Abstract
Vagal afferent nerves are essential for optimal neural regulation of visceral organs, but are not often considered important for their defense. However, there are well-defined subsets of vagal afferent nerves that have activation properties indicative of specialization to detect potentially harmful stimuli (nociceptors). This is clearly exemplified by the vagal bronchopulmonary C-fibers that are quiescent in healthy lungs but are readily activated by noxious chemicals and inflammatory molecules. Vagal afferent nerves with similar activation properties have been also identified in the esophagus and probably exist in other visceral tissues. In addition, these putative vagal C-fibers often initiate defensive reflexes, can be sensitized, and have the capacity to induce central sensitization. This set of properties is characteristic of nociceptors in somatic tissues.
Introduction
The term nociceptor is commonly used to imply that the nerve in question initiates pain, although this assumption is not always validated. To our knowledge there is no conclusive evidence that the vagal primary afferent nerves initiate pain. This function seems to be reserved for a subset of afferent nerves originating in the spinal dorsal root ganglia. Vagal inputs likely integrate with the inputs from the spinal pathways in the central nervous system to modulate perception of pain. However, this potential role hardly qualifies vagus nerves as pain pathways. Thus, if the common usage of term nociceptor is followed, it is at present difficult to argue that any particular subset of vagal nerve fibers should be labeled as nociceptors.
In this text we evaluate the question whether there are vagal afferent nerves that specialize on the detection of stimuli associated with impeding or actual tissue damage and initiate responses aimed to prevent or limit tissue damage. We review the evidence that there are well-characterized examples of vagal afferent nerves with the properties that typically characterize certain types of somatosensory nociceptors. While the nomenclature of these vagal nerves continues to be debated, we argue that there are practical advantages in conceptualizing these vagal afferent nerves as nociceptors.
In the Integrated Action of the Nervous System, Sherrington discussed specific types of sensory nerve fibers in the skin that, ”Instead of but one kind of stimulus being their adequate excitant, they may be regarded as adapted to a whole group of excitants, a group of excitants which has in relations to the organism one feature common to all its components, namely a nocuous character” (Sherrington, 1906). Sherrington argued that such nerve fibres… “under selective adaptation, attach to the skin a so-to-say specific sense of its own injuries” and termed this special type of afferent nerve nociceptor. In contrast, in modern literature the term nociceptor is generally used to refer to the nerves that mediate pain. Thus, it appears that compared to the Sherrington original description, the defining property of the nociceptor has shrunk from the nature of its activators to a single specific consequence of its activation (pain). This complicates the discussion of the afferent nerves that fit the Sherrington’s characterization of the nociceptors, but whose role in pain has not been established. Such nerves include large numbers of vagal afferent nerve fibers innervating the viscera.
Vagal afferent nerves are traditionally thought of as the afferent pathways that detect physiologic information from the viscera while a subset of the spinal afferent nerves is implicated in detection of “noxious” visceral information (Blackshaw et al., 2007; Grundy, 2002). Indeed, there is extensive evidence of the well-defined vagal afferent nerve fibers specialized to detect stimuli associated with physiological activity of visceral tissues (reviewed elsewhere in this issue). The examples include the vagal low threshold mechanosensitive nerve terminals in the lungs (“stretch receptors”) and in the esophagus (tension mechano-receptors), and the vagal afferent nerves specialized to detect nutrients. On the other hand, a subset of vagal afferent nerves have sensory transduction properties allowing for selective detection of stimuli associated with tissue inflammation and damage. For the purpose of this discussion we will term these nerves “putative vagal nociceptors” referring to the Sherrington’s original definition above. In the instances when a commonly accepted term is available to denote a specific group of putative vagal nociceptors (such as bronchopulmonary C-fibers) this term will be preferred.
The classically described somatosensory nociceptor in the skin is an unmyelinated afferent nerve fiber (C-fiber) that is polymodal, i.e. responsive to various physical stimuli in an intensity range associated with impending or actual tissue damage as well as to noxious chemical stimuli including endogenous molecules produced in tissue injury and inflammation (Julius et al., 2001). The C-fiber nociceptor is relatively unresponsive to innocuous stimulation thus displaying little activity in a healthy uninjured skin not exposed to noxious factors. Typical phenomena observed in C-fiber somatosensory nociceptors are peripheral and central sensitization. Peripheral sensitization denotes an increase of the primary afferent nerve sensitivity and is typically initiated by inflammatory mediators. Central sensitization is the capacity of nociceptors to induce an increase in the synaptic efficacy on the second order sensory neurons. Central sensitization in the somatosensory system results in reduced threshold for pain, an amplification of pain responses and a spread of pain hypersensitivity to non-injured areas. The consequences of C-fiber nociceptor activation are nocifensive reflexes and a major sensation attributed to the skin C-fiber nociceptors is pain.
Similarly to somatosensory nociceptors, certain vagal afferent nerves are selectively responsive to noxious stimuli, initiate defensive reflexes and contribute to warning sensations, and can be sensitized and have capacity to induce central sensitization. It should be kept in mind that the definition of noxious stimuli may be context dependent. For example, certain mechanical force may be considered noxious in some tissue, and innocuous in another more distensible tissue. It should be also noted that even though vagal nociceptors and somatosensory nociceptors in the skin share certain fundamental properties this does not imply that they are identical in all aspects. It has been long appreciated that the nociceptors in the skin (and likely elsewhere in somatic tissues) can be divided into distinct classes with certain class-specific properties. Similarly, as discussed below, the putative vagal nociceptors can be segregated into distinct subtypes, but the basis of the subdivision may differ between vagal and somatosensory nociceptors.
Putative vagal nociceptive nerve fibers in the lungs
Vagal afferent nerves innervating the mammalian lungs can be divided into two general categories: the A-fiber mechanosensors sensitive to lung distention (also termed pulmonary stretch receptors) and bronchopulmonary C-fibers (Canning et al., 2006). In addition, several other types of vagal afferent nerve fibers can be found in the respiratory system such as myelinated fibers innervating the neuroepithelial bodies (Adriaensen et al., 2006) and the touch-sensitive A-fibers in the large conducting airways (Riccio et al., 1996). The unmyelinated fibers (C-fibers) comprise majority (>75%) of the afferent nerve fibers innervating the lungs (Agostoni et al., 1957). Vagal C-fibers innervate all compartments of the lungs from the large conducting airways to alveoli (Coleridge et al., 1984; Lee et al., 2001b).
General anatomical and functional properties of the bronchopulmonary C-fibers are reasonably similar in all species in which these nerves have been studied including mouse, rat, guinea pig, rabbit, cat, dogs and monkey (reviewed in (Canning et al., 2009)). Anatomically, the vagal C-fibers in the airway have been described as extensively branched nerve fibers that form dense plexus (Baluk et al., 1992; Watanabe et al., 2005). Thus the vagal C-fibers in the respiratory mucosa structurally resemble C-fiber nociceptors in the skin. A proportion of the vagal C-fibers in all species contain neuropeptides such as substance P, neurokinin A and calcitonin gene related peptide, although the neuropeptides are more commonly found in the C-fibers of some rodents.
The primary function of the respiratory system is gas exchange. This function critically depends on airway patency and functional integrity of the alveolar membrane. Hypoxia resulting from a serious limitation to alveolar gas exchange is an imminent danger to the organism. Given a relatively delicate biology of the airway epithelium and alveolar membrane, and the fact that they come to extensive contact with the components of outside environment (inhaled air) it is relatively straightforward to identify exogenous noxious factors with the potential to compromise lung function. Examples of such noxious factors are sulfur dioxide, acids, cigarette smoke, ozone, acrolein, ammonia and particulate matter. Similarly, inflammation in the lungs can limit alveolar gas exchange through a number of mechanisms deteriorating ventilation-perfusion ratio such as edema, infiltration and impaired mucociliary transport. Thus, and similar to the situation elsewhere in the body, inflammatory mediators signal the presence of noxious factors in the lungs.
Vagal bronchopulmonary C-fibers are stimulated by a wide range of exogenous noxious stimuli including those mentioned above, as well as by endogenous inflammatory mediators. The receptors responsible for the activation have been identified for a number of these noxious stimuli. Available data indicate that certain members of the TRP ion channel family, namely TRPV1 and TRPA1, play a prominent role in the activation of bronchopulmonary C-fibers by noxious stimuli.
The selective TRPV1 agonist capsaicin has long been used to stimulate the vagal bronchopulmonary C-fibers for the study of respiratory reflexes and in electrophysiological experiments. Indeed, a majority of the bronchopulmonary C-fibers in all species are activated by capsaicin (Lee et al., 2001b; Lee et al., 2003). TRPV1 directly mediates or contribute to sensory transduction of acid, certain lipid mediators such 12-lipoxygenase products and anandamide, bradykinin and others (Clapham et al., 2005). More recently, TRPA1 has been shown to mediate robust activation in the vagal bronchopulmonary C-fibers (Nassenstein et al., 2008; Taylor-Clark et al., 2009b). TRPA1 has attracted much attention in the pain field (Jordt et al., 2004; Story et al., 2003). Due to an atypical mechanism of TRPA1 activation that involves covalent modification of specific cysteine residues, TRPA1 confers sensitivity to a long list of reactive electrophilic compounds (Hinman et al., 2006; Macpherson et al., 2007). Some examples of the exogenous noxious chemicals relevant for the lung include environmental pollutants acrolein, toluene diisocyanate, and presumably ozone and nitrogen oxides (Taylor-Clark et al., 2009b). Endogenous TRPA1 activators can be produced by oxidative stress (nitrated fatty acids, 4-hydroxynonenal, 4-oxononenal) or during prostaglandin metabolism (i.e. the PGD2 metabolite 15-deoxy-Δ(12,14)-prostaglandin J2) (Andersson et al., 2008; Bessac et al., 2008; Taylor-Clark et al., 2009a; Taylor-Clark et al., 2008a; Taylor-Clark et al., 2008b). Thus, combined expression of TRPV1 and TRPA1 channels makes vagal C-fibers in the lungs wide range chemosensors for noxious chemicals. In addition, other specific receptors such as the adenosine A1 and A2A receptors and nicotinic receptors can mediate activation of the vagal C-fibers (Chuaychoo et al., 2006; Kou et al., 1989).
In contrast to their robust activation by noxious stimuli, the vagal bronchopulmonary C-fibers are rather quiescent in the healthy uninjured lungs. A recent quantitative analysis of data from >20 studies in 5 species (rats, guinea pig, rabbits, cats and dogs) (reviewed in Canning) documents that the bronchopulmonary C-fibers are either silent or display a low level of irregular activity during normal breathing. This is consistent with their mechanosensitive properties. Typically, high levels of lung inflation (deemed non-physiologic) are required to induce meaningful action potential discharge in the naive unsensitized vagal bronchopulmonary C-fibers (Fig. 1D–E). The low resting activity of the vagal C-fibers also implies that these fibers are not effectively stimulated by chemicals comprising the physiological milieu in the environment of their nerve terminals.
Figure 1. Distinct properties of the vagal mechanosensors and putative nociceptors in the respiratory system.

Nerve terminals of the low threshold mechanosensitive vagal A-fiber (A) and putative nociceptive vagal C-fiber (B) in the guinea pig trachea. Nerve terminals were visualized by eGFP expression following the transfection of vagal sensory neurons by eGFP encoding AAV2/8 virus vector injected into vagal ganglia. Computer assisted drawings are shown for clarity. The A-fiber terminals formed regular defined structures arranged parallel to the tracheal muscle fibers as described before (Mazzone et al., 2006; Mazzone et al., 2008). The structure of the A-fibers terminals in the lung is similar (Brouns et al., 2006; Yu et al., 2003).. A single A-fiber was found to supply 1–5 structures shown in (A). A typical C-fiber branching extensively and irregularly over large areas (Watanabe et al., 2005). Note tenfold difference in the scale between (A) and (B). Response of the A-fiber (C) and putative nociceptive vagal bronchopulmonary C-fiber (D) in the lung to the lung inflation during eupneic breathing and supraphysiological lung inflation in rat (group data in (E)). During eupneic breathing majority of the A-fibers in the lung are cyclically active, while majority of the bronchopulmonary C-fibers are quiescent. C-fibers in this study were defined by conduction velocity <2m/s) (REF). Bronchopulmonary C-fibers but not A-fibers are stimulated by capsaicin (F). (Data in C–F adapted from Lee et al, Anat Rec A Discov Mol Cell Evol Biol. 270(1):17–24)(Lee et al., 2003)
The activation properties of vagal bronchopulmonary C-fibers are in stark contrast with the activation profile of the non-nociceptive vagal A-fiber mechanosensors in the lungs (reviewed in (Canning et al., 2006)). The majority of the lung mechanosensors display regular discharge in phase with the cyclic lung distention during eupneic breathing (Fig.1)(Lee et al., 2003) (Bergren, 1997). Unlike bronchopulmonary C-fibers, the A-fiber mechanosensors lack the TRPV1 receptor (and TRPA1 receptor, thus far shown in the mouse) (Bergren, 1997; Nassenstein et al., 2008).
A common feature of the reflexes initiated by the stimulation of the bronchopulmonary C-fibers is that they act to remove and/or limit the effects of noxious factors (Coleridge et al., 1984; Lee et al., 2001b). Perhaps the most prominent example of a C-fiber triggered reflex is cough that can be initiated by the vagal C-fibers in the large airways (Canning et al., 2009). Since cough is a rapid response that limits the exposure of the tissue to potentially harmful factors, it is probably comparable to the withdrawal reflex initiated by somatic nociceptors. The C-fiber induced reflex increase in mucus secretion aids the protection of airway mucosa. Increased breathing rate (tachypnea) mediated by the vagal C-fibers innervating the lung parenchyma is believed to enhance the removal of the air containing noxious factors from the alveolar compartment. Reflex bronchoconstriction due to C-fiber activation is thought to mechanically support the airways and adjust the distribution of ventilation in pathological states, although the functional importance of these effects is not universally accepted. The bronchopulmonary C-fibers are thought to contribute to sensations from the lung including “urge to cough”, dyspnea, breathlessness and chest tightness (Lee, 2008). Apart from initiation and regulation of the responses to potentially harmful situations, other functions of bronchopulmonary C-fibers have not been defined.
In addition to overt activation, certain inflammatory mediators induce peripheral sensitization of the vagal bronchopulmonary C-fibers. For example, a classical pro-nociceptive mediator prostaglandin E2 sensitizes the vagal C-fiber terminals to physical and chemical stimuli via direct action on the EP2 receptor in their neuronal membrane (Ho et al., 2000; Lee et al., 1995; Zhang et al., 2007). Mechanistically, the PGE2–induced sensitization has been attributed to the combination of receptor-specific sensitization (such as TRPV1 sensitization) and an increase in non-specific sensory excitability (Kwong et al., 2002). This is similar to the PGE2 effects on somatic nociceptors. The sensitization of the vagal C-fibers translates into exaggerated reflexes mediated by these nerves. Unlike C-fibers, A-fiber mechanosensors are not sensitized by PGE2 (Lee et al., 1995). Other mediators, such as histamine, adenosine and eosinophilic cationic protein have been also shown to induce C-fiber sensitization (Gu et al., 2003; Lee et al., 2001a; Lee et al., 1993). An important aspect of the C-fiber sensitization demonstrated in these studies is that their mechanical threshold can be lowered to the extent that the C-fibers become responsive to mechanical forces generated in the lung tissue by eupneic breathing. Inasmuch that breathing does not stop, the sensitization is predicted to result into continuous afferent input from the bronchopulmonary C-fibers. As expected, vagal C-fibers are sensitized and their reflexes exaggerated in the models of acute and chronic inflammation relevant for the human diseases such as the allergic inflammation and chronic exposure to cigarette smoke (Bergren, 2001; Zhang et al., 2008).
In addition to peripheral sensitization, the major mechanisms inducing hyperalgesia and allodynia (painful perception of normally innocuous stimulus) in the somatosensory system is central sensitization (Julius et al., 2001). In the most commonly described scenario, persistent C-fiber activation leads to increase in the synaptic efficacy on the second order sensory neurons. A number of receptors, intracellular pathways and neurotransmitters including neuropeptides have been implicated in this process. Central sensitization of at least two vagal reflexes by C-fiber activation has been described. Basal bronchial tone in the guinea pig is determined by a parasympathetic cholinergic reflex driven by the activity in a subset of the vagal A-fiber mechanosensors (termed rapidly adapting stretch receptors, abbreviated SARs). Activation of the C-fibers in the proximal airways induces robust sensitization of this reflex that is mediated by a neurokinin-dependent central interaction of the afferent input from the vagal A-fiber mechanosensors and C-fibers (Mazzone et al., 2002). This leads to the potentiation of bronchoconstriction. Another example is the sensitization of the cough reflex evoked from one location in the respiratory system by C-fiber input from a separate distant location. The cough reflex induced from the proximal airways (trachea) can be sensitized by the C-fiber input from a distal lung (Mazzone et al., 2005).
Presented evidence demonstrates that the vagal bronchopulmonary C-fibers have a number of properties characteristic of somatosensory nociceptors. Bronchopulmonary C-fibers are essentially unresponsive to physiological events in respiratory system, but are readily activated by exogenous and endogenous bona fide noxious stimuli, this fulfilling Sherrington’s criteria defining nociceptors. These afferent nerves can be sensitized and can induce central sensitization. Bronchopulmonary C-fibers initiate defensive reflexes and contribute to warning sensations, but appear to have no role in maintaining homeostasis in physiological conditions. It is unlikely that only respiratory system receives these putative vagal nociceptors. We next review the evidence that vagal afferent nerves with properties characteristic for nociceptors are also found in the esophagus.
Putative vagal nociceptors in the esophagus
Arguably, the most thoroughly characterized vagal afferent nerves in the esophagus are the low threshold mechanosensors also termed tension mechano-receptors (Falempin et al., 1978; Medda et al., 2005; Page et al., 1998; Page et al., 2002; Satchell, 1984; Sengupta et al., 1989; Yu et al., 2008; Yu et al., 2005; Zagorodnyuk et al., 2000; Zagorodnyuk et al., 2003). The neurobiology of these afferent nerves is reviewed in detail elsewhere. Here we briefly summarize their activation properties in the guinea pig in order to contrast them to the putative nociceptive vagal afferent nerve types in the esophagus. The mechanical threshold of tension mechanosensors to esophageal distention is exquisitely low, in fact, the majority of these nerves are stimulated by the resting tension in the wall of non-distended esophagus. Esophageal distention with increasing intraesophageal pressure evokes initially an increase in their activity that is proportional to the degree of esophageal distention up the pressure 30–60mmHg depending on distention protocol. Further increase in the intraesophageal pressure >30–60 mmHg typically results only in a marginal increase in the activity. This is illustrated in the tension mechanosensors (Fig. 2B). The distention pressure <60 mmHg is considered an innocuous in humans and experimental animals. Thus the pattern of mechanical activation of tension mechanosensors indicates, that they are ideally suited to encode physiological, but less capable to encode potentially noxious levels of esophageal distention. Neurophysiologically, the activity of tension mechanosensors is characterized by the regular high frequency action potential discharge in response to constant intensity mechanical stimuli (for example, esophageal distention to 30 mmHg evokes sustained non-adapting discharge >30Hz in the guinea pig). This pattern of discharge resembles the activity recorded from the somatic mechanosensors. In the guinea pig and dog, the nerve axons of esophageal tension mechanosensors conduct action potentials in the A-fiber range, although slower conducting velocities have been noted in a proportion of tension mechanosensors in other species.
Figure 2. Distinct mechanical activation properties of tension mechanosensors and putative nociceptive nerve fibers in the guinea pig esophagus.
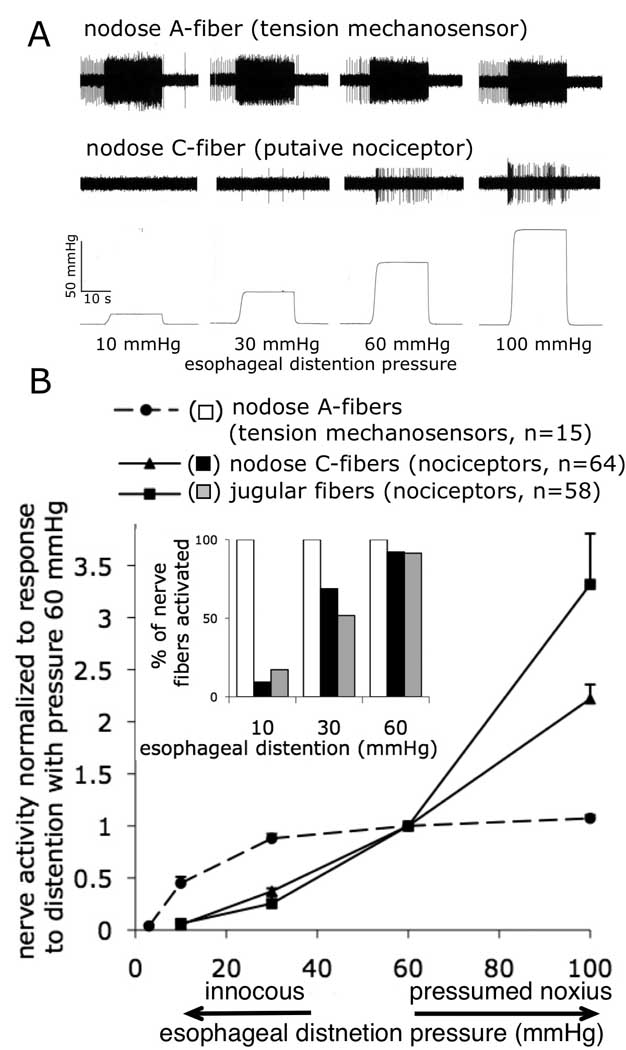
Representative traces of the response of a tension mechanosensor and a putative nodose nociceptor to esophageal distention (A) and distention pressure-activity curves (data are normalized to 60mmHg) (B). Note that tension mechanosensors saturate at presumed innocuous distention pressure ≈ 30mmHg while both nodose and jugular putative nociceptors encode esophageal distention in the presumed noxious range (up to 100mmHg). Inset: Percentage of the nerve fibers that were overtly activated with indicated distention pressure. Note that 40–50% of the presumed nociceptive fibers have threshold≥30 mmHg while all of tension mechanosensors have threshold ≤10 mmHg. Extracellular recordings were made from the vagal afferent nerve fibers projecting mechanosensitive nerve terminals into the esophagus in the isolated vagally-innervated guinea pig esophagus. Vagal afferent nerve fibers innervating the guinea pig esophagus originate from the nodose and jugular vagal afferent ganglia. Tension mechanosensors originate exclusively from the nodose ganglia and their axons conduct action potentials in Aδ-fiber range (2–10m/s). In addition to Aδ-fiber tension mechanosensors, nodose ganglia also project putative nociceptive C-fibers (<1 m/s) into the esophagus. Jugular ganglion project only nociceptive fibers into the esophagus (the majority of jugular fibers are also C-fibers) See text for more details. Data combined from (Yu et al., 2008; Yu et al., 2005) and unpublished studies.
Noxious chemicals and mediators associated with tissue injury and inflammation typically do not activate tension mechanosensors directly via the action on the receptors in their terminal membrane. However, because of the exquisite low mechanical threshold, the activity of mechanosensors can be easily modulated by stimuli that lead to changes in the esophageal muscle tension (Zagorodnyuk et al., 2003). Indeed, the capacity of some chemicals to modulate the activity of tension mechanosensors has been attributed to their direct or reflex effect on the esophageal muscle (Sengupta et al., 1992). In addition, certain chemicals have been reported to modulate the activity of mechanosensors by inducing the production of secondary mediators from non-neuronal tissue (McIlwrath et al., 2009). In guinea pigs, tension mechanosensors are not directly activated by the prototypical stimulators of nociceptive nerves including bradykinin (Yu et al., 2009b), histamine (Yu et al., 2009a), the chest pain mediator adenosine (abstract (Ru et al., 2006)), the TRPV1 receptor activators including capsaicin and acid (Kollarik et al., 2007; Yu et al., 2008; Yu et al., 2005), and the TRPA1 receptor agonists (Yu et al., 2009b). This relative chemical insensitivity of tension mechanosensors supports the notion of their non-nociceptive function. Indeed, it is widely accepted that tension mechanosensors regulate physiological motor reflexes comprising esophageal motility (Sengupta et al., 2005).
We have reported that in addition to tension mechanosensors, another large population of distention-sensitive afferent nerve fibers innervates the guinea pig esophagus (Yu et al., 2008; Yu et al., 2005). Using the caveats discussed above we will refer to those fibers as putative vagal esophageal nociceptive nerve fibers. Although putative vagal nociceptive nerve fibers can be classified into at least two large subtypes (discussed below), they share certain common mechano- and chemo-sensitive properties clearly distinguishing them from tension mechanosensors. Firstly, the pattern of the response of the putative vagal nociceptors to esophageal distention is notably different from tension mechanosensors (Yu et al., 2008; Yu et al., 2005). These nerve fibers are not effectively stimulated by low levels of esophageal distention. Overall, their population threshold is approximately 30 mmHg (Figure 2). Interestingly, similar threshold has been reported for the esophageal spinal nociceptors (Sengupta et al., 1990; Sengupta et al., 1992). Unlike the activity of tension mechanosensors that saturates at innocuous intraesophageal pressure, putative vagal nociceptors linearly encode esophageal distention in the noxious range (up to 100mmHg).
Secondly, the putative vagal nociceptors in the guinea pig esophagus are stimulated by noxious chemicals directly via the receptors in their neuronal membrane (Kollarik et al., 2007; Yu et al., 2009b; Yu et al., 2008; Yu et al., 2005). The putative nociceptive nerve fibers in the esophagus express TRPV1 and TRPA1 receptors and are stimulated by the TRPV1 receptor activators including acid and capsaicin, and the TRPA1 agonists. Bradykinin acting on the bradykinin B2 receptor (Yu et al., 2009b) and the chest pain mediator adenosine acting on the adenosine A1 and A2A receptors (unpublished data) also effectively stimulate vagal nociceptors in the esophagus. Neurophysiologically, the vast majority of guinea pig putative nociceptors in the esophagus conduct action potential in the C-fiber range (Yu et al., 2008; Yu et al., 2005). This includes all putative nociceptors derived from the vagal nodose ganglia and over two thirds of nociceptors derived from the vagal jugular ganglia (discussed below). A relatively low frequency (as compared to mechanosensors) irregular action potential discharge elicited in putative vagal nociceptors by mechanical or chemical stimuli is reminiscent of the activity pattern observed in somatosensory nociceptors.
The reflexes mediated or modulated by the vagal putative nociceptive nerve fibers have not been defined yet. Better understanding of activation properties of these fibers will likely help to gain more insight into the functional consequences of their activation. Similarly, it is at present unknown whether vagal putative nociceptive nerve fibers mediate or modulate conscious perceptions.
Vagal putative nociceptive fibers in the esophagus can be sensitized by inflammatory mediators. Bradykinin induces sensitization of the guinea pig putative vagal nociceptive fibers via a pathway that involves bradykinin B2 receptor and TRPA1 (Yu et al., 2009b). Stimulation of the histamine H1 receptor and PAR2 receptor by mediators released during mast cell degranulation has been reported to sensitize the nodose subset of putative esophageal vagal nociceptors to mechanical stimuli (Yu et al., 2009a; Yu et al., 2007). In contrast, bradykinin and mast cell mediators failed to sensitize the guinea pig tension mechanosensors (Yu et al., 2009a; Yu et al., 2007; Yu et al., 2009b).
At lease circumstantial evidence suggests that putative vagal nociceptive fibers in the esophagus can induce central sensitization. Clinical and animal experiments showed that the cough reflex can be sensitized by nociceptive activators delivered into the esophagus. Infusion of acid into the esophagus of patients with GERD and chronic cough or in patients with asthma does not trigger cough, but lowers the cough threshold to inhaled irritants (Javorkova et al., 2008; Wu et al., 2002). This is similar to the reduction of mechanical threshold for pain in hyperalgesia. The sensitization of the cough reflex can be reproduced in the guinea pig model by esophageal infusion of acid or capsaicin (Canning BJ, personal communication). While the mechanisms of this sensitization have not been elucidated, it seems that the vagal putative nociceptive fibers are ideal candidates for this function. Firstly, the gross anatomical proximity of the central projections of the vagal cough-triggering nerves from the airways and the vagal nociceptive fibers from the esophagus in the nucleus tractus solitarius (NTS) may provide substrate for central sensitization (analogous to the arrangement in the spinal dorsal horn). Indeed, the cough sensitization can be mimicked by injection of capsaicin into the discrete area of NTS (Mazzone et al., 2005). This also indicates that the vagal rather than spinal pathways sensitize the cough reflex. Secondly, as discussed above, acid and capsaicin are effective direct activators of putative nociceptors in the esophagus, although they also influence tension mechanosensors in vivo (Peles et al., 2009). Finally, precedents for sensitization of the respiratory reflexes by vagal C-fibers have been established (Mazzone et al., 2002; Mazzone et al., 2005).
Thus, the available evidence shows that the guinea pig esophagus is innervated by a large number of vagal afferent nerve fibers with properties similar to somatosensory nociceptors. These putative vagal nociceptors have polymodal discriminative responsiveness to potentially noxious stimuli, can be sensitized, and probably can induce central sensitization. Vagal afferent nerves with properties consistent with nociceptors have also been described in rat (Lennerz et al., 2007). The mucosa of the proximal esophagus in this species contains large numbers of polymodal vagal afferent nerve fibers supplied by the vagal branches (superior laryngeal nerves). Over 30% of these polymodal afferent nerves are sensitive to acid. Similar to the guinea pig, two types of distention-sensitive vagal afferent nerve fibers were described in the ferret esophagus: typical tension mechanosensors and a fiber type termed tension-mucosal receptor (Page et al., 1998; Page et al., 1999). It is possible that at least a proportion of tension mucosal receptors are similar to the guinea pig vagal esophageal nociceptors. However, direct comparison is difficult due to different experimental designs. However, putative vagal nociceptors sensitive to esophageal distention may be less abundant in some species. Page and coworkers reported that virtually all vagal afferent nerves sensitive to circular tension in the mouse esophagus are tension mechanosensors (Page et al., 2002). Consistent with this conclusion we found that distention-sensitive putative vagal nociceptors were relatively rare compared to tension mechanosensors in the mouse esophagus (unpublished data). Similarly, only tension mechanosensors were recorded from the vagal pathways in the distal esophagus of opossum, although nociceptive nerve fibers could be recorded from the spinal (sympathetic) in this species. Differences in the stimulation protocols may also influence detection and analysis of the nerve types (Phillips et al., 2000).
It is possible that other visceral organs also receive putative vagal nociceptors. For example, anatomical studies argue that there are two fundamentally different types of vagal afferent nerve terminals in the muscle of the stomach (IGLEs and intramuscular arrays), although only one type is typically functionally recorded (Phillips et al., 2000). Interestingly, a proportion of neurons identified functionally in the mouse stomach showed a combination of neurochemical markers often found in somatic nociceptors (Bielefeldt et al., 2006). Future studies will likely provide more definitive answer this question.
Subtypes of vagal putative nociceptive nerve fibers
It has been recognized for some time that spinal DRG nociceptors in the somatosensory system are not a homogeneous group of nerves (Woolf et al., 2007). At least two distinct somatosensory C-fiber nociceptive subtypes have been described based initially on the isolectin B4 (IB4) binding and expression of neuropeptides (Molliver et al., 1995). The IB4-postive nociceptive neurons do not express neuropeptides, whereas the majority of IB4-negative nociceptive neurons contain calcitonin gene related peptide (CGRP) and tachykinins. The nociceptive subtypes differentiated based on IB4 have different sensory properties and project centrally to different layers in the dorsal horn of the spinal cord (Hunt et al., 1985). Peripherally, a topographic segregation of the projections of peptidergic and nonpeptidergic nociceptive sensory neurons to the epidermis has been described (Zylka et al., 2005). These findings suggest that the nociceptive subtypes in somatosensory system have different functional roles. Recent studies have begun to elucidate the transcription factors (for example Runx1) and grow factor receptors (for example Trk-A and Ret) that govern complex development of somatosensory nociceptive subtypes (Woolf et al., 2007). Subtypes of visceral spinal DRG neurons innervating colon have also been proposed (Malin et al., 2009). Much less is known about the subtypes of the vagal nociceptive nerve fibers.
Vagal afferent nerves originate from two different embryonic sources: epibranchial placodes and neural crest (Baker et al., 2001). The neurons derived from placodes are located in the nodose (inferior) vagal ganglia, while the neurons derived from neural crest are located in the jugular (supranodose or superior) vagal ganglia. Anatomically discernable nodose and jugular ganglia are found in humans and many laboratory species including guinea pigs (Fig. 1 in (Christianson et al., 2009)), dogs (Chibuzo et al., 1981) and cats (Lucier et al., 1986). In the mouse, jugular and nodose neurons are located in a single ganglion termed jugular/nodose complex or more often simply nodose ganglion (Joachim et al., 2006). Recent analysis of the vagal neurons in the Wnt1Cre/R26R mice that express transgenic marker beta-galactosidase only in the neural-crest derived neurons, confirmed that the jugular/nodose complex comprises of many neural-crest (i.e. jugular) neurons ((Nassenstein et al., 2009), published in abstract form). Some authors report jugular and nodose neurons also in rats (Gu et al., 2006; Wank et al., 2001).
Both vagal nodose and jugular neurons project nerve fibers into the esophagus. Elegant anatomical studies combining neuronal tracing and neurectomy have indicated that the nodose and jugular neurons projecting into the rat proximal esophagus have different neurochemical phenotypes (Yu et al., 2008; Yu et al., 2005) and different central projections (Wank et al., 2001). Our studies showed that both jugular and nodose ganglia project putative nociceptors into the guinea pig esophagus (Yu et al., 2008; Yu et al., 2005). (In contrast, nodose ganglia are an exclusive source of tension mechanosensors.) Nodose and jugular nociceptors in the guinea pig esophagus share the defining nociceptive properties including non-saturating response to esophageal distention (Fig. 2) and expression of the TRPV1 and TRPA1 channels. However, they can be differentiated by activation profile and neurochemistry.
The available data from the guinea pig studies suggest that the nodose nociceptive subtype has a wider range of sensitivity to endogenous mediators (Tab. 1). For example, a major gastrointestinal signaling molecule serotonin (5-HT) is an effective stimulant of the nodose nociceptors (via the 5-HT3 receptor), but not jugular nociceptors (Yu et al., 2008). Similarly, ATP that is implicated in sensory signaling, activates the nodose, but not jugular nociceptors (Yu et al., 2005). We found that this difference can be attributed to the differential expression of the purinergic receptor subunits (Kwong et al., 2008). Nodose nociceptive neurons express a combination of the P2X2 and P2X3 purinergic receptor subunits. These two subunits form heteromeric P2X2/3 receptor that mediates non-inactivating current in response to ATP or other purinergic agonists. At the nerve terminals of nodose nociceptors this translates into an overt sustained action potential discharge. In contrast, jugular nociceptors express the P2X3 subunit, but lack the P2X2 subunit. Consistent with the expression of homomeric P2X3 channels, purinergic agonists evoke only transient currents in the jugular nociceptive neurons that fail to evoke overt action potential discharge in their nerve terminals. The expression of the neuropeptide substance P also differentiates nociceptive subtypes and is more common in jugular nociceptors (Yu et al., 2005).
Table 1.
Comparison of the vagal nodose and jugular nociceptors innervating the guinea pig esophagus.
| Nodose nociceptors |
Jugular nociceptors |
|
|---|---|---|
| Embryonic origin | Epibranchial placodes |
Neural crest |
| Discrimination of noxious esophageal distention |
Yes | Yes |
| Activation | ||
| Capsaicin (TRPV1 receptor) | Yes | Yes |
| Bradkykinin (B2 receptor) | Yes | Yes |
| Serotonin | Yes | No |
| The ATP purinergic p2X receptors agonist α,β-methylelene-ATP (P2X receptors subunits) |
Yes (P2X3, P2X2) |
No (P2X3) |
| Adenosine (adenosine receptor subunits)* |
Yes (A1, A2A) |
Yes (A1) |
| Neuropeptide expression | ||
| Substance P | +/− | +++ |
Data from (Yu et al., 2008; Yu et al., 2005) (Kwong et al., 2008) (Yu et al., 2009b) and *unpublished data.
Both nodose and jugular nociceptive subtypes have been also demonstrated in the guinea pig lungs (Undem et al., 2004). Bronchopulmonary C-fibers are traditionally divided into the bronchial C-fibers and pulmonary C-fibers (Coleridge et al., 1984). In vivo studies in larger mammals showed that the nerve terminals of one subset of C-fibers are preferentially accessible to chemicals from bronchial circulation, while the other subset is more accessible from pulmonary circulation. It was inferred from that the former are located in the airways including intrapulmonary small airways (bronchial C-fibers), while the latter are located in the lung parenchyma (pulmonary C-fibers). C-fibers in the guinea pig trachea and extrapulmonary bronchi originate exclusively from the jugular ganglia, but both jugular and nodose C-fibers innervate the lung (Riccio et al., 1996; Undem et al., 2004). While it is likely that within the lungs jugular C-fibers terminate in the small intrapulmonary airways (bronchial C-fibers) and nodose C-fibers terminate in the lung parenchyma (pulmonary C-fibers), this assumption has not yet been validated.
Importantly, the difference in the activation profile observed between nodose and jugular nociceptors in the esophagus is also found in the lungs (Undem et al., 2004). Thus, the nodose C-fibers, but not jugular C-fibers in the lungs are responsive to 5-HT and ATP (the pattern of P2X subunits expression in the lung nociceptors and esophageal nociceptors is identical) (Chuaychoo et al., 2005; Kwong et al., 2008). Similarly to the situation in the esophagus, the neuropeptides are more often expressed in the jugular than the nodose subtype in the lungs. These observations support the notion that certain features of the vagal nociceptive subtypes depend on the ganglionic (and therefore embryonic) origin and are independent on the tissue innervated. Whether the nodose or jugular nociceptive subtypes in different organs share additional properties or have any organ-specific properties remains to be determined.
The vagal nociceptive subtypes were identified also in the mouse. Preliminary data in Wnt1Cre/R26R mice show that the pattern of the P2X2 subunit expression (restricted to nodose placodes-derived nociceptive neurons) and neuropeptide expression (predominantly in the jugular neural crest-derived nociceptive neurons) in vagal system innervating lungs is identical to the guinea pig ((Nassenstein et al., 2009), abstract). The studies in the rat showing preferential expression of neuropeptides in the esophagus-specific neurons of the cranial (jugular) portion of the vagal ganglia are also similar to the findings in the guinea pig (Wank et al., 2001). Nodose innervation of the visceral organs is well established. Whether the jugular nociceptive fibers innervate visceral organs other than lungs and esophagus has not been to our knowledge reported.
Apart from the tracheal jugular C-fibers, the exact anatomical location of the nerve terminals of the vagal nociceptive nerve subtypes is unknown at present. Putative location of the bronchopulmonary C-fibers in the lungs is discussed above. A recent exciting study published thus far in abstract form reports that at least some of the guinea pig putative nociceptive fibers terminate in the esophageal muscle (described previously as intramuscular arrays)(Brookes et al., 2009). The pattern of the mechanical response to rapid esophageal distention may also provide a clue to the location of the nerve terminal. The guinea pig nodose putative nociceptive fibers are very sensitive to the dynamic phase of esophageal distention. The action potential discharge of the nodose putative nociceptors during the dynamic phase is 3–4 times higher than the discharge during sustained distention (Fig. 1 in (Yu et al., 2008)) suggesting location of their nerve terminals in a highly compliant compartment of the esophagus, most likely the esophageal muscle. In contrast, jugular putative nociceptors are relatively insensitive to the dynamic phase (Fig. 1 in (Yu et al., 2008)) indicating location in a different, less compliant compartment, perhaps esophageal mucosa/submucosa. Interestingly, combination of retrograde tracing and denervation studies indicated that jugular neurons terminate in the mucosa in the rat (Wank et al., 2001).
Given that the vagal nociceptive subtypes have distinct activation and neurochemical profiles, and likely different peripheral and central projections, it is tempting to assume that they play differential roles in the detection of noxious stimuli and regulate different aspects of the defensive responses. As it becomes available, better understanding of the vagal nociceptive subtypes will likely enable studies aimed to elucidate their function. Indeed, a recent study utilizing this information has shown that the activation of nodose and jugular C-fibers in the respiratory system has opposite effects on breathing rate (Chou et al., 2008).
CONCLUSION
The lungs and esophagus are innervated by clearly defined subsets of vagal afferent nerves with sensory properties that typically characterize certain somatosensory nociceptors. The characteristics of the putative vagal nociceptors include discriminative responsiveness to potentially noxious physical and chemical stimuli, peripheral sensitization and capacity to induce central sensitization. In the lungs the activation of putative vagal nociceptive nerves triggers defensive reflexes (cough, mucus hypersecretion) and is linked to conscious sensations warning of impeding harm (dyspnea, chest tightness). The putative vagal nociceptors in the lung and esophagus can be classified into at least two developmentally distinct subtypes that differ in activation properties, topographical distribution in the tissue, and may differentially regulate nociceptive reflexes. The evidence therefore forces an affirmative answer to the question “Are there any vagal afferent nerves that specialize on the detection of stimuli associated with impeding or actual tissue damage, initiate responses aimed to prevent or limit tissue damage, and have no other known function?” Should these vagal nerve fibers be called nociceptors? We argue that for the purpose of better understanding of their neurobiology, there is a distinctive advantage in conceptualizing these vagal afferent nerves as nociceptors.
Acknowledgement
M.K. is supported by NIH DK074480 and HL062296, and AstraZeneca IRUSESOM0568/0403
M.B. is supported by VEGA 1/0018/08 and Department of Health (Slovakia) Grant 2007/54-UK-15
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adriaensen D, Brouns I, Pintelon I, De Proost I, Timmermans JP. Evidence for a role of neuroepithelial bodies as complex airway sensors: comparison with smooth muscle-associated airway receptors. J Appl Physiol. 2006;101:960–970. doi: 10.1152/japplphysiol.00267.2006. [DOI] [PubMed] [Google Scholar]
- Agostoni E, Chinnock JE, De Daly MB, Murray JG. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol. 1957;135:182–205. doi: 10.1113/jphysiol.1957.sp005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Baluk P, Nadel JA, McDonald DM. Substance P-immunoreactive sensory axons in the rat respiratory tract: a quantitative study of their distribution and role in neurogenic inflammation. J Comp Neurol. 1992;319:586–598. doi: 10.1002/cne.903190408. [DOI] [PubMed] [Google Scholar]
- Bergren DR. Sensory receptor activation by mediators of defense reflexes in guinea-pig lungs. Respir Physiol. 1997;108:195–204. doi: 10.1016/s0034-5687(97)00030-3. [DOI] [PubMed] [Google Scholar]
- Bergren DR. Enhanced lung C-fiber responsiveness in sensitized adult guinea pigs exposed to chronic tobacco smoke. J Appl Physiol. 2001;91:1645–1654. doi: 10.1152/jappl.2001.91.4.1645. [DOI] [PubMed] [Google Scholar]
- Bessac BF, Jordt SE. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 2008;23:360–370. doi: 10.1152/physiol.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeldt K, Zhong F, Koerber HR, Davis BM. Phenotypic characterization of gastric sensory neurons in mice. Am J Physiol Gastrointest Liver Physiol. 2006;291:G987–G997. doi: 10.1152/ajpgi.00080.2006. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:1–19. doi: 10.1111/j.1365-2982.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- Brookes SJ, Montes NA, Reynolds BR. Digestive Disease Week. Chicago: 2009. Identification of Vagal Mechano-Nociceptor Endings in the Guinea Pig Esophagus. http://www.ddw.org/ [Google Scholar]
- Brouns I, De Proost I, Pintelon I, Timmermans JP, Adriaensen D. Sensory receptors in the airways: neurochemical coding of smooth muscle-associated airway receptors and pulmonary neuroepithelial body innervation. Auton Neurosci. 2006;126–127:307–319. doi: 10.1016/j.autneu.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Chou YL. Cough sensors. I. Physiological and pharmacological properties of the afferent nerves regulating cough. Handb Exp Pharmacol. 2009:23–47. doi: 10.1007/978-3-540-79842-2_2. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Mori N, Mazzone SB. Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol. 2006;152:223–242. doi: 10.1016/j.resp.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Chibuzo GA, Cummings JF. The origins of the afferent fibers to the lingual muscles of the dog, a retrograde labelling study with horseradish peroxidase. Anat Rec. 1981;200:95–101. doi: 10.1002/ar.1092000109. [DOI] [PubMed] [Google Scholar]
- Chou YL, Scarupa MD, Mori N, Canning BJ. Differential effects of airway afferent nerve subtypes on cough and respiration in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1572–R1584. doi: 10.1152/ajpregu.90382.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JA, Bielefeldt K, Altier C, Cenac N, Davis BM, Gebhart GF, High KW, Kollarik M, Randich A, Undem B, Vergnolle N. Development, plasticity and modulation of visceral afferents. Brain Res Rev. 2009;60:171–186. doi: 10.1016/j.brainresrev.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuaychoo B, Lee MG, Kollarik M, Pullmann R, Jr, Undem BJ. Evidence for both adenosine A1 and A2A receptors activating single vagal sensory C-fibres in guinea pig lungs. J Physiol. 2006;575:481–490. doi: 10.1113/jphysiol.2006.109371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuaychoo B, Lee MG, Kollarik M, Undem BJ. Effect of 5-hydroxytryptamine on vagal C-fiber subtypes in guinea pig lungs. Pulm Pharmacol Ther. 2005;18:269–276. doi: 10.1016/j.pupt.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57:427–450. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol. 1984;99:1–110. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- Falempin M, Mei N, Rousseau JP. Vagal mechanoreceptors of the inferior thoracic oesophagus, the lower oesophageal sphincter and the stomach in the sheep. Pflugers Arch. 1978;373:25–30. doi: 10.1007/BF00581145. [DOI] [PubMed] [Google Scholar]
- Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut. 2002;51 Suppl 1:i2–i5. doi: 10.1136/gut.51.suppl_1.i2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Lee LY. Characterization of acid signaling in rat vagal pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol. 2006;291:L58–L65. doi: 10.1152/ajplung.00517.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Ruan T, Hong JL, Burki N, Lee LY. Hypersensitivity of pulmonary C fibers induced by adenosine in anesthetized rats. J Appl Physiol. 2003;95:1315–1324. doi: 10.1152/japplphysiol.00107.2003. discussion 1314. [DOI] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CY, Gu Q, Hong JL, Lee LY. Prostaglandin E(2) enhances chemical and mechanical sensitivities of pulmonary C fibers in the rat. Am J Respir Crit Care Med. 2000;162:528–533. doi: 10.1164/ajrccm.162.2.9910059. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Rossi J. Peptide- and non-peptide-containing unmyelinated primary afferents: the parallel processing of nociceptive information. Philos Trans R Soc Lond B Biol Sci. 1985;308:283–289. doi: 10.1098/rstb.1985.0028. [DOI] [PubMed] [Google Scholar]
- Javorkova N, Varechova S, Pecova R, Tatar M, Balaz D, Demeter M, Hyrdel R, Kollarik M. Acidification of the oesophagus acutely increases the cough sensitivity in patients with gastro-oesophageal reflux and chronic cough. Neurogastroenterol Motil. 2008;20:119–124. doi: 10.1111/j.1365-2982.2007.01020.x. [DOI] [PubMed] [Google Scholar]
- Joachim RA, Cifuentes LB, Sagach V, Quarcoo D, Hagen E, Arck PC, Fischer A, Klapp BF, Dinh QT. Stress induces substance P in vagal sensory neurons innervating the mouse airways. Clin Exp Allergy. 2006;36:1001–1010. doi: 10.1111/j.1365-2222.2006.02533.x. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kollarik M, Ru F, Undem BJ. Acid-sensitive vagal sensory pathways and cough. Pulm Pharmacol Ther. 2007;20:402–411. doi: 10.1016/j.pupt.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou YR, Frazier DT, Lee LY. The stimulatory effect of nicotine on vagal pulmonary C-fibers in dogs. Respir Physiol. 1989;76:347–356. doi: 10.1016/0034-5687(89)90075-3. [DOI] [PubMed] [Google Scholar]
- Kwong K, Kollarik M, Nassenstein C, Ru F, Undem BJ. P2X2 receptors differentiate placodal vs. neural crest C-fiber phenotypes innervating guinea pig lungs and esophagus. Am J Physiol Lung Cell Mol Physiol. 2008;295:L858–L865. doi: 10.1152/ajplung.90360.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong K, Lee LY. PGE(2) sensitizes cultured pulmonary vagal sensory neurons to chemical and electrical stimuli. J Appl Physiol. 2002;93:1419–1428. doi: 10.1152/japplphysiol.00382.2002. [DOI] [PubMed] [Google Scholar]
- Lee LY. Respiratory sensations evoked by activation of bronchopulmonary C-fibers. Respir Physiol Neurobiol. 2008 doi: 10.1016/j.resp.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LY, Gu Q, Gleich GJ. Effects of human eosinophil granule-derived cationic proteins on C-fiber afferents in the rat lung. J Appl Physiol. 2001a;91:1318–1326. doi: 10.1152/jappl.2001.91.3.1318. [DOI] [PubMed] [Google Scholar]
- Lee LY, Morton RF. Histamine enhances vagal pulmonary C-fiber responses to capsaicin and lung inflation. Respir Physiol. 1993;93:83–96. doi: 10.1016/0034-5687(93)90070-q. [DOI] [PubMed] [Google Scholar]
- Lee LY, Morton RF. Pulmonary chemoreflex sensitivity is enhanced by prostaglandin E2 in anesthetized rats. J Appl Physiol. 1995;79:1679–1686. doi: 10.1152/jappl.1995.79.5.1679. [DOI] [PubMed] [Google Scholar]
- Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol. 2001b;125:47–65. doi: 10.1016/s0034-5687(00)00204-8. [DOI] [PubMed] [Google Scholar]
- Lee LY, Shuei Lin Y, Gu Q, Chung E, Ho CY. Functional morphology and physiological properties of bronchopulmonary C-fiber afferents. Anat Rec A Discov Mol Cell Evol Biol. 2003;270:17–24. doi: 10.1002/ar.a.10005. [DOI] [PubMed] [Google Scholar]
- Lennerz JK, Dentsch C, Bernardini N, Hummel T, Neuhuber WL, Reeh PW. Electrophysiological characterization of vagal afferents relevant to mucosal nociception in the rat upper oesophagus. J Physiol. 2007;582:229–242. doi: 10.1113/jphysiol.2007.130823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucier GE, Egizii R, Dostrovsky JO. Projections of the internal branch of the superior laryngeal nerve of the cat. Brain Res Bull. 1986;16:713–721. doi: 10.1016/0361-9230(86)90143-7. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- Malin SA, Christianson JA, Bielefeldt K, Davis BM. TPRV1 expression defines functionally distinct pelvic colon afferents. J Neurosci. 2009;29:743–752. doi: 10.1523/JNEUROSCI.3791-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone SB, Canning BJ. Synergistic interactions between airway afferent nerve subtypes mediating reflex bronchospasm in guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2002;283:R86–R98. doi: 10.1152/ajpregu.00007.2002. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, McGovern AE. Na+K+2Cl- cotransporters and Cl- channels regulate citric acid cough in guinea pigs. J Appl Physiol. 2006;101:635–643. doi: 10.1152/japplphysiol.00106.2006. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, McGovern AE. Immunohistochemical characterization of nodose cough receptor neurons projecting to the trachea of guinea pigs. Cough. 2008;4:9. doi: 10.1186/1745-9974-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone SB, Mori N, Canning BJ. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J Physiol. 2005;569:559–573. doi: 10.1113/jphysiol.2005.093153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwrath SL, Davis BM, Bielefeldt K. Deletion of P2X3 receptors blunts gastro-oesophageal sensation in mice. Neurogastroenterol Motil. 2009 doi: 10.1111/j.1365-2982.2009.01292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medda BK, Sengupta JN, Lang IM, Shaker R. Response properties of the brainstem neurons of the cat following intra-esophageal acid-pepsin infusion. Neuroscience. 2005;135:1285–1294. doi: 10.1016/j.neuroscience.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Radeke MJ, Feinstein SC, Snider WD. Presence or absence of TrkA protein distinguishes subsets of small sensory neurons with unique cytochemical characteristics and dorsal horn projections. J Comp Neurol. 1995;361:404–416. doi: 10.1002/cne.903610305. [DOI] [PubMed] [Google Scholar]
- Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, Undem BJ. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol. 2008;586:1595–1604. doi: 10.1113/jphysiol.2007.148379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassenstein C, Taylor-Clark TE, Myers AC, Ru F, Bettner W, Undem B. Placodal and neural crest vagal C-fibers innervating murine airways: localization and phenotypic differences. 104th Annual Meeting of the Anatomische Gesellschaft; Antwerpen, Belgium. 2009. http://www.anatomische-gesellschaft.de/abstract_archiv/1237905980.pdf. [Google Scholar]
- Page AJ, Blackshaw LA. An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. J Physiol. 1998;512(Pt 3):907–916. doi: 10.1111/j.1469-7793.1998.907bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Blackshaw LA. GABA(B) receptors inhibit mechanosensitivity of primary afferent endings. J Neurosci. 1999;19:8597–8602. doi: 10.1523/JNEUROSCI.19-19-08597.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Martin CM, Blackshaw LA. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol. 2002;87:2095–2103. doi: 10.1152/jn.00785.2001. [DOI] [PubMed] [Google Scholar]
- Peles S, Medda BK, Zhang Z, Banerjee B, Lehmann A, Shaker R, Sengupta JN. Differential effects of transient receptor vanilloid one (TRPV1) antagonists in acid-induced excitation of esophageal vagal afferent fibers of rats. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000;34:1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Riccio MM, Kummer W, Biglari B, Myers AC, Undem BJ. Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol. 1996;496(Pt 2):521–530. doi: 10.1113/jphysiol.1996.sp021703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru F, Kollarik M. Adenosine activates a subset of nociceptive vagal sensory nerves in the esophagus. Gastroenterology. 2006;130:A-252. [Google Scholar]
- Satchell PM. Canine oesophageal mechanoreceptors. J Physiol. 1984;346:287–300. doi: 10.1113/jphysiol.1984.sp015022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta JN, Kauvar D, Goyal RK. Characteristics of vagal esophageal tension-sensitive afferent fibers in the opossum. J Neurophysiol. 1989;61:1001–1010. doi: 10.1152/jn.1989.61.5.1001. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Saha JK, Goyal RK. Stimulus-response function studies of esophageal mechanosensitive nociceptors in sympathetic afferents of opossum. J Neurophysiol. 1990;64:796–812. doi: 10.1152/jn.1990.64.3.796. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Saha JK, Goyal RK. Differential sensitivity to bradykinin of esophageal distension-sensitive mechanoreceptors in vagal and sympathetic afferents of the opossum. J Neurophysiol. 1992;68:1053–1067. doi: 10.1152/jn.1992.68.4.1053. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Shaker R. Vagal afferent nerve stimulated reflexes in the GI tract. In: Undem BJ, Weinreich D, editors. Advances in vagal afferent neurobiology. New York: CRC Taylor & Francis; 2005. pp. 379–401. [Google Scholar]
- Sherrington SC. The integrative action of the nervous system. New Haven: Yale University Press; 1906. [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, Ghatta S, Bettner W, Undem BJ. Nitrooleic acid, an endogenous product of nitrative stress, activates nociceptive sensory nerves via the direct activation of TRPA1. Mol Pharmacol. 2009a;75:820–829. doi: 10.1124/mol.108.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark TE, McAlexander MA, Nassenstein C, Sheardown SA, Wilson S, Thornton J, Carr MJ, Undem BJ. Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. J Physiol. 2008a;586:3447–3459. doi: 10.1113/jphysiol.2008.153585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark TE, Nassenstein C, McAlexander MA, Undem BJ. TRPA1: A potential target for anti-tussive therapy. Pulm Pharmacol Ther. 2009b;22:71–74. doi: 10.1016/j.pupt.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, Undem BJ, Macglashan DW, Jr, Ghatta S, Carr MJ, McAlexander MA. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1) Mol Pharmacol. 2008b;73:274–281. doi: 10.1124/mol.107.040832. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol. 2004;556:905–917. doi: 10.1113/jphysiol.2003.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wank M, Neuhuber WL. Local differences in vagal afferent innervation of the rat esophagus are reflected by neurochemical differences at the level of the sensory ganglia and by different brainstem projections. J Comp Neurol. 2001;435:41–59. doi: 10.1002/cne.1192. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Horie S, Michael GJ, Spina D, Page CP, Priestley JV. Immunohistochemical localization of vanilloid receptor subtype 1 (TRPV1) in the guinea pig respiratory system. Pulm Pharmacol Ther. 2005;18:187–197. doi: 10.1016/j.pupt.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Ma Q. Nociceptors--noxious stimulus detectors. Neuron. 2007;55:353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Wu DN, Yamauchi K, Kobayashi H, Tanifuji Y, Kato C, Suzuki K, Inoue H. Effects of esophageal acid perfusion on cough responsiveness in patients with bronchial asthma. Chest. 2002;122:505–509. doi: 10.1378/chest.122.2.505. [DOI] [PubMed] [Google Scholar]
- Yu J, Wang YF, Zhang JW. Structure of slowly adapting pulmonary stretch receptors in the lung periphery. J Appl Physiol. 2003;95:385–393. doi: 10.1152/japplphysiol.00137.2003. [DOI] [PubMed] [Google Scholar]
- Yu S, Gao G, Peterson BZ, Ouyang A. TRPA1 in Mast Cell Activation-induced Long-lasting Mechanical Hypersensitivity of Vagal Afferent C fibers in Guinea Pig Esophagus. Am J Physiol Gastrointest Liver Physiol. 2009a doi: 10.1152/ajpgi.00068.2009. [DOI] [PubMed] [Google Scholar]
- Yu S, Kollarik M, Ouyang A, Myers AC, Undem BJ. Mast cell-Mediated Long-Lasting Increases in Excitability of Vagal C-Fibers in Guinea Pig Esophagus. Am J Physiol Gastrointest Liver Physiol. 2007 doi: 10.1152/ajpgi.00277.2007. [DOI] [PubMed] [Google Scholar]
- Yu S, Ouyang A. TRPA1 in bradykinin-induced mechanical hypersensitivity of vagal C fibers in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol. 2009b;296:G255–G265. doi: 10.1152/ajpgi.90530.2008. [DOI] [PubMed] [Google Scholar]
- Yu S, Ru F, Ouyang A, Kollarik M. 5-Hydroxytryptamine selectively activates the vagal nodose C-fibre subtype in the guinea-pig oesophagus. Neurogastroenterol Motil. 2008;20:1042–1050. doi: 10.1111/j.1365-2982.2008.01136.x. [DOI] [PubMed] [Google Scholar]
- Yu S, Undem BJ, Kollarik M. Vagal afferent nerves with nociceptive properties in guinea-pig oesophagus. J Physiol. 2005;563:831–842. doi: 10.1113/jphysiol.2004.079574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Brookes SJ. Transduction sites of vagal mechanoreceptors in the guinea pig esophagus. J Neurosci. 2000;20:6249–6255. doi: 10.1523/JNEUROSCI.20-16-06249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Costa M, Brookes SJ. Mechanotransduction by intraganglionic laminar endings of vagal tension receptors in the guinea-pig oesophagus. J Physiol. 2003;553:575–587. doi: 10.1113/jphysiol.2003.051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Lee LY. Prostaglandin E2 enhances the sensitizing effect of hyperthermia on pulmonary C-fibers in rats. Respir Physiol Neurobiol. 2007;156:241–249. doi: 10.1016/j.resp.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Lin RL, Wiggers ME, Lee LY. Sensitizing effects of chronic exposure and acute inhalation of ovalbumin aerosol on pulmonary C fibers in rats. J Appl Physiol. 2008;105:128–138. doi: 10.1152/japplphysiol.01367.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]


