Abstract
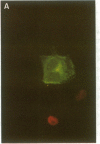
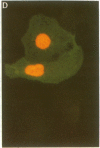
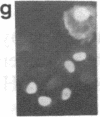
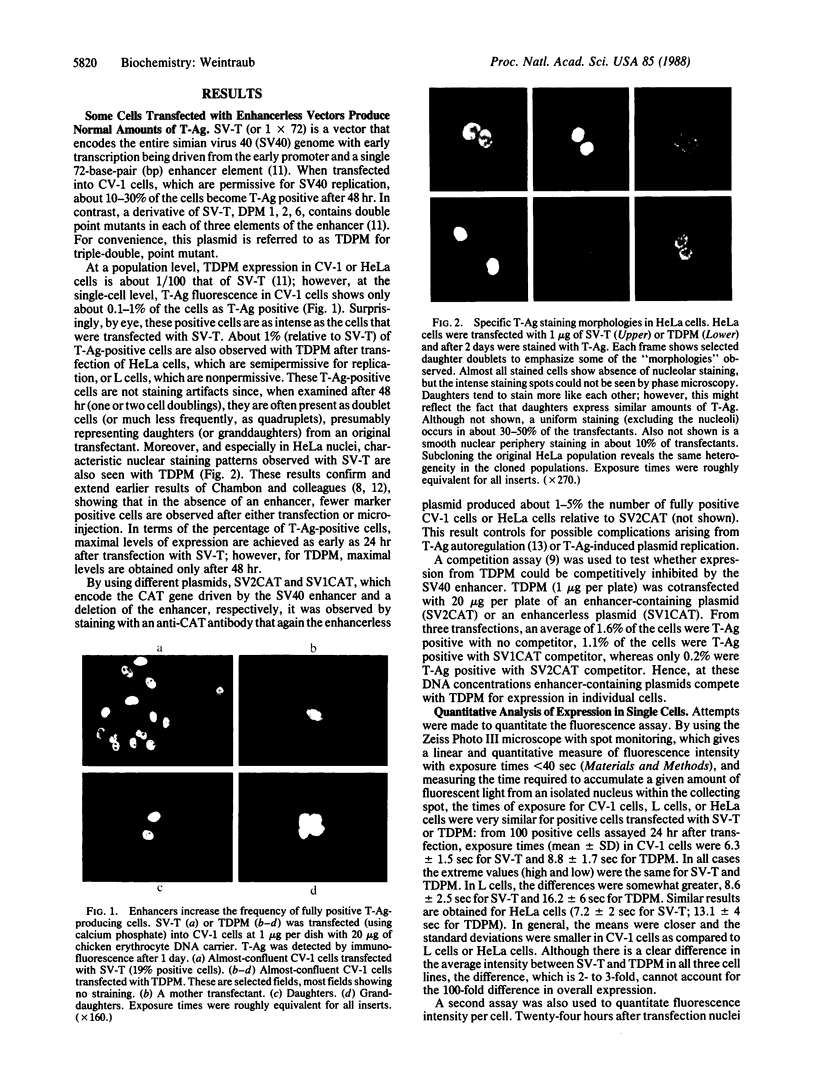
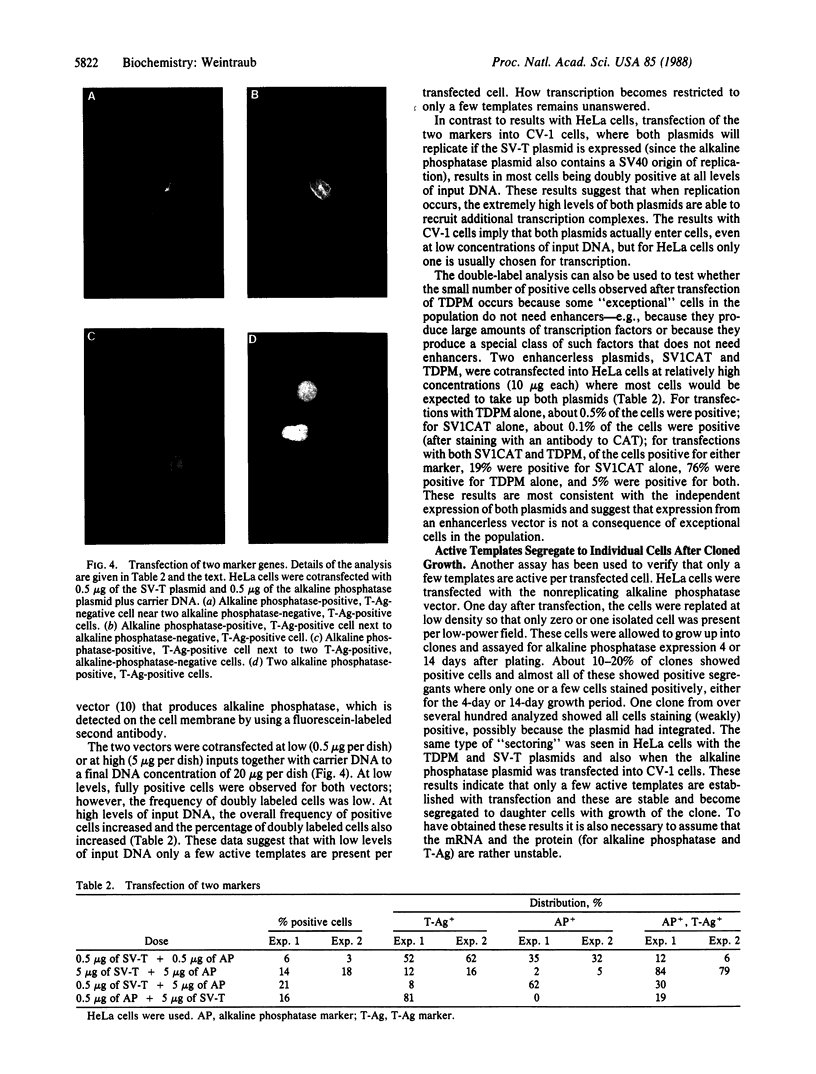
Conditions were established where transient transfection of two marker genes resulted in the expression of one or the other, but not both, in individual cells as assayed by immunofluorescence. Thus, the expression from a single cell reflects the activity of single active transcription templates. Under these conditions, a vector encoding the simian virus 40 large tumor antigen (SV40 T-Ag) driven by the SV40 enhancer and early promoter was transfected into CV-1, L, or HeLa cells yielding, for all three cell types, about 10-30% T-Ag-positive cells as assayed by immunofluorescence. Similar vectors containing either mutated or deleted SV40 enhancers also gave T-Ag-positive cells, but at about 1/100 the frequency. Quantitative analysis showed that T-Ag-positive cells produced about the same amount of T-Ag whether or not an active enhancer was present. Chloramphenicol acetyltransferase-encoding vectors gave the same result. The data are consistent with the hypothesis that at a low, but finite, probability, fully functional transcription complexes can form on a given active template in the absence of enhancer DNA. Enhancers seem to increase the number of active templates. Subcloning experiments suggest that these transcription complexes can be surprisingly stable.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atchison M. L., Perry R. P. The role of the kappa enhancer and its binding factor NF-kappa B in the developmental regulation of kappa gene transcription. Cell. 1987 Jan 16;48(1):121–128. doi: 10.1016/0092-8674(87)90362-x. [DOI] [PubMed] [Google Scholar]
- Berger J., Howard A. D., Gerber L., Cullen B. R., Udenfriend S. Expression of active, membrane-bound human placental alkaline phosphatase by transfected simian cells. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4885–4889. doi: 10.1073/pnas.84.14.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli E., Hen R., Chambon P. Adenovirus-2 E1A products repress enhancer-induced stimulation of transcription. Nature. 1984 Dec 13;312(5995):608–612. doi: 10.1038/312608a0. [DOI] [PubMed] [Google Scholar]
- Chalifour L. E., Wirak D. O., Hansen U., Wassarman P. M., DePamphilis M. L. cis- and trans-acting sequences required for expression of simian virus 40 genes in mouse oocytes. Genes Dev. 1987 Dec;1(10):1096–1106. doi: 10.1101/gad.1.10.1096. [DOI] [PubMed] [Google Scholar]
- Dierich A., Gaub M. P., LePennec J. P., Astinotti D., Chambon P. Cell-specificity of the chicken ovalbumin and conalbumin promoters. EMBO J. 1987 Aug;6(8):2305–2312. doi: 10.1002/j.1460-2075.1987.tb02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echols H. Multiple DNA-protein interactions governing high-precision DNA transactions. Science. 1986 Sep 5;233(4768):1050–1056. doi: 10.1126/science.2943018. [DOI] [PubMed] [Google Scholar]
- Gaub M. P., Dierich A., Astinotti D., Touitou I., Chambon P. The chicken ovalbumin promoter is under negative control which is relieved by steroid hormones. EMBO J. 1987 Aug;6(8):2313–2320. doi: 10.1002/j.1460-2075.1987.tb02506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Clarke J. The SV40 enhancer is composed of multiple functional elements that can compensate for one another. Cell. 1986 May 9;45(3):461–470. doi: 10.1016/0092-8674(86)90332-6. [DOI] [PubMed] [Google Scholar]
- Klein S., Sablitzky F., Radbruch A. Deletion of the IgH enhancer does not reduce immunoglobulin heavy chain production of a hybridoma IgD class switch variant. EMBO J. 1984 Nov;3(11):2473–2476. doi: 10.1002/j.1460-2075.1984.tb02158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj I. W., Lienhard S., Jiricny J., De Robertis E. M. An enhancer-like sequence within the Xenopus U2 gene promoter facilitates the formation of stable transcription complexes. Nature. 1985 Jul 11;316(6024):163–167. doi: 10.1038/316163a0. [DOI] [PubMed] [Google Scholar]
- Moreau P., Hen R., Wasylyk B., Everett R., Gaub M. P., Chambon P. The SV40 72 base repair repeat has a striking effect on gene expression both in SV40 and other chimeric recombinants. Nucleic Acids Res. 1981 Nov 25;9(22):6047–6068. doi: 10.1093/nar/9.22.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. Gene regulation by proteins acting nearby and at a distance. Nature. 1986 Aug 21;322(6081):697–701. doi: 10.1038/322697a0. [DOI] [PubMed] [Google Scholar]
- Schöler H. R., Gruss P. Specific interaction between enhancer-containing molecules and cellular components. Cell. 1984 Feb;36(2):403–411. doi: 10.1016/0092-8674(84)90233-2. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Maniatis T. Simian virus 40 enhancer increases number of RNA polymerase II molecules on linked DNA. Nature. 1985 May 2;315(6014):73–75. doi: 10.1038/315072a0. [DOI] [PubMed] [Google Scholar]
- Wabl M. R., Burrows P. D. Expression of immunoglobulin heavy chain at a high level in the absence of a proposed immunoglobulin enhancer element in cis. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2452–2455. doi: 10.1073/pnas.81.8.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. F., Calame K. SV40 enhancer-binding factors are required at the establishment but not the maintenance step of enhancer-dependent transcriptional activation. Cell. 1986 Oct 24;47(2):241–247. doi: 10.1016/0092-8674(86)90446-0. [DOI] [PubMed] [Google Scholar]
- Weber F., Schaffner W. Simian virus 40 enhancer increases RNA polymerase density within the linked gene. Nature. 1985 May 2;315(6014):75–77. doi: 10.1038/315075a0. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Cheng P. F., Conrad K. Expression of transfected DNA depends on DNA topology. Cell. 1986 Jul 4;46(1):115–122. doi: 10.1016/0092-8674(86)90865-2. [DOI] [PubMed] [Google Scholar]
- Yakobson E., Revel M., Winocour E. Inhibition of simian virus 40 replication by interferon treatment late in the lytic cycle. Virology. 1977 Jul 1;80(1):225–228. doi: 10.1016/0042-6822(77)90397-x. [DOI] [PubMed] [Google Scholar]
- Zinn K., Maniatis T. Detection of factors that interact with the human beta-interferon regulatory region in vivo by DNAase I footprinting. Cell. 1986 May 23;45(4):611–618. doi: 10.1016/0092-8674(86)90293-x. [DOI] [PubMed] [Google Scholar]