Abstract
When coupled with a microelectrode, background-subtracted fast scan cyclic voltammetry (FSCV) allows fast, sensitive and selective determination of analytes within a small spatial location. For the past 30 years experiments using this technique have been largely confined to recordings at a single microelectrode. Arrays with closely separated microelectrodes would allow researchers to gain more informative data as well as probe regions in close spatial proximity. This work presents one of the first FSCV microelectrode arrays (MEA) implemented in vivo with the ability to sample from different regions in close spatial proximity (equidistant within 1 mm). The array is manufactured from fused silica capillaries and a microfabricated electrode spacer. The functionality of the array is assessed by simultaneously monitoring electrically stimulated dopamine (DA) release in the striatum of anesthetized rat. As expected, heterogeneous dopamine release was simultaneously observed. Additionally, the pharmacological effect of raclopride (D2 receptor antagonist) and cocaine (monoamine uptake blocker) on the heterogeneity of DA release, in spatially different brain regions was shown to alter neurotransmitter release at all four electrode sites.
1. Introduction
For years researchers have been able to monitor neurotransmitter concentrations in vivo and in vitro with carbon fiber microelectrodes coupled with electrochemical techniques such as background-subtracted fast scan cyclic voltammetry (FSCV) (Ewing et al., 1983; Kita and Wightman, 2008; Wightman, 2006). The FSCV technique allows real-time acquisition of quantitative and qualitative information about chemical microenvironment of the brain via redox reactions at an electrode surface. In this work, all observed signals are from redox reactions of easily oxidizable neurotransmitter released by neurons (i.e. catecholamines) rather than electrical signals (e.g. action potentials). The data is collected in the form of a voltammogram which helps to establish the chemical nature of the species of interest and their concentration. Using this technique fast, transient events such as exocytosis from single cells in vitro as well as naturally occurring in vivo neurotransmitter release have been observed (Sombers et al., 2009; Travis and Wightman, 1998; Troyer and Wightman, 2002; Wightman et al., 2007).
Generally monitoring neurotransmitter release is done using a single carbon fiber microelectrode in a single brain region. FSCV experiments linked with microelectrode array (MEA) technology would enable integrative experiments to be performed. Such experiments would elucidate information regarding the link between neurotransmission across multiple brain compartments. Additionally, neurotransmitter release has been shown to be heterogeneous within a single brain area possessing several “hot” and “cold” spots (Garris et al., 1994; Venton et al., 2003a; Wightman et al., 2007). Such areas could be probed with a microelectrode array to provide information regarding that single brain region under normal conditions or under pharmacological alterations. MEAs could also be useful in monitoring multiple neurotransmitters simultaneously. Using FSCV, sensitivity and selectivity towards a neurotransmitter or analyte of interest can be optimized by applying a specific potential waveform to a working electrode. For example, such “waveforms” have been designed and optimized for the detection of oxygen (Venton et al., 2003b; Zachek et al., 2009; Zimmerman and Wightman, 1991), adenosine (Swamy and Venton, 2007), tyramine and octopamine (Cooper and Venton, 2009), serotonin (Jackson et al., 1995), and epinephrine (Pihel et al., 1994). If each of these waveforms were implemented on separate electrodes integrative information regarding multiple neurotransmitters could be gathered simultaneously in real-time.
Neurobiological research implementing MEAs has been previously accomplished using techniques such as electrophysiology and amperometry. For example, the Nicolelis group has successfully implemented tungsten microwire arrays that provide eletrophysiological data regarding the functionality of motor cortex (Nicolelis, 2008). Carbon fiber based arrays have been developed as well. Zhang et. al has developed a 7 disk-electrode array capable of amperometrically monitoring heterogeneous exocytotic release in PC12 cells in vitro (Zhang et al., 2008). However, such micro-disk electrodes would be unsuitable for in vivo, as the chemical diffusion length in solution is much longer than the distance between electrodes (Zhang et al., 2008). Barring any diffusional barrier, this lack of spatial separation would essentially cause the electrode array to behave as a single unit rather than individual electrodes in a bulk solution. A similar method was employed by Dressman et. al. to record in vivo dopamine release from a single brain area with FSCV (Dressman et al., 2002). Again as noted by the authors, this array can only be used to monitor the same neurochemical event rather than multiple, spatially different, simultaneous events.
The sensitivity of a FSCV electrochemical sensor is directly proportional to the exposed surface area (Bard and Faulkner, 2001); therefore, detecting small concentrations of neurotransmitter release in vivo has been typically done at cylindrical electrodes. This work presents a method of preparing cylindrical carbon fiber microelectrode arrays that can are spatially localized 250 μm apart on a microfabricated electrode holder; thus avoiding any electrical or diffusional crosstalk. The MEAs were then used to monitor electrically stimulated dopamine release in vivo, under normal and pharmacologically altered states, in heterogeneous regions of rat striatum.
2. Materials and Methods
2.1 Chemicals
All chemicals and drugs used herein were obtained from Sigma-Aldrich (St. Louis, MO) and were used as delivered. Raclopride-HCl and cocaine-HCl dissolved in saline were administered intraperitoneally (i.p.). Post-in vivo electrode calibrations were done as previously described (Heien et al., 2005; Heien et al., 2003; Sombers et al., 2009) in a flow injection system with a Tris buffer solution (pH 7.4) consisting of 15 mM Tris, 140 mM NaCl, 3.25 mM KCl, 1.2 mM CaCl2, 1.25 mM NaH2PO4, and 2.0 mM Na2SO4. Stock solutions of dopamine were made using 0.1 N HClO4 and were diluted immediately prior to calibration.
2.2 Microfabrication of Electrode Holders
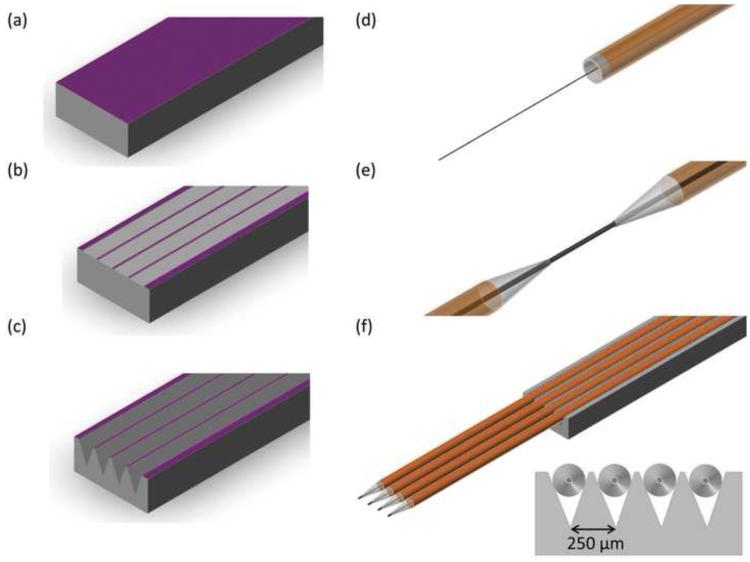
Figure 1 describes the microfabrication processes involved in the manufacture of the capillary electrode holders. All fabrication was done using 380 μm thick, p-type, <100> silicon wafers (University Wafer, Boston, MA). Figure 1A describes the low pressure chemical vapor deposition (LPCVD) of a silicon nitride etch mask on the wafer. The deposition was done using a three-zone, quartz tube LPCVD system. Films were deposited at a temperature of 775°C and a pressure of 300 mTorr in ammonia (NH3) and dichlorosilane (SiH2Cl2) atmosphere. These parameters enabled a nitride deposition rate of approximately 3.2 nm/min and a final thickness of 1000 Å. Film thicknesses were verified using a Nanometrics Nanospec/AFT interferometer (Nanometrics, Inc., Milpitas, CA).
Figure 1.
Fabrication of microelectrode spacer and fused silica capillary microelectrodes. (A) A layer of Si3N4 is deposited on <100> Si via LPCVD. (B) Si3N4 is etched using RIE (C) Si is etched in 40% KOH and device is diced into final form. (D) Carbon fiber is loaded in fused silica capillary (160 μm OD × 100 μm ID) (E) Capillary is pulled using a laser puller and carbon fiber is cut to a length of 150 μm. (F) Microelectrodes are lowered into the spacer, and secured in place. Connections are made with a tungsten rod (50 μm diameter) and silver epoxy.
Nitride films were subsequently patterned and etched to enable silicon etching (Figure 1B). The grove areas were first defined using standard photolithography techniques. A reactive ion etching (RIE) system (SemiGroup, Inc.) was used at an RF power of 100 W and a pressure of 60 mTorr to etch the nitride film under a trifluoromethane (40 sccm) and oxygen (5.0 sccm) atmosphere. The nitride etch rate under these conditions is 200Å/min. The photoresist was subsequently stripped using a Nanostrip solution (Cyantek, Inc., Freemont, CA).
Anisotropic etching of silicon was finally performed to fabricate the V-groves that secure the electrodes in place (Figure 1C). This was accomplished by placing the nitride masked silicon wafer in a heated (90°C) aqueous solution of potassium hydroxide (33% KOH). The etch rate of this solution is approximately 1.5 μm/min, and it is highly selective toward <110> and <100> crystallographic directions over the <111> direction (Madou, 2002). This selectivity results in 54° anisotropic sidewalls that are 90 μm deep. The spacing between groves was measured to be 250 μm. Measurements were done using a Tencor P-6 Profilometer (KLA-Tencor, Inc.). The devices were cut into their final form using a dicing saw.
2.3 Electrode Fabrication
Figure 1 (D-F) describes the electrode fabrication process. Fused silica capillaries (165 μm OD, 100 μm ID) (Polymicro Technologies, Phoenix, AZ), were cut to a length of 8 cm and loaded with an individual T-650 carbon fiber (Figure 1D). This loading step was facilitated through the use of thin tungsten rods (50 μm in diameter) (AM systems, Sequim, WA) and a cyanoacrylate adhesive. The loaded capillary was then pulled to a fine tip using a CO2 laser puller (Figure 1E) (Sutter Instruments, Novato, CA). As previously reported (Heien et al., 2003), the carbon fiber was sealed in the capillary through the use of a low-viscosity epoxy (Epon 828 resin with 14% m-phenylenediamine by weight, Miller-Stephenson Chemical Co., Danbury, CT). Excess epoxy was removed by acetone immersion. The epoxy was then allowed to cure (room temperature for 12 hrs, 100°C for 12 hrs, 150°C for 2 hrs). Electrical contact was made through the use of a small tungsten wire with a thin coating of silver epoxy (Epoxy Technologies, Billerica, MA). The protruding carbon fiber was cut to ~150μm in length.
Electrodes are loaded into the capillary array holder, microscopically aligned, and secured using a cyanoacrylate adhesive (Figure 1F). The modular design of the MEA allows different numbers of electrodes to be used in addition to different spacing along the Z-direction, depending on the experimental setup. All MEA experiments here, however, were done with the electrodes at the same relative depth. The holder was secured on a PC board (Express PCB, Santa Barbara, CA ) where subsequent electrical connections were made using silver epoxy and Molex connectors (Molex, Inc., Lisle, IL).
Utilization of T-650 carbon fibers as the active portion of the working electrodes enables the information gained through years of experience with single electrode experiments to be extended to a multi-electrode format. Previous research has used the same fibers (Heien et al., 2005; Heien et al., 2003), the same methods for sealing electrodes (Heien et al., 2003; Kawagoe et al., 1993) and the same electrochemical monitoring parameters (Heien et al., 2003). The carbon fiber microelectrode has been well characterized in vivo as well as in vitro via flow injection analysis (Bath et al., 2001; Bath et al., 2000; Heien et al., 2003; Hermans et al., 2006). Therefore, little in vitro electrode characterization was necessary. Additionally, similar fused silica electrodes for neurochemical recordings have been previously described (Swiergiel et al., 1997), but never used in an array format.
2.4 Data Acquisition and Analysis
The multi-electrode data collection for the FSCV experiments was done as previously reported (Zachek et al., 2009). Briefly, a customized version of TH-1 software (ESA, Inc., Chelmsford, MA) was used in conjunction with a “Quad UEI” headstage (University of North Carolina Department of Chemistry Electronics shop) to simultaneously apply the FSCV technique to multiple working electrodes. The small size of the electrodes enabled them to be used without a counter electrode. For all FSCV experiments the “extended” waveform using a scan rate of 400 V/s from −0.4 V to +1.3 V at 10 Hz was implemented. This particular waveform has been shown to increase the electrodes sensitivity toward catecholamines in vivo and in vitro (Heien et al., 2003). The waveform was digitally generated and then low-pass filtered at 2 kHz to eliminate undesirable capacitive effects from digital steps.
Background subtraction and further data analysis was done in the TH-1 Software. In vivo voltammetric data was represented through the use of color plots (Michael et al., 1998), peak voltammetric current vs. time traces and traditional voltammograms. Color plots are generated by stacking voltammograms temporally. Consequently, time is represented on the x-axis, potential is on the y-axis and current is represented as a false color. Oxidation currents observed in vivo were converted to concentration through the use of a calibration factor obtained via flow injection analysis.
2.5 In vivo Experiments
Male Sprague-Dawley rats (250-400 g; Charles River Laboratories, Wilmington, MA) were used for in vivo experiments. Rats were anesthetized with urethane in saline solution (50% w/w) and mounted in a stereotaxic frame (Kopf instruments, Tujunga, CA). Holes were precisely drilled in the skull to allow for microelectrode array placement in dopamine rich brain areas (caudate-putamen, nucleus accumbens core and shell). The stereotaxic coordinates served as the reference point for the working electrodes and were 2.2 mm anterior and 1.5 mm lateral from bregma (Paxinos and Watson, 2007). A Ag/AgCl reference electrode was implanted contralateral to the working electrodes of the MEA beneath the meningeal membrane. This Ag/AgCl electrode acted as an inert reference point for the measurements.
Bipolar stimulations of the medial forebrain bundle (MFB) were done to electrically elicit dopamine responses in the brain area of interest. Stimulation pulses were software generated and synchronized to occur between voltammetric scans to avoid crosstalk. Bipolar stimulating electrode and voltage-to-current converter (NeuroLog System, Hertfordshire, England) were used. Unless otherwise noted 40 pulses, 60 Hz biphasic stimulations were used (± 300 μA, 2 ms per phase.
2.6 Histological Preperations and Carbon Fiber Microlesioning
To confirm the MEA placement lesions were produced at the completion at the end of the experiment. Two methods were used to produce lesions in this work. In the initial experiments, traditional lesioning with a tungsten microelectrode was done (Sombers et al., 2009). This enabled a post-calibration of the electrodes in the array to ensure they were behaving as expected. In later experiments, the lesions were produced at the completion at the experiment by supplying the carbon fiber with a sufficient current to cause tissue destruction (Garris and Wightman, 1994). This destroys the carbon fiber, however, precluding post-calibration.
Following a lethal dose of urethane, brains were perfused transcardially with saline and subsequently with 10% formalin. Then, brains were removed from the skull, stored in 10% formaldehyde for at least 3 days, and coronally sectioned into 45 μm slices with a cryostat (Leica Microsystems, Bannockburn, IL). The sections were mounted on slides, stained with 0.2% thionin, and coverslipped before viewing under a light microscope.
3. Results
3.1 Simultaneous recordings of dopamine release in the nucleus accumbens (NAc)
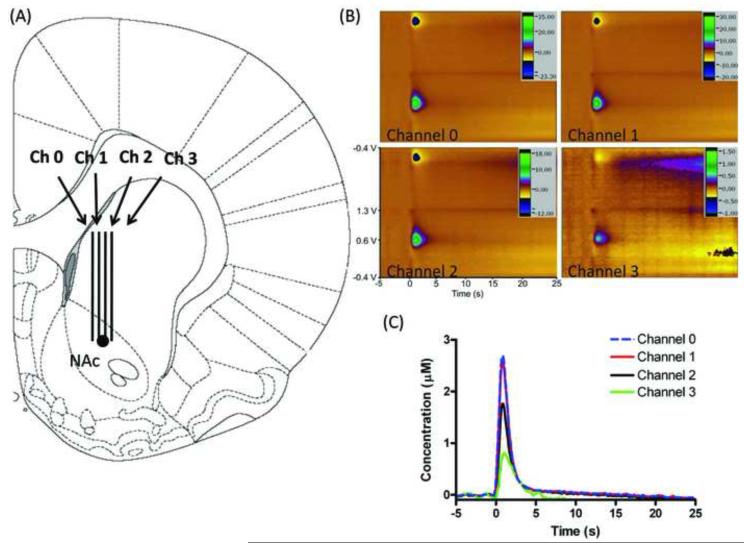
Initial experiments were performed, and repeated, to verify that simultaneous, decoupled monitoring of dopamine was possible. For this experiment the microelectrode array was lowered to the nucleus accumbens, a brain region that is known to support electrically stimulated release of dopamine (Wightman et al., 2007). Once in place, electrodes were electrochemically pretreated for 15 minutes at 60 Hz to optimize electrode sensitivity (Heien et al., 2003). After pretreatment the MFB stimulation and simultaneous monitoring in the NAc were performed. Figure 2B shows stimulated dopamine release measured simultaneously at four separate spatial locations in the nucleus accumbens (NAc). As done previously, the identity of the released species was verified to be dopamine from the cyclic voltammogram, the peak dopamine currents (for dopamine this corresponds to the current collected when the working electrode was at ~0.6 V) were plotted vs. time, and subsequently multiplied by an in vitro calibration factor to obtain the concentration vs. time graph shown in Figure 2C. While dopamine release is observed with each electrode, it varies in amplitude.
Figure 2.
Simultaneous in vivo dopamine detection. (A) Histological placement of MEA as confirmed by lesioning with a tungsten microelectrode post-experimentation (marked with a black circle). (B) Simultaneously collected color plots of electrically stimulated dopamine release. Stimulation parameters: 60 Hz, 40 pulses (± 300 μA, 2 ms per pulse). (C) Dopamine oxidation current vs. time traces at 4 channels. Stimulation delivered at t = 0 s.
3.2 Verifying Electrode Placement through Histology
After the experiment the positions of the electrodes could be confirmed using the traditional lesioning techniques for FSCV experiments. Figure 2A describes the histological placement of the microelectrode array. To confirm the MEA placement, lesioning with a tungsten microelectrode was done as described. Lesioning was done with a tungsten wire microelectrode to enable post-experiment in vitro calibration factors for the carbon fiber MEA to be obtained. The lesioning resulted in four separate electrode tracks being visible indicating that the electrodes in the MEA were placed to enable sampling from four spatially different locations.
3.3 Effect of raclopride and cocaine on dopamine overflow at four separate channels
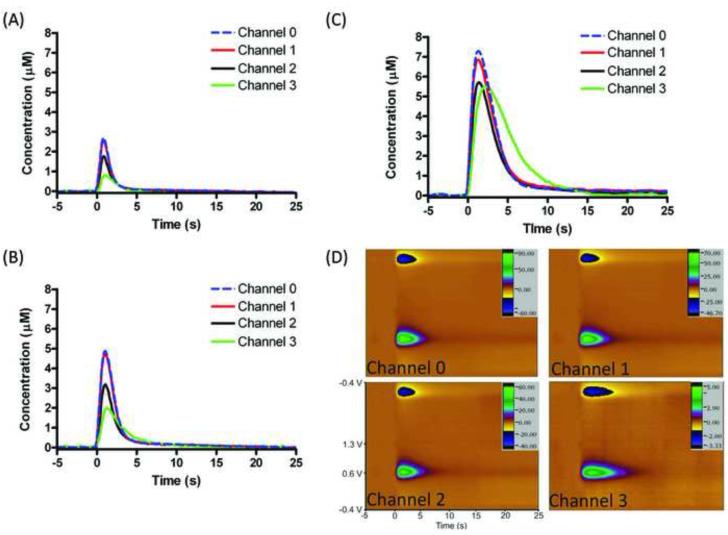
Pharmacological experiments were performed to ensure that the dopamine release observed on the MEA was consistent with previous studies. After obtaining stable pre-drug dopamine release, a D2 dopamine autoreceptor antagonist (raclopride; 2 mg/kg) followed by a monoamine transporter (i.e. DAT) inhibitor (cocaine; 15 mg/kg) was administered via intraperitoneal (i.p.) injection. Figure 3A represents simultaneous electrically stimulated peak dopamine concentration vs. time traces without pharmacological alteration. Simultaneous electrically stimulated dopamine release was monitored following subsequent i.p. injections of raclopride (dopamine concentration vs. time traces; Figure 3B) and cocaine (dopamine concentration vs. time traces; Figure 3C). The electrochemical color plots for simultaneous release following a raclopride-cocaine injections are shown in figure 3D. Consistent with prior work, raclopride caused an increase in dopamine release (Kita et al., 2007; Wu et al., 2002) with a further increase caused by cocaine administration (Wu et al., 2001). Additionally, cocaine caused the rate of disappearance of dopamine to decrease.
Figure 3.
Raclopride and cocaine effect on dopamine response at four channels. Dopamine oxidation current vs. time traces (A) prior to drug administration, (B) after raclopride (2 mg/kg) and (C) after raclopride-cocaine cocktail (cocaine 15 mg/kg) (D) Color plots of stimulated dopamine release at 4 channels after raclopride and cocaine. Stimulation parameters: 60 Hz, 40 pulses (± 300 μA, 2 ms per pulse).
3.4 Dopamine release in different brain environments
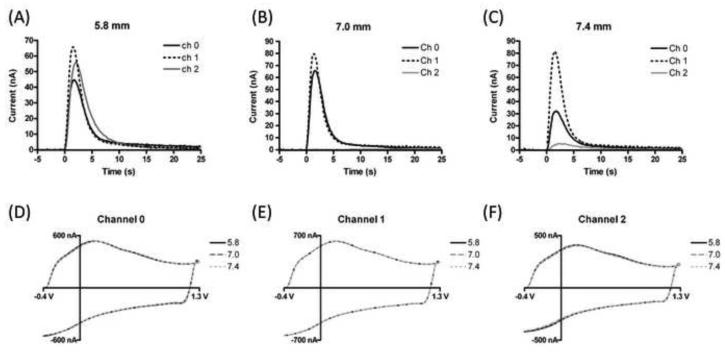
The modular design of the MEA allows the electrode number and position to be modified. Therefore, we used three electrodes to investigate simultaneously brain regions that are both dopamine releasing and non-releasing. Figure 4 highlights the ability of the arrays to be used in heterogeneous brain areas of that are close in spatial proximity. In this particular experiment, the MEA was sequentially lowered to three different depths. For a more pronounced effect, rats were again administered raclopride and cocaine as done previously. At a depth of 5.8 mm dopamine release was elicited via electrical stimulation and observed at the three electrodes (Figure 4A). The MEA was then lowered to a depth of 7.0 mm. At this point we expected to encounter a small area without dopamine release, the anterior commissure (Paxinos and Watson, 1986), a major fiber bundle in the brain Figure 4B shows that we were able to electrically stimulate and monitor dopamine release at two electrodes while the third electrode, located in the anterior commissure, showed no change in signal. At a depth of 7.4 mm (Figure 4C) dopamine detection was restored all three electrodes. To ensure that electrode area remained consistent throughout these experiments, “background” voltammograms, which are proportional to the electrode area, were examined and showed no change with depth (Figure 4D-F).
Figure 4.
Monitoring release in different brain regions. Stimulated dopamine release was monitored with a 3 electrode MEA at three different depths (A) 5.8 mm (B) 7.0 mm (Channel 2 is on x-axis) (C) 7.4 mm. (D-F) Electrochemical “background” currents at each channel for the three depths. Waveform: −0.4V to 1.3 V at 400 V/s. Background currents are proportional to electrode area.
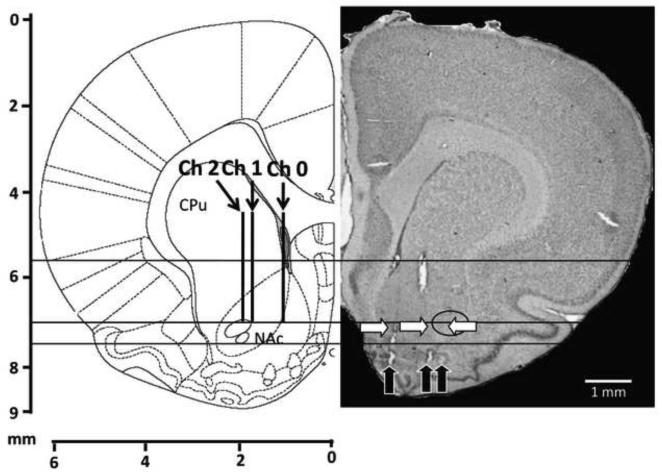
Electrode placement was confirmed via histology of the coronal brain slices, using carbon fiber microlesioning to identify the exact spatial locations of the electrodes. Figure 5 depicts three separate carbon fiber lesions that relate to the electrode dimensions and configuration that was chosen for this experiment. Again separate electrode tracks were seen, each leading to a microlesion.
Figure 5.
Histology and carbon fiber microlesioning of an MEA. (Left pane) histological diagram of a coronal rat brain slice at 2.28 mm anterior from bregma. (Right pane) thionin stained coronal brain slice with carbon fiber micro lesions (black arrows) and electrode tracks (white arrows). The anterior commissure is indicated with a black circle. The three different depths are indicated by a solid black line.
4. Discussion
4.1 Simultaneous observation of dopamine release in the NAc
The dopamine terminal rich nucleus accumbens is a particularly important neurobiological region that has been heavily implicated in a variety of behavioral and cognitive functions; most notably for its importance regarding motivation and reward (Ikemoto, 2007; Schultz, 2000; Schultz and Dayan, 1997; Wightman et al., 2007). Electrically stimulated dopamine release in this area, however, is considered to be heterogeneous; containing “hot” and “cold” spots” of release (Garris et al., 1997; Garris et al., 1994; Wightman et al., 2007). MEAs located within this brain region enable sampling from a variety of these microenvironments; thus obtaining an increase in number of samples per animal. This allows for the acquisition of more representative data. Additionally, an array of microelectrodes is able to elucidate the temporal dependence of the signals in relation to one another.
Figure 2C illustrates peak dopamine currents at each of the four electrodes (electrode positions are shown in Figure 2A). The dopamine release in the area seems to vary medial to lateral, although separate electrode tracks were observed in the histological preparations. While heterogeneity of dopamine terminals might be a possible explanation for this release pattern (Jones et al., 1995), this medial to lateral distribution likely stems from a spatially asymmetric electrical stimulation of the MFB. Indeed, the mediolateral topography of dopaminergic projections (Ikemoto, 2007) to the NAc would suggest that a more medial stimulation would yield an increased medial release compared to lateral. This illustrates the need for such arrays even in a single brain area, as more representative data can be obtained from the average signal of each electrode. Without the use of an MEA such heterogeneity would have gone unnoticed.
4.2 Effect of cocaine and raclopride on dopamine release at four separate channels
Figure 3 describes the observation of electrically stimulated dopamine on four separate channels while under pharmacological alteration with raclopride and raclopride-cocaine drug cocktail. Consistent with other pharmacological studies (Aragona et al., 2008; Venton and Wightman, 2007; Wightman and Zimmerman, 1990), inhibiting negative feedback via a D2 antagonist caused a significant increase in stimulated dopamine release across each channel without visually affecting the parameters of uptake kinetics (Figure 3B). Cocaine is an effective DAT blocker (Kitayama et al., 1992; Rocha et al., 1998) and therefore should cause a significant delay in uptake, and synaptic overflow, at each channel (Figure 3C). While the observed dopamine responded to pharmacological alteration as expected; interestingly, the electrode that reported the largest increase in dopamine concentration also experienced the largest delay in uptake. This electrode also observed the lowest dopamine concentration without any pharmacological alteration. One possible explanation for these results is that this particular electrode is located further away from a dopamine releasing terminal (in addition to the asymmetric stimulation). At channels 0-2, increasing the synaptic overflow with raclopride caused a twofold increase in the amount of dopamine seen at each electrode, and a threefold increase was observed with raclopride-cocaine cocktail. Channel 3 also observed a twofold increase in dopamine levels following raclopride administration; however it experienced a 7 fold increase in response to cocaine. It is plausible that this extra distance, and axonally located dopamine transporters, would cause the diffusing dopamine to become increasingly dilute at the electrode surface under normal conditions. The subsequent blocking of these transporters with cocaine, coupled with an increase in the amount of dopamine from the synapse via negative feedback inhibition (raclopride), would cause such an increase in concentration at this particular electrode (Cragg and Rice, 2004; Moquin and Michael, 2009).
4.3 Dopamine release in different brain environments
A major advantage of these MEAs is their ability to probe different brain areas that are in close spatial proximity. Valuable information has been gained using a single electrode in multiple dopamine releasing regions such as the nucleus accumbens core and nucleus accumbens shell (Aragona et al., 2008; Owesson-White et al., 2008). These arrays could be used to probe multiple dopamine releasing regions (too close for multiple traditional electrodes) simultaneously rather than using multiple animals utilizing single electrode experiments. Additionally the probes could be used to study multiple neurotransmitters in different brain regions. Serotonin releasing and dopamine releasing nerve terminals in the substantia nigra, for example, occur in areas that are separated by less than 1 mm mediolaterally (Paxinos and Watson, 2007). Information regarding the integration of these two neurotransmitter systems could be vastly important in the study of many diseases and disorders including Parkinson’s disease (Scatton et al., 1983).
Figure 4 illustrates the ability to probe different brain regions. In this experiment a three electrode MEA was placed so that two of the electrodes were in separate dopamine releasing regions (NAc core and NAc shell), while another was in a non-releasing region (anterior commissure). To exacerbate the effect at each electrode raclopride and cocaine were again administered. Prior to placement at the separate locations, dopamine release was observed at a depth of 5.8 mm, in the caudate putamen (CPu). Consistent stimulated dopamine release was observed at all three electrodes in this region (Figure 4A). At a depth of 7.0 mm, the electrodes were located at separate locations, and as expected, observed release in the NAc core and shell but not in the anterior commissure (Figure 4B). Figure 4C shows that after the electrodes are moved to a depth of 7.4, past the depth of the anterior commissure, the third electrode was again able to observe dopamine release. To ensure that the electrode surfaces were not blocked or damaged, background voltammograms were recorded for each electrode at each depth (Figures 4D-F). Each electrode showed no change in background current. This background current is proportional to electrode area; therefore, any change in the amount of dopamine is expected to be purely due to the amount of neurotransmitter released in the vicinity of each electrode.
Post-experiment histology was preformed to ensure MEA placement, as well as to verify that the relative spatial separation of the electrodes remained intact during in vivo implementation. Figure 5 illustrates the results of the histological preparation. The black arrows indicate the separate microlesions that were preformed post-experimentation (at 8 mm), while the white arrows indicate the position of observed electrode tracks. The black circle highlights the position of the the anterior commissure. Measured from the optical microscopy micrographs, the microlesions indicate that the electrode spacing was around 1 mm total. This measurement was larger than the expected 0.75 mm, showing that the electrodes had slightly moved during implantation; however still yielded three separate mircolesions that were in a configuration consistent with the experimental design. Additionally, histology verified that the third electrode did in fact pass through the anterior commissure at 7.0 mm, explaining the lack of dopamine observed at that electrode.
5. Conclusion
This work has demonstrated a method for producing the first microelectrode array capable of simultaneously distinguishing different neurochemical events in close proximity using fast scan cyclic voltammetry. The functionality of the arrays were assessed through monitoring dopamine release in the same brain region under normal, and pharmacologically altered, conditions. Additionally, the ability of the MEA to monitor electrically stimulated dopamine fluctuations in different brain regions was accomplished and supported with histology. The functionality of these arrays in vivo will allow important questions to be answered regarding the integrative nature of neurobiology with higher throughput.
Acknowledgements
The authors would like to acknowledge financial support from NIH (Grant NS 15841) to R.M.W. and from NIH (Grant DA023586) to M.K.Z as well as Eli Lilly Fellowship to P.T. We would like to thank the Allbritton lab for the access to the laser puller and Ramsey lab for the access to their dicing saw. Also, we would like to acknowledge Sumaiya Sarwar for her help in constructing the microelectrode arrays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. Journal of Neuroscience. 2008;28:8821–31. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard AJ, Faulkner LR. Electrochemical methods : fundamentals and applications. 2nd ed Wiley; New York: 2001. [Google Scholar]
- Bath BD, Martin HB, Wightman RM, Anderson MR. Dopamine adsorption at surface modified carbon-fiber electrodes. Langmuir. 2001;17:7032–9. [Google Scholar]
- Bath BD, Michael DJ, Trafton BJ, Joseph JD, Runnels PL, Wightman RM. Subsecond adsorption and desorption of dopamine at carbon-fiber microelectrodes. Analytical Chemistry. 2000;72:5994–6002. doi: 10.1021/ac000849y. [DOI] [PubMed] [Google Scholar]
- Cooper S, Venton B. Fast-scan cyclic voltammetry for the detection of tyramine and octopamine. Analytical and Bioanalytical Chemistry. 2009 doi: 10.1007/s00216-009-2616-0. (in press) [DOI] [PubMed] [Google Scholar]
- Cragg SJ, Rice ME. DAncing past the DAT at a DA synapse. Trends in Neurosciences. 2004;27:270–7. doi: 10.1016/j.tins.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Dressman SF, Peters JL, Michael AC. Carbon fiber microelectrodes with multiple sensing elements for in vivo voltammetry. Journal of Neuroscience Methods. 2002;119:75–81. doi: 10.1016/s0165-0270(02)00180-2. [DOI] [PubMed] [Google Scholar]
- Ewing AG, Bigelow JC, Wightman RM. Direct Invivo Monitoring of Dopamine Released from 2 Striatal Compartments in the Rat. Science. 1983;221:169–71. doi: 10.1126/science.6857277. [DOI] [PubMed] [Google Scholar]
- Garris PA, Christensen JRC, Rebec GV, Wightman RM. Real-time measurement of electrically evoked extracellular dopamine in the striatum of freely moving rats. Journal of Neurochemistry. 1997;68:152–61. doi: 10.1046/j.1471-4159.1997.68010152.x. [DOI] [PubMed] [Google Scholar]
- Garris PA, Ciolkowski EL, Wightman RM. Heterogeneity of Evoked Dopamine Overflow within the Striatal and Striatoamygdaloid Regions. Neuroscience. 1994;59:417–27. doi: 10.1016/0306-4522(94)90606-8. [DOI] [PubMed] [Google Scholar]
- Garris PA, Wightman RM. In-Vivo Voltammetric Measurement of Evoked Extracellular Dopamine in the Rat Basolateral Amygdaloid Nucleus. Journal of Physiology-London. 1994;478:239–49. doi: 10.1113/jphysiol.1994.sp020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien MLAV, Khan AS, Ariansen JL, Cheer JF, Phillips PEM, Wassum KM, Wightman RM. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10023–8. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien MLAV, Phillips PEM, Stuber GD, Seipel AT, Wightman RM. Overoxidation of carbon-fiber microelectrodes enhances dopamine adsorption and increases sensitivity. Analyst. 2003;128:1413–9. doi: 10.1039/b307024g. [DOI] [PubMed] [Google Scholar]
- Hermans A, Seipel AT, Miller CE, Wightman RM. Carbon-fiber microelectrodes modified with 4-sulfobenzene have increased sensitivity and selectivity for catecholamines. Langmuir. 2006;22:1964–9. doi: 10.1021/la053032e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Research Reviews. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson BP, Dietz SM, Wightman RM. Fast-scan cyclic voltammetry of 5-hydroxytryptamine. Anal Chem. 1995;67:1115–20. doi: 10.1021/ac00102a015. [DOI] [PubMed] [Google Scholar]
- Jones SR, Garris PA, Kilts CD, Wightman RM. Comparison of dopamine uptake in the basolateral amygdaloid nucleus, caudate-putamen, and nucleus accumbens of the rat. J.Neurochem. 1995;64:2581–9. doi: 10.1046/j.1471-4159.1995.64062581.x. [DOI] [PubMed] [Google Scholar]
- Kawagoe KT, Zimmerman JB, Wightman RM. Principles of Voltammetry and Microelectrode Surface-States. Journal of Neuroscience Methods. 1993;48:225–40. doi: 10.1016/0165-0270(93)90094-8. [DOI] [PubMed] [Google Scholar]
- Kita JM, Parker LE, Phillips PE, Garris PA, Wightman RM. Paradoxical modulation of short-term facilitation of dopamine release by dopamine autoreceptors. J Neurochem. 2007;102:1115–24. doi: 10.1111/j.1471-4159.2007.04621.x. [DOI] [PubMed] [Google Scholar]
- Kita JM, Wightman RM. Microelectrodes for studying neurobiology. Current Opinion in Chemical Biology. 2008;12:491–6. doi: 10.1016/j.cbpa.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama S, Shimada S, Xu H, Markham L, Donovan DM, Uhl GR. Dopamine Transporter Site-Directed Mutations Differentially Alter Substrate Transport and Cocaine Binding. PNAS. 1992;89:7782–5. doi: 10.1073/pnas.89.16.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madou MJ. Fundamentals of microfabrication : the science of miniaturization. 2nd ed CRC Press; Boca Raton: 2002. [Google Scholar]
- Michael D, Travis ER, Wightman RM. Color images for fast-scan CV measurements in biological systems. Anal.Chem. 1998;70:586A–92A. doi: 10.1021/ac9819640. [DOI] [PubMed] [Google Scholar]
- Moquin KF, Michael AC. Tonic autoinhibition contributes to the heterogeneity of evoked dopamine release in the rat striatum. J Neurochem. 2009 doi: 10.1111/j.1471-4159.2009.06254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis MAL. Methods for neural ensemble recordings. 2nd ed. CRC Press; Boca Raton: 2008. [PubMed] [Google Scholar]
- Owesson-White CA, Cheer JF, Beyene M, Carelli RM, Wightman RM. Dynamic changes in accumbens dopamine correlate with learning during intracranial self-stimulation. PNAS. 2008;105:11957–62. doi: 10.1073/pnas.0803896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd ed Academic Press; Sydney ; Orlando: 1986. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Elsevier; Amsterdam: 2007. [DOI] [PubMed] [Google Scholar]
- Pihel K, Schroeder TJ, Wightman RM. Rapid and Selective Cyclic Voltammetric Measurements of Epinephrine and Norepinephrine as a Method to Measure Secretion from Single Bovine Adrenal-Medullary Cells. Analytical Chemistry. 1994;66:4532–7. [Google Scholar]
- Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, Miller GW, Caron MG. Cocaine self-administration in dopamine-transporter knockout mice. Nature Neuroscience. 1998;1:132–7. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Research. 1983;275:321–8. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nature Reviews Neuroscience. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P. A neural substrate of prediction and reward. (Cover story) Science. 1997;275:1593. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sombers LA, Beyene M, Carelli RM, Wightman RM. Synaptic Overflow of Dopamine in the Nucleus Accumbens Arises from Neuronal Activity in the Ventral Tegmental Area. Journal of Neuroscience. 2009;29:1735–42. doi: 10.1523/JNEUROSCI.5562-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy BEK, Venton BJ. Subsecond Detection of Physiological Adenosine Concentrations Using Fast-Scan Cyclic Voltammetry. Analytical Chemistry. 2007;79:744–50. doi: 10.1021/ac061820i. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Palamarchouk VS, Dunn AJ. A new design of carbon fiber microelectrode for in vivo voltammetry using fused silica. Journal of Neuroscience Methods. 1997;73:29–33. doi: 10.1016/s0165-0270(96)02207-8. [DOI] [PubMed] [Google Scholar]
- Travis ER, Wightman RM. Spatio-temporal resolution of exocytosis from individual cells. Annual Review of Biophysics and Biomolecular Structure. 1998;27:77–103. doi: 10.1146/annurev.biophys.27.1.77. [DOI] [PubMed] [Google Scholar]
- Troyer KP, Wightman RM. Temporal separation of vesicle release from vesicle fusion during exocytosis. Journal of Biological Chemistry. 2002;277:29101–7. doi: 10.1074/jbc.M202856200. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Hui Z, Paul AG, Paul EMP, David S, Wightman RM. Real-time decoding of dopamine concentration changes in the caudate-putamen during tonic and phasic firing. Journal of Neurochemistry. 2003a;87:1284–95. doi: 10.1046/j.1471-4159.2003.02109.x. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Michael DJ, Wightman RM. Correlation of local changes in extracellular oxygen and pH that accompany dopaminergic terminal activity in the rat caudate-putamen. Journal of Neurochemistry. 2003b;84:373–81. doi: 10.1046/j.1471-4159.2003.01527.x. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Wightman RM. Pharmacologically induced, subsecond dopamine transients in the caudate-putamen of the anesthetized rat. Synapse. 2007;61:37–9. doi: 10.1002/syn.20343. [DOI] [PubMed] [Google Scholar]
- Wightman RM. Probing cellular chemistry in biological systems with microelectrodes. Science. 2006;311:1570–4. doi: 10.1126/science.1120027. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Heien MLAV, Wassum KM, Sombers LA, Aragona BJ, Khan AS, Ariansen JL, Cheer JF, Phillips PEM, Carelli RM. Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. European Journal of Neuroscience. 2007;26:2046–54. doi: 10.1111/j.1460-9568.2007.05772.x. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Zimmerman JB. Control of dopamine extracellular concentration in rat striatum by impulse flow and uptake. Brain Research Reviews. 1990;15:135–44. doi: 10.1016/0165-0173(90)90015-g. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Kuhar MJ, Carroll FI, Garris PA. Preferential increases in nucleus accumbens dopamine after systemic cocaine administration are caused by unique characteristics of dopamine neurotransmission. J.Neurosci. 2001;21:6338–47. doi: 10.1523/JNEUROSCI.21-16-06338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Walker QD, Kuhn CM, Carroll FI, Garris PA. Concurrent autoreceptor-mediated control of dopamine release and uptake during neurotransmission: an in vivo voltammetric study. J Neurosci. 2002;22:6272–81. doi: 10.1523/JNEUROSCI.22-14-06272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachek MK, Takmakov P, Moody B, Wightman RM, McCarty GS. Simultaneous Decoupled Detection of Dopamine and Oxygen Using Pyrolyzed Carbon Microarrays and Fast-Scan Cyclic Voltammetry. Analytical Chemistry. 2009;81:6258–65. doi: 10.1021/ac900790m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Adams KL, Luber SJ, Eves DJ, Heien ML, Ewing AG. Spatially and Temporally Resolved Single-Cell Exocytosis Utilizing Individually Addressable Carbon Microelectrode Arrays. 2008. pp. 1394–400. [DOI] [PMC free article] [PubMed]
- Zimmerman JB, Wightman RM. Simultaneous Electrochemical Measurements of Oxygen and Dopamine Invivo. Analytical Chemistry. 1991;63:24–8. doi: 10.1021/ac00001a005. [DOI] [PubMed] [Google Scholar]