; [F(1,33)= 2.64, p=0.11]).
Subjective effects
Table 1 shows the zolpidem-induced increases in ratings of “dizzy”, “nauseous”, “confused”, and “bad effects”. Ratings of “anxious”, “high”, “sleepy”, “good effects”, “loose”, “carefree”, “restless”, “like”, “willing to take again”, “willing to pay for”, and “mentally slow” were not affected significantly. The ARCI was affected such that ratings on the PCAG [F(1,37)= 15.39, p< 0.001] and LSD [F(1,36)= 7.90, p= 0.008] scales were increased, while there was no change in ratings on the BG, MBG, and AMPH scales (data not shown). Out of 19 participants, 4 vomited approximately 2–3 hrs following administration of zolpidem.
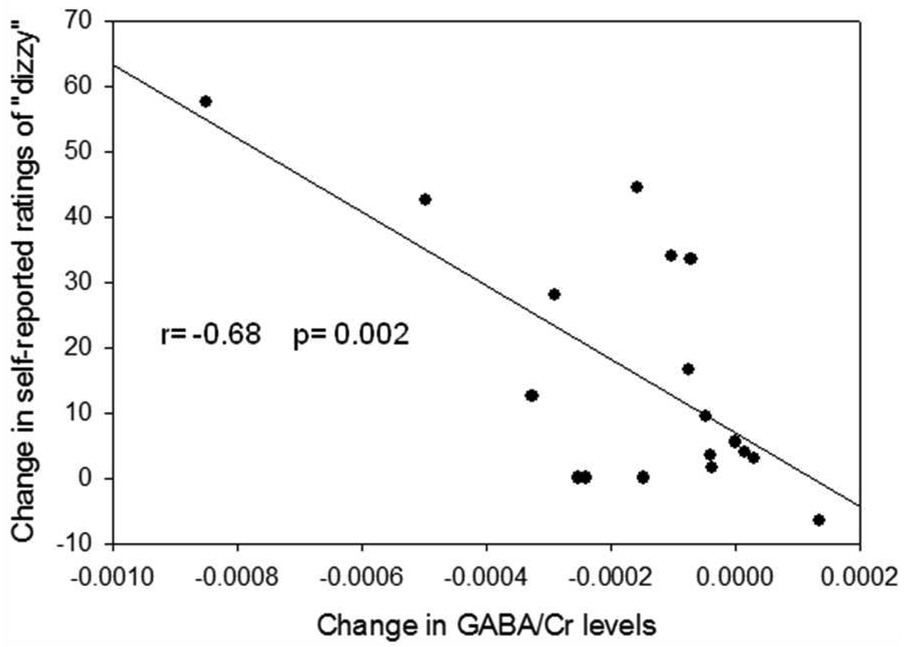
Correlation analyses between zolpidem-induced differences in thalamic GABA levels and changes in subjective effects revealed a significant relationship for “dizzy” (r= −0.68, p= 0.002; Figure 4). The reduction in GABA was not correlated significantly with any other subjective effects.
Fig. 4.
Negative correlation between zolpidem-induced feelings of “dizzy” and zolpidem-induced changes in thalamic GABA/Cr levels.
Discussion
In healthy volunteers with no history of drug abuse, acute oral administration of a therapeutic dose of zolpidem (10 mg) reduced thalamic GABA levels. Self-reported ratings of “dizzy”, “nauseous”, “confused”, and “bad effects” were increased, as were sedation/intoxication and psychotomimetic/dysphoric effects. Moreover, there was a negative correlation between the change in GABA and the ratings of “dizzy”.
Benzodiazepine-type drug effects within the thalamus and ACC
The 25% reduction in thalamic GABA observed here following acute administration of zolpidem is in agreement with the 24% reduction in GABA observed in the occipital cortex following acute administration of the benzodiazepine clonazepam (Goddard et al. 2004). The present result also is consistent with previous studies demonstrating a decrease in cerebral metabolic activity within the thalamus following administration of benzodiazepine-type drugs. For example, studies employing positron emission tomography (PET) imaging in healthy volunteers have reported a reduction in regional cerebral blood flow of approximately 20% (Matthew et al. 1995; Veselis et al. 1997) and a 23% reduction in glucose metabolism (Volkow et al. 1995; Wang et al. 1996) upon administration of benzodiazepines. Similarly, local cerebral glucose metabolism was decreased in subcortical areas when participants were administered zolpidem (Gillin et al. 1996). Together, these results suggest that the thalamus is a prominent effector site for the action of benzodiazepines and the newer non-benzodiazepine hypnotics.
In the present study zolpidem had no effect on GABA in the ACC. Previously, zolpidem caused a decrease in regional cerebral blood flow in the ACC of healthy volunteers following zolpidem administration (Finelli et al. 2000), while in a patient with akinetic mutism a paradoxical increase in cerebral metabolism was observed that was accompanied by an improvement in motor and cognitive functioning (Brefel-Courbon et al. 2007). The temporal resolution of this particular experiment may have obscured the effects of zolpidem in the ACC; the scanning protocol began 30 min following drug administration, but data acquisition in the ACC began approximately 15 min after that, and the two separate sequences together (2D-JPRESS and MEGAPRESS) lasted approximately 26 min before a voxel was placed in the thalamus. Therefore, metabolite measurement in the ACC occurred approximately 45–70 min following drug administration, while the thalamus was measured approximately 80–105 min after zolpidem. Alternatively, it is possible that the low baseline levels of GABA within the ACC prevented further reductions. In other words, there may have been a floor effect in this brain region relative to the thalamus.
Functional output of GABAergic modulation by zolpidem
In the present study, a therapeutic dose of zolpidem increased ratings of feelings that may be considered unpleasant such as “dizzy”, “nauseous”, “confused”, and “bad effects”, in healthy drug-naïve participants. These results agree with previous studies in which similar effects were reported under comparable conditions (Licata et al. 2008; Mintzer et al. 1998; Rush et al. 1997). Other consistent changes in self-reported subjective effects of zolpidem in healthy volunteers included higher scores on the PCAG (sedation/intoxication) and LSD (psychotomimetic/dysphoric) scales of the ARCI (Licata et al. 2008; Rush and Griffiths 1996; Rush et al. 1997; Rush et al. 1998; Stoops and Rush 2003).
Despite the persistence of these zolpidem-induced dysphoric and sedative effects across studies, only the effect of “dizzy” was correlated significantly with the decrease in GABA observed in the thalamus. This finding was evident by many participants’ inability to balance without difficulty when walking from the scanning suite to the behavioral research laboratory, and is consistent with previous empirical studies demonstrating zolpidem’s impairment of balance independent of muscle relaxation (Licata et al. in press; Nakamura et al. 2005). Importantly, it is in agreement with a body of literature indicating not only that GABA is a primary neurotransmitter of the vestibular nuclei in many different species (Tighilet and Lacour 2001), but that the thalamus is an important relay structure in the pathway between the brainstem and the vestibular cortex (e.g., Karnath et al. 2000).
Interestingly, zolpidem had no effect on “sleepy”. While this dose of zolpidem is the recommended dose prescribed to treat insomnia, participants often needed to be awoken after their scanning sessions, and scores on the sedation (PCAG) scale of the ARCI were elevated relative to placebo, this finding is in agreement with other reports demonstrating that when compared, benzodiazepines engendered increases in ratings of “sleepy” but zolpidem did not (Evans et al. 1990; Mintzer et al. 1998). Moreover, given that zolpidem modulated GABAergic tone in the thalamus, and the thalamus is an integral component of the complex circuitry regulating sleep/wakefulness (e.g., see review by Merica and Fortune 2004), it is surprising that participants consistently have failed to report feelings of sleepiness. This is in contrast to the benzodiazepine lorazepam, which induced an increase in ratings of sleepiness and tiredness that correlated with a decrease in thalamic glucose metabolism (Volkow et al. 1995). Although the results obtained with lorazepam were not compared to zolpidem directly, these seemingly discordant findings suggest that either there is some subtle divergence in zolpidem’s underlying mechanism of action relative to that of classical benzodiazepines, or the sensitivity of the VAS is insufficient to access this subjective state.
Circuitry mediating observed effects of zolpidem in the thalamus
The thalamus is an integral component within the brain that receives sensory input, sends appropriate motor output, and also regulates sleep states. The circuitry mediating thalamic function is extensive, and the profound effects of zolpidem in this region may arise not only as a result of several systems projecting to and converging upon the thalamus, but also as a function of zolpidem’s interactions with specific GABAA receptors therein. Unlike classical benzodiazepines, zolpidem binds selectively to α1GABAA receptors, moderately at α2GABAA and α3GABAA receptors, and inappreciably at α5GABAA receptors (Hadingham et al. 1993; Sanna et al. 2002).
For instance, one main GABAergic projection into the thalamus originates from the cerebellum, which receives afferents from the pontine nuclei (Brodal and Bjaalie 1992). This connection is particularly relevant with respect to the putative involvement of vestibular pathways in mediating zolpidem’s ability to engender “dizzy” because cerebellar nuclei not only supply the thalamus with this type of information (Meng et al. 2007), but they are plentiful with α1GABAA receptors (Fritschy and Mohler 1995). Similarly, α1GABAA receptors are distributed abundantly throughout the cortex (Pirker et al. 2000) raising the possibility that zolpidem’s influence in the thalamus may be cortical in origin. Enhanced cortical inhibition may lead to a reduction in glutamatergic drive that may not only diminish GABA synthesis and/or release from thalamic neurons (Goddard et al. 2004), but it could help to explain the other metabolic effects observed in the thalamus following administration of this type of drug (Gillin et al. 1996; Matthew et al. 1995; Veselis et al. 1997; Volkow et al. 1995; Wang et al. 1996). In contrast, the distribution of GABAA receptor subtypes within the thalamus makes it unlikely that the present results could be explained by direct thalamic stimulation. Although α1GABAA receptors are highly expressed in thalamus (Wisden et al. 1992), α4GABAA receptors (i.e., benzodiazepine-type drug-insensitive receptors) also are abundant (Khan et al. 1996; Sur et al. 1999). Furthermore, distribution of the γ subunit is scant in the thalamus, thereby precluding the binding of benzodiazepine-type ligands (Sur et al. 1999; Wisden et al. 1992). Although GABAA receptor localization data all have been derived from rodent studies, together they suggest that zolpidem’s effect on GABA was most likely consequent to activation of the ascending system and/or corticothalamic pathways in the brains of healthy human volunteers.
Limitations
There are several limitations to this study that should be considered when interpreting the findings. First, only two voxels of interest were assessed. Although the cerebellum was discussed as a potential site of interaction between zolpidem GABAA receptors, there were no a priori hypotheses regarding the cerebellum, and therefore changes in neurochemistry were not evaluated there. A second limitation concerns the relative percentage of grey matter in the voxel that was placed in the thalamus. This voxel was large and covered a fair amount of the left thalamic lobe, thereby encompassing a substantial amount of white matter in addition to grey. While it will be important to optimize this voxel placement and subsequent segmentation in future studies, it should be noted the relative percentage of thalamic grey matter did not change upon administration of zolpidem. Finally, the data were normalized to Cr despite the inherent disadvantage that its concentration may not be stable across brain regions, between GM and WM, or in disease states. Without a tissue biopsy, there is no way to determine precisely Cr concentration, thus preventing estimation of absolute millimolar concentrations of metabolites. However, internal Cr does correct for important factors such as voxel partial-volume effects and B1-inhomogeneity, thus reducing the variance in the data. This is a crucial step in quantifying low-abundance metabolites such as GABA and Gln. In the present study, using Cr as an internal reference was sufficient because relative metabolite changes following drug administration were the measures of interest, and each participant served as his/her own control. If acute administration of zolpidem had caused a transient shift in neuronal bioenergetics, this likely would have translated into a shift of the creatine-kinase reaction while preserving the total sum of Cr and Pcr (which has been termed “Cr” in the present study). Therefore, regardless of any potential zolpidem-induced alterations in energetics, total Cr would be expected to remain unchanged. Indeed, the present data support that hypothesis, as the raw Cr integral was unchanged following the zolpidem challenge.
Conclusions
A therapeutic dose of the popular non-benzodiazepine hypnotic zolpidem had a profound effect in drug-naïve individuals, engendering primarily dysphoric-like effects. GABA levels were reduced concomitantly with an increase in feelings of “dizzy”, suggesting that zolpidem interacts with the vestibular system at the dose recommended to treat insomnia. Further study is necessary to determine if these effects are unique to this dose and to this population, and if abuse-related effects that typically occur in experienced drug-using individuals will be associated with a different neurochemical outcome.
Acknowledgments
This study was funded by the National Institutes on Drug Abuse grants K01 DA023659 (Dr. Licata), K05 DA000343 (Dr. Lukas), and K24 DA151116 (Dr. Renshaw). The authors have no financial relationships with the National Institutes on Drug Abuse. The experiments described herein complied with the current laws of the United States of America.
The authors thank R. Ross MacLean for his expert assistance. The authors also thank Dr. George Trksak for helpful discussion regarding statistical analyses.
References
- Burau T, Schilling AM, Seyfert S, Spies C, Wolf KJ. Cerebral drug effects investigated by 1H MR spectroscopy in volunteers and patients [abstract] Proceedings of the International Society for Magnetic Resonance in Medicine. 1997;5:1244. [Google Scholar]
- Benavides J, Peny B, Dubois A, Perrault G, Morel E, Zivkovic B, Scatton B. In vivo interaction of zolpidem with central benzodiazepine (BZD) binding sites (as labeled by [3H]Ro 15–1788) in the mouse brain. Preferential affinity of zolpidem for the omega 1 (BZD1) subtype. J Pharmacol Exp Ther. 1988;245:1033–1041. [PubMed] [Google Scholar]
- Brambilla P, Stanley JA, Nicoletti M, Harenski K, Forster Wells K, Mallinger AG, Keshavan MS, Soares JC. 1H MRS brain measures and acute lorazepam administration in healthy human subjects. Neuropsychopharmacology. 2002;26:546–551. doi: 10.1016/S0893-133X(01)00388-8. [DOI] [PubMed] [Google Scholar]
- Brefel-Courbon C, Payoux P, Ory F, Sommet A, Slaoui T, Raboyeau G, Lemesle B, Puel M, Montastruc JL, Demonet JF, Cardebat D. Clinical and imaging evidence of zolpidem effect in hypoxic encephalopathy. Ann Neurol. 2007;62:102–105. doi: 10.1002/ana.21110. [DOI] [PubMed] [Google Scholar]
- Brodal P, Bjaalie JG. Organization of the pontine nuclei. Neurosci Res. 1992;13:83–118. doi: 10.1016/0168-0102(92)90092-q. [DOI] [PubMed] [Google Scholar]
- Brodeur MR, Stirling AL. Delirium associated with zolpidem. Ann Pharmacother. 2001;35:1562–1654. doi: 10.1345/aph.10385. [DOI] [PubMed] [Google Scholar]
- Davanzo P, Oshiro T, Thomas MS, Shah B, Belin T, McCracke J, Ke Y. 1H MR Spectroscopy in human brain with and without lorazepam [abstract] Proceedings of the International Society for Magnetic Resonance in Medicine. 1997;5:1226. [Google Scholar]
- Evans SM, Funderburk FR, Griffiths RR. Zolpidem and triazolam in humans: behavioral and subjective effects and abuse liability. J Pharmacol Exp Ther. 1990;255:1246–1255. [PubMed] [Google Scholar]
- Finelli LA, Landolt HP, Buck A, Roth C, Berthold T, Borbély AA, Achermann P. Functional neuroanatomy of human sleep states after zolpidem and placebo: a H215O-PET study. J Sleep Res. 2000;9:161–173. doi: 10.1046/j.1365-2869.2000.00191.x. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Ganzoni E, Santoni JP, Chevillard V, Sébille M, Mathy B. Zolpidem in insomnia: a 3-year post-marketing surveillance study in Switzerland. J Int Med Res. 1995;23:61–73. doi: 10.1177/030006059502300108. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Buchsbaum MS, Valladares-Neto DC, Hong CC-H, Hazlett E, Langer SZ, Wu J. Effects of zolpidem on local cerebral glucose metabolism during non-REM sleep in normal volunteers: A positron emission tomography study. Neuropsychopharmacology. 1996;15:302–313. doi: 10.1016/0893-133X(95)00234-5. [DOI] [PubMed] [Google Scholar]
- Goddard AW, Mason GF, Appel M, Rothman DL, Gueorguieva R, Behar KL, Krystal JH. Impaired GABA neuronal response to acute benzodiazepine administration in panic disorder. Am J Psychiatry. 2004;161:2186–2193. doi: 10.1176/appi.ajp.161.12.2186. [DOI] [PubMed] [Google Scholar]
- Hadingham KL, Wingrove P, Le Bourdelles B, Palmer KJ, Ragan CI, Whiting PJ. Cloning of cDNA sequences encoding human alpha 2 and alpha 3 gamma-aminobutyric acidA receptor subunits and characterization of the benzodiazepine pharmacology of recombinant alpha 1-, alpha 2-, alpha 3-, and alpha 5-containing human gamma-aminobutyric acidA receptors. Mol Pharmacol. 1993;43:970–975. [PubMed] [Google Scholar]
- Haertzen CA. Development of scales based on patterns of drug effects, using the Addiction Research Center Inventory (ARCI) Psychol Rep. 1966;18:163–194. doi: 10.2466/pr0.1966.18.1.163. [DOI] [PubMed] [Google Scholar]
- Hajak G, Bandelow B. Safety and tolerance of zolpidem in the treatment of disturbed sleep: a post-marketing surveillance of 16944 cases. Int Clin Psychopharmacol. 1998;13:157–167. doi: 10.1097/00004850-199807000-00002. [DOI] [PubMed] [Google Scholar]
- Harazin J, Berigan TR. Zolpidem tartrate and somnambulism. Mil Med. 1999;164:669–670. [PubMed] [Google Scholar]
- Hoehns JD, Perry PJ. Zolpidem: a nonbenzodiazepine hypnotic for treatment of insomnia. Clin Pharm. 1993;12:814–828. [PubMed] [Google Scholar]
- Huang CL, Chang CJ, Hung CF, Lin HY. Zolpidem-induced distortion in visual perception. An Pharmacother. 2003;37:683–686. doi: 10.1345/aph.1C318. [DOI] [PubMed] [Google Scholar]
- Jasinski DR. Assessment of the abuse potential of morphine-like drugs (methods used in man) In: Martin WR, editor. Drug Addiction I. Vol 45/1. Springer-Verlag: Heidelberg; 1977. pp. 197–258. [Google Scholar]
- Karnath HO, Ferber S, Dichgans J. The neural representation of postural control in humans. Proc Natl Acad Sci USA. 2000;98:13931–13936. doi: 10.1073/pnas.240279997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem MN, Bartha R. Quantitative proton short-echo-time LASER spectroscopy of normal human white matter and hippocampus at 4 Tesla incorporating macromolecule subtraction. Magn Reson Med. 2003;49:918–927. doi: 10.1002/mrm.10443. [DOI] [PubMed] [Google Scholar]
- Khan ZU, Gutierrez A, Mehta AK, Miralles CP, De Blas AL. The α4 subunit of the GABAA receptors from rat brain and retina. Neuropharmacology. 1996;35:1315–1322. doi: 10.1016/s0028-3908(96)00033-0. [DOI] [PubMed] [Google Scholar]
- Langtry HD, Benfield P. Zolpidem: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential. Drugs. 1990;40:291–313. doi: 10.2165/00003495-199040020-00008. [DOI] [PubMed] [Google Scholar]
- Liappas IA, Malitas PN, Dimopoulos NP, Gitsa OE, Liappas AI, Nikolau ChK, Christodoulou GN. Zolpidem dependence case series: possible neurobiological mechanisms and clinical management. J Psychopharmacol. 2003;17:131–135. doi: 10.1177/0269881103017001723. [DOI] [PubMed] [Google Scholar]
- Licata SC, Penetar DM, Dunlap S, Lukas SE. A therapeutic dose of zolpidem has limited abuse-like effects in drug-naïve females: A pilot study. Eur J Pharmacol. 2008;598:64–67. doi: 10.1016/j.ejphar.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata SC, Platt DM, Cook JM, Van Linn ML, Rowlett JK. Contribution of α1 subunit-containing γ-aminobutyric acidA (GABAA) receptors to motor-impairing effects of benzodiazepines in squirrel monkeys. Psychopharmacology. doi: 10.1007/s00213-008-1401-7. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz JS, Brewerton TD. Zolpidem-induced psychosis. Ann Clin Psychiatry. 1996;8:89–91. doi: 10.3109/10401239609148806. [DOI] [PubMed] [Google Scholar]
- Matthew E, Andreason P, Pettigrew K, Carson RE, Herscovitch P, Cohen R, King C, Johanson CE, Greenblatt DJ, Paul SM. Benzodiazepine receptors mediate regional blood flow changes in the living human brain. Proc Natl Acad Sci U S A. 1995;92:2775–2779. doi: 10.1073/pnas.92.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor alpha 1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- Meng H, May PJ, Dickman JD, Angelaki DE. Vestibular signals in primate thalamus: Properties and origins. J Neurosci. 2007;27:13590–13602. doi: 10.1523/JNEUROSCI.3931-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merica H, Fortune RD. State transitions between week and sleep, and within the ultradian cycle, with focus on the link to neuronal activity. Sleep Med Rev. 2004;8:473–485. doi: 10.1016/j.smrv.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Mescher M, Merkle M, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Frey JM, Griffiths RR. Zolpidem is differentiated from triazolam in humans using a three-response drug discrimination procedure. Behav Pharmacol. 1998;9:545–559. doi: 10.1097/00008877-199811000-00010. [DOI] [PubMed] [Google Scholar]
- Morgenthaler TI, Silber MH. Amnestic sleep-related eating disorder associated with zolpidem. Sleep Med. 2002;3:323–327. doi: 10.1016/s1389-9457(02)00007-2. [DOI] [PubMed] [Google Scholar]
- Morlock RJ, Tan M, Mitchell DY. Patient characteristics and patterns of drug use for sleep complaints in the United States: analysis of National Ambulatory Medical Survey Data, 1997–2002. Clin Ther. 2006;28:1044–1053. doi: 10.1016/j.clinthera.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Ishii M, Niwa Y, Yamazaki M, Ito H. Temporal changes in postural sway caused by ultrashort-acting hypnotics: triazolam and zolpidem. ORL J Otorhinolaryngol Relat Spec. 2005;67:106–112. doi: 10.1159/000084998. [DOI] [PubMed] [Google Scholar]
- Najjar M. Zolpidem and amnestic sleep related eating disorder. J Clin Sleep Med. 2007;15:637–638. [PMC free article] [PubMed] [Google Scholar]
- Öngür D, Jensen JE, Prescott AP, Stork C, Lundy M, Cohen BM, Renshaw PF. Abnormal glutamatergic neurotransmission and neuronal glial interactions in acute mania. Biol Psychiatry. 2008;64:718–726. doi: 10.1016/j.biopsych.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Seeburg PH. Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science. 1990;245:1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Roger M, Attali P, Coquelin JP. Multicenter, double-blind, controlled comparison of zolpidem and triazolam in elderly patients with insomnia. Clin Ther. 1993;15:127–136. [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bleuthmann H, Möhler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acid (A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Rush CR. Behavioral pharmacology of zolpidem relative to benzodiazepines: a review. Pharmacol Biochem Behav. 1998;61:253–269. doi: 10.1016/s0091-3057(98)00102-6. [DOI] [PubMed] [Google Scholar]
- Rush CR, Armstrong DL, Ali JA, Pazzaglia PJ. Benzodiazepine-receptor ligands in humans: acute performance-impairing, subject-rated and observer-rated effects. J Clin Psychopharmacol. 1998;18:154–165. doi: 10.1097/00004714-199804000-00008. [DOI] [PubMed] [Google Scholar]
- Rush CR, Baker RW, Wright K. Acute behavioral effects and abuse potential of trazodone, zolpidem and triazolam in humans. Psychopharmacology. 1999;144:220–233. doi: 10.1007/s002130050997. [DOI] [PubMed] [Google Scholar]
- Rush CR, Griffiths RR. Zolpidem, triazolam, and temazepam: behavioral and subject-rated effects in normal volunteers. J Clin Psychopharmacol. 1996;16:146–157. doi: 10.1097/00004714-199604000-00007. [DOI] [PubMed] [Google Scholar]
- Rush CR, Madakasira S, Goldman NH, Woolverton WL, Rowlett JK. Discriminative stimulus effects of zolpidem in pentobarbital-trained subjects: II. Comparison with triazolam and caffeine in humans. J Pharmacol Exp Ther. 1997;280:174–188. [PubMed] [Google Scholar]
- Salva P, Costa J. Clinical pharmacokinetics and pharmacodynamics of zolpidem. Clin Pharmacokinet. 1995;29:142–153. doi: 10.2165/00003088-199529030-00002. [DOI] [PubMed] [Google Scholar]
- Sanger DJ, Perrault G, Morel E, Joly D, Zivkovic B. The behavioral profile of zolpidem, a novel hypnotic drug of imidazopyridine structure. Physiol Behav. 1987;41:235–240. doi: 10.1016/0031-9384(87)90359-3. [DOI] [PubMed] [Google Scholar]
- Sanna E, Busonero F, Talani G, Carta M, Massa F, Peis M, Maciocco E, Biggio G. Comparison of the effects of zaleplon, zolpidem, and triazolam at various GABA(A) receptor subtypes. Eur J Pharmacol. 2002;451:103–110. doi: 10.1016/s0014-2999(02)02191-x. [DOI] [PubMed] [Google Scholar]
- Schulte RF, Boesiger P. ProFit: two-dimensional prior knowledge fitting of the J-resolved spectra. NMR Biomed. 2006;19:255–263. doi: 10.1002/nbm.1026. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dewan VK. A case report of zolpidem-induced somnambulism. Prim Care Companion J Clin Psychiatry. 2005;7:74. doi: 10.4088/pcc.v07n0207a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Levante TO, Meier BH, Ernst RR. Computer simulations in magnetic resonance. An object oriented programming approach. J Magn Reson A. 1994;106:75–105. [Google Scholar]
- Stoops WW, Rush CR. Differential effects in humans after repeated administrations of zolpidem and triazolam. Am J Drug Alcohol Abuse. 2003;29:281–299. doi: 10.1081/ada-120020513. [DOI] [PubMed] [Google Scholar]
- Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of α4 and δ subunits of the γ-aminobutyric acidA receptor in rat thalamus. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- Théberge J, Menon RS, Williamson PC, Drost DJ. Implementation issues of multivoxel STEAM-localized 1H spectroscopy. Magn Reson Med. 2005;53:713–718. doi: 10.1002/mrm.20350. [DOI] [PubMed] [Google Scholar]
- Tighilet B, Lacour M. Gamma amino butyric acid (GABA) immunoreactivity in the vestibular nuclei of normal and unilateral vestibular neurectomized cats. Eur J Neurosci. 2001;13:2255–2267. doi: 10.1046/j.0953-816x.2001.01622.x. [DOI] [PubMed] [Google Scholar]
- Toner LC, Tsambiras BM, Catalano G, Catalano MC, Cooper DS. Central nervous system side effects associated with zolpidem treatment. Clin Neuropharmacol. 1999;23:54–58. doi: 10.1097/00002826-200001000-00011. [DOI] [PubMed] [Google Scholar]
- Troy SM, Lucki I, Unruh MA, Cevallos WH, Leister CA, Martin PT, Furlan PM, Mangano R. Comparison of the effects of zaleplon, zolpidem, and triazolam on memory, learning, and psychomotor performance. J Clin Psychopharmacol. 2000;20:328–337. doi: 10.1097/00004714-200006000-00007. [DOI] [PubMed] [Google Scholar]
- Tsai MJ, Tsai YH, Huang YB. Compulsive activity and anterograde amnesia after zolpidem use. Clin Toxicol. 2007;45:179–181. doi: 10.1080/15563650600956741. [DOI] [PubMed] [Google Scholar]
- Tsai MJ, Huang YB, Wu PC. A novel clinical pattern of visual hallucination after zolpidem use. J Toxicol Clin Toxicol. 2003;41:869–872. doi: 10.1081/clt-120025354. [DOI] [PubMed] [Google Scholar]
- Veselis RA, Reinsel RA, Beattie BJ, Mawlawi OR, Feshchenko VA, DiResta GR, Larson SM, Blasberg RG. Midazolam changes cerebral blood flow in discrete brain regions: an H2(15)O positron emission tomography study. Anesthesiology. 1997;87:1106–1117. doi: 10.1097/00000542-199711000-00015. [DOI] [PubMed] [Google Scholar]
- Victorri-Vigneau C, Dailly E, Veyrac G, Jolliet P. Evidence of zolpidem abuse and dependence: results of the French Centre for Evaluation and Information on Pharmacodependence (CEIP) network survey. Br J Clin Pharmacol. 2007;64:198–209. doi: 10.1111/j.1365-2125.2007.02861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Hitzemann R, Fowler JS, Pappas N, Lowrimore P, Burr G, Pascani K, Overall J, Wolf AP. Depression of thalamic metabolism by lorazepam is associated with sleepiness. Neuropsychopharmacology. 1995;12:123–132. doi: 10.1016/0893-133X(94)00068-B. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Overall J, Hitzemann RJ, Pappas N, Pascani K, Fowler JS. Reproducibility of regional brain metabolic responses to lorazepam. J Nucl Med. 1996;37:1609–1613. [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Dollear M, Muthukrishnan SR. One rare side effect of zolpidem—sleepwalking: a case report. Arch Phys Med Rehabil. 2005;86:1265–1266. doi: 10.1016/j.apmr.2004.11.022. [DOI] [PubMed] [Google Scholar]