Abstract
Background:
Lymphangioleiomyomatosis (LAM) is a rare multisystem disorder affecting primarily women of child-bearing age, and characterized by cystic lung destruction, tumors of the kidney (angiomyolipomas [AMLs]), and involvement of the axial lymphatics (lymphangioleiomyomas). Patients with LAM experience loss of pulmonary function attributed to the proliferation of abnormal-appearing smooth muscle-like cells (LAM cells). It is possible to group the LAM population by the presence or absence of extrapulmonary involvement (eg, AMLs, lymphangioleiomyomas, chylous effusions). Serum vascular endothelial growth factor (VEGF)-D, a lymphangiogenic factor, is higher in LAM patients than in healthy volunteers and has been proposed as a tool in the differential diagnosis of cystic lung disease. We assessed serum VEGF-D concentrations in relationship to clinical phenotype in LAM patients.
Methods:
Serum VEGF-D levels were quantified by enzyme immunosorbent assay for 111 patients with LAM and 40 healthy volunteers. VEGF-D levels in patients with pulmonary LAM, with or without extrapulmonary manifestations, were compared to those of healthy volunteers.
Results:
Serum VEGF-D levels were greater in patients with LAM compared to those of healthy volunteers (p < 0.001). However, when patient samples were grouped based on the extent of lymphatic extrapulmonary involvement (eg, lymphangioleiomyomas and adenopathy), the statistical difference was maintained only for patients with LAM with lymphatic involvement (p < 0.001), not for those patients whose disease was restricted to the lung. Serum VEGF-D levels are a good biomarker for lymphatic involvement (area under the curve [AUC], 0.845; p < 0.0001), and a fair predictor for LAM disease (AUC, 0.751; p < 0.0001). Serum levels correlated to CT scan grade (p = 0.033).
Conclusions:
Serum VEGF-D concentration is a measure of lymphatic involvement in patients with LAM.
Keywords: adenopathy, angiomyolipoma, cystic lung disease, lymphangioleiomyoma, lymphatics, tuberous sclerosis complex, vascular endothelial growth factor-D
Lymphangioleiomyomatosis (LAM), a rare multisystem disorder that affects primarily women of child-bearing age, is characterized by cystic lung destruction, abdominal tumors (eg, angiomyolipomas [AMLs]), and infiltration of the axial lymphatics in the thorax and abdomen (eg, adenopathy and lymphangioleiomyomas).1–5 Cystic lung destruction can result in a decline in lung function, leading to respiratory failure, the need for supplemental oxygen, and, in some cases, lung transplantation or death.5 High-resolution CT (HRCT) scans reveal thin-walled cysts homogeneously distributed throughout the lung parenchyma1,3–5; ventilation/perfusion scans indicate airtrapping in the cystic areas.6
The preeminent histologic feature of LAM is the presence of smooth muscle-like LAM cells in pulmonary and extrapulmonary lesions.7 In the lung, the morphologically heterogeneous LAM cells are found in nodular clusters in the interstitium and walls of cystic lesions.7 LAM lung nodules contain channels lined by lymphatic endothelial cells.7,8 Extrapulmonary LAM cells in the lymphatics are arranged in fascicular and papillary patterns, and are commonly present in lymph nodes of the mediastinum and retroperitoneum.8
One third of women with tuberous sclerosis complex (TSC), an autosomal-dominant disorder associated with benign, hamartomatous lesions, present with pulmonary cysts.9–11 Identical TSC2 mutations and/or loss of heterozygosity in cells of the kidney, lung, and lymph nodes of patients with sporadic LAM having no identifiable germline TSC2 mutation indicate a probable common genetic association.12–14
A metastatic model for LAM has been proposed15,16 based on genetic evidence that the cells of LAM lesions that recurred in transplanted lungs, originated in the recipient. Further, LAM cells were identified in blood and other body fluids (ie, urine, chylous pleural effusions, ascites).17 These data are consistent with the metastatic spread of LAM cells among organs.
Rates of LAM progression differ among patients; 3 to 6 years may elapse between the onset of symptoms and diagnosis.1,5 Prognostic indicators would be beneficial for early diagnosis and decisions concerning treatment. Serum vascular endothelial growth factor (VEGF)-D levels have been shown18 to be higher in patients with LAM than in healthy volunteers. Since VEGF-D is a lymphangiogenic growth factor, we questioned whether VEGF-D levels might reflect lymphatic involvement.
Materials and Methods
Participants
The research was approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute (protocols 95-H-0186 and 96-H-0100); all participants gave written informed consent. The diagnosis of LAM was made by tissue biopsy and/or clinical and roentgenographic data. The presence or absence of lymphangioleiomyoma and adenopathy in 111 patients with LAM was determined by screening CT scan (model 9800 and Lightspeed scanners; General Electric; Milwaukee, WI) and/or sonography (abdominal and/or pelvic) [ATL 4000 and 5000; Philips Medical Systems; Amsterdam, the Netherlands; and Acuson 128XP, Aspen, and Sequoia scanners; Siemens Medical Solutions; Munich, Germany]. All patients underwent a CT scan of the chest, abdomen, and pelvis to determine the extent of lung disease, and the presence of AMLs, thoracic and abdominal adenopathy, and lymphangioleiomyomas. All patients underwent renal ultrasounds to look for AMLs. Patients who had lymphangioleiomyomas seen on a CT scan were evaluated with ultrasound of the abdomen/pelvis to further characterize the masses. The method for defining regional adenopathy is based on that of Eisenstein et al.19 Round or elliptical solid masses in the distribution of lymph node chains were interpreted as lymph nodes. Short-axis diameters of 0.6, 1.0, and 1.5 cm, respectively, were the cutoff criteria for lymph node enlargement in the retrocrural space, upper abdomen, and pelvis.
Under protocol 96-H-0100, the exclusion criteria for participants in the healthy volunteer group were as follows: age < 18 or > 80 years; serum test positive for HIV; serum test positive for hepatitis virus; and the inability to perform reliable pulmonary function tests (PFTs). Applicants with minor health problems were reviewed on a case-by-case basis by the principal investigator.
Quantification of Serum VEGF-D Levels
Serum was stored at − 80°C. Data from serum samples were correlated with the results of PFTs and CT scans at the time of the visit. Serum VEGF-D levels were measured by an enzyme-linked immunosorbent assay (R&D Systems; Minneapolis, MN). To test the reproducibility of serum levels of VEGF-D, 12 serum samples, chosen randomly from the original data set, were retested in duplicate using the same conditions as for the initial testing, as follows: first assay average, 2,048 pg/mL; second assay average, 1,800 pg/mL (n = 12; p > 0.05).
Assessment of Disease Severity
The severity of lung disease was determined by visual inspection of HRCT images, which received one of three grades: mild, if pulmonary cysts involved less than one third (CT scan grade I) of the lung parenchyma; moderate, if pulmonary cysts involved one third to two thirds (CT scan grade II) of the lung parenchyma; or severe, if pulmonary cysts involved two thirds or more (CT scan grade III) of the lung parenchyma.6
PFTs
The American Thoracic Society standards were used as the criteria for evaluating PFTs (Gold Standard Plus; Warren E. Collins; Braintree, MA; or Master Screen PFT; Erich Jaeger; Würzburg, Germany).
Statistical Analysis
A Student t test or linear regression analysis was used to evaluate the differences among serum VEGF-D levels of different groups. Variables found to be statistically significant (at p < 0.05) were further tested by multivariate analysis. A statistical software package (SPSS, version 14.0; SPSS; Chicago, IL) was used for the analysis. Logistic regression was used to explore the diagnostic ability of serum VEGF-D levels and the following two binary outcomes: LAM disease or not; and lymphatic involvement or not. Variables that were statistically significant (at the p < 0.05 level) for each of the outcomes were considered for inclusion in a multivariate analysis. Using the final logistic regression model, we derived estimates of sensitivity (Se) and specificity (Sp) values, and the receiver operating characteristic (ROC) curve. The ROC curve was also used to choose the optimal cutoff point at which the Se and Sp were maximized.
Results
The study group was composed of 111 patients with sporadic LAM (all women; mean age, 51.4 years) and 40 healthy volunteers (all women; mean age, 49.6 years) [Table 1]. The diagnosis of LAM was confirmed by biopsy or by roentgenographic evidence of characteristic cystic lung lesions and extrapulmonary manifestations (eg, AMLs lymphangioleiomyomas). Subjects presenting with pulmonary involvement alone can be considered as possible false-positive findings. Of the 111 patients in the LAM cohort, only 27 did not have extrapulmonary involvement characteristic of LAM. The diagnosis of LAM was confirmed by biopsy for 22 of those 27 patients. To ensure that possible false-positive findings could not have skewed the data, the statistics were recalculated, deleting the data from the remaining five patients. The statistical significance of the data was preserved.
Table 1.
Characteristics of Patients With LAM and Healthy Volunteer Cohorts
| Variables | LAM Patients (n = 111) | Healthy Volunteers(n = 40) |
|---|---|---|
| Average age, yr* | 51 | 49.6 |
| Gender | Female | Female |
| Race, % | ||
| White | 86 | 73 |
| Asian/Pacific Islander | 3 | 10 |
| Hispanic | 0.9 | 5 |
| African American | 9 | 10 |
| Unknown | 0.9 | 3 |
*Age as of January 1, 2006.
Based on HRCT scan data, 39% of patients in the study group had mild disease (n = 43), 27% had moderate disease (n = 30), and 34% had severe disease (n = 38). The mean (± SE) serum VEGF-D levels of patients with sporadic LAM (1,869 ± 145 pg/mL) were higher compared to levels in healthy volunteers (657 ± 43 pg/mL; p < 0.001) [Fig 1, top, A]. Patients with LAM were grouped according to thoracic or abdominal lymphatic involvement (ie, the presence or absence of adenopathy and/or lymphangioleiomyomas). The mean serum VEGF-D concentrations of patients with lymphatic involvement (2,277 ± 173 pg/mL) were higher than the concentrations of healthy volunteers (657 ± 43 pg/mL; p < 0.001) [Fig 1, center, B]. There was, however, no significant difference in mean serum VEGF-D levels between the LAM group without lymphatic involvement (945 ± 186 pg/mL) and healthy volunteers (657 ± 43 pg/mL; p = 0.14) [Fig 1, center, B].
Figure 1.
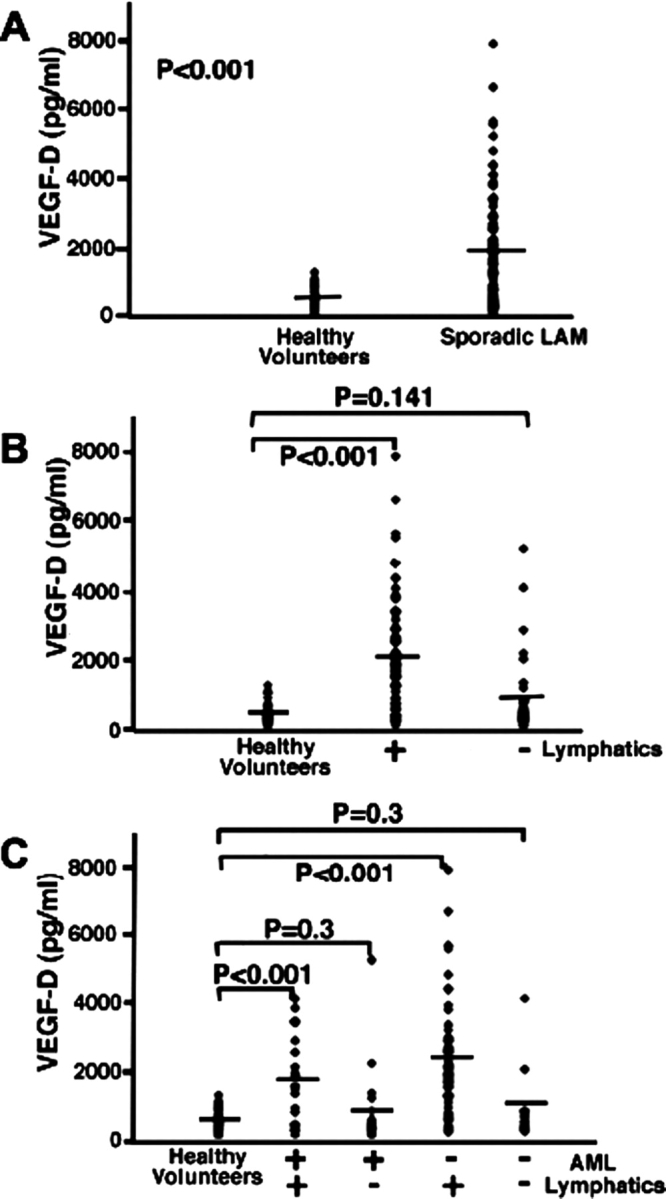
Serum levels of VEGF-D in LAM. Serum VEGF-D levels in all patients with sporadic LAM (n = 111) were compared to those of healthy volunteers (n = 40) [top, A]. Patient samples were further grouped and compared on the basis of thoracic or abdominal lymphatic involvement (presence (n = 77) or absence (n = 34) of lymphangioleiomyomas and/or adenopathy) [center, B] and the presence (n = 40) or absence (n = 71) of renal AMLs [bottom, C]. All groups were compared to healthy volunteers (n = 40). + = presence; − = absence; ♦ = serum measurement of VEGF-D from one patient or healthy volunteer; lines = mean values.
We found AMLs in 36% of patients with sporadic LAM, and further divided the sporadic LAM population into those with or without AMLs and compared those groups to healthy volunteers. Regardless of the presence of AMLs, the VEGF-D levels of the sporadic LAM group with lymphatic involvement were higher than those of healthy volunteers (positive for AMLs/positive for lymphatic involvement: LAM group, 1,900 ± 285 pg/mL; healthy volunteers, 657 ± 43 pg/mL (p < 0.001); negative for AMLs/positive for lymphatic involvement: LAM group, 2,419 ± 211 pg/mL; healthy volunteers, 657 ± 43 pg/mL (p < 0.001) [Fig 1, bottom, C]. There was no significant difference between the groups without lymphatic involvement (positive or negative for AMLs) and healthy volunteers (Fig 1, bottom, C). Serum VEGF-D concentrations were higher, however, in patients with sporadic LAM and without AMLs than in those with sporadic LAM plus AMLs (negative for AMLs, 2,108 ± 186 pg/mL; positive for AMLs, 1,445 ± 209 pg/mL; p = 0.02). These data indicate that increased amounts of serum VEGF-D in patients with LAM are not a consequence of the presence of AMLs.
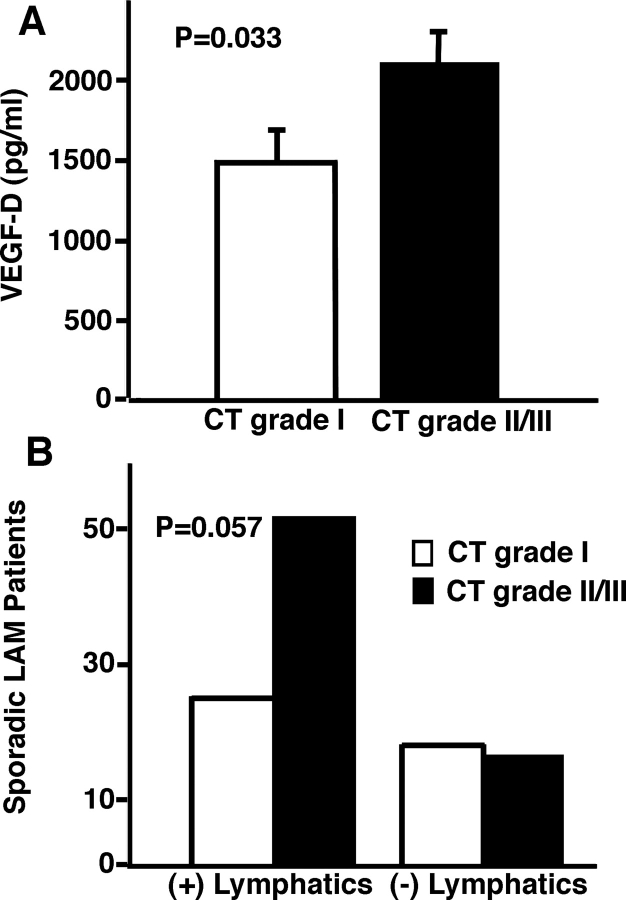
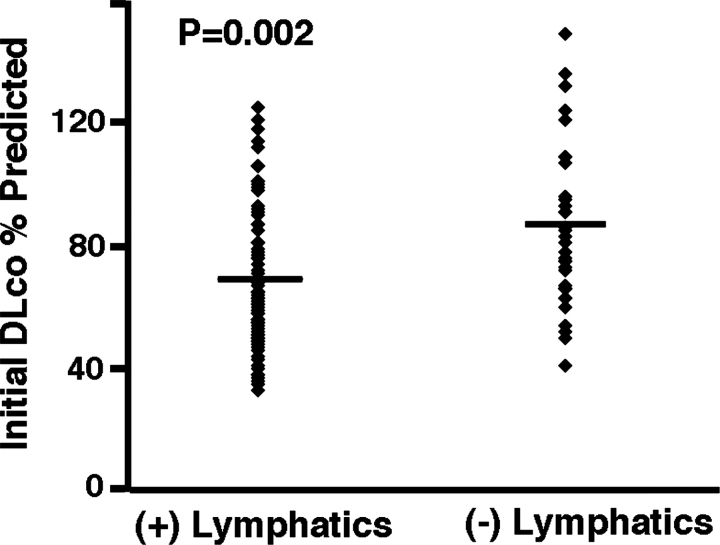
Levels of VEGF-D did not correlate with the initial FEV1 percent predicted, initial total lung capacity (TLC) percent predicted, initial FVC percent predicted, FEV1/FVC percent predicted, decline in FEV1 percentage per year, decline in diffusing capacity of the lung for carbon monoxide (Dlco) percentage per year, or residual volume (RV) percent predicted (Table 2). Higher VEGF-D levels were associated with increased severity of disease by CT scan (CT grade II and III, p = 0.033; n = 111) [Fig 2, top, A], lower initial Dlco percent predicted (p = 0.0012) [Fig 3, top, A, Table 2], and Dlco/alveolar volume (VA) percent predicted (p = 0.028) [Table 2]. Using a multivariate model, VEGF-D levels correlated to initial Dlco percent predicted only for patients with LAM with lymphatic involvement (p = 0.018) [Fig 3, center, B, and bottom, C], not to those patients with only pulmonary LAM. The presence of lymphatic involvement in patients with LAM was also associated with lower initial Dlco percent predicted (p = 0.002) [Fig 4, Table 3]. There was a trend toward a correlation between lymphatic involvement and severity of disease as assessed by CT scan (CT scan grade II and III, p = 0.057) [Fig 2, bottom, B]. Patients with lymphatic involvement were less likely to have AMLs (p = 0.004).
Table 2.
Correlation of Serum VEGF-D Levels and Clinical Characteristics in Patients With LAM*
| Variables | p Value |
|---|---|
| Initial FVC % predicted | NS† |
| Initial FEV1 % predicted | NS |
| Initial TLC % predicted | NS |
| Initial Dlco % predicted | 0.0012 |
| Initial FEV1/FVC % predicted | NS |
| Decline in FEV1 %/yr | NS |
| Decline Dlco %/yr | NS |
| Age as of January 2006, years | NS |
| PFT years | 0.008 |
| Duration of disease‡ | NS |
| Dlco/VA % predicted | 0.028 |
| RV % predicted | NS |
*NS = not significant.
†NS = p > 0.05.
‡Years from the time of diagnosis until December 2005 (n = 100).
Figure 2.
Correlation of VEGF-D serum levels or lymphatic involvement and disease severity in patients with LAM. Top, A: comparison of serum VEGF-D levels of patients with LAM between CT scan grade I and CT scan grade II and III (n = 111). The area outlined by the column represents the mean serum VEGF-D level of patients with LAM grouped by designated CT scan grade. Standard error bars. Bottom, B: comparison of the number of LAM patients, with (+) or without (−) lymphatic involvement, having CT scan grade I and CT grade II and III (n = 111). The area outlined by the column represents the number of patients with LAM grouped by designated CT scan grade and status of lymphatic involvement.
Figure 3.
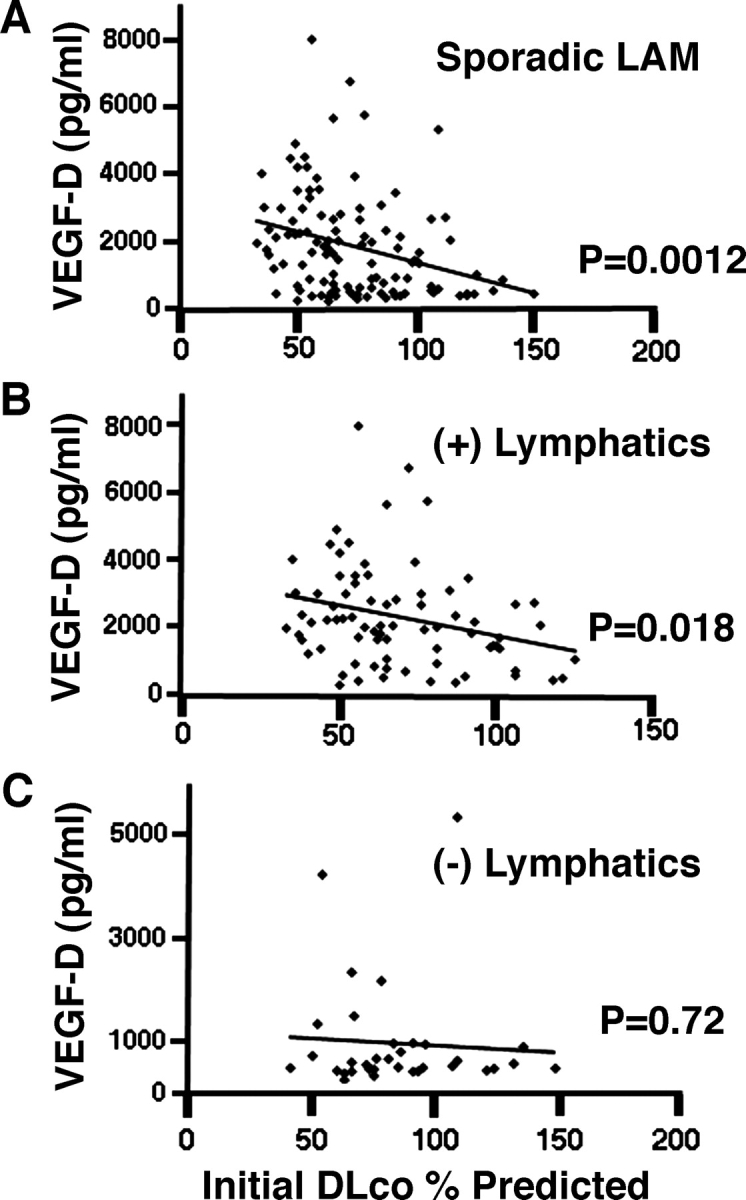
Association between serum VEGF-D levels in patients with LAM and initial Dlco percent predicted. Top, A: univariate analysis of correlation between the serum VEGF-D levels in 111 patients with LAM and the initial Dlco percent predicted. Center, B, and bottom, C: for multivariate analysis, LAM patients were grouped by lymphatic involvement. Serum VEGF-D levels correlated with initial Dlco percent predicted for patients with lymphatic involvement. (+) lymphatics = patients with lymphangioleimyomas and/or adenopathy; (-) lymphatics = patients without lymphangioleiomyomas or adenopathy; ♦ = serum measurement of VEGF-D from one patient with LAM.
Figure 4.
Association of lymphatic involvement in patients with LAM and initial Dlco percent predicted. The presence of lymphatic involvement in patients with LAM is correlated to lower Dlco percent predicted (p = 0.002; n = 111). ♦ = one patient with LAM. See the legend of Figure 3 for explanation of symbols not used in the text.
Table 3.
Comparison of Clinical Characteristics: LAM Patients With and Without Lymphatic Involvement*
| Lymphatics |
|||
|---|---|---|---|
| Variables | + | − | p Value |
| Initial FVC % predicted | 88.0 | 92.6 | NS† |
| Initial FEV1 % predicted | 74.6 | 78.6 | NS |
| Initial TLC % predicted | 91.6 | 94.9 | NS |
| Initial Dlco % predicted | 69.0 | 84.9 | 0.002 |
| Initial FEV1/FVC, % | 0.7 | 1.5 | NS |
| Decline FEV1 %/yr | − 2.6 | − 2.1 | NS |
| Decline Dlco %/yr | − 2.9 | − 2.8 | NS |
| Average age in yr | 51.3 | 51.6 | NS |
| PFT years | 5.1 | 6.3 | 0.005 |
| Duration of disease yr‡ | 9.6 | 8.1 | 0.055 |
| Dlco/VA % predicted | 87 | 98 | NS |
| RV % predicted | 99 | 97 | NS |
*+ = presence; − = absence. See Table 2 for abbreviation not used in the text.
†NS = p > 0.05.
‡Time in years from date of diagnosis until December 2005 (n = 100).
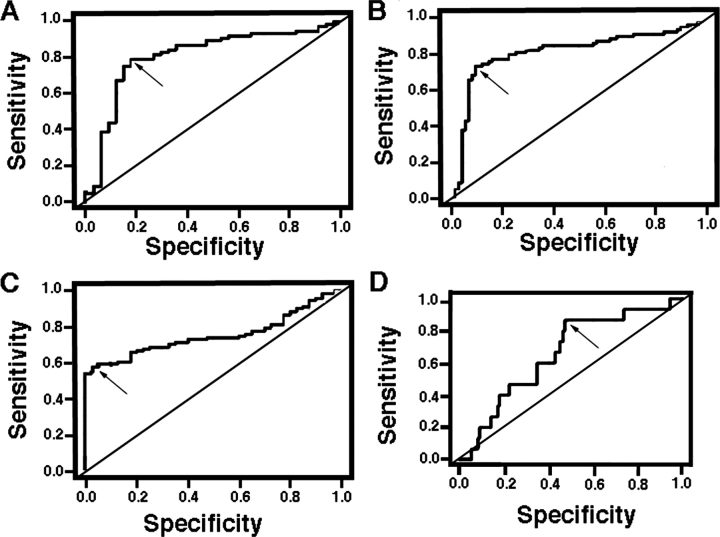
To assess serum VEGF-D levels as a biomarker both for LAM and for lymphatic involvement, we derived a model of prediction by plotting the Se (the proportion of subjects who have the predicted condition, LAM or lymphatic involvement) vs Sp (the proportion of subjects not having the predicted condition), with the curve of the plot (ROC) defining the optimal cutoff point (ie, serum VEGF-D levels above which the diagnosis of LAM disease or lymphatic involvement may be accepted). The area under the curve (AUC) was used as a measure of accuracy for the ROC. The closer the AUC is to 1, the better the diagnostic test result, which, for this study, was the ability to use serum levels of VEGF-D to classify patients with or without LAM or with or without lymphatic involvement. We based the model on Se and Sp, rather than on positive predictive value, as the positive predictive value depends on the prevalence of disease as well as the Sp of the test, and LAM is a rare disease. In evaluating lymphatic involvement among LAM patients, serum VEGF-D levels have a good discriminating ability (AUC, 0.813 [p < 0.0001]; Se, 0.792; Sp, 0.824; cutoff point, 949 pg/mL) [Fig 5, top left, A]. When the analysis group included healthy volunteers, the predictability increased (AUC, 0.845 [p < 0.0001]; Se, 0.753; Sp, 0.919; cutoff point, 1,317 pg/mL) [Fig 5, top right, B] The discriminating ability of serum VEGF-D level to predict LAM disease is not as robust (AUC, 0.751 [p < 0.0001]; Se, 0.577; Sp, 0.975; cutoff, 1,239 pg/mL) [Fig 5, bottom left, C] and is poor for predicting LAM disease for patients with only cystic lung disease (AUC, 0.66 [p = 0.046]; Se, 0.867; Sp, 0.529; cutoff, 1,202 pg/mL) [Fig 5, bottom right, D].
Figure 5.
Model representing the diagnostic ability of serum VEGF-D levels for predicting lymphatic involvement or LAM disease. A larger AUC indicates a higher measure of accuracy of the diagnostic test. Se, Sp, and predicted values are given at the optimal cutoff point where the Se and Sp are maximized (arrows) [n = 111]. Top left, A: ROC curve for detecting lymphatic involvement in a sample of LAM patients only. The AUC is 0.813 (p < 0.0001). The threshold VEGF-D value is 949 pg/mL. Top right, B: ROC curve for detecting lymphatic involvement in a combined sample of LAM patients and healthy volunteers. The AUC is 0.845 (p < 0.0001). The threshold VEGF-D value is 1,317 pg/mL. Bottom left, C: ROC curve for diagnosing LAM in a combined sample of LAM patients and healthy volunteers. The AUC is 0.751 (p < 0.0001). The threshold VEGF-D value is 1,239 pg/mL. Bottom right, D: ROC curve for diagnosing only pulmonary LAM in a combined sample of pulmonary LAM, extrapulmonary LAM, and healthy volunteers. The AUC is 0.66 (p = 0.046). The threshold VEGF-D value is 1,202 pg/mL.
It is of interest to note that, in our cohort, there were four patients with LAM who had pleural effusions and no evidence of lymphatic involvement. The average serum level of VEGF-D for these patients was 466 pg/mL, which is well below the serum VEGF-D cutoff point of 1,347 pg/mL for predicting lymphatic involvement and is significantly lower than the levels for healthy volunteers (p < 0.001).
Discussion
A patient with cystic lung disease is likely to have LAM if presenting with extrapulmonary manifestations (eg, AML lymphangioleiomyoma). It is in patients whose disease is restricted to the lung that the diagnostic dilemma occurs. Similar thin-walled cysts may be seen in other diseases characterized by lung involvement. In these cases, an open-lung biopsy is often recommended to confirm LAM. The discovery of a biomarker for diagnosis might obviate the need for an invasive biopsy procedure. Young et al20 compared serum VEGF-D levels in 38 patients with LAM to those in healthy volunteers and to those in patients with pulmonary Langerhans cell histiocytosis, LAM, and emphysema. Serum VEGF-D concentrations in LAM patients were greater than those in control subjects and patients with other cystic lung diseases. It was proposed20 that serum VEGF-D levels might be used as a potential biomarker in the differential diagnosis of LAM. Our data substantiate the difference in VEGF-D levels between healthy volunteers and patients with LAM but also reveal a large overlap of data points in the two groups (Fig 1, top, A). Furthermore, no difference in levels was apparent in patients with lung disease and AMLs or in those with disease restricted to the pulmonary parenchyma compared to concentrations in healthy volunteers (Fig 1, center, B, and bottom, C). Thus, normal VEGF-D levels do not exclude LAM, especially in patients who present with lung cysts only or lung cysts plus AMLs.
In this study, we report that VEGF-D levels were significantly greater than those in volunteers only in patients with LAM who had lymphatic involvement (Fig 1, center, B). Our VEGF-D predictive model shows that serum levels of VEGF-D are a good biomarker for predicting lymphatic involvement but are only fair for diagnosing LAM disease. In our population, those patients with LAM having only pulmonary symptoms had serum VEGF-D levels ranging from 235 to 5,322 pg/mL, but only 11% of this subgroup had levels above the predictive cutoff range of 1,239 pg/mL. Chylous effusions alone were not associated with elevated VEGF-D levels in serum. Therefore, we conclude that serum VEGF-D levels appear to reflect lymphatic involvement, as defined by the presence of adenopathy or lymphangioleiomyoma.
As an indicator of lymphatic involvement, we queried whether serum VEGF-D levels would have correlations to clinical phenotypes in LAM. The LAM patients in our study with high serum VEGF-D levels had decreased Dlco. These findings are consistent with the data from Seyama et al18 who found that serum VEGF-D levels negatively correlated to pulmonary function in patients with LAM. In addition, we report new data indicating that patients with LAM with high serum VEGF-D levels are more likely to have severe disease. Consistently, patients with LAM and lymphatic involvement had significantly decreased pulmonary function and trended toward more severe disease. These correlations would suggest that lymphatic involvement might develop during disease progression.
The mechanism by which VEGF-D might affect disease severity in LAM is as yet unknown. It has been reported that LAM cells express VEGF-D.21 Kumasaka et al22 postulated that LAM cells might spread to other organs by dissemination via lymphangiogenesis-mediated shedding of LAM cell clusters (LAM cells surrounded by lymphatic endothelial cells), a process that might result in the development of new lesions.
In conclusion, we show evidence that serum levels of VEGF-D indicate lymphatic involvement in patients with LAM. We have proposed alternative biomarkers. Plasma levels of osteopontin (a protein that binds to CD44) were higher in LAM patients than in healthy volunteers,23 and LAM cells from blood or chyle can be characterized for TSC2 LOH.17 These, in addition to VEGF-D, may aid in the difficult diagnosis of a patient who presents with only cystic lung disease.
Acknowledgment:
We thank Dr. Martha Vaughan, Dr. Wendy Steagall, Dr. Souheil El-Chemaly, and Dr. Gustavo Pacheco-Rodriguez (Translational Medicine Branch, National Heart, Lung, and Blood Institute, National Institutes of Health) for helpful discussions and critical review of the manuscript.
Abbreviations:
- AML
angiomyolipoma
- AUC
area under the curve
- Dlco
diffusing capacity of the lung for carbon monoxide
- HRCT
high-resolution CT
- LAM
lymphangioleiomyomatosis
- PFT
pulmonary function test
- ROC
receiver operating characteristic
- RV
residual volume
- Se
sensitivity
- Sp
specificity
- TLC
total lung capacity
- TSC
tuberous sclerosis complex
- VA
alveolar volume
- VEGF
vascular endothelial growth factor
Footnotes
Funding/Support: This research was funded by the Intramural Research Program, National Institutes of Health, National Heart, Lung, and Blood Institute.
Financial/nonfinancial disclosures: The authors have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/site/misc/reprints.xhtml).
References
- 1.Taylor JR, Ryu J, Colby TV, et al. Lymphangioleiomyomatosis: clinical course in 32 patients. N Engl J Med. 1990;323:1254–1260. doi: 10.1056/NEJM199011013231807. [DOI] [PubMed] [Google Scholar]
- 2.Ryu JH, Moss J, Beck GJ, et al. The NHLBI lymphangioleiomyomatosis registry: characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006;173:105–111. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitaichi M, Nishimura K, Itoh H, et al. Pulmonary lymphangioleiomyomatosis: a report of 46 patients including a clinicopathologic study of prognostic factors. Am J Respir Crit Care Med. 1995;151:527–533. doi: 10.1164/ajrccm.151.2.7842216. [DOI] [PubMed] [Google Scholar]
- 4.Johnson SR, Tattersfield AE. Lymphangioleiomyomatosis. Semin Respir Crit Care Med. 2002;23:85–92. doi: 10.1055/s-2002-25298. [DOI] [PubMed] [Google Scholar]
- 5.Taveira-DaSilva AM, Steagall WK, Moss J. Lymphangioleiomyomatosis. Cancer Control. 2006;13:276–285. doi: 10.1177/107327480601300405. [DOI] [PubMed] [Google Scholar]
- 6.Avila NA, Chen CC, Chu SC, et al. Pulmonary lymphangioleiomyomatosis: correlation of ventilation-perfusion scintigraphy, chest radiography, and CT with pulmonary function tests. Radiology. 2000;214:441–446. doi: 10.1148/radiology.214.2.r00fe41441. [DOI] [PubMed] [Google Scholar]
- 7.Ferrans VJ, Zu-Xi Y, Nelson WK, et al. Lymphangioleiomyomatosis (LAM): a review of clinical and morphological features. J Nippon Med Sch. 2000;67:311–329. doi: 10.1272/jnms.67.311. [DOI] [PubMed] [Google Scholar]
- 8.Matsui K, Tatsuguchi A, Valencia JC, et al. Extrapulmonary lymphangioleiomyomatosis (LAM): clinicopathologic features in 22 cases. Hum Pathol. 2000;31:1242–1248. doi: 10.1053/hupa.2000.18500. [DOI] [PubMed] [Google Scholar]
- 9.Costello LC, Hartman TE, Ryu JH. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin Proc. 2000;75:591–594. doi: 10.4065/75.6.591. [DOI] [PubMed] [Google Scholar]
- 10.Franz DN, Brody A, Meyer C, et al. Mutational and radiographic analysis of pulmonary disease consistent with lymphangioleiomyomatosis and micronodular pneumocyte hyperplasia in women with tuberous sclerosis. Am J Respir Crit Care Med. 2001;164:661–668. doi: 10.1164/ajrccm.164.4.2011025. [DOI] [PubMed] [Google Scholar]
- 11.Moss J, Avila NA, Barnes PM, et al. Prevalence and clinical characteristics of lymphangioleiomyomatosis (LAM) in patients with tuberous sclerosis complex. Am J Respir Crit Care Med. 2001;163:669–671. doi: 10.1164/ajrccm.164.4.2101154. [DOI] [PubMed] [Google Scholar]
- 12.Smolarek TA, Wessner LL, McCormack FX, et al. Evidence that lymphangiomyomatosis is caused by TSC2 mutations: chromosome 16p13 loss of heterozygosity in angiomyolipomas and lymph nodes from women with lymphangiomyomatosis. Am J Hum Genet. 1998;62:810–815. doi: 10.1086/301804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA. 2000;97:6085–6090. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato T, Seyama K, Fujii H, et al. Mutation analysis of the TSC1 and TSC2 genes in Japanese patients with pulmonary lymphangioleiomyomatosis. J Hum Genet. 2002;47:20–28. doi: 10.1007/s10038-002-8651-8. [DOI] [PubMed] [Google Scholar]
- 15.Bittmann I, Rolf B, Amann G, et al. Recurrence of lymphangioleiomyomatosis after single lung transplantation: new insights into pathogenesis. Hum Pathol. 2003;34:95–98. doi: 10.1053/hupa.2003.50. [DOI] [PubMed] [Google Scholar]
- 16.Karbowniczek M, Astrinidis A, Balsara BR, et al. Recurrent lymphangiomyomatosis after transplantation: genetic analyses reveal a metastatic mechanism. Am J Respir Crit Care Med. 2003;167:976–982. doi: 10.1164/rccm.200208-969OC. [DOI] [PubMed] [Google Scholar]
- 17.Crooks DM, Pacheco-Rodriguez G, DeCastro RM, et al. Molecular and genetic analysis of disseminated neoplastic cells in lymphangioleiomyomatosis. Proc Natl Acad Sci USA. 2004;101:17462–467. doi: 10.1073/pnas.0407971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seyama K, Kumasaka T, Souma S, et al. Vascular endothelial growth factor-D is increased in serum of patients with lymphangioleiomyomatosis. Lymphat Res Biol. 2006;3:143–152. doi: 10.1089/lrb.2006.4.143. [DOI] [PubMed] [Google Scholar]
- 19.Eisenstein DM, Singer AA, Chicolte WA, et al. Abdominal lymphadenopathy: spectrum of CT findings. Radiographics. 1991;11:457–472. doi: 10.1148/radiographics.11.3.1852937. [DOI] [PubMed] [Google Scholar]
- 20.Young LR, Inoue Y, McCormack FX. Diagnostic potential of serum VEGF-D for lymphangioleiomyomatosis. N Engl J Med. 2008;358:199–200. doi: 10.1056/NEJMc0707517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumasaka T, Seyama K, Mitani K, et al. Lymphangiogenesis in lymphangioleiomyomatosis: its implication in the progression of lymphangioleiomyomatosis. Am J Surg Pathol. 2004;28:1007–1016. doi: 10.1097/01.pas.0000126859.70814.6d. [DOI] [PubMed] [Google Scholar]
- 22.Kumasaka T, Seyama K, Mitani K, et al. Lymphangiogenesis-mediated shedding of LAM cell clusters as a mechanism for dissemination in lymphangioleiomyomatosis. Am J Surg Pathol. 2005;10:1356–1366. doi: 10.1097/01.pas.0000172192.25295.45. [DOI] [PubMed] [Google Scholar]
- 23.Pacheco-Rodriguez G, Steagall WK, Crooks DM, et al. TSC2 loss in lymphangioleiomyomatosis cells correlated with expression of CD44v6, a molecular determinant of metastasis. Cancer Res. 2007;67:10573–10581. doi: 10.1158/0008-5472.CAN-07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]