Abstract
Nitric oxide and other reactive nitrogen species target multiple sites in the mitochondria to impact cellular bioenergetics and survival. Kinetic imaging studies revealed that NO from either activated macrophages or donor compounds rapidly diffuses to the mitochondria, causing a dose dependent progressive increase in NO-dependent DAF fluorescence that corresponded to mitochondrial membrane potential loss, and initiated alterations in cellular bioenergetics that ultimately led to necrotic cell death. Cellular dysfunction is mediated by an elevated 3-nitrotyrosine signature of the mitochondrial complex I subunit NDUFB8, which is vital for normal mitochondrial function as evidenced by selective knockdown via siRNA. Overexpression of mitochondrial superoxide dismutase substantially decreased NDUFB8 nitration and restored mitochondrial homeostasis. Further, treatment of cells with either necrostatin-1 or siRNA knockdown of RIP1 and RIP3 prevented NO-mediated necrosis. This work demonstrates that the interaction between NO and mitochondrially-derived superoxide alters mitochondrial bioenergetics and cell function, thus providing a molecular mechanism for reactive oxygen and nitrogen species-mediated alterations in mitochondrial homeostasis.
Introduction
Nitric Oxide (NO) is a short-lived reactive radical species produced constitutively in endothelial cells by the enzyme endothelial nitric oxide synthase (eNOS) [1]. Upon formation, endothelial-derived NO diffuses to vascular smooth muscle cells, where it activates guanylate cyclase and mediates vascular relaxation. During vascular insult, both endothelial cells and circulating macrophages can increase NO levels through the inducible form of nitric oxide synthase (iNOS). Aside from its role in vascular tone, NO can also mediate multiple cellular processes including inflammation [2], vascular proliferation [3], and even cell survival and death [4, 5], depending on localization or magnitude of production. The effects of NO are attributed to its interaction with multiple cellular targets such as proteins, thiols, and superoxide (O2․-) [6-10].
One of the prominent sites where NO interacts with cells is mitochondria. In fact, NO directly and indirectly interacts with multiple targets within the mitochondrial respiratory chain, including NADH:ubiquinone oxidoreductase (Complex I) [11, 12] and the terminal enzyme cytochrome C oxidase (Complex IV) [13]. Mitochondrial Complex I is a membrane bound multimeric enzyme that consists of approximately 45 independent protein subunits, some of which are potential targets for reactive oxygen and nitrogen species. While our knowledge is still limited, NO appears to persistently inhibit Complex I, indicating an important role in the control of mitochondrial respiration and energy production under physiological conditions [14, 15]. Mechanistically, NO induces reversible inhibition of Complex I via S-nitrosation of a critical thiol residue in a 75kDa subunit, which limits electron entry into the mitochondrial electron transport chain [16]. However, rising NO levels irreversibly inactivate Complex I [17], triggering mitochondrial ROS generation and apoptosis [18] that has been implicated in numerous pathological conditions including sepsis and cardiac failure [19-21]. In addition to programmed apoptosis, mitochondrial-derived ROS also play a vital role in necrosis [22, 23]. An emerging modulator of necrotic cell death is the receptor interacting protein (RIP) kinases [24-29]. Blockade of RIPK1 by the specific inhibitor necrostatin-1 and/or reduction of RIP1 and RIP3 expression limited necrosis brought about by proinflammatory cytokines in a ROS-dependent manner [29]. Given the well-known interactions between NO and ROS, and the intriguing links between ROS, and RIP kinase in necrosis, we hypothesized that these two pathways may functionally interact.
Our present study examined the effects of NO on mitochondrial function and bioenergetics. Previous studies show that mitochondria and submitochondrial particles from tissues exposed to NO resulted in a progressive nitration of tyrosine residues of mitochondrial proteins [11]. Here, we used intact cells to identify a precise molecular target of tyrosine nitration that facilitates caspase-independent cell death. Molecularly, we show that reactive nitrogen species (RNS) induce nitration of the complex I subunit NDUFB8 in endothelial cells and that NDUFB8 nitration coincided with mitochondrial membrane potential alterations and inhibition of mitochondrial bioenergetics (reduction of complex I activity, mitochondrial oxygen consumption and change of ADP/ATP ratio) that resulted in necrotic cell death. Targeting of NO to the NDUFB8 was dependent upon mitochondrial O2․- generation and could be reversed by overexpression of the mitochondrial isoform of superoxide dismutase (Manganese Superoxide Dismutase; MnSOD). Our study also revealed that NO-induced caspase-independent cell death could be blocked by either inhibiting RIP kinases or overexpression of mitochondrial antioxidant, indicating that NDUFB8 nitration is an important physiologic mechanism that evokes bioenergetic collapse and necrotic cell death.
Experimental procedures
Cell Culture
Rat pulmonary microvascular endothelial cells (RPMVECs) (obtained from Troy Stevens, University of South Alabama) [30] were maintained in Dulbecco's Modified Eagle's Medium (GIBCO) supplemented with 10% FBS, endothelial cell growth supplement, nonessential amino acids and antibiotics at 37°C, 5%CO2. Human pulmonary microvascular ECs (obtained from C.J. Kirkpatrick, Johannes-Gutenberg University) [31] were cultured in M199 supplemented with 15% FCS, L-glutamine and antibiotics. J774.1 murine macrophages were cultured in RPMI 1640 supplemented with 10% FCS and antibiotics. HEK293T and murine embryonic fibroblast (MEF) cells were cultured in DMEM supplemented with 10% FBS and 1% antibiotics.
Preparation of NO solution
An ice-chilled, dual-pore, glass vessel apparatus containing double distilled water was set up in a fume hood. The two pores were sealed with rubber septums and the water was de-aerated for 20 minutes by bubbling with nitrogen gas via a 22 gauge needle while excess gas escaped through a needle inserted in the other septum. Following de-aeration, pure nitric oxide gas was slowly bubbled into the water for 20 minutes to saturate the water with NO (1.91 mM) [32]. Known amounts of the saturated solution were then introduced into the experimental medium via gas-tight syringe to observe the effects of NO.
Detection of Reactive Oxygen/Nitrogen Species
Detailed description is provided in supplemental data. Briefly, RPMVECs were loaded with either DAF-FM (5 μM; Invitrogen), H2DCF-DA (10μM; Invitrogen), or dihydroethidine (DHE - 5 μM; Invitrogen) to visualize NO and reactive oxygen species, respectively, in HBSS, pH 7.4. Coverslips were then mounted in a PDMI-2 open perfusion micro-incubator (Harvard apparatus, MA) and maintained at 37°C on a Nikon TE300 inverted microscope. Varying concentrations of the nitric oxide donor DeaNONOate (Cayman Chemicals, Ann Arbor, MI; 0.5-10 μM) or NO solution (1.0-10.0 μM) was added at the indicated times. For co-culture studies, J774.1 murine macrophages were labeled with Cell Tracker Red (1 μM, Invitrogen) for 15 min, washed twice, and resuspended in serum-free medium. Macrophages were activated by exposure to Lipopolysaccharide (1 μg/ml) for 14 hr at 37°C in order to induce significant iNOS expression (data not shown). Macrophages were added onto DAF-FM-loaded HPMVECs as indicated. Images were recorded every 5 sec via a BioRad Radiance 2000 confocal imaging system (excitation at 488 and 568 for DAF-FM and Cell Tracker Red, respectively).
Intracellular localization of DAF-NO adduct
RPMVECs loaded with DAF-FM were co-labeled with either Lysotracker Red (Invitrogen; 1 μM) or brefeldin A BODIPY® 558/568 conjugate (Invitrogen; 0.25 μg/ml) to visualize the lysosomes or golgi apparatus, respectively, at 568/610 nm excitation/emission. To visualize the mitochondria, RPMVECs transfected with pDsRed2-Mito Vector (Clontech) by electroporation (Amaxa Inc., Gaithersburg, MD) were loaded with DAF-FM. Images were collected 20 min following addition of 5 μM DEANONOate via confocal microscopy.
Kinetics of NO production and mitochondrial membrane potential
Cells cultured on 25 mm glass coverslips were loaded for 15 min with the NO sensitive membrane permeable fluorescent indicator DAF-FM at room temperature. For measurement of membrane potential, the cationic potentiometric dye TMRE (50 nM) was added to the loading medium and allowed to equilibrate for an additional 15 min. After dye loading, cells were washed and resuspended in HBSS. Cells were then exposed to NO donor and cells imaged simultaneously for DAF and TMRE at 488 and 568 nm every 3 sec using the Radiance 2000 imaging system. Upon alterations in mitochondrial membrane potential, TMRE dissociates from the mitochondria and rapidly redistributes into the cytoplasm, and can therefore be detected in the nucleus. DAF and TMRE fluorescence changes were determined by nuclear masking of all cells in the field and expressed as arbitrary units.
Measurement of ADP/ATP ratio
ADP and ATP were measured using ApoGlow assay kit (Cambrex) according to manufacturer's instructions.
Adenoviral MnSOD overexpression
Approximately 1×107 endothelial cells were infected with an adenoviral virus that overexpresses MnSOD (Ad5CMVSOD2; University of Iowa Gene Transfer Vector Core) at an MOI of 2,000 particles/cell as described [33]. On day 2 post infection, transduction of MnSOD was verified by western blot analysis (BD Biosciences Pharmingen).
Complex I immunocapture, Immunoprecipitation and immunoblot assay
The isolation of Complex I was achieved by using permeabilized endothelial cells (HPMVEC) with the complex I Immunocapture beads (Mitosciences) according to the manufacturer's recommendations and probed with an anti-nitrotyrosine antibody (Invitrogen). At 48 hrs post infection with Ad5CMVSOD2, HPMVECs were exposed to various doses of DEANONOate at different time intervals. After the treatment, cells were lysed and subjected to immunoprecipitation and immunoblotting and probed with anti-nitrotyrosine and anti-NDUFB8 antibodies (MitoSciences).
RNA interference
Small interfering RNA (siRNA) containing a mixture of two targeted siRNAs for human NDUFB8 (sc-90752 A (5′-GAGAGAGAUCCAUGGUAUAtt-3′) and sc-90752 B (5′-GACCAAAGCAGUAUCCUUAtt-3′)) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). HPMVECs cultured in 60 mm petridishes were transfected with the appropriate siRNA (100nM) using TransIT-siQUEST® transfection reagent (Mirus Bio LLC, Madison, WI). Control siRNA duplex containing scrambled sequence (Santa Cruz Biotechnology) was used in parallel experiments. After 48 hr of transfection, cells were harvested for immunoblotting and oxygen consumption measurement. Human RIPK1 and RIPK3 siRNAs were obtained from Dharmacon ON-TARGET plus siRNA pools of 4 oligoes. RIPK1-1 (5′-CCACUAGUCUGACGGAUAA-3′), RIPK1-2 (5′-UGAAUGACGUCAACGCAAA-3′), RIPK1-3 (5′-GCACAAAUACGAACUUCAA-3′) and RIPK1-4 (5′-GAUGAAAUCCAGUGACUUC-3′) and RIPK3-1 (5′-CCACAGGGUUGGUAUAAUC-3′), RIP3K-2 (5′-AACCAGCACUCUCGUAAUG-3′), RIP3K-3 (5′-GCUACGAUGUGGCGGUCAA-3′) and RIPK3-4 (5′-GACCGCUCGUUAACAUAUA-3′)). Control siRNA (5′-AACGUACGCGGAAUACUUCGA-3′) was synthesized by Dharmacon. RIPK1 and RIPK3 transfected 293T cells were harvested after 48 hr and effective gene knockdown was determined by immunoblotting.
Measurement of mitochondrial Complex I activity
The activity of complex I was determined by monitoring the reduction of NADH at 340 nm and at 30 °C according to the procedure by [34], using cytochrome c as an electron acceptor. Permeabilized cells were pretreated with NO donor for indicated times and the complex I activity was measured. This activity was 99% inhibited by rotenone.
Oxygen electrode measurements
HPMVECs were permeabilized with digitonin (final concentration 0.01%), and substrates and inhibitors were added in the following order and final concentrations: malate + pyruvate, 5 mM each; rotenone, 100 nm; succinate, 5 mM; antimycin A, 50 nM; ascorbate, 2.5 mM with TMPD (tetramethyl-p-phenylenediamine) 0.4 mM; and FCCP 1 μM. MT200A MitoCell Clark-type electrode (Strathkelvin Instruments) was used to measure the oxygen consumption. Rate of oxygen consumption was expressed as nmoles oxygen/1×106 cells/min.
Immunocytochemistry
MEFs cultured on 25 mm glass coverslips were transfected with Mito-DsRed. After 48 hr, cells were exposed to 10 μM DeaNONOate or the necroptosis inducing agonist combination of TNFα (100 ng) and the pan caspase inhibitor Z-VAD-FMK (50 μM) for 6 hr. Cells were then fixed with 3% paraformadehyde and permeabilized with digitonin (200 μg/ml). MEFS were probed for RIP3 (Imgenex, Sorrento Valley, CA) and visualized using an AlexaFluor-488 secondary antibody (Invitrogen). DAPI was used as a nuclear counterstain. AlexaFluor-488 levels were set using TNFα+z-VAD-FMK controls and maintained for all experiments. Post acquisition brightness was adjusted in the DsRed and DAPI channels to account for differences in protein expression and dye loading, respectively. Images are representative of 3 independent experiments.
Data analysis
Fluorescent tracings are representative of the mean fluorescence value of all cells in one field and are indicative of at least 3 independent experiments. Data are presented as the mean ± SEM.
Results
Nitric oxide triggers morphological changes in endothelial cells
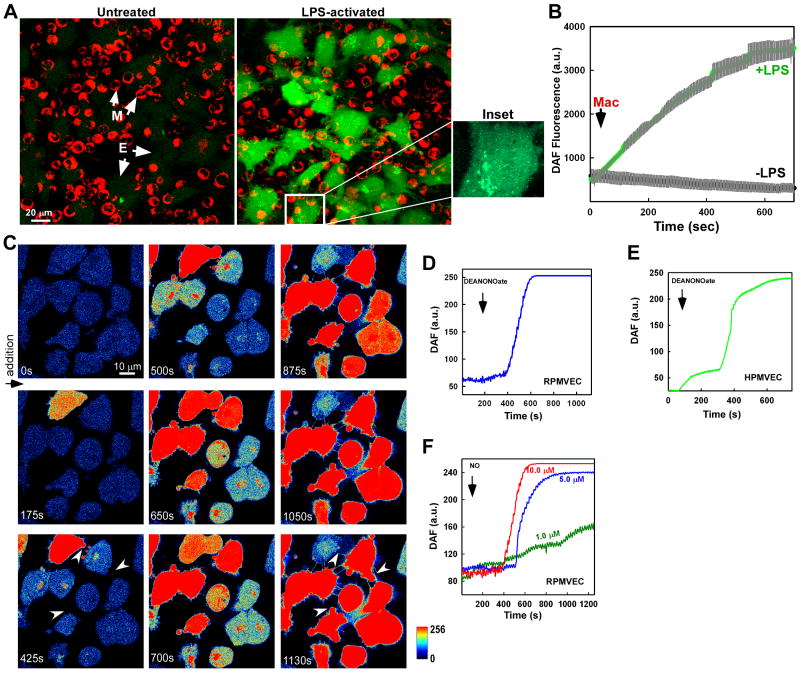
Inflammatory cells including macrophages play an important early defense mechanism against infection. Under physiological conditions, macrophages produce nominal levels of reactive oxygen and nitrogen species but in response to an infection, activated macrophages trigger an elevation of oxidative and nitrosative agents which may impact vascular function. One protein up-regulated during bacterial infection is iNOS, which greatly increases macrophage NO production and has been implicated in the pathogenesis of endothelial dysfunction during sepsis [35]. To investigate whether NO generated in activated macrophages can transfer to adjacent endothelial cells, LPS-challenged macrophages were added onto endothelial cells loaded with the NO indicator DAF-FM. Macrophage-derived NO greatly elevated the endothelial NO signature as detected by DAF fluorescence versus non-activated macrophages (Fig. 1A and B). Interestingly, elevated DAF fluorescence presented in a perinuclear pattern in many cells (Fig. 1A inset, macrophage overlay removed), indicating potential NO targeting to distinct subcellular organelles.
Figure 1.
Transmission of macrophage-derived NO to adjacent endothelial cells can be detected in discrete cellular organelles and facilitates morphological plasma membrane modifications. (A) Murine macrophages (J774A.1) were stimulated with LPS (1μg/ml) for 14 hr. Macrophages were added to the DAF-FM loaded Human pulmonary microvascular endothelial cells (HPMVECs) to monitor the DAF fluorescence change via confocal microscopy. An individual endothelial cell is enlarged and the macrophage overlay removed to illustrate discrete subcellular localization of DAF fluorescence following addition of activated macrophages. (B) Real time DAF fluorescence changes of three individual experiments following addition of control and LPS-activated macrophages. Gray bars indicate standard error. (C) Rat pulmonary microvascular endothelial cells (RPMVECs) loaded with DAF-FM were exposed to 10 μM of the NO donor DeaNONOate and fluorescence changes recorded over time. White arrows at 425 and 1130 s represent morphological plasma membrane changes following NO delivery. Representative mean fluorescence changes in all (D) RPMVEC and (E) HPMVEC cells in the microscopic field after addition of 10 μM DeaNONOate. (F) Exogenous delivery of an NO solution results in a dose-dependent progressive increase in DAF fluorescence in RPMVECs.
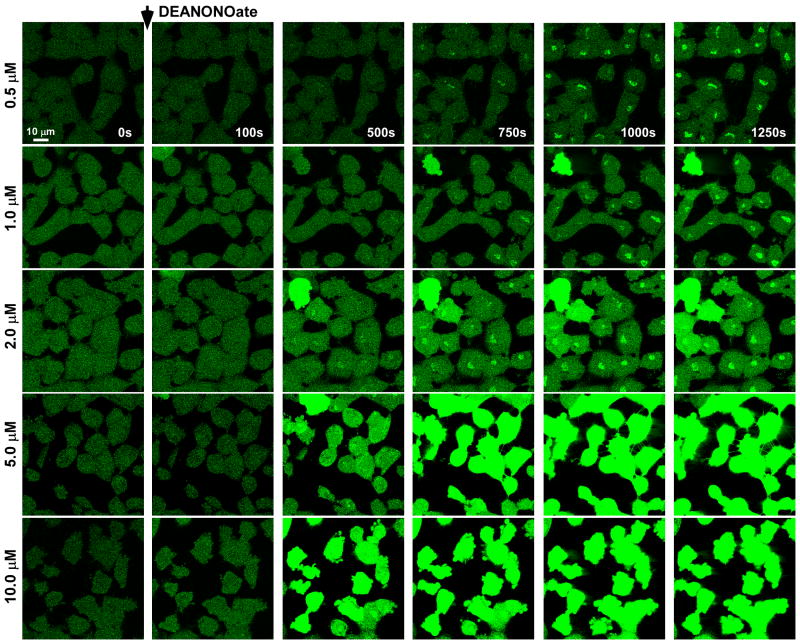
In addition to NO, activated macrophages generate a number of reactive species as well as inflammatory cytokines such as TNFα. To evaluate the subcellular targeting of NO and its endothelial effect using a pure NO source, the donor compound DeaNONOate was applied to cultured RPMVECs loaded with the NO indicator DAF-FM. Addition of DeaNONOate (10 μM) revealed subcellular localization and a progressive increase in DAF fluorescence both in rat pulmonary microvascular endothelial cells (RPMVECs; Fig. 1C and D) and human pulmonary microvascular endothelial cells (HPMVECs; Fig. 1E). Furthermore, a dose-dependent increase in DAF fluorescence was also observed in RPMVECs exposed to a NO solution (1.0-10.0 μM) (Fig. 1F), suggesting that DAF is sensitive to NO derived from other sources. Interestingly, plasma membrane protrusions were observed following the increase in DAF fluorescence (Fig. 1C; arrows marked at 425 s and 1130 s), indicative of the early stages of cell death. To determine the sensitivity of DAF in our cell culture model, we exposed DAF-loaded RPMVECs to varying concentrations of NO and assessed both the progressive increase in DAF fluorescence and subsequent morphological changes. Similar to previous published work [36]., an increase in DAF fluorescence was detected at concentrations as low as 0.5 μM of NO (Fig. 2). As in Fig. 1, we observed an accumulation of the DAF-NO adduct at these lower NO concentrations independent of global cellular DAF fluorescence. This accumulation pattern was not visible at higher NO concentrations, and indicates that cellular organelles may serve as a “sink” to buffer cellular NO. Elevated doses of NO (10 μM) consistently produced plasma membrane protrusions. These “blebs” suggest that RNS may have an important pathophysiologic role in endothelial function (i.e. cell death).
Figure 2.
Dose-dependent increase in DAF fluorescence indicates localization of DAF-NO adduct. RPMVECs loaded with DAF-FM were imaged following addition of increasing doses of DeaNONOate. Lower doses elicit punctate DAF-NO adduct accumulation (0.5 μM – 10 μM). Higher doses triggered robust and global DAF fluorescence followed by morphological changes.
NO scavenging by O2․- ablated DAF fluorescence
Both in vivo and in vitro, both endothelial cells and macrophages produce the free radical O2․-, which can react with NO to produce peroxynitrite. We therefore tested to see whether production of peroxynitrite will affect DAF fluorescence. DAF-loaded RPMVECS were exposed to 5 μM NO or to a combination of 5 μM NO and the O2․- generating system xanthine + xanthine oxidase (X + XO; 100 μM and 10 mU/ml). Similar to Fig. 1, addition of 5.0 μM NO resulted in a progressive increase in DAF fluorescence in RPMVECs (Fig. S1A and D). Scavenge of NO by O2․- resulted in a complete ablation of DAF fluorescence (Fig. S1B and E), validating the use of DAF under our experimental conditions. Similarly, treating the cells with Antimycin A, a mitochondrial Complex III inhibitor that triggers O2․- generation (Dihydroethidine fluorescence; DHE), also abolished the observed increase in DAF fluorescence (Fig. S1C and F); indicating that the observed alterations in DAF fluorescence are due to NO. It was interesting to note that O2․- delivery abolished the DAF fluorescence increase but did not prevent the endothelial plasma membrane changes.
Subcellular localization of DAF-NO fluorescence
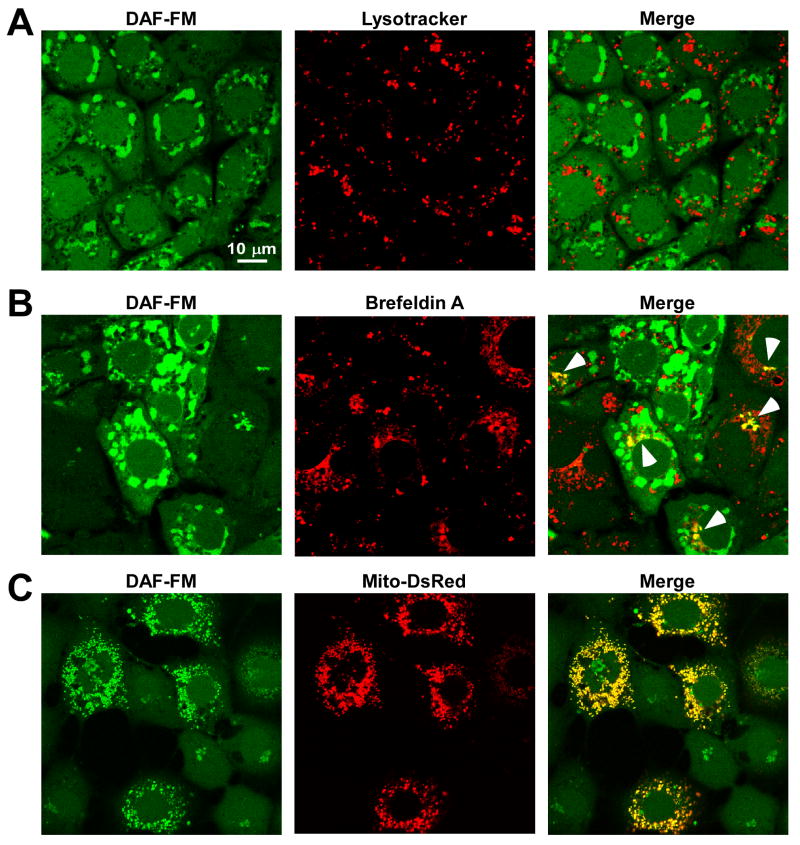
Having confirmed that DAF is both sensitive and specific for detecting NO in endothelial cell culture model, we next sought to identify the subcellular structure(s) where the DAF-NO fluorescence increase occurs both temporally and spatially (see Fig. 1 and 2). RPMVECs loaded with DAF were colabeled with conjugated fluorophores that localize to specific organelles (Lysotracker Red for lysosomes or brefeldin A BODIPY® for Golgi). Following addition of 5 μM DeaNONOate, we simultaneously visualized localization of the DAF-NO adduct with these organelle-specific fluorophores. No DAF-NO adduct was detected in lysosomal-like compartments (Fig. 3A). In contrast, NO partially accumulated in golgi-like structures as shown by its colocalization with the golgi-specific fluorophore Brefeldin A (Fig. 3B). This is in agreement with studies in which NO was shown to target golgi-like vesicles [37, 38]. Since NO has been proposed to regulate mitochondrial function, we next assessed whether mitochondria act as a NO sink, and therefore are a site where the DAF-NO adduct accumulates along with the golgi. RPMVECs expressing a mitochondrial-targeted DsRed protein displayed strong colocalization with DAF following addition of DeaNONOate (Fig. 3C). The observed DAF fluorescence increase is probably largely restricted to the mitochondrial pool, demonstrating that, in addition to the proposed role for NO in regulating mitochondrial function, mitochondria act as a primary site for NO accumulation. The altered mitochondrial morphology observed in Fig. 4A and B are likely attributed to the presence of the labeled fluorophores, which are known to affect mitochondrial function [39].
Figure 3.
Subcellular localization of DAF-NO adduct following NO delivery. DAF-loaded RPMVECs exposed to DeaNONOate (5 μM) were stained with (A) Lysotracker Red and (B) brefeldin A- BODIPY 558/568 conjugate to simultaneously assess DAF-NO adduct localization in the lysosomes and golgi apparatus, respectively. (C) RPMVECs transfected with mitochondrially-targeted DsRed (Mito-DsRed) were also loaded with DAF to assess DAF-NO adduct localization in the mitochondria following DeaNONOate application.
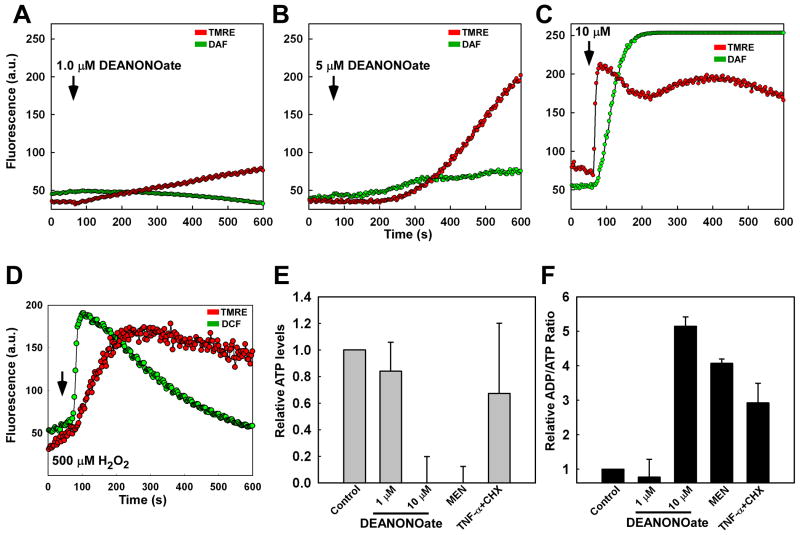
Figure 4.
NO alters mitochondrial function and bioenergetics in endothelial cells. RPMVECs were simultaneously stained with DAF-FM and the mitochondrial membrane potential indicator dye tetramethyl-rhodamine-ethyl ester (TMRE; 50 nM) and imaged continuously via confocal microscopy. Mean representative traces of DAF and TMRE fluorescence were quantitated following application of (A) 1.0 μM, (B) 5.0 μM, and (C) 10.0 μM DeaNONOate. (D) The kinetics of hydrogen peroxide (H2O2)-induced fluorescence (CMH2DCF-DA) and mitochondrial membrane potential alterations. (E) ATP levels were assessed 12 hr following NO addition and normalized to untreated RPMVECs. (F) Ratio of ADP/ATP in RPMVECs. Menadione (20 μM) and TNF-α (20 ng/ml) + cycloheximide (CHX; 1 μM) were used as positive controls for necrosis and apoptosis, respectively.
NO-linked signaling alters mitochondrial function and bioenergetics in endothelial cells
Both apoptotic and necrotic cell death are dependent upon mitochondrial functional alterations [30, 31, 33, 40-42]. These functional alterations are manifested by mitochondrial membrane depolarization, and result in dramatic changes in mitochondrial ATP production. It is plausible from our data that NO targets to the mitochondria and leads to endothelial dysfunction. Therefore, we sought to establish the link between mitochondrial-targeted NO and bioenergetic alterations. To assess mitochondrial alterations associated with NO, RPMVECs were simultaneously loaded with DAF and the mitochondrial membrane potential indicator tetramethylrhodamine ethyl ester perchlorate (TMRE) and exposed to increasing doses of DeaNONOate (1.0, 5.0, and 10.0 μM). Time-lapse image recording revealed a dose-dependent increase in both DAF and TMRE nuclear fluorescence (Fig. 4A-C), which indicates a dissociation of TMRE from the mitochondria upon membrane potential loss. In contrast, ROS-mediated mitochondrial membrane potential loss occurred secondary to an elevation in DCF fluorescence (Fig. 4D), underscoring mitochondria as a rapid cellular sink for NO.
NO regulates the activity of mitochondrial respiratory complexes I and IV, and therefore plays a crucial role in mitochondrial bioenergetics [43]. Our work clearly demonstrates that NO targets to the mitochondria and alters mitochondrial membrane potential. It is therefore likely that these alterations in membrane potential result in endothelial bioenergetic changes. A high dose (10.0 μM) of DeaNONOate caused a significant reduction in ATP levels (Fig. 4E) as well as an elevation in the ADP/ATP ratio (Fig. 4F) compared to untreated RPMVECs. Alternatively, a low concentration of DeaNONOate (1.0 μM) neither appreciably reduced ATP levels nor affected the ADP/ATP ratio. Interestingly, 10.0 μM DEANONOate reduced ATP levels and elevated the ADP/ATP ratio in a similar fashion to that of the necrosis-inducing agent Menadione (20 μM) in contrast to cells treated with TNF-α + cycloheximide, in which cells undergo energy-dependent apoptotic cell death (Fig. 4E and F). Taken together, we conclude that elevated RNS levels induce perturbations of mitochondrial function and bioenergetics, resulting in endothelial dysfunction.
MnSOD limits the NO-mediated complex I subunit NDUFB8 nitration and restores endothelial function
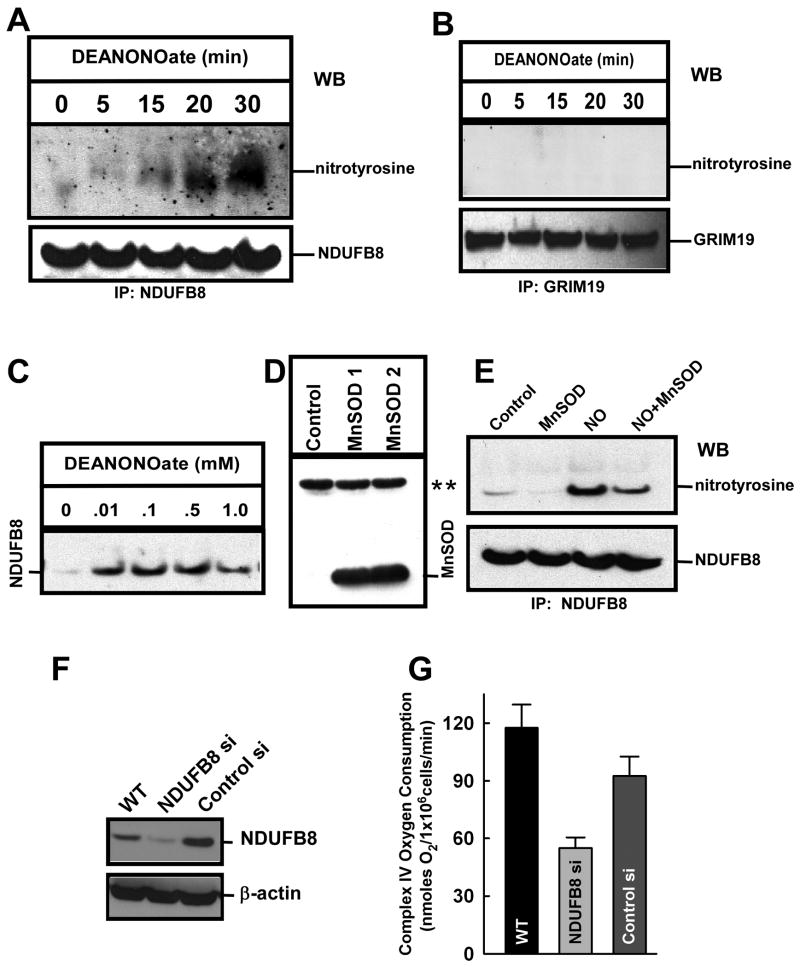
Mitochondrial respiratory chain complexes serve both as a source of O2․- and as a potential target. Mechanistically, mitochondrial-derived O2․- can react with NO to form highly reactive peroxynitrite even at nominal O2․- generation due to its rapid kinetics. Peroxynitrite can in turn irreversibly damage mitochondrial complex proteins via nitration of tyrosine residues [44]. In fact, isolated mitochondria exposed to peroxynitrite exhibited increased nitration of nuclear-encoded mitochondrial complex I subunits and a concomitant inhibition in complex I activity [12]. However, it is unknown if physiological generation of O2․- by mitochondria is sufficient to react with NO to alter mitochondrial function. The results described above suggest that human pulmonary microvascular endothelial cells (HPMVECs) exposed to DeaNONOate showed a progressive increase in DAF fluorescence (Fig. 1E). Since both mitochondrial complexes I and IV are targets for NO and complex IV has been extensively studied, we examined the multisubunit complex I. We therefore searched for a mitochondrial complex I target of the RNS-induced oxidative signal by a complex I immunocapture approach. Human endothelial cells were exposed to two different doses of DeaNONOate (0.01 and 0.1 mM). Immunocaptured complex I proteins were probed with anti-nitrotyrosine antibody for the detection of nitrotyrosine. Suppl Fig. 2 depicts nitrotyrosine modified proteins after NO exposure. Two low molecular weight (between 20-30 kDa) nitrotyrosine positive bands appeared in 0.1 mM DeaNONOate treated cells. Based on the recent mitocarta chart [45], we investigated NDUFB8 and GRIM 19 proteins which separate by SDS-PAGE at the above mentioned molecular weight range. HPMVECs exposed to DeaNONOate (0.1 mM) display a progressive increase in nitration of the previously undescribed complex I subunit NDUFB8 (Fig. 5A) but not GRIM19, indicating that it may not be the target of NO (Fig. 5B). Further, a NDUFB8 nitrotyrosine signature was evident at all doses tested (Fig. 5C). To investigate whether mitochondrial-derived O2․- is crucial for NO-linked NDUFB8 nitration, HPMVECs were transfected with an adenoviral construct that overexpresses MnSOD (Figs. 5D and S3). A reduction in mitochondrial O2․- via MnSOD overexpression (Fig. S4) resulted in a significant decrease in NDUFB8 nitration (Fig. 5E and 6A). This data suggests that mitochondrial-derived O2․- is crucial for the observed elevation in complex I nitration. However, because NO can target multiple proteins, it is not clear whether an elevated NDUFB8 nitrotyrosine signature has a causative or merely correlative effect on complex I activity. Therefore, siRNA technology was employed to test the importance of the NDUFB8 subunit in mitochondrial function. HPMVECs expressing less NDUFB8 (Fig 5F) exhibited significantly reduced overall mitochondrial oxygen consumption when compared to untreated or scrambled siRNA expressed cells (Fig. 5G), indicating the importance of this subunit in mitochondrial function.
Figure 5.
Induction of NO-mediated complex I subunit NDUFB8 nitration in endothelial cells requires mitochondrial O2․-. (A) HPMVECs were permeabilized with ICM Buffer containing digitonin [40] and exposed to DeaNONOate (100 μM) for the indicated times. Immunoprecipitation was performed with NDUFB8 antibody and immunoblotted with an anti-nitrotyrosine antibody. Bound NDUFB8 protein was probed for specificity and equal loading. (B) Similarly, HPMVECs exposed to DeaNONOate (100 μM) for the indicated times were immunoprecipitated with 19 kDa Complex I (NADH dehydrogenase) subunit GRIM-19 and probed with anti-nitrotyrosine antibody. (C) Dose-dependent nitration of NDUFB8 by DeaNONOate. (D) HPMVECs were infected with Ad5CMVMnSOD and cell lysate analyzed by immunoblotting with anti-MnSOD antibody. ** indicates the nonspecific protein band as loading control. (E) HPMVECs overexpressing MnSOD and control cells were stimulated with 100 μM DeaNONOate for 30 min. Cell lysates were then immunoreacted with NDUFB8 antibody to detect NDUFB8 nitration. (F) Western immunoblotting demonstrated a significant reduction in NDUFB8 protein expression when HPMVECs were transfected with NDUFB8 siRNA as compared to control-siRNA transfected cells 48 hr post transfection. Representative immunoblot is shown of 2 independent measurements. (G) Mitochondrial oxygen consumption in response to the Complex IV substrates TMPD/Asc (0.4 mM/2.5 mM) was measured in digitonin-permeabilized HPMVECs (mean±S.E.M., n=3).
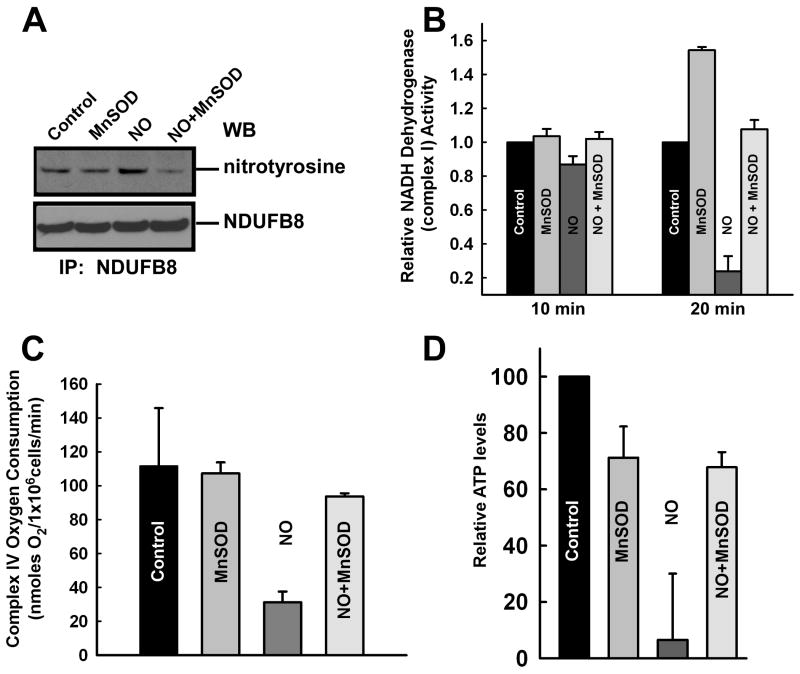
Figure 6.
Reduction of mitochondrial O2․- suppresses NO-mediated mitochondrial dysfunction. Permeabilized HPMVECs were exposed to DeaNONOate (10 μM) for 30 min. HPMVECs were infected with Ad5CMVMnSOD for 48 hr and these cells were exposed to DeaNONOate. (A) Immunoprecipitation was performed with NDUFB8 antibody and immunoblotted with an anti-nitrotyrosine antibody. (B) DeaNONOate induced a significant reduction in complex I activity that was restored by overexpression of MnSOD (mean±S.E.M., n=3). (C) Endothelial cells overexpressing MnSOD prevented the DeaNONOate-induced reduction in complex IV mitochondrial oxygen consumption. (D) MnSOD overexpressing HPMVECs were exposed to 10 μM DeaNONOate and ATP levels were assessed 12 hr following NO addition and normalized to untreated RPMVECs.
Measuring complex I activity specifically, nitration of NDUFB8 by 10 μM DeaNONOate (Fig. 6A) was associated with a progressive inhibition in complex I activity that reached 60-70% after 20 min (Fig. 6B). Overexpression of MnSOD prevented the NO-induced decrease in Complex I activity, suggesting that mitochondrial-derived O2․- is the key determinant that dictates the mitochondrial targeting action of NO. One predicted consequence of decreased mitochondrial complex activity is a depletion of cellular ATP levels and a reduction in oxygen consumption. MnSOD overexpression similarly rescued the NO-mediated reduction in overall mitochondrial oxygen consumption and ATP production (Fig. 6C and D), indicating that the NO-induced endothelial cell dysfunction is due to mitochondrial formation of peroxynitrite. Moreover, this data strongly suggest that the mitochondrial membrane depolarization, and resultant decreases in oxygen consumption and ATP production can be attributed not to NO per se, but rather to the interaction of NO with mitochondrially-derived O2․- and the resultant formation of peroxynitrite.
ROS and RIP1 and RIP3 are requisite for NO-mediated cell death
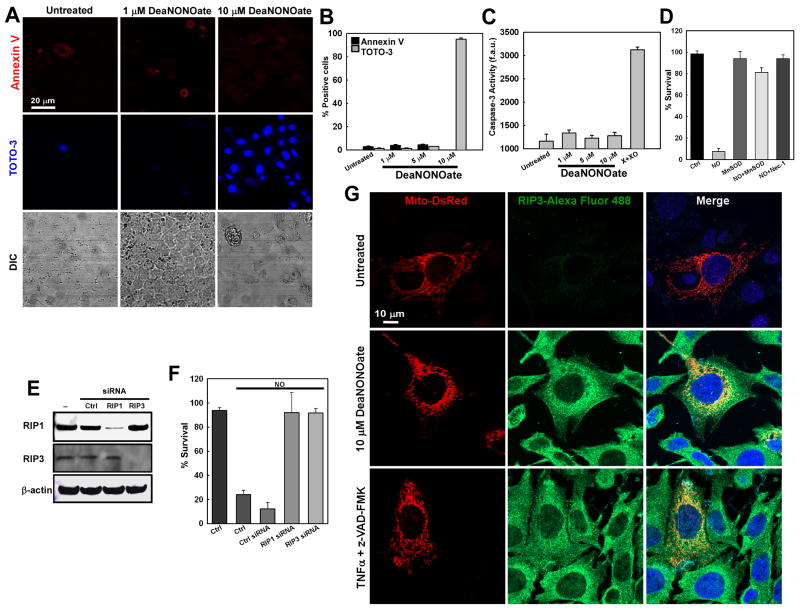
Plasma membrane protrusions, as observed in Fig. 1 and 2, are indicative of cell death. Coupled with mitochondrial depolarization (Fig. 4A – C) and reductions in ATP production (Fig. 4E and F), our studies suggest that mitochondrial NO-targeting triggers bioenergetic collapse and cell death. Because NO can contribute to both apoptosis and necrosis, we next investigated the effects of varying concentrations of NO on cell viability. Cells treated with NO for 14 hr were stained for the early apoptotic marker annexin V and the necrotic or late apoptotic marker TOTO-3. NO treatment (10 μM) resulted in significant cell death as evidenced by positive TOTO-3 staining, indicative of either late stage apoptosis or necrosis (Fig. 7A and B). Lower concentrations of NO (1.0 and 5.0 μM) did not trigger cell death, and treated cells were similar to untreated cells (Fig. 7B). To delineate whether NO-induced death occurred through apoptotic or necrotic pathways, we next measured caspase-3 activation following NO exposure. Caspase-3 activity was unaltered by NO treatment, and was similar to that of untreated cells (Fig. 7C). Together, these data indicate that NO-induced cell death results primarily from necrosis.
Figure 7.
ROS, RIP1 and RIP3 are necessary for NO-mediated necrotic cell death. (A) RPMVECs were exposed to 1 and 10 μM DeaNONOate for 14 hr and stained for phosphatidylserine externalization (Annexin V) and plasma membrane permeabilization (TOTO-3), indicative of early stage apoptosis and late stage apoptosis or necrosis, respectively. (B) Quantitation of endothelial cell death in 3 independent experiments (mean±S.E.M.). (C) After 14 hr exposure, RPMVEC lysates were assessed for caspase-3 activity (mean±S.E.M., n=3). (D) HPMVECs were treated with DeaNONOate (10 μM) for hr with or without Ad5CMVMnSOD and 30 μM Necrostatin-1. Cell viability was assessed by propidium iodide uptake (mean ± S.E.M.; n=6). (E) RIP1 and RIP3 protein levels following siRNA gene silencing 48 hr postransfection in HEK 293T cells. (F) 293T cells were transfected with control or RIP1 and RIP3 siRNAs. Forty-eight hours postransfection, cells were treated with DeaNONOate (10 μM) for additional 14 hr. The cell viability was determined by PI uptake. Data are represented as mean ± S.E.M., n=6. (G) Immunocytochemistry of RIP3 expression and localization in Mito-DsRed transfected MEFs exposed to either 10 μM DeaNONOate or TNFα+z-VAD-FMK (100 ng/ml and 50 μM). RIP3 was imaged using an Alexa Fluor 488-conjugated secondary antibody.
Receptor interacting protein (RIP) kinases are a family of proteins that serve as crucial regulators of cell survival and cell death [46]. In response to inflammatory cytokines or pathogens, RIP1 utilizes its kinase function to initiate cell survival through NF-κB activation. When cellular energy levels decrease, that same kinase function activates caspase-8 and initiates apoptotic cell death. However, when cellular ATP diminishes further, the kinase function of RIP1 is inactive, and necrotic cell death ensues. While the exact mechanism whereby RIP1 governs cell fate is incompletely understood, RIP1-mediated cell death is thought to be dependent upon mitochondrial function and ROS generation [27, 28]. As our studies demonstrate mitochondrial dysfunction and a decrease in cellular energy production, we next sought to identify whether NO-mediated necrotic cell death is regulated by RIP kinases. MnSOD overexpression inhibited NO-dependent necrosis (Fig. 7D). Similarly, treatment of cells with Nec-1 attenuated NO-elicited necrosis. Recent reports have implicated both RIP1 and RIP3 for death receptor-induced cell death [25-27, 29]. Since MnSOD and Nec-1 abolished the NO-induced necrosis, we next examined whether knockdown of RIP 1 and 3 kinases also have a role in NO-induced necrosis. siRNA silencing of RIP1 and RIP3 with four combined human siRNA oligos demonstrated a efficient knockdown of both kinases and each serves as a control (Fig. 7E). Consistent with the observed resistance to necrosis following Nec-1, RIP1 and RIP3 knockdown cells were more resistant to necrosis induced by NO (Fig. 7F) indicating that cellular resistance to necrosis requires RIP kinases and antioxidants. To date, RIP1 and RIP3-mediated cell death has been linked to receptor-mediated signaling cascades. However, our data demonstrate a receptor-independent, RIP1 and RIP3-mediated mode of programmed necrosis via NO. The necroptosis initiating combination of TNFα+z-VAD-FMK resulted in a robust increase in RIP3 expression in MEFs [28] that was similar to that observed following exposure to NO (Fig. 7G). While increased globally, a discrete perinuclear RIP3 localization was observed that colocalized with the mitochondria (Mito-DsRed expression). No increase in mitochondrial association of RIP1 was noted (data not shown), suggesting that RIP3 is the key initiator of NO-mediated necrotic cell death. Together these results show that overexpression of MnSOD and inhibition of RIP3 (via RIP1) by Nec-1 modulate necrosis in human cells induced by RNS and ROS.
Discussion
It has been shown that NO interacts with the mitochondrion [47] and can influence cellular bioenergetics [14, 15] and oxygen consumption [48, 49]. In the present study, we utilized intact cells to identify a novel molecular mitochondrial target of NO and how nitration of this target impacts cell bioenergetics. We demonstrate that mitochondrial-generated peroxynitrite targets the NADH dehydrogenase (ubiquinone) I beta subcomplex 8 (NDUFB8) in intact cells; which is associated with alterations in complex I enzymatic activity and endothelial bioenergetics. NDUFB8 is a nuclear-encoded component of mitochondrial complex I with no known function, and is primarily thought to be an accessory subunit not involved in catalysis [50]. However, genetic screening has implicated that polymorphisms in NDUFB8 may be involved in neurodegeneration [51]. To our knowledge, this is the first study indicating an important role for NDUFB8 in mitochondrial function.
Potential molecular targets for NO in the mitochondria include heme proteins, thiols and O2․-. The reaction of NO with O2․- is particularly intriguing, since neither NO nor O2․- is particularly toxic in vivo. However, the reaction of the two molecules forms the much more powerful oxidant peroxynitrite [52], which inhibits electron transport chain complexes more potently than NO [53, 54]. In our studies, O2․- formed as a byproduct of mitochondrial respiration is sufficient to generate peroxynitrite in the presence of NO. In support, NO rapidly targets to the mitochondria in unstimulated endothelial cells (Fig. 3). In contrast, the increase in DAF fluorescence was abolished by overproduction of O2․- in both the extracellular (X + XO) (Fig. S1B and E) and intracellular (antimycin A) milieu (Fig. S1C and F). Therefore, exaggerated production of O2․- serves to limit the ability of NO to target the mitochondria, likely by facilitating nitrosative modification of additional, non-specific proteins. Importantly, only physiologic mitochondrial O2․- generation by intact mitochondria led to mitochondrial localization of DAF fluorescence following NO exposure. In contrast to O2․- generation under physiologic conditions, elevated mitochondrial ROS production during inflammatory conditions may lead to increased NDUFB8 nitration and inactivation of complex I. In support, overexpression of MnSOD has previously been shown to ameliorate cellular inflammation and injury [33].
Donor compounds such as DeaNONOate are commonly added to cell culture models to elucidate the role of NO in various cellular pathways. Chronic (24 hrs) exposure of endothelial cells to 500 μM of a longer acting NO donor (DetaNONOate) was recently shown to alter both mitochondrial complex I and IV activity without affecting cell viability [43]. In this regard, NO donors may afford cellular protection against ischemic insult by decreasing tissue oxygen demand [17, 55]. In contrast, our present work demonstrates mitochondrial functional alterations that triggered endothelial dysfunction with 10 μM NO by the rapid NO donor DeaNONOate. Factors that may contribute to these differential responses include the concentration, duration, kinetics of NO liberation by a particular NO donor, cell type, and the specific reactive nitrogen species employed. Indeed, our studies utilized activated macrophages as a NO source, which generate significantly higher NO levels via iNOS than constitutive endothelial specific eNOS, and suggest that small amounts or NO may be endothelial protective. However, exaggerated NO levels during inflammatory conditions may induce deleterious NO-mediated protein modifications that contribute to endothelial dysfunction rather than offer protection from insult. Further, our studies were conducted in serum-free conditions. In fact, when identical experiments were conducted using serum-containing buffer, we required a ∼50 fold higher NO concentration to elicit similar a DAF fluorescence increase and alterations in mitochondrial membrane potential (Fig. S6). The concentration needed to evoke such a response far exceeds NO levels attained during septic shock [56], and thus may be of limited physiological relevance. Because a large fraction of the proteins found in serum is albumin, a known sink for NO [57], it is plausible that the difference in effective NO concentrations could be due to NO consumption by serum proteins.
Mitochondrial membrane potential loss and consequent depletion of intracellular ATP are hallmarks of both apoptotic and necrotic cell death. In our studies, exposure of cells to lower levels of DeaNONOate did not cause mitochondrial membrane potential alterations or cellular injury. However, 10 μM DeaNONOate triggered plasma membrane bleb-like protrusions (Fig. 1) that correlated with mitochondrial NO accumulation and a loss in membrane potential (Figs. 3 and 4, respectively). While individually non-specific for apoptosis and necrosis, these protrusions led us to hypothesize that NO may evoke endothelial dysfunction. Interestingly, NO evoked mediated mitochondrial membrane potential loss (Fig. 4C), indicating that NO sequestration in the mitochondria may be a key factor in NO-induced alterations in endothelial bioenergetics. We do not suggest that our work implicates constitutive endothelial NO production in endothelial dysfunction. Rather, our co-culture studies indicate that macrophage-derived mitochondrial NO accumulation, NDUFB8 nitration, and endothelial cell death may be an important mechanism during inflammatory conditions.
Endothelial cells are a major source of both ROS and RNS, and excess peroxynitrite formation evokes endothelial dysfunction. In vitro, peroxynitrite preferentially reacts with cysteine and tyrosine residues in the mitochondrial respiratory chain complexes. However, mitochondrial complex I inhibition is normally observed using higher peroxynitrite concentrations [12]. In the present study, mitochondrial-derived O2․- is the key determinant of nitrotyrosine formation even in the presence of excess NO, as it has a shorter biological half-life than NO [58] and is selectively permeable to membranes [31]. Therefore, sites of O2․- generation serve to target specific proteins for nitration. This is especially true of complex I, which is a major site of O2․- production in endothelial cells (Fig. S6). MnSOD is a mitochondrial-specific antioxidant enzyme that is essential for maintaining normal cell development and function, and plays a central role in protecting cells and tissues from oxidant stress [59, 60]. A reduction in O2․- bioavailability by overexpression of MnSOD (Figs. 5D Fig. S3) decreased tyrosine nitration of NDUFB8 and prevented bioenergetic collapse in endothelial cells exposed to high doses of NO. Peroxynitrite (and likely, other NO species) has been shown to inhibit complex I activity [11] and in the present study, decreased complex I activity could be inhibited by overexpression of MnSOD, which effectively limits peroxynitrite formation.
Complex I activity is considered an important factor in the regulation of mitochondrial respiration, and therefore a reduction in activity should correspond to a decrease in oxygen consumption. We found a significant reduction in mitochondrial oxygen consumption at complex IV by NO that could be reversed by overexpression of MnSOD, indicating that mitochondrial reactive oxygen species are the key determinant that dictates the effect of NO on the mitochondria. Physiologically, mitochondrial oxygen consumption occurs at complex IV in the mitochondrial electron transport chain, and we observed only minor differences in oxygen consumption following addition of either the complex I substrates malate and pyruvate or the complex II substrate succinate in permeabilized endothelial cells, possibly owing to a higher reliance on glycolysis for energy production [61]. However, dramatic and consistent differences were observed by the addition of the complex IV substrates TMPD and ascorbate. These differences in complex IV oxygen consumption were also noted when NDUFB8 expression was inhibited, even in the absence of NO (Fig. 5F and 5G). Recent data has demonstrated the formation of mitochondrial supercomplexes [62] that are necessary for mitochondrial respiration. We therefore hypothesize that NDUFB8 is a vital structural component in mitochondrial supercomplex formation, and that selective nitration of NDUFB8 leads to dissociation of mitochondrial supercomplexes and the disruption of complex I activity and mitochondrial function.
Serine-threonine RIP kinases play an essential in necrotic cell death [25, 27, 29]. Specifically, RIP1 kinase and RIP3 complex is necessary for vaccinia virus infection-mediated programmed necrosis [28]. It has been implicated that ROS play a crucial role in proinflammatory cytokine-induced necrosis via RIP complex dependent manner [29, 63], and allosteric RIP1 inhibition by necrostatins [26, 64] abolished Fas/TNFα+z-VAD-FMK induced necrosis. Our results introduce two key concepts: One, that mitochondrial-derived ROS is involved in NO-induced necrotic cell death and can be prevented by overexpression of MnSOD or inihibition of RIP1 by nec-1 (Figure 7D), and two, that RIP kinases can function in a receptor-independent manner. In support, receptor-independent NO treatment induced a similar increase in RIP3 expression as the necroptosis initiating combination of TNFα+z-VAD-FMK. Interestingly, RIP3 appeared to associate with the mitochondria, implicating the mitochondria as an important regulator of RIP-mediated necrosis, possibly through interactions with the Bcl2 family proteins Bax and Bak (Fig. S7). Our study has not excluded the potential contribution of nonmitochondrial-derived ROS in RIP1 and 3-mediated necrosis. Rather, our results indicate that mitochondrial ROS is requisite specifically for the NO-induced, RIP-mediated necrosis in response to elevated NO levels that would occur during the inflammatory response.
Our study establishes that mitochondrial O2․- is necessary for the signaling process that links the NO dependent reduction in mitochondrial complex I activity to necrotic cell death. Therefore, it is likely that mitochondrial-derived O2․- functions as a pathologic signaling molecule that facilitates the nitration of the complex I subunit NDUFB8. Our data also suggest nitration of complex I leads to loss of activity that is crucial for mitochondrial depolarization, energy imbalance, and cell viability. Together with the known protective effects of low concentrations of NO, our work now defines a ROS/RNS/RIP interplay that serves as a switch between physiologic and pathologic cell function.
Supplementary Material
Acknowledgments
We thank Drs. Harry Ischiropoulos and Aron B. Fisher for their helpful thoughts and suggestions, Dr. Craig B. Thompson for discussion and the Bax/Bak double knockout MEFs, and Dr. Troy Stevens for RPMVECS. We thank the Vector Core Facility of the University of Iowa for AdMnSOD construct. This work was supported by an AHA Scientist Development Grant (0530087N), National Institutes of Health Grants (HL086699, 1S10RR022511), and a University Research Foundation awarded to M.M. C.W.D. was supported by a NHLBI NRSA postdoctoral fellowship (T32 HL7748). B.J.H. was supported by a postdoctoral fellowship from the Pulmonary Hypertension Association and a National Institutes of Health K99/R00 grant (K99HL094536).
Abbreviations List
- NO
Nitric Oxide
- NDUFB8
NADH dehydrogenase (ubiquinone) 1 beta subcomplex 8
- O2․-
Superoxide
- MnSOD
Manganese-Superoxide Dismutase
- eNOS
Endothelial nitric oxide synthase
- ROS
Reactive oxygen species
- RNS
Reactive nitrogen species
- RPMVECs
Rat pulmonary microvascular endothelial cells
- HPMVECs
Human pulmonary microvascular endothelial cells
- DeaNONOate
Diethylamine NONOate
- X+XO
Xanthine + Xanthine Oxidase
- TMRE
Tetramethylrhodamine ethyl ester perchlorate
- DAF
Diaminofluorescein
- ΔΨm
Mitochondrial membrane potential
- nec-1
necrostatin-1
- RIPK
receptor interacting protein kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bredt DS. Endogenous nitric oxide synthesis: biological functions and pathophysiology. Free Radic Res. 1999;31:577–596. doi: 10.1080/10715769900301161. [DOI] [PubMed] [Google Scholar]
- 2.van der Vliet A, Eiserich JP, Cross CE. Nitric oxide: a pro-inflammatory mediator in lung disease? Respir Res. 2000;1:67–72. doi: 10.1186/rr14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng J, Wen Y, Austin JL, Chen DB. Exogenous nitric oxide stimulates cell proliferation via activation of a mitogen-activated protein kinase pathway in ovine fetoplacental artery endothelial cells. Biol Reprod. 2006;74:375–382. doi: 10.1095/biolreprod.105.043190. [DOI] [PubMed] [Google Scholar]
- 4.Dimmeler S, Zeiher AM. Nitric oxide-an endothelial cell survival factor. Cell Death Differ. 1999;6:964–968. doi: 10.1038/sj.cdd.4400581. [DOI] [PubMed] [Google Scholar]
- 5.Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 6.Gow AJ, Ischiropoulos H. Nitric oxide chemistry and cellular signaling. J Cell Physiol. 2001;187:277–282. doi: 10.1002/jcp.1085. [DOI] [PubMed] [Google Scholar]
- 7.Khan SA, Lee K, Minhas KM, Gonzalez DR, Raju SV, Tejani AD, Li D, Berkowitz DE, Hare JM. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc Natl Acad Sci U S A. 2004;101:15944–15948. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madesh M, Balasubramanian KA. Nitric oxide inhibits enterocyte mitochondrial phospholipase D. FEBS Lett. 1997;413:269–272. doi: 10.1016/s0014-5793(97)00903-4. [DOI] [PubMed] [Google Scholar]
- 9.Dhanasekaran A, Kotamraju S, Karunakaran C, Kalivendi SV, Thomas S, Joseph J, Kalyanaraman B. Mitochondria superoxide dismutase mimetic inhibits peroxide-induced oxidative damage and apoptosis: role of mitochondrial superoxide. Free Radic Biol Med. 2005;39:567–583. doi: 10.1016/j.freeradbiomed.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Bhargava K, Keszler A, Feix J, Hogg N, Joseph J, Kalyanaraman B. Transmembrane nitration of hydrophobic tyrosyl peptides. Localization, characterization, mechanism of nitration, and biological implications. J Biol Chem. 2003;278:8969–8978. doi: 10.1074/jbc.M211561200. [DOI] [PubMed] [Google Scholar]
- 11.Riobo NA, Clementi E, Melani M, Boveris A, Cadenas E, Moncada S, Poderoso JJ. Nitric oxide inhibits mitochondrial NADH:ubiquinone reductase activity through peroxynitrite formation. Biochem J. 2001;359:139–145. doi: 10.1042/0264-6021:3590139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray J, Taylor SW, Zhang B, Ghosh SS, Capaldi RA. Oxidative damage to mitochondrial complex I due to peroxynitrite: identification of reactive tyrosines by mass spectrometry. J Biol Chem. 2003;278:37223–37230. doi: 10.1074/jbc.M305694200. [DOI] [PubMed] [Google Scholar]
- 13.Cooper CE, Davies NA, Psychoulis M, Canevari L, Bates TE, Dobbie MS, Casley CS, Sharpe MA. Nitric oxide and peroxynitrite cause irreversible increases in the K(m) for oxygen of mitochondrial cytochrome oxidase: in vitro and in vivo studies. Biochim Biophys Acta. 2003;1607:27–34. doi: 10.1016/j.bbabio.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Beltran B, Mathur A, Duchen MR, Erusalimsky JD, Moncada S. The effect of nitric oxide on cell respiration: A key to understanding its role in cell survival or death. Proc Natl Acad Sci U S A. 2000;97:14602–14607. doi: 10.1073/pnas.97.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci U S A. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chinta SJ, Andersen JK. Reversible inhibition of mitochondrial complex I activity following chronic dopaminergic glutathione depletion in vitro: implications for Parkinson's disease. Free Radic Biol Med. 2006;41:1442–1448. doi: 10.1016/j.freeradbiomed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Burwell LS, Brookes PS. Mitochondria as a target for the cardioprotective effects of nitric oxide in ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10:579–599. doi: 10.1089/ars.2007.1845. [DOI] [PubMed] [Google Scholar]
- 18.Brookes PS, Levonen AL, Shiva S, Sarti P, Darley-Usmar VM. Mitochondria: regulators of signal transduction by reactive oxygen and nitrogen species. Free Radic Biol Med. 2002;33:755–764. doi: 10.1016/s0891-5849(02)00901-2. [DOI] [PubMed] [Google Scholar]
- 19.Nojiri H, Shimizu T, Funakoshi M, Yamaguchi O, Zhou H, Kawakami S, Ohta Y, Sami M, Tachibana T, Ishikawa H, Kurosawa H, Kahn RC, Otsu K, Shirasawa T. Oxidative stress causes heart failure with impaired mitochondrial respiration. J Biol Chem. 2006;281:33789–33801. doi: 10.1074/jbc.M602118200. [DOI] [PubMed] [Google Scholar]
- 20.Popovich M, Kostin S, Branishte T, Kobets V, Kapelko V. Cellular hypertrophy in cardiomyopathic patients is associated with lower creatine-stimulated mitochondrial respiration. Mol Cell Biochem. 1995;143:1–5. doi: 10.1007/BF00925920. [DOI] [PubMed] [Google Scholar]
- 21.Davies NA, Cooper CE, Stidwill R, Singer M. Inhibition of mitochondrial respiration during early stage sepsis. Adv Exp Med Biol. 2003;530:725–736. doi: 10.1007/978-1-4615-0075-9_73. [DOI] [PubMed] [Google Scholar]
- 22.Goossens V, Grooten J, De Vos K, Fiers W. Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proc Natl Acad Sci U S A. 1995;92:8115–8119. doi: 10.1073/pnas.92.18.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madesh M, Zong WX, Hawkins BJ, Ramasamy S, Venkatachalam T, Mukhopadhyay P, Doonan PJ, Irrinki KM, Rajesh M, Pacher P, Thompson CB. Execution of superoxide-induced cell death by the proapoptotic Bcl-2-related proteins Bid and Bak. Mol Cell Biol. 2009;29:3099–3112. doi: 10.1128/MCB.01845-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 25.Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 30.Madesh M, Hawkins BJ, Milovanova T, Bhanumathy CD, Joseph SK, Ramachandrarao SP, Sharma K, Kurosaki T, Fisher AB. Selective role for superoxide in InsP3 receptor-mediated mitochondrial dysfunction and endothelial apoptosis. J Cell Biol. 2005;170:1079–1090. doi: 10.1083/jcb.200505022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawkins BJ, Madesh M, Kirkpatrick CJ, Fisher AB. Superoxide flux in endothelial cells via the chloride channel-3 mediates intracellular signaling. Mol Biol Cell. 2007;18:2002–2012. doi: 10.1091/mbc.E06-09-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thom SR, Fisher D, Zhang J, Bhopale VM, Ohnishi ST, Kotake Y, Ohnishi T, Buerk DG. Stimulation of perivascular nitric oxide synthesis by oxygen. Am J Physiol Heart Circ Physiol. 2003;284:H1230–1239. doi: 10.1152/ajpheart.01043.2002. [DOI] [PubMed] [Google Scholar]
- 33.Hawkins BJ, Solt LA, Chowdhury I, Kazi AS, Abid MR, Aird WC, May MJ, Foskett JK, Madesh M. G protein-coupled receptor Ca2+-linked mitochondrial reactive oxygen species are essential for endothelial/leukocyte adherence. Mol Cell Biol. 2007;27:7582–7593. doi: 10.1128/MCB.00493-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birch-Machin MA, Turnbull DM. Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. Methods Cell Biol. 2001;65:97–117. doi: 10.1016/s0091-679x(01)65006-4. [DOI] [PubMed] [Google Scholar]
- 35.Titheradge MA. Nitric oxide in septic shock. Biochim Biophys Acta. 1999;1411:437–455. doi: 10.1016/s0005-2728(99)00031-6. [DOI] [PubMed] [Google Scholar]
- 36.Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- 37.Iwakiri Y, Satoh A, Chatterjee S, Toomre DK, Chalouni CM, Fulton D, Groszmann RJ, Shah VH, Sessa WC. Nitric oxide synthase generates nitric oxide locally to regulate compartmentalized protein S-nitrosylation and protein trafficking. Proc Natl Acad Sci U S A. 2006;103:19777–19782. doi: 10.1073/pnas.0605907103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sessa WC, Garcia-Cardena G, Liu J, Keh A, Pollock JS, Bradley J, Thiru S, Braverman IM, Desai KM. The Golgi association of endothelial nitric oxide synthase is necessary for the efficient synthesis of nitric oxide. J Biol Chem. 1995;270:17641–17644. doi: 10.1074/jbc.270.30.17641. [DOI] [PubMed] [Google Scholar]
- 39.Chami M, Oules B, Szabadkai G, Tacine R, Rizzuto R, Paterlini-Brechot P. Role of SERCA1 truncated isoform in the proapoptotic calcium transfer from ER to mitochondria during ER stress. Mol Cell. 2008;32:641–651. doi: 10.1016/j.molcel.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madesh M, Hajnoczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J Cell Biol. 2001;155:1003–1015. doi: 10.1083/jcb.200105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Csordas G, Madesh M, Antonsson B, Hajnoczky G. tcBid promotes Ca(2+) signal propagation to the mitochondria: control of Ca(2+) permeation through the outer mitochondrial membrane. EMBO J. 2002;21:2198–2206. doi: 10.1093/emboj/21.9.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McClintock DS, Santore MT, Lee VY, Brunelle J, Budinger GR, Zong WX, Thompson CB, Hay N, Chandel NS. Bcl-2 family members and functional electron transport chain regulate oxygen deprivation-induced cell death. Mol Cell Biol. 2002;22:94–104. doi: 10.1128/MCB.22.1.94-104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramachandran A, Ceaser E, Darley-Usmar VM. Chronic exposure to nitric oxide alters the free iron pool in endothelial cells: role of mitochondrial respiratory complexes and heat shock proteins. Proc Natl Acad Sci U S A. 2004;101:384–389. doi: 10.1073/pnas.0304653101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 45.Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell's decision to live or die. Cell Death Differ. 2007;14:400–410. doi: 10.1038/sj.cdd.4402085. [DOI] [PubMed] [Google Scholar]
- 47.Ghafourifar P, Schenk U, Klein SD, Richter C. Mitochondrial nitric-oxide synthase stimulation causes cytochrome c release from isolated mitochondria. Evidence for intramitochondrial peroxynitrite formation. J Biol Chem. 1999;274:31185–31188. doi: 10.1074/jbc.274.44.31185. [DOI] [PubMed] [Google Scholar]
- 48.Cooper CE, Giulivi C. Nitric oxide regulation of mitochondrial oxygen consumption II: Molecular mechanism and tissue physiology. Am J Physiol Cell Physiol. 2007;292:C1993–2003. doi: 10.1152/ajpcell.00310.2006. [DOI] [PubMed] [Google Scholar]
- 49.Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol. 2002;3:214–220. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- 50.Loeffen JL, Triepels RH, van den Heuvel LP, Schuelke M, Buskens CA, Smeets RJ, Trijbels JM, Smeitink JA. cDNA of eight nuclear encoded subunits of NADH:ubiquinone oxidoreductase: human complex I cDNA characterization completed. Biochem Biophys Res Commun. 1998;253:415–422. doi: 10.1006/bbrc.1998.9786. [DOI] [PubMed] [Google Scholar]
- 51.Emahazion T, Jobs M, Howell WM, Siegfried M, Wyoni PI, Prince JA, Brookes AJ. Identification of 167 polymorphisms in 88 genes from candidate neurodegeneration pathways. Gene. 1999;238:315–324. doi: 10.1016/s0378-1119(99)00330-3. [DOI] [PubMed] [Google Scholar]
- 52.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 53.Cassina A, Radi R. Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys. 1996;328:309–316. doi: 10.1006/abbi.1996.0178. [DOI] [PubMed] [Google Scholar]
- 54.Castro LA, Robalinho RL, Cayota A, Meneghini R, Radi R. Nitric oxide and peroxynitrite-dependent aconitase inactivation and iron-regulatory protein-1 activation in mammalian fibroblasts. Arch Biochem Biophys. 1998;359:215–224. doi: 10.1006/abbi.1998.0898. [DOI] [PubMed] [Google Scholar]
- 55.Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E. Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res. 2007;101:1155–1163. doi: 10.1161/CIRCRESAHA.107.155879. [DOI] [PubMed] [Google Scholar]
- 56.Santak B, Radermacher P, Iber T, Adler J, Wachter U, Vassilev D, Georgieff M, Vogt J. In vivo quantification of endotoxin-induced nitric oxide production in pigs from Na15NO3-infusion. Br J Pharmacol. 1997;122:1605–1610. doi: 10.1038/sj.bjp.0701553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keaney JF, Jr, Simon DI, Stamler JS, Jaraki O, Scharfstein J, Vita JA, Loscalzo J. NO forms an adduct with serum albumin that has endothelium-derived relaxing factor-like properties. J Clin Invest. 1993;91:1582–1589. doi: 10.1172/JCI116364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33:1451–1464. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- 59.Fridovich I, Freeman B. Antioxidant defenses in the lung. Annu Rev Physiol. 1986;48:693–702. doi: 10.1146/annurev.ph.48.030186.003401. [DOI] [PubMed] [Google Scholar]
- 60.Tsan MF. Superoxide dismutase and pulmonary oxygen toxicity. Proc Soc Exp Biol Med. 1997;214:107–113. doi: 10.3181/00379727-214-44076. [DOI] [PubMed] [Google Scholar]
- 61.Quintero M, Colombo SL, Godfrey A, Moncada S. Mitochondria as signaling organelles in the vascular endothelium. Proc Natl Acad Sci U S A. 2006;103:5379–5384. doi: 10.1073/pnas.0601026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Acin-Perez R, Fernandez-Silva P, Peleato ML, Perez-Martos A, Enriquez JA. Respiratory active mitochondrial supercomplexes. Mol Cell. 2008;32:529–539. doi: 10.1016/j.molcel.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 63.Kim YS, Morgan MJ, Choksi S, Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007;26:675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 64.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.