Abstract
While S-nitrosothiols are regarded as important elements of many NO-dependent signal transduction pathways, the physiological mechanism of their formation remains elusive. Here, we demonstrate a novel mechanism by which cytochrome c may represent an efficient catalyst of S-nitrosation in vivo. In this mechanism, initial binding of GSH to ferric cytochrome c is followed by reaction of NO with this complex, yielding ferrous cytochrome c and GSNO. We show that when submitochondrial particles or cell lysates are exposed to NO in the presence of cytochrome c, there is a robust formation of protein S-nitrosothiols. In the case of submitochondrial particles protein S-nitrosation is paralleled with an inhibition of mitochondrial complex I. These observations raise the possibility that cytochrome c is a mediator of S-nitrosation in biological systems, particularly during hypoxia, and that release of cytochrome c in to the cytosol during apoptosis potentially releases a GSNO synthase activity which could modulate apoptotic signaling.
Keywords: nitric oxide, nitrosation, nitrosylation, cytochrome c, mitochondria
Introduction
S-Nitrosation, also referred to as S-nitrosylation, is thought to be a major mechanism of NO-mediated signaling in pathology and physiology [1]. S-Nitrosated proteins have been found in many tissues [2], and proteins reported to be S-nitrosated are involved in a wide range of physiological functions including transcription, channel activity, response to hypoxia, and cell death [1]. It has been proposed that dysregulation of S-nitrosation is involved in many pathological states and that control of S-nitrosation could lead to new therapeutic strategies [1].
Although it is widely accepted that S-nitrosation occurs in vivo, mechanistic details have been elusive. NO is synthesized from nitric oxide synthase, but does not directly react with thiols to form S-nitrosothiols (RSNO) [3]. Several mechanisms of S-nitrosation have been proposed that fall into three main categories: (i) formation of nitrosating species from NO oxidation by a molecular oxygen-dependent process [4, 5] (ii) thiol oxidation to form a thiyl radical, which can combine with NO to form RSNO [5, 6] and (iii) direct addition of NO to RSH to form the radical intermediate RSNOH followed by oxidation of this radical by oxygen or some other one-electron acceptor [7]. While the first two mechanisms have been shown to be viable in vitro, evidence for the third reaction in vitro is equivocal. Redox-active metal ions [8], metalloproteins [9] and hydrophobic environments [10, 11] have been implicated as elements that enhance S-nitrosation via the above mechanisms. However, no specific intracellular S-nitrosothiol synthetic pathway has been established. Although S-nitrosation has been thought to be largely oxygen dependent, recent evidence points to hypoxia as being a stimulus of S-nitrosothiol synthesis [12, 13].
In this study we show that ferric cytochrome c (cyt c) is an efficient S-nitrosoglutathione (GSNO) synthase in both the presence and absence of oxygen. Ferric cyt c is reduced by NO. At pH below 7, NO reversibly binds ferric cytochrome c, but at higher pH, it will slowly reduce ferric cytochrome c to the ferrous form [14]. This process is believed to involve nucleophilic attack by OH− on the ferric nitrosyl which is expected to have considerable FeII-NO+ character, forming nitrite and the ferrous heme. In the case of other heme proteins like hemoglobin and myoglobin, excess NO in solution rapidly binds to the reduced heme so that the process is often referred to as reductive nitrosylation [14]. In the case of cytochrome c, NO doesn t avidly bind the ferrous heme and, therefore, mainly ferrous cyt c rather than the ferrous nitrosyl form is made [14]. It has been known for many years that ferric cyt c can also be reduced by thiols to the ferrous form [15–20]. The reaction is thought to involve both metal-catalyzed and metal-independent pathways [15]. The metal-independent pathway involves a glutathione-cyt c intermediate where GSH is loosely bound to the heme [20]. The end products reported in these reactions are ferrous cyt c and glutathione disulfide (GSSG) [15, 19]. We show here that the rate of reduction of cyt c by GSH is increased at least 10 fold by NO and that the major product of this reaction is GSNO. The reaction is most efficient at low NO concentrations and at higher concentrations a secondary GSSG-forming reaction occurs that lowers efficiency. This represents the first report of a specific intracellular S-nitrosothiol synthetic mechanism.
Experimental Procedures
PROLI/NO was purchased from Cayman Chemicals (Ann Arbor, MI) and 1,2-Dioleoyl-sn-glycero-3 phosphocholine and 1,1′,2,2′-tetraoleoyl cardiolipin (sodium salt) were purchased from Avanti Polar Lipids (Albaster, AL). We conducted experiments using three different cyt c preparations from Sigma (bovine heart C-2037, horse heart C-7752, and horse heart C-2506) with no qualitative differences in observed phenomenon. To insure that the purity of our samples was not likely to contribute to our results we included experiments using horse heart cytochrome c (C-7752) which has been shown to be substantially devoid of impurities [1, 2]. Experiments were performed using this preparation following centrifugation at 16,000 g to sediment any aggregates and further purification using G-25 columns. Similar results were obtained with all cyt c preparations. All other chemicals were purchased from Sigma Chemicals unless otherwise noted. Liposomes were prepared by sonication of a 1:1 molar ratio of the two lipids as described [21]. All experiments were performed in either 0.1 M phosphate buffer or 2-(N-morpholino)ethanesulfonic acid buffer (MES) adjusted to the appropriate pH with NaOH or HCl. Metal chelating agents diethylenetriamine penta-acetic acid (DTPA, 100 μM) or ethylenediaminetetraacetic acid (EDTA, 100 μM) or both were added to all reaction mixtures.
Absorption spectroscopy was conducted either on a Cary 50, Perkin Elmer Lambda 9, or an Agilent 8452 diode array UV-Vis spectrophotometer. The Perkin Elmer instrument is equipped with an integrating sphere detector so that scattered light from turbid samples, such as those containing liposomes, is collected. Time-resolved absorption was analyzed by global analysis using Specfit (Spectrum Software Associates, Chapel Hill, NC) where singular value decomposition is applied and the absorption data at all wavelengths and times are fit to a kinetic model, such as a single exponential process or double exponential process in order to obtain observed rate constants.
Anaerobic conditions were achieved by dilution of reagents into deoxygenated buffer following purging of the buffer and reagents with an inert gas (argon or nitrogen). The samples were transferred using Hamilton air-tight syringes to septum-capped absorption cuvettes and a positive pressure of inert gas was maintained during the measurements. For some experiments anaerobiosis was achieved using a Coy anaerobic chamber. Samples were prepared within the chamber and rapidly transferred to the spectrophotometer for kinetic analysis.
Low oxygen (1%) conditions were achieved by adding air to samples that had been prepared anaerobically while monitoring the oxygen pressure using a Unisense (Aahrus, Denmark) Oxy-meter with a needle electrode.
The concentration of nitrosothiols was determined by either chemiluminescence or HPLC. To determine the concentration of nitrosothiols using chemiluminesence measurements at a particular time point in the reaction, 5 mM N-ethylmaleimide (NEM) was added to the reaction mixture to prevent any subsequent nitrosation reactions during analysis. After a forty minute incubation, low molecular weight thiols were separated from the protein using Microcon centrifugal filter devices with a molecular weight cutoff of 10,000 Da (Millipore, Billerica, MA). The concentration of low molecular weight S-nitrosothiols was then determined using a Sievers 280i nitric oxide analyzer employing the copper/cysteine assay described previously [22]. The authenticity of the nitrosothiols was confirmed by verification that addition of 5 mM mercury chloride eliminated the chemiluminescent signal.
All HPLC analyses were performed on a Kromasil C-18 column (25 cm length, 0.46 cm internal diameter, 0.5 μm particle size). GSNO was eluted isocratically with a mobile phase consisting of methanol and 0.05% trifluoro-acetic acid (TFA) in a ratio of 6:94. GSSG was analyzed by an ion-pair method, using 3% acetonitrile and 97% 10 mM K2HPO4 and 10 mM tetra-butyl-ammoniumhydrogensulfite (TBAHS) as eluent. The effluents were monitored with a diode array spectrophotometer at 336 and 210 nm for GSNO and GSSG, respectively [23].
Submitochondrial particles (prepared from rat liver by sonication and centrifugation [24]) were incubated anaerobically with ferric cyt c, GSH, and PROLI/NO for 0–120 minutes, after which the samples were centrifuged (3 min. at 10,000 g) to separate GSH from SMP protein. Successful separation was confirmed by measuring the absence of complex I activity in the supernatant, but not in the pellet, as well as the presence of reduced thiol in the supernatant (measured using Ellman s reagent). Both fractions were subjected to copper cysteine based reductive chemiluminescence to measure GSNO and protein SNO levels. Complex I activity was also measured in the SMP fraction by monitoring the rotenone sensitive oxidation of NADH at 340 nm as previously described [25].
RAW 264.7 cells were lysed under anaerobic conditions in lysis buffer (50 mM Tris, pH 7.4, 1 mM DTPA, 0.5 % Tween-20, protease inhibitors) and incubated with PROLI/NO in the presence or absence of ferric cyt c for 30 minutes. To prevent any subsequent S-nitrosation reactions free thiols were blocked with 50 mM NEM in the presence of 6.5 M urea and 3 % CHAPS. Samples were precipitated with trichloroacetic acid to separate proteins from low molecular weight compounds and S-nitrosothiol levels in protein fraction of cell lysate were assessed by tri-iodide-based chemiluminescence.
All data are reported as a mean ± one standard deviation unless otherwise designated.
Results
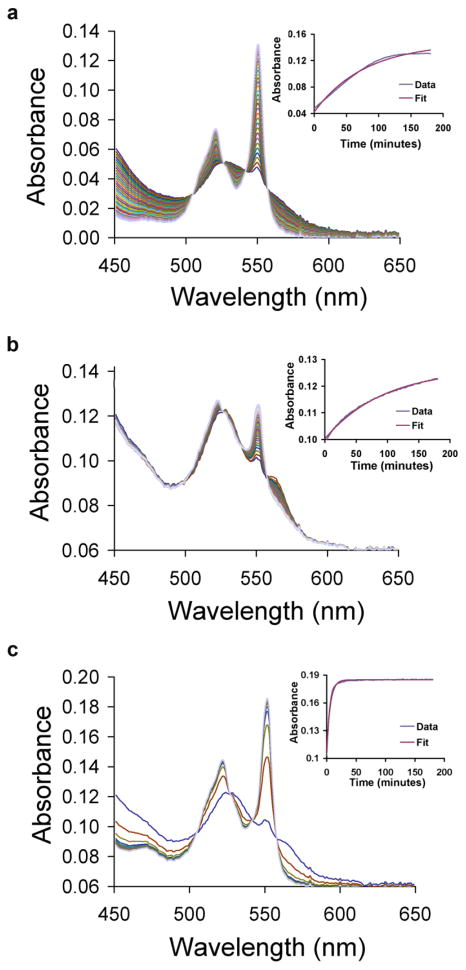
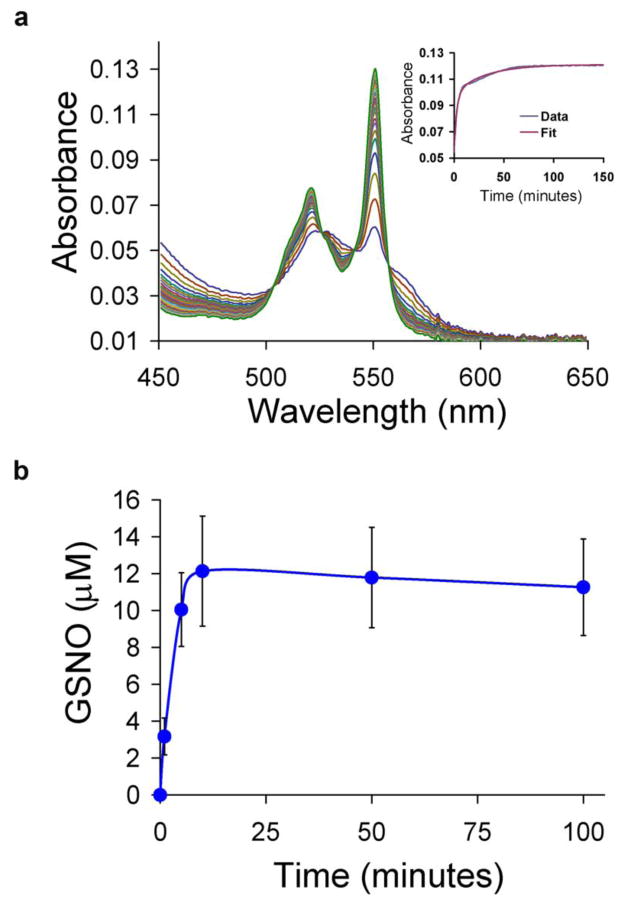
Both NO and glutathione alone reduce cyt c. The reduction of cyt c (50 μM) by GSH (1 mM) at pH 7.4, followed by absorption spectroscopy, is shown in Fig. 1a. The spectrum of ferric cyt c consists of a single broad absorption centered at 530 nm. Reduction to the ferrous hexacoordinate heme-protein results in the evolution of two absorption peaks at 525 and 550 nm. The time course of absorbance changes at 550 nm is shown in the inset to Fig. 1a. A global fit to the data (fitting all wavelengths simultaneously) using a single exponential model (inset of Fig. 1a) exhibits a relatively poor fit with a kobs of 0.25 ± 0.16 × 10−3 s−1 (n=6). Previous studies have demonstrated that the reduction of ferric cyt c by GSH is complex and may be autocatalyzed by the formation of GSSG resulting in non exponential kinetics [15–20].
Fig. 1.
GSH/NO-dependent reduction of ferric cytochrome c Time-resolved absorption spectra following combination of cyt c (50 μM) and (a) GSH (1 mM), (b) PROLI/NO (25 μM), or (c) both of these under anaerobic conditions at room temperature and pH 7.4. Spectra were collected every two minutes following mixture of the reagents in a 0.1 cm pathlength cell. Insets show kinetic traces of absorption at 550 nm and the theoretical fit to a single exponential decay with observed rate constants of 0.22 × 10−3 s−1, 0.18 × 10−3 s−1, and 2.8 × 10−3 s−1 for conditions in (a), (b), and (c). The results shown here were for bovine heart cyt c. Similar results are shown in a supplementary figure (Figure S2) for horse heart cyt c (C-7752) that had been further purified using sedimentation and a G-25 column.
Cyt c reduction by NO at pH 7.4, under anaerobic conditions, was followed by adding the rapid-release NO donor proliNONOate (PROLI/NO, 25 μM, t½ of approximately 2 s) to ferric cyt c (50 μM, Fig. 1b). The first spectrum, taken immediately after NO addition, indicates a mixture of the ferric and ferric nitrosyl forms of the heme protein. The time course of reduction fits well to a single exponential process (inset of Fig. 1b) with an observed rate constant of 0.27 ± 0.16 × 10−3 s−1 (n=3).
The synergistic enhancement of cyt c reduction by the combination of NO and GSH is shown in Fig. 1c and inset. The time-course again fits well to a single exponential process with an observed rate constant of 4.2 ± 1.1 × 10−3 s−1 (n=7). The additive rate constant under these conditions is 0.52 × 10−3 s−1, indicating an almost 10-fold increase in rate constant over the expected value.
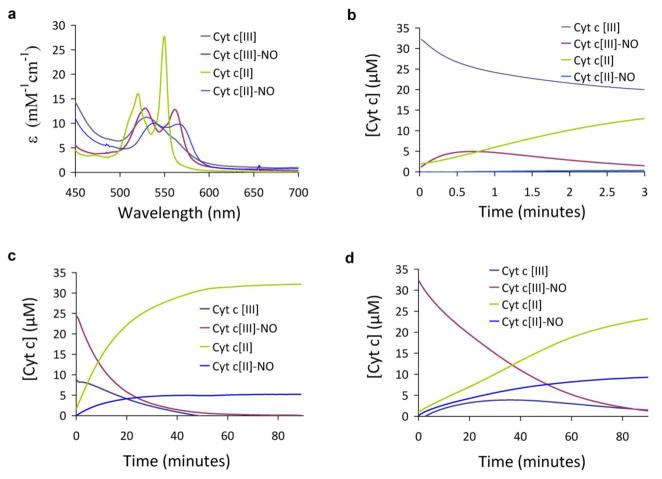
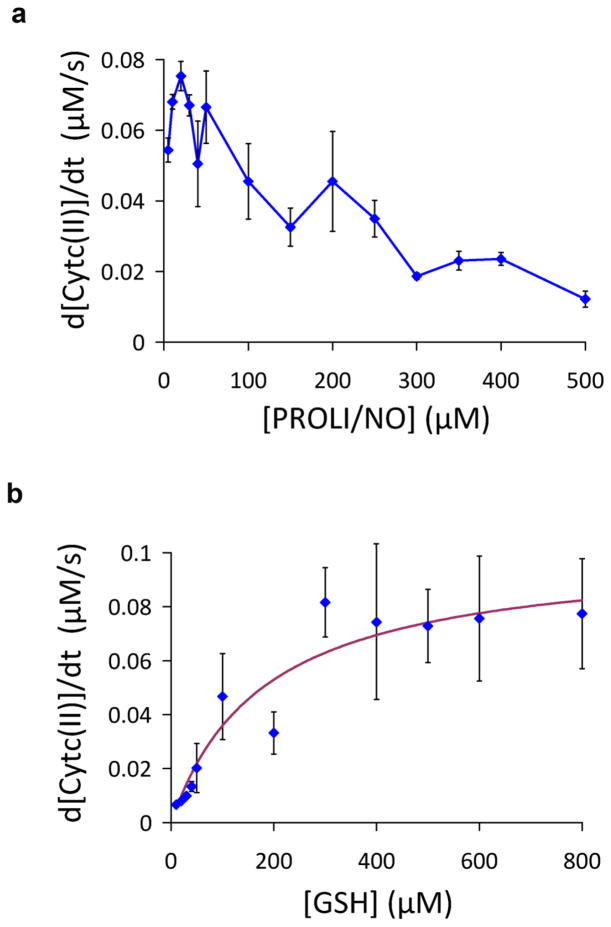
Full analysis of the spectral changes that occur during the reduction of cyt c by GSH/NO indicates that at least four spectrally distinct species are involved. These four spectra are shown in Fig. 2a and are used as basis spectra for multiple linear regression analysis of the data. The spectra of the ferric and ferrous forms of cytochrome c are well established and spectral subtraction revealed the presence of the two distinct NO-bound species that have been previously observed and refered to as compound 1 and compound 2. Compound 1 is formed upon addition of NO to ferric cyt c and is either ferric nitrosyl cyt c or a derivative thereof, and compound 2 is formed from treating compound 1 with sodium dithionite and is likely ferrous nitrosyl cyt c [26]. Deconvolution of the spectral changes that occur during reduction of cyt c by 20 μM, 100 μM and 500 μM PROLI/NO are shown in Figs. 2b to d respectively. At low NO concentration there is an initial formation of ferric nitrosyl cyt c, which then decays in parallel with the remaining ferric cyt c to generate ferrous cyt c as the only heme product. Under these conditions the reaction is almost complete within 3 minutes. At intermediate and high NO levels, there is a much greater initial formation of ferric nitrosyl cyt c, and ferrous nitrosyl cyt c is generated as a product. Importantly, at high levels of NO the rate of cyt c reduction decreases. The initial rate of cyt c reduction (from the sum of both ferrous and ferrous nitrosyl cyt c), as a function of PROLI/NO concentration, is shown in Fig. 3a. The maximal initial rate of cyt c reduction occurs at an NO concentration approximately stoichiometric with cyt c. This unexpected behavior indicates underlying mechanistic complexity at higher non-physiological levels of NO. The fact that high initial levels of ferric nitrosyl cyt c, as occurs at high PROLI/NO concentrations, result in a slower reaction suggests that ferric nitrosyl cyt c is not an intermediate in the reduction of cyt c, but rather represents a complex that inhibits the reaction by reducing the amount of available ferric cyt c and NO. This would suggest a mechanism whereby GSH initially binds to cyt c, and NO reacts with this complex to result in reduction of the heme iron. In favor of this mechanism the initial rate of cyt c reduction appears to follow a hyperbolic dependence as a function of GSH concentration (Fig. 3b) indicative of saturable binding. The apparent Km for GSH derived from a hyperbolic fit to these data (shown on Fig. 3b) is 181 ± 68 μM. This behavior also favors reaction of NO with GSH in an active site of cyt c, rather than the mechanism similar to that proposed by Gow et al [7] where GSH and NO would combine in solution to form a radical intermediate which would then reduce an available oxidant.
Fig. 2.
Spectral analysis of cyt c during reduction by NO/GSH. (a) Basis spectra of 4 species used in multiple linear regression analysis: ferric cyt c, ferric nitrosyl cyt c, ferrous cyt c, and ferrous nitrosyl cyt c. b-d) Multiple linear regression analysis of spectral changes occurring during incubation of ferric cyt c (35 μM), GSH (1 mM) and PROLI/NO at a concentration of (b) 20 μM, (c) 100 μM and (d) 500 μM. Reactions were performed anaerobically at room temperature, in 100 mM phosphate buffer, pH 7.4 containing 100 μM DTPA.
Fig. 3.
Concentration dependences of anaerobic cyt c reduction. (a) Initial rate of cyt c (35 μM) reduction (sum of ferrous cyt c and ferrous nitrosyl cyt c) by GSH (1 mM) as a function of PROLI/NO concentration, taken from similar data to that shown in Fig. 2. (b) Dependence of cyt c (35 μM) reduction (sum of ferrous cyt c and ferrous nitrosyl cyt c) by PROLI/NO (50 μM) as a function of with GSH concentration taken from similar data to that shown in fig 2. Data represent mean ± standard deviation (n=3)
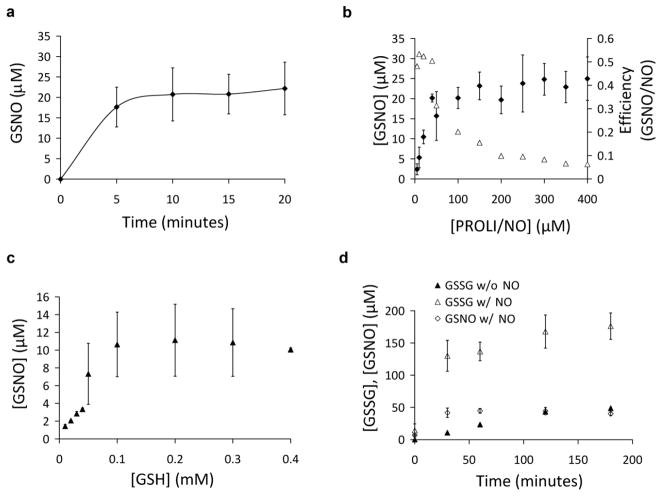
In agreement with published data,[3] the anaerobic incubation of GSH with NO in the absence of cyt c did not result in GSNO formation (data not shown). However, the inclusion of cyt c resulted in robust glutathione S-nitrosation that occurs on the same time scale as cyt c reduction (Fig 4a). To examine if glutathionyl radical is an intermediate in GSNO formation experiments were performed in the presence and absence of the radical scavenger 5,5-dimethyl-1-pyrroline N-oxide (DMPO). No significant difference in GSNO formation or the observed rate constant for cyt c reduction were observed (21 ± 3 μM GSNO vs 20 ± 3 μM final GSNO yield and observed rate constant of 3.3 ± 0.1 × 10−3 s−1 vs 3.3 ± 0.5 × 10−3 s−1 when 50 μM PROLI/NO is added to 1 mM GSH and 50 μM cyt c without and with 10 mM DMPO, n=3). The dependence of GSNO yield on NO concentration is shown in Fig. 4b. It is clear from these data that the formation of GSNO is not catalytic and that the amount of GSNO formed is limited by either the concentration of NO or the concentration of cyt c, whichever is the smaller. The efficiency of GSNO formation from NO decreases as NO concentration is increased (Fig 4b), suggesting a competing reaction that involves GSH-dependent NO consumption but that does not generate GSNO or reduce cyt c, and this is likely related to the slower kinetics of cyt c reduction observed at higher NO concentrations (Fig. 3). Efficiency values were calculated based on the measured release of NO from PROLI/NO. While it is usually assumed that PROLI/NO generates 2 NO/molecule, our measurements, conducted independently in two laboratories (using oxy hemoglobin oxidation and direct NO-dependent chemiluminescence as observables), indicated that the yield of NO from PROLI/NO is close to 1:1 (see Supplementary Figure 1) and this value was used for efficiency calculations. The yield of GSNO formation as a function of GSH concentration is shown in Fig 4c. This somewhat mirrors the GSH-dependence of cyt c reduction shown in Fig 3b, and again indicates that the GSNO-generating reaction is a saturable phenomenon. The diminished efficiency of GSNO formation at higher NO levels suggests that alternative glutathione-derived products are being formed. An ion-pair HPLC method (TBAHS method) was employed to assess GSH oxidation products. It was observed that at low NO, GSNO was the only detectable product of GSH (data not shown), but at high NO the major product was GSSG (Fig. 4d). In fact NO results in a large increase in GSSG formation over that observed in the absence of NO. It is of interest to note that at higher NO concentrations, although the rate of cyt c reduction is diminished (Fig 3a), there is a higher initial level of ferric nitrosyl cyt c (Figure 2d) and GSSG is additionally formed (figure 4d), and the yield of GSNO is not decreased (Figure 4b). This suggests that at high NO, the yield of GSNO depends largely on the initial level of ferric cyt c, and that other side reactions may interfere kinetically with the formation of GSNO, but do not ultimately change the yield of S-nitrosation.
Fig. 4.
Cyt c-mediated GSNO synthesis (a) Cyt c (50 μM), PROLI/NO (25 μM) and GSH (1 mM) were incubated under anaerobic conditions at pH 7.4 and samples removed every 5 minutes. S-Nitrosothiol content was determined by chemiluminescence. (b) Cyt c (35 μM) was incubated with GSH (1 mM) and increasing amounts of PROLI/NO under anaerobic conditions in 100 mM phosphate buffer pH 7.4 containing 100 μM DTPA at room temperature for 1 hour. GSNO levels (◆) were determined by HPLC (TFA method). Efficiency (△) was calculated as the ratio of GSNO formed divided by NO added, assuming 1 NO formed from each PROLI/NO molecule added. (c) Conditions as in panel “b”, but with 25 μM PROLI/NO and variable GSH. (d) Cyt c (75 μM) was incubated with GSH (1 mM) and GSSG formation was determined by HPLC (▲,TBAH method). An identical incubation was performed in the presence of PROLI/NO (500 μM) and both GSSG (△) and GSNO (◇) were measured by HPLC (TBAH method). Data represent mean ± standard deviation (n=3).
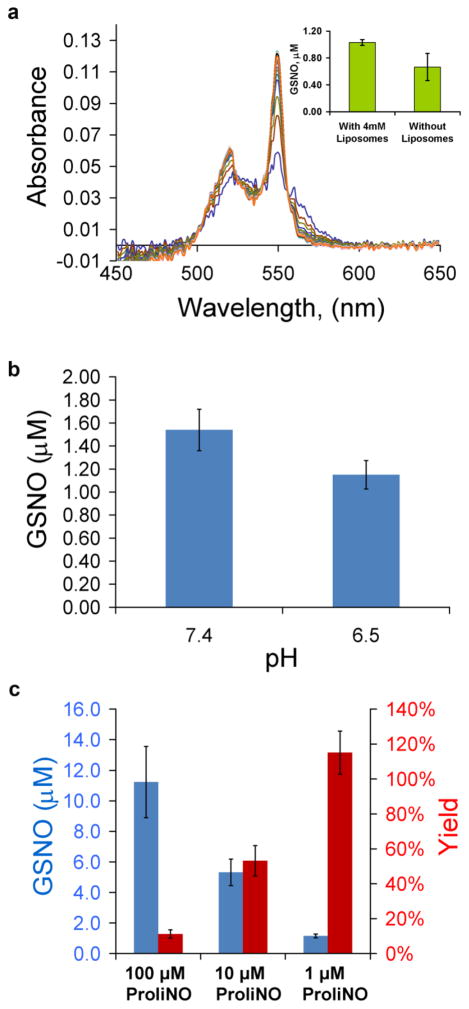
The anionic phospholipid cardiolipin has been shown to interact with cyt c, leading to pentacoordination of the cyt c heme iron and subsequent alterations in its chemical reactivity.[27–31] To examine if cardiolipin affects the GSNO synthase activity of cyt c, the reaction was examined in the presence of cardiolipin-containing liposomes. As shown in Fig. 5a, the GSNO synthase activity was apparent in the presence of cardiolipin albeit with a slightly lower kobs. We found that, as is the case for when liposomes are absent, the combination of GSH and NO greatly enhanced the rate of cyt c reduction compared to when only one of these reagents is present (observed rate constants of 1.8 ± 1.0 × 10−3 s−1 with both NO and GSH present compared to 0.18 ± 0.07 × 10−3 s−1 with only GSH and too slow to fit with only NO, n=3). Interestingly, the amount of GSNO generated is greater when anionic phospholipids are present compared to when they are absent (Fig. 5a, inset), suggesting that pentacoordination may modulate GSNO synthesis.
Fig. 5.
Dependence of GSNO formation on the presence of anionic phospholipids and pH. (a) Liposomes (2 mM) were added (40:1 lipid:protein) and the reduction of cyt c (50 μM) was followed by time-resolved absorption in the presence of GSH (1 mM) and PROLI/NO (25 μM) in a 0.1 cm pathlength cell. The observed rate constant for cyt c reduction obtained by global analysis was 2.0 × 10−3 s−1. The inset shows the amount of GSNO made in the presence and absence of liposomes after a five minute incubation of GSH (1 mM) and PROLI/NO (1 μM), pH 6.5 (p = 0.019) (b) GSNO yield after mixing 1 μM PROLI/NO, 100 μM cyt c, 4 mM liposomes, and 1 mM GSH at pH shown, (p = 0.018). After five minutes, NEM was added and the sample was incubated for 40 minutes followed by filtration of low molecular weight compounds and GSNO analysis by chemiluminescence. (c) Dependence of GSNO formation (blue) in experiments as described in panel “b” at pH 6.5 as a function of NO. GSNO yield (red) is calculated as a percentage of GSNO made compared to PROLI/NO added.
The effect of local acidity may also be an important effector of cyt c activity in mitochondria, especially during hypoxia. It was observed that GSNO synthesis is slower at pH 6.5, but yields are not substantially affected. (Fig. 5b and 5c).
To examine if oxygen affects GSNO formation the reaction was performed in a 1% oxygen atmosphere. The time course of cyt c reduction under these conditions fitted well to a bi-exponential model (Fig. 6a). The majority of the cytochrome is reduced during the fast phase which occurs with an observed rate constant of 7.5. ± 2.1 × 10−3 s−1 while reduction in the slow phase is described by an observed rate constant of 0.53 ± 0.02 × 10−3 s−1 (n=3). Importantly, substantial GSNO is formed even when oxygen is present (Fig. 6b).
Fig. 6.
Effect of Oxygen. (a) Cyt c (50 μM) was mixed with 1 mM GSH and 25 μM PROLI/NO under a 1% oxygen atmosphere. Absorption spectra were collected in a 0.1 cm pathlength cell as a function of time, every 0.5 minutes for 10 minutes and then every two minutes until 170 minutes after mixing. The kinetics are shown in the inset and could not be fit well to a single exponential process but did fit to two exponentials with observed rate constants of 6.3 × 10−3 s−1 for the fast phase and 0.55 × 10−3 s−1 for the slow phase. (b) GSNO formation for the reaction described in (a) measured by chemiluminescence.
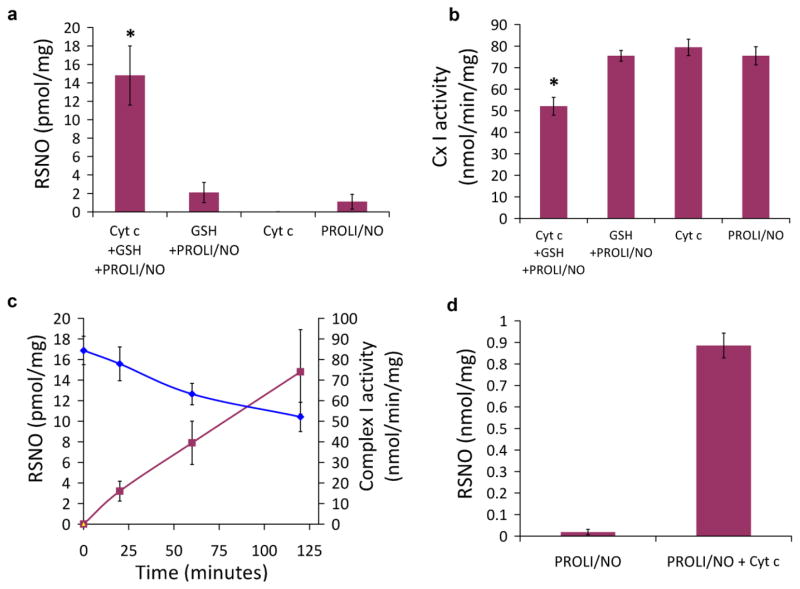
To determine whether the GSH/NO/cyt c system could lead to protein S-nitrosation in a model of a biological system, submitochondrial particles (SMPs), which are devoid of cyt c and mitochondrial matrix proteins, were incubated with NO, GSH and ferric cyt c, under anaerobic conditions, and both total S-nitrosation and complex I activity were determined. Robust S-nitrosation of SMP proteins was only observed in the complete system, whereas much lower levels were observed when individual components were omitted (Fig. 7a). Parallel measurements of complex I activity, which has been shown to be inhibited by S-nitrosation, demonstrated that all reaction components were necessary for an inhibition of activity (Fig. 7b), and that the magnitude of inhibition of activity was temporally correlated to S-nitrosothiol formation (Fig. 7c). The yield for protein S-nitrosation is slightly lower than that for GSNO formation in the absence of SMPs (compare Fig. 7c and Fig. 4a), and the rate of S-nitrosation is considerably slower. This is consistent with cyt c-mediated GSNO formation followed by slower GSNO-mediated transnitrosation of SMP protein thiols.
Fig. 7.
S-nitrosation and catalytic activity in submitochondrial particles and S-nitrosation of cellular proteins. Submitochondrial particles (SMP; 2mg/ml) were incubated anaerobically with or without ferric cyt c (50μM), GSH (1mM), and PROLI/NO (25μM) for 0–120 minutes. (a) The S-nitrosothiol concentration in the SMPs at 120 minutes. (b) Complex I catalytic activity in the SMPs at 120 minutes, *p< 0.001 (c) Time course of S-nitrosothiol formation in the SMPs (maroon line) and Complex I inhibition (blue line). (n ≥ 3 independent experiments) (d) Cell lysates (1.5 mg/ml) were incubated anaerobically with PROLI/NO (100 μM) in the absence or presence of ferric cyt c (100 μM) for 30 minutes. S-Nitrosothiol levels in the high molecular weight fraction were determined by tri-iodide-dependent chemiluminescence.
The ability of cyt c to generate protein S-nitrosothiols in cell lysate under strictly anaerobic conditions was determined by incubating PROLI/NO (100 μM) and ferric cyt c (100 μM) with lysate from RAW 264.7 cells (1.5 mg/ml). Proteins were precipitated and S-nitrosothiol content determined by chemiluminescence. As shown in Fig 7d, in the absence of cyt c, a small but measureable level of S-nitrosation was observed. In the presence of added cyt c, the level of S-nitrosation was greatly enhanced approaching 1nmol/mg protein. Filtration of cell lysate using a centricon 10 KDa cut-off filter revealed about 40% of total RSNO associated with the high molecular weight fraction and 60% associated with low molecular weight component (data not shown).
Discussion
In this study we have demonstrated that ferric cyt c can act as an electron acceptor allowing the efficient formation of GSNO from a mixture of GSH and NO. Coupled with cyt c oxidation, this observation suggests that cyt c may represent an important catalyst of S-nitrosation in vivo. The yield in terms of GSNO formed compared to NO added is the largest of any proposed mechanism that is likely to occur under physiological conditions. While protein S-nitrosation has been proposed as an important intracellular signaling paradigm, and there are many examples of proteins that have been reported to be S-nitrosated under various conditions and stresses, no concerted and efficient S-nitrosothiol synthetic machinery has been described in cells. Most discussion of S-nitrosothiol formation has focused on its intrinsic chemistry with oxygen. This reaction is known to generate S-nitrosothiols in vitro through the intermediacy of nitrogen dioxide. However there is significant doubt, based mainly on kinetic arguments, of the validity of this mechanism at physiological oxygen and NO levels. Recent reports have suggested S-nitrosation can occur and may even be enhanced under anaerobic conditions [12, 13]. Proposed mechanisms for anaerobic S-nitrosation include the reductive bioactivation of nitrite by heme proteins [32], and the intermediacy of dinitrosyl iron complexes [12]. Since NO has a short biological half-life, its chemical conversion to a nitrosothiol must compete with its other reactions and we suggest the GSH/NO/cyt c reactions demonstrated in this paper may constitute such a mechanism.
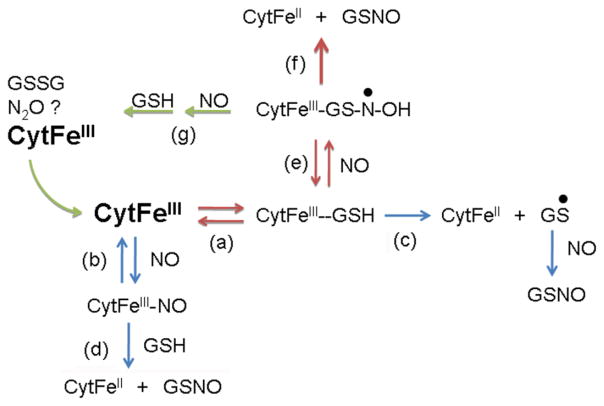
The proposed mechanism of GSNO formation is shown in Fig 8. The characteristics of GSNO formation from ferric cyt c, NO and GSH suggests that the mechanism involves initial binding of GSH to the heme protein followed by reaction of NO with this complex. The rate of cyt c reduction saturates at high GSH and is diminished at high NO. The loose association of GSH with the cyt c heme has been previously described [20], and our data indicates an apparent Km of about 200 μM for GSH. We propose that the reaction of NO with GSH-bound cyt c generates a ternary complex that can be thought of as a hydroxyl amino radical (or a related tautomer as described previously [33]) bound in the vicinity of the cyt c heme. This bound radical then reduces the heme to the ferrous state, resulting in the generation and release of GSNO (Fig. 8 red arrows). Alternatively, and more effectively at higher NO concentrations, NO can react with the bound radical leading to GSSG formation and presumably nitrous oxide, and regenerate ferric cyt c (step g, green arrows, in Fig. 8). As the concentration of NO increases, the unproductive pathway via step g becomes more favorable compared to step f, slowing down cyt c reduction.
Fig. 8.
Proposed kinetic scheme. The favored pathway responsible for GSNO formation is shown with red arrows. The non-productive pathway that becomes important with high [NO] is shown with green arrows. This results in a catalytic cycle producing GSSG and reduced NO forms which could oxidize ferrous cyt c. See text for further details.
Since many ferric heme nitrosyl species have FeII-NO+ character, direct transfer of the NO+ to a reduced thiol has been considered as a viable nitrosation pathway [34, 35]. This pathway is shown in steps b and d of Fig. 8, but is not consistent with the observed dependence of cyt c reduction on NO concentration. One would expect that as the concentration of NO increases, more ferric nitrosyl cyt c would form (Fig. 3) and the reaction pathway depicted in steps b and d would result in an increase, as opposed to a decrease, in the rate of reaction. Thus, NO binding to the ferric cyt c (step b in Fig. 8) actually represents a non-productive competitive pathway in GSH/NO-dependent cytochome c reduction and GSNO production. One could consider an electron transfer as depicted in step c, yielding the reduced heme and a thiyl radical that could form GSNO upon reaction with NO (step c in Fig. 8). However, our finding that addition of the thiyl radical scavenger DMPO (10 mM) did not affect GSNO yield suggests strongly that thiyl radical is not an intermediate.
S-Nitrosation of GSH to form GSNO will not directly serve as a signal. However, it is well established that GSNO can participate in transnitrosation reactions where the S-nitroso functional group is reversibly transferred to another thiol [36, 37]. Such transfer of nitroso groups is responsible for the very large increase in protein S-nitrosation that occurs when cells are exposed to S-nitrosocysteine [38]. We show here that when both SMPs and cell lysate are exposed to NO in the presence of cyt c, there is a robust formation of protein S-nitrosothiols. In the case of SMPs we show that protein S-nitrosation is paralleled with an inhibition of mitochondrial complex I, an enzyme complex known to be inhibited by thiol S-nitrosation. Although S-nitrosation of complex I has been implicated in a number of signaling pathways, it is very important to note that there are several aspects of our model SMP system that do not apply to normal physiology of the intact mitochondria. Superoxide generation by the mitochondrion, is likely to mitigate protein S-nitrosation. In addition, the localization of cytochrome c in the intermembrane space, where the concentration of GSH is relatively low may not be optimal for this nitrosative reaction to proceed. Thus, our experiments performed using the SMP system should be viewed as a demonstration that the cyt c/GSH/NO combination can result in protein S-nitrosation, but much more work is needed to demonstrate that this actually occurs in the healthy mitochondria and there are several reasons for believing that it would not.
The experiments in this study are largely performed under anaerobic conditions. This was done largely to inhibit oxygen-dependent S-nitrosation. However, anaerobiosis is not required for this activity as NO still enhances cyt c reduction in the presence of 1% oxygen and robust S-nitrosation is still observed under these conditions. Acidification to pH 6.5 appears to result in a small but significant decrease in the yield of GSNO, indicating that the lower pH of the mitochondrial inter-membrane space will still allow for GSNO formation through this mechanism (Fig. 5). S-Nitrosation may also be turned on in hypoxia through an increase in NO via nitrite reduction by hemoglobin, [39, 40] myoglobin [41, 42] or cytochrome c oxidase, [43, 44] potentially linking SNO/NO signaling to nitrite reduction. Nitrite reduction by the mitochondria results in the S-nitrosation of complex I [45]. Interestingly, cyt c acts as a nitrite reductase when the heme iron-methionine bond is broken whether through oxidative stress or interaction with anionic phospholipids such as those present in the inner membrane of the mitochondria as occurs during apoptosis [24, 46]. In addition the release of cyt c in to the cytosol during apoptosis potentially releases a GSNO synthase activity and this may at least partially explain the observed inhibitory effects of NO on apoptosis. It is conceivable that cytochrome-c dependent protein S-nitrosation could be occurring in the apoptosome after the association of cyt c with apaf, and that such S-nitrosation could negatively influence apoptotic signaling. While methemoglobin and metmyoglobin do not appear to support efficient GSNO synthesis, it is possible that other heme proteins, or iron complexes are also able to generate S-nitrosothiols [12].
Cyt c-dependent GSNO formation clearly merits additional investigations to place this reaction in the context of physiology and pathophysiology. We demonstrate here a simple and efficient biologically relevant mechanism of S-nitrosothiol synthesis and the previous lack of such a mechanism has been a handicap to a full understanding of NO-signaling through S-nitrosothiol formation.
Supplementary Material
Acknowledgments
This work was supported by NIH grants HL58091 (DK-S) and GM55792 (NH). DK-S gratefully acknowledges further support from NIH grant K02 HL078706
Abbreviations
- RSNO
S-nitrosothiols
- cyt c
cytochrome c
- GSNO
S-nitrosoglutathione
- GSSG
glutathione disulfide
- PROLI/NO
proliNONOate
- DMPO
5,5-dimethyl-1-pyrroline N-oxide
- TBAHS
tetra-butyl-ammoniumhydrogensulfite
- SMP
submitochondrial particle
- DTPA
diethylenetriamine penta-acetic acid
- EDTA
ethylenediaminetetraacetic acid
- NEM
5 mM N-ethylmaleimide
- TFA
trifluoro-acetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: Purview and parameters. Nature Reviews Molecular Cell Biology. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 2.Bryan NS, Rassaf T, Maloney RE, Rodriguez CM, Saijo F, Rodriguez JR, Feelisch M. Cellular targets and mechanisms of nitros(yl)ation: An insight into their nature and kinetics in vivo. Proc Natl Acad Sci USA. 2004;101:4308–4313. doi: 10.1073/pnas.0306706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hogg N, Singh RJ, Kalyanaraman B. The role of glutathione in the transport and catabolism of nitric oxide. FEBS Lett. 1996;382:223–228. doi: 10.1016/0014-5793(96)00086-5. [DOI] [PubMed] [Google Scholar]
- 4.Wink DA, Nims RW, Darbyshire JF, Christodoulou D, Hanbauer I, Cox GW, Laval F, Laval J, Cook JA, Krishna MC, Degraff WG, Mitchell JB. Kinetics for nitrosation of cysteine and glutathione in aerobic nitric-oxide solutions at neutral pH Insights into the date and physiological effects of intermediates generated in the NO/O2 reaction. Chem Res Toxicol. 1994;7:519–525. doi: 10.1021/tx00040a007. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein S, Czapski G. Mechanism of the Nitrosation of Thiols and Amines by Oxygenated NO Solutions: the Nature of the Nitrosating Intermediates. J Am Chem Soc. 1996;118:3419–3425. [Google Scholar]
- 6.Jourd’heuil D, Jourd’heuil FL, Feelisch M. Oxidation and nitrosation of thiols at low micromolar exposure to nitric oxide - Evidence for a free radical mechanism. J Biol Chem. 2003;278:15720–15726. doi: 10.1074/jbc.M300203200. [DOI] [PubMed] [Google Scholar]
- 7.Gow AJ, Buerk DG, Ischiropoulos H. A novel reaction mechanism for the formation of S-nitrosothiol in vivo. J Biol Chem. 1997;272:2841–2845. doi: 10.1074/jbc.272.5.2841. [DOI] [PubMed] [Google Scholar]
- 8.Stubauer G, Giuffre A, Sarti P. Mechanism of S-nitrosothiol formation and degradation mediated by copper ions. J Biol Chem. 1999;274:28128–28133. doi: 10.1074/jbc.274.40.28128. [DOI] [PubMed] [Google Scholar]
- 9.Inoue K, Akaike T, Miyamoto Y, Okamoto T, Sawa T, Otagiri M, Suzuki S, Yoshimura T, Maeda H. Nitrosothiol formation catalyzed by ceruloplasmin - Implication for cytoprotective mechanism in vivo. J Biol Chem. 1999;274:27069–27075. doi: 10.1074/jbc.274.38.27069. [DOI] [PubMed] [Google Scholar]
- 10.Moller MN, Li Q, Vittur DA, Robinson JM, Lancaster JR, Denicola A. Membrane “lens” effect: Focusing the formation of reactive nitrogen oxides from the (NO)-N-center dot/O-2 reaction. Chem Res Toxicol. 2007;20:709–714. doi: 10.1021/tx700010h. [DOI] [PubMed] [Google Scholar]
- 11.Nedospasov A, Rafikov R, Beda N, Nudler E. An autocatalytic mechanism of protein nitrosylation. Proc Natl Acad Sci USA. 2000;97:13543–13548. doi: 10.1073/pnas.250398197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosworth CA, Toledo JC, Zmijewski JW, Li Q, Lancaster JR. Dinitrosyliron complexes and the mechanism(s) of cellular protein nitrosothiol formation from nitric oxide. Proc Natl Acad Sci USA. 2009;106:4671–4676. doi: 10.1073/pnas.0710416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster MW, Stamler JS. New insights into protein S-nitrosylation - Mitochondria as a model system. J Biol Chem. 2004;279:25891–25897. doi: 10.1074/jbc.M313853200. [DOI] [PubMed] [Google Scholar]
- 14.Hoshino M, Maeda M, Konishi R, Seki H, Ford PC. Studies on the reaction mechanism for reductive nitrosylation of ferrihemoproteins in buffer solutions. J Am Chem Soc. 1996;118:5702–5707. [Google Scholar]
- 15.Froede HC, Hunter EH. Catalytic effect of GSSG on Reduction of Cytochrome C by GSH - Possible Model for Facilitation of Electron Transfer and Energy Conservation by Sulfonium Ion Formation. Biochem Biophys Res Commun. 1970;38:954–961. doi: 10.1016/0006-291x(70)90814-4. [DOI] [PubMed] [Google Scholar]
- 16.Prutz WA, Butler J, Land EJ. The glutathione free-radical equilibrium, GS(.)+GS(−)REVERSIBLE-ARROW-GSS(.−)G, mediating electron-transfer to the Fe(III)-cytochrome c. Biophys Chem. 1994;49:101–111. [Google Scholar]
- 17.Subudhi U, Chainy GBN, Mohanty P. Kinetics and mechanism of reduction of ferricytochrome c by glutathione and L-cysteine: A comparative study. Indian J Biochem Bio. 2006;43:37–40. [PubMed] [Google Scholar]
- 18.Birus M, Romic Z. Kinetics and mechanism of the reduction of cytochrome-c with L-cysteine. Acta Pharmaceutica. 1992;42:231–237. [Google Scholar]
- 19.Deng HT. Characterization of the reaction products of cytochrome c with glutathione by mass spectrometry. Biochem Biophys Res Commun. 2006;342:73–80. doi: 10.1016/j.bbrc.2006.01.108. [DOI] [PubMed] [Google Scholar]
- 20.Everse J, Kujundzic N. Kinetics and mechanism of the reduction of horse heart ferricytochrome-c by glutathione. Biochemistry-US. 1979;18:2668–2673. doi: 10.1021/bi00579a037. [DOI] [PubMed] [Google Scholar]
- 21.Kapralov AA, Kurnikov IV, Vlasova II, Belikova NA, Tyurin VA, Basova LV, Zhao Q, Tyurina YY, Jiang JF, Bayir H, Vladimirov YA, Kagan VE. The hierarchy of structural transitions induced in cytochrome c by anionic phospholipids determines its peroxidase activation and selective peroxidation during apoptosis in cells. Biochemistry-US. 2007;46:14232–14244. doi: 10.1021/bi701237b. [DOI] [PubMed] [Google Scholar]
- 22.Fang KZ, Ragsdale NV, Carey RM, MacDonald T, Gaston B. Reductive assays for S-nitrosothiols: Implications for measurements in biological systems. Biochem Biophys Res Commun. 1998;252:535–540. doi: 10.1006/bbrc.1998.9688. [DOI] [PubMed] [Google Scholar]
- 23.Tsikas D, Denker K, Frolich JC. Artifactual-free analysis of S-nitrosoglutathione and S-nitroglutathione by neutral-pH, anion-pairing, high-performance liquid chromatography - Study on peroxynitrite-mediated S-nitration of glutathione to S-nitroglutathione under physiological conditions. Journal of Chromatography A. 2001;915:107–116. doi: 10.1016/s0021-9673(01)00634-3. [DOI] [PubMed] [Google Scholar]
- 24.Basu S, Azarova NA, Font MD, King SB, Hogg N, Gladwin MT, Shiva S, Kim-Shapiro DB. Nitrite Reductase Activity of Cytochrome c. J Biol Chem. 2008;283:32590–32597. doi: 10.1074/jbc.M806934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang XD, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butt WD, Keilin D. Absorption spectra and some other properties of cytochrome c and its compounds with lgands. Proc Royal Soc London - Series B, Biol Sci. 1962;156:429–458. doi: 10.1098/rspb.1962.0049. [DOI] [PubMed] [Google Scholar]
- 27.Belikova NA, Vladimirov YA, Osipov AN, Kapralov AA, Tyurin VA, Potapovich MV, Basova LV, Peterson J, Kurnikov IV, Kagan VE. Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry-US. 2006;45:4998–5009. doi: 10.1021/bi0525573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 29.Rytomaa M, Kinnunen PKJ. Reversibility of the Binding of Cytochrome-C to Liposomes - Implications for Lipid-Protein Interactions. J Biol Chem. 1995;270:3197–3202. doi: 10.1074/jbc.270.7.3197. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalvez F, Gottlieb E. Cardiolipin: Setting the beat of apoptosis. Apoptosis. 2007;12:877–885. doi: 10.1007/s10495-007-0718-8. [DOI] [PubMed] [Google Scholar]
- 31.Nantes IL, Zucchi MR, Nascimento OR, Faljoni-Alario A. Effect of heme iron valence state on the conformation of cytochrome c and its association witt membrane interfaces - A CD and EPR investigation. J Biol Chem. 2001;276:153–158. doi: 10.1074/jbc.M006338200. [DOI] [PubMed] [Google Scholar]
- 32.Basu S, Grubina R, Huang J, Conradie J, Huang Z, Jeffers A, Jiang A, He X, Azarov I, Seibert R, Mehta A, Patel R, King SB, Hogg N, Ghosh A, Gladwin MT, Kim-Shapiro DB. Catalytic generation of N2O3 by a concerted nitrite reductase and anhydrase activity of hemoglobin. Nature Chemical Biology. 2007;3:785–794. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 33.Zhao YL, Houk KN. Thionitroxides, RSNHO center dot: The structure of the SNO moiety in “S-nitrosohemoglobin”, a possible NO reservoir and transporter. J Am Chem Soc. 2006;128:1422–1423. doi: 10.1021/ja057097f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luchsinger BP, Rich EN, Gow AJ, Williams EM, Stamler JS, Singel DJ. Routes to S-nitroso-hemoglobin formation with heme redox and preferential reactivity in the beta subunits. Proc Natl Acad Sci USA. 2003;100:461–466. doi: 10.1073/pnas.0233287100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herold S, Rock G. Reactions of deoxy-, oxy-, and methemoglobin with nitrogen monoxide - Mechanistic studies of the S-nitrosothiol formation under different mixing conditions. J Biol Chem. 2003;278:6623–6634. doi: 10.1074/jbc.M210275200. [DOI] [PubMed] [Google Scholar]
- 36.Arnelle DR, Stamler JS. No+, No(Center-Dot), and No- Donation by S-Nitrosothiols - Implications for Regulation of Physiological Functions by S-Nitrosylation and Acceleration of Disulfide Formation. Arch Biochem Biophys. 1995;318:279–285. doi: 10.1006/abbi.1995.1231. [DOI] [PubMed] [Google Scholar]
- 37.Singh RJ, Hogg N, Joseph J, Kalyanaraman B. Mechanism of nitric oxide release from S-nitrosothiols. J Biol Chem. 1996;271:18596–18603. doi: 10.1074/jbc.271.31.18596. [DOI] [PubMed] [Google Scholar]
- 38.Zhang YH, Hogg N. The mechanism of transmembrane S-nitrosothiol transport. Proc Natl Acad Sci USA. 2004;101:7891–7896. doi: 10.1073/pnas.0401167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu XL, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 40.Nagababu E, Ramasamy S, Abernethy DR, Rifkind JM. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J Biol Chem. 2003;278:46349–46356. doi: 10.1074/jbc.M307572200. [DOI] [PubMed] [Google Scholar]
- 41.Shiva S, Huang Z, Grubina R, Sun JH, Ringwood LA, MacArthur PH, Xu XL, Murphy E, Darley-Usmar VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 42.Rassaf T, Flogel U, Drexhage C, Hendgen-Cotta U, Kelm M, Schrader J. Nitrite reductase function of deoxymyoglobin - Oxygen sensor and regulator of cardiac energetics and function. Circ Res. 2007;100:1749–1754. doi: 10.1161/CIRCRESAHA.107.152488. [DOI] [PubMed] [Google Scholar]
- 43.Castello PR, David PS, McClure T, Crook Z, Poyton RO. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: Implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metabolism. 2006;3:277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Castello PR, Woo DK, Ball K, Wojcik J, Liu L, Poyton RO. Oxygen-regulated isoforms of cytochrome c oxidase have differential effects on its nitric oxide production and on hypoxic signaling. Proceedings of the National Academy of Sciences. 2008;105:8203–8208. doi: 10.1073/pnas.0709461105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shiva S, Gladwin M. Nitrite mediates cytoprotection after ischemia/reperfusion by modulating mitochondrial function. Basic Research in Cardiology. 2009;104:113–119. doi: 10.1007/s00395-009-0009-3. [DOI] [PubMed] [Google Scholar]
- 46.Chen YR, Chen LC, Liu X, Li HT, Zweier JL, Mason MP. Involvement of protein radical, protein aggregation, and effects on NO metabolism in the Hypochlorite-mediated oxidation of mitochondrial cytochrome c. Free Radical Biol Med. 2004;37:1591–1603. doi: 10.1016/j.freeradbiomed.2004.07.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.