Abstract
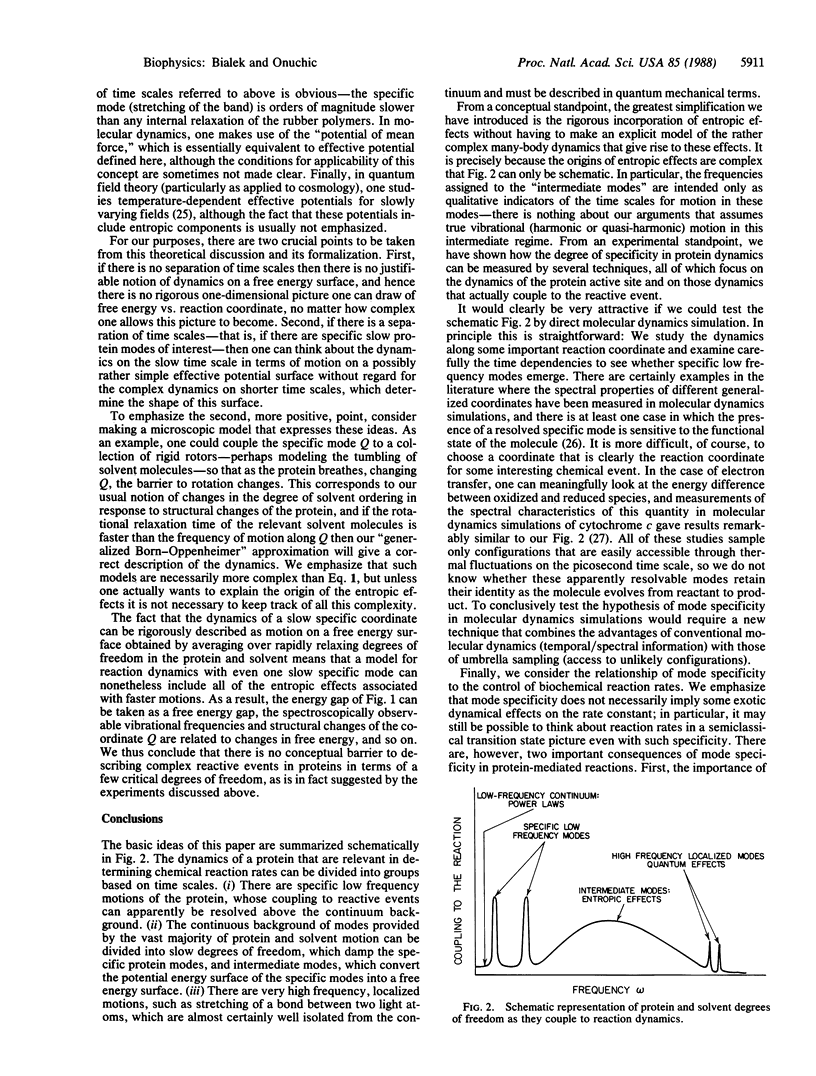
Reactive events in proteins may be strongly coupled to a few specific modes of protein motion or they may couple nonspecifically to the dense continuum of protein and solvent modes. We summarize the evidence that at least some biologically important reactions can be described in terms of a few specific modes, and we propose experiments to quantify the strength of coupling to the continuum. We also show that large entropic effects--solvent ordering, for example--can be rigorously incorporated in few-mode models without losing mode specificity. Within our description, the dynamics that determine chemical reaction rates can be summarized by a small number of parameters directly related to spectroscopic and thermodynamic data. Mode specificity allows protein dynamics to contribute directly to the control and specificity of biochemical reaction rates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberding N., Austin R. H., Beeson K. W., Chan S. S., Eisenstein L., Frauenfelder H., Nordlund T. M. Tunneling in ligand binding to heme proteins. Science. 1976 Jun 4;192(4243):1002–1004. doi: 10.1126/science.1273579. [DOI] [PubMed] [Google Scholar]
- Aqvist J., Sandblom P., Jones T. A., Newcomer M. E., van Gunsteren W. F., Tapia O. Molecular dynamics simulations of the holo and apo forms of retinol binding protein. Structural and dynamical changes induced by retinol removal. J Mol Biol. 1986 Dec 5;192(3):593–603. doi: 10.1016/0022-2836(86)90279-2. [DOI] [PubMed] [Google Scholar]
- Brooks B., Karplus M. Harmonic dynamics of proteins: normal modes and fluctuations in bovine pancreatic trypsin inhibitor. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6571–6575. doi: 10.1073/pnas.80.21.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVault D., Chance B. Studies of photosynthesis using a pulsed laser. I. Temperature dependence of cytochrome oxidation rate in chromatium. Evidence for tunneling. Biophys J. 1966 Nov;6(6):825–847. doi: 10.1016/s0006-3495(66)86698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield J. J. Electron transfer between biological molecules by thermally activated tunneling. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3640–3644. doi: 10.1073/pnas.71.9.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middendorf H. D. Biophysical applications of quasi-elastic and inelastic neutron scattering. Annu Rev Biophys Bioeng. 1984;13:425–451. doi: 10.1146/annurev.bb.13.060184.002233. [DOI] [PubMed] [Google Scholar]
- Okamura M. Y., Feher G. Isotope effect on electron transfer in reaction centers from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8152–8156. doi: 10.1073/pnas.83.21.8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant J. M. Heat capacity and entropy changes in processes involving proteins. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2236–2240. doi: 10.1073/pnas.74.6.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]


