Abstract
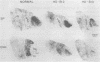
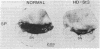
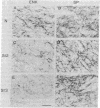
Huntington disease (HD) is characterized by the loss of striatal projection neurons, which constitute the vast majority of striatal neurons. To determine whether there is differential loss among different populations of striatal projection neurons, the integrity of the axon terminal plexuses arising from the different populations of substance P-containing and enkephalin-containing striatal projection neurons was studied in striatal target areas by immunohistochemistry. Analysis of 17 HD specimens indicated that in early and middle stages of HD, enkephalin-containing neurons projecting to the external segment of the globus pallidus were much more affected than substance P-containing neurons projecting to the internal pallidal segment. Furthermore, substance P-containing neurons projecting to the substantia nigra pars reticulata were more affected than those projecting to the substantia nigra pars compacta. At the most advanced stages of the disease, projections to all striatal target areas were depleted, with the exception of some apparent sparing of the striatal projection to the substantia nigra pars compacta. These findings may explain some of the clinical manifestations and pharmacology of HD. They also may aid in identifying the neural defect underlying HD and provide additional data with which to evaluate current models of HD pathogenesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronin N., Difiglia M., Graveland G. A., Schwartz W. J., Wu J. Y. Localization of immunoreactive enkephalins in GABA synthesizing neurons of the rat neostriatum. Brain Res. 1984 May 23;300(2):376–380. doi: 10.1016/0006-8993(84)90850-3. [DOI] [PubMed] [Google Scholar]
- Beal M. F., Kowall N. W., Ellison D. W., Mazurek M. F., Swartz K. J., Martin J. B. Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature. 1986 May 8;321(6066):168–171. doi: 10.1038/321168a0. [DOI] [PubMed] [Google Scholar]
- Beckstead R. M., Cruz C. J. Striatal axons to the globus pallidus, entopeduncular nucleus and substantia nigra come mainly from separate cell populations in cat. Neuroscience. 1986 Sep;19(1):147–158. doi: 10.1016/0306-4522(86)90012-6. [DOI] [PubMed] [Google Scholar]
- Bouras C., Schulz P., Constantinidis J., Tissot R. Differential effects of acute and chronic administration of haloperidol on substance P and enkephalins in diverse rat brain areas. Neuropsychobiology. 1986;16(4):169–174. doi: 10.1159/000118321. [DOI] [PubMed] [Google Scholar]
- Buck S. H., Burks T. F., Brown M. R., Yamamura H. I. Reduction in basal ganglia and substantia nigra substance P levels in Huntington's disease. Brain Res. 1981 Mar 30;209(2):464–469. doi: 10.1016/0006-8993(81)90171-2. [DOI] [PubMed] [Google Scholar]
- DiFiglia M., Aronin N., Martin J. B. Light and electron microscopic localization of immunoreactive Leu-enkephalin in the monkey basal ganglia. J Neurosci. 1982 Mar;2(3):303–320. doi: 10.1523/JNEUROSCI.02-03-00303.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emson P. C., Arregui A., Clement-Jones V., Sandberg B. E., Rossor M. Regional distribution of methionine-enkephalin and substance P-like immunoreactivity in normal human brain and in Huntington's disease. Brain Res. 1980 Oct 13;199(1):147–160. doi: 10.1016/0006-8993(80)90237-1. [DOI] [PubMed] [Google Scholar]
- Ferrante R. J., Beal M. F., Kowall N. W., Richardson E. P., Jr, Martin J. B. Sparing of acetylcholinesterase-containing striatal neurons in Huntington's disease. Brain Res. 1987 May 12;411(1):162–166. doi: 10.1016/0006-8993(87)90694-9. [DOI] [PubMed] [Google Scholar]
- Féger J., Crossman A. R. Identification of different subpopulations of neostriatal neurones projecting to globus pallidus or substantia nigra in the monkey: a retrograde fluorescence double-labelling study. Neurosci Lett. 1984 Aug 24;49(1-2):7–12. doi: 10.1016/0304-3940(84)90127-7. [DOI] [PubMed] [Google Scholar]
- Gale J. S., Bird E. D., Spoke E. G., Ivejsen L. L., Jessel T. Human brain substance P: distribution in controls and Huntington's chorea. J Neurochem. 1978 Mar;30(3):633–634. doi: 10.1111/j.1471-4159.1978.tb07818.x. [DOI] [PubMed] [Google Scholar]
- Grafe M. R., Forno L. S., Eng L. F. Immunocytochemical studies of substance P and Met-enkephalin in the basal ganglia and substantia nigra in Huntington's, Parkinson's and Alzheimer's diseases. J Neuropathol Exp Neurol. 1985 Jan;44(1):47–59. doi: 10.1097/00005072-198501000-00004. [DOI] [PubMed] [Google Scholar]
- Graveland G. A., Williams R. S., DiFiglia M. Evidence for degenerative and regenerative changes in neostriatal spiny neurons in Huntington's disease. Science. 1985 Feb 15;227(4688):770–773. doi: 10.1126/science.3155875. [DOI] [PubMed] [Google Scholar]
- Gusella J. F., Wexler N. S., Conneally P. M., Naylor S. L., Anderson M. A., Tanzi R. E., Watkins P. C., Ottina K., Wallace M. R., Sakaguchi A. Y. A polymorphic DNA marker genetically linked to Huntington's disease. Nature. 1983 Nov 17;306(5940):234–238. doi: 10.1038/306234a0. [DOI] [PubMed] [Google Scholar]
- Haber S. N., Nauta W. J. Ramifications of the globus pallidus in the rat as indicated by patterns of immunohistochemistry. Neuroscience. 1983 Jun;9(2):245–260. doi: 10.1016/0306-4522(83)90291-9. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Yoshikawa K., Kanamatsu T., Sabol S. L. Modulation of striatal enkephalinergic neurons by antipsychotic drugs. Fed Proc. 1985 Jun;44(9):2535–2539. [PubMed] [Google Scholar]
- JERVIS G. A. HUNTINGTON'S CHOREA IN CHILDHOOD. Arch Neurol. 1963 Sep;9:244–257. doi: 10.1001/archneur.1963.00460090050005. [DOI] [PubMed] [Google Scholar]
- Jackson A., Crossman A. R. Experimental choreoathetosis produced by injection of a gamma-aminobutyric acid antagonist into the lentiform nucleus in the monkey. Neurosci Lett. 1984 Apr 20;46(1):41–45. doi: 10.1016/0304-3940(84)90196-4. [DOI] [PubMed] [Google Scholar]
- Kanazawa I., Sasaki H., Muramoto O., Matsushita M., Mizutani T., Iwabuchi K., Ikeda T., Takahata N. Studies on neurotransmitter markers and striatal neuronal cell density in Huntington's disease and dentatorubropallidoluysian atrophy. J Neurol Sci. 1985 Sep;70(2):151–165. doi: 10.1016/0022-510x(85)90084-x. [DOI] [PubMed] [Google Scholar]
- Lange H., Thörner G., Hopf A., Schröder K. F. Morphometric studies of the neuropathological changes in choreatic diseases. J Neurol Sci. 1976 Aug;28(4):401–425. doi: 10.1016/0022-510x(76)90114-3. [DOI] [PubMed] [Google Scholar]
- Lasker A. G., Zee D. S., Hain T. C., Folstein S. E., Singer H. S. Saccades in Huntington's disease: initiation defects and distractibility. Neurology. 1987 Mar;37(3):364–370. doi: 10.1212/wnl.37.3.364. [DOI] [PubMed] [Google Scholar]
- Marshall P. E., Landis D. M., Zalneraitis E. L. Immunocytochemical studies of substance P and leucine-enkephalin in Huntington's disease. Brain Res. 1983 Dec 19;289(1-2):11–26. doi: 10.1016/0006-8993(83)90003-3. [DOI] [PubMed] [Google Scholar]
- Martin J. B., Gusella J. F. Huntington's disease. Pathogenesis and management. N Engl J Med. 1986 Nov 13;315(20):1267–1276. doi: 10.1056/NEJM198611133152006. [DOI] [PubMed] [Google Scholar]
- Pan H. S., Penney J. B., Young A. B. Gamma-aminobutyric acid and benzodiazepine receptor changes induced by unilateral 6-hydroxydopamine lesions of the medial forebrain bundle. J Neurochem. 1985 Nov;45(5):1396–1404. doi: 10.1111/j.1471-4159.1985.tb07205.x. [DOI] [PubMed] [Google Scholar]
- Penney J. B., Jr, Young A. B. Striatal inhomogeneities and basal ganglia function. Mov Disord. 1986;1(1):3–15. doi: 10.1002/mds.870010102. [DOI] [PubMed] [Google Scholar]
- Reiner A., Carraway R. E. Immunohistochemical and biochemical studies on Lys8-Asn9-neurotensin8-13 (LANT6)-related peptides in the basal ganglia of pigeons, turtles, and hamsters. J Comp Neurol. 1987 Mar 15;257(3):453–476. doi: 10.1002/cne.902570312. [DOI] [PubMed] [Google Scholar]
- Reiner A. The distribution of proenkephalin-derived peptides in the central nervous system of turtles. J Comp Neurol. 1987 May 1;259(1):65–91. doi: 10.1002/cne.902590106. [DOI] [PubMed] [Google Scholar]
- Shoulson I. Huntington's disease. A decade of progress. Neurol Clin. 1984 Aug;2(3):515–526. [PubMed] [Google Scholar]
- Spokes E. G. Neurochemical alterations in Huntington's chorea: a study of post-mortem brain tissue. Brain. 1980 Mar;103(1):179–210. doi: 10.1093/brain/103.1.179. [DOI] [PubMed] [Google Scholar]
- Walker F. O., Young A. B., Penney J. B., Dovorini-Zis K., Shoulson I. Benzodiazepine and GABA receptors in early Huntington's disease. Neurology. 1984 Sep;34(9):1237–1240. doi: 10.1212/wnl.34.9.1237. [DOI] [PubMed] [Google Scholar]
- Williams R. G., Dockray G. J. Distribution of enkephalin-related peptides in rat brain: immunohistochemical studies using antisera to met-enkephalin and met-enkephalin Arg6Phe7. Neuroscience. 1983 Jul;9(3):563–586. doi: 10.1016/0306-4522(83)90175-6. [DOI] [PubMed] [Google Scholar]
- Young A. B., Shoulson I., Penney J. B., Starosta-Rubinstein S., Gomez F., Travers H., Ramos-Arroyo M. A., Snodgrass S. R., Bonilla E., Moreno H. Huntington's disease in Venezuela: neurologic features and functional decline. Neurology. 1986 Feb;36(2):244–249. doi: 10.1212/wnl.36.2.244. [DOI] [PubMed] [Google Scholar]
- Zech M., Bogerts B. Methionine-enkephalin and substance P in the basal ganglia of normals, Parkinson patients, Huntington patients, and schizophrenics. A qualitative immunohistochemical study. Acta Neuropathol. 1985;68(1):32–38. doi: 10.1007/BF00688953. [DOI] [PubMed] [Google Scholar]