SUMMARY
Several members of the RecQ family of DNA helicases are known to interact with DNA topoisomerase III. Here we show that the S. cerevisiae Sgs1 and Top3 proteins physically interact in cell extracts and bind directly in vitro. Sgs1 and Top3 proteins co-immunoprecipitate from cell extracts under stringent conditions, indicating that Sgs1 and Top3 are present in a stable complex. The domain of Sgs1 that interacts with Top3 was identified by expressing Sgs1 truncations in yeast. The results indicate that the N-terminal 158 amino acids of Sgs1 are sufficient for the high-affinity interaction between Sgs1 and Top3. In vitro assays using purified Top3 and N-terminal Sgs1 fragments demonstrate that at least part of the interaction is through direct protein-protein interactions with these 158 amino acids. Consistent with these physical data, we find that mutant phenotypes caused by a point mutation or small deletions in the Sgs1 N-terminus can be suppressed by Top3 overexpression. We conclude that Sgs1 and Top3 form a tight complex in vivo and that the first 158 amino acids of Sgs1 are necessary and sufficient for this interaction. Thus, a primary role of the Sgs1 amino terminus is to mediate the Top3 interaction.
INTRODUCTION
The Saccharomyces cerevisiae SGS1 gene encodes a member of the RecQ family of DNA helicases. In addition to the RecQ protein of E. coli, this family includes the human BLM, WRN, RECQL4 and RECQ5 proteins as well as Rqh1 from S. pombe (1–7). These proteins play an important role in DNA metabolism as mutations in the human genes give rise to diseases characterized by genome instability and a predisposition to cancer. Werner’s syndrome cells, which result from mutations in WRN (2), display a genomic instability termed variegated translocation mosaicism (8). Bloom’s syndrome cells, which result from mutations in BLM (1), are characterized by increased rates of sister chromatid exchange and sensitivity to DNA damaging agents (9). Mutations in RECQL4 are found in a subset of Rothmund-Thomson syndrome cases. These cells are characterized by elevated rates of chromosomal breaks and rearrangements (5,10). All the members of this family contain a C-terminal domain with homology to RecQ, and all those that have been tested exhibit a 3’ to 5’ DNA helicase activity (11–15). In addition to the helicase domain, the eukaryotic proteins contain a large N-terminal domain of about 650 amino acids whose sequence is poorly conserved between members. The N-terminal domain is important for activity in yeast (16), but with the exception of the 3’–5’ exonuclease domain of WRN (17,18) the biochemical function of the N-terminal domain is unknown.
A subset of the eukaryotic RecQ family members has been shown to interact with DNA topoisomerase III (Top3) (19–22). Eukaryotic DNA topoisomerase III was first identified as a hyper-recombination mutant in yeast that also displayed a slow-growth phenotype (23). Top3 has since been identified in several organisms including S. pombe (21,24), C. elegans (25), and humans (26,27). Like the bacterial enzyme, eukaryotic topoisomerase III is a type I 5’ DNA topoisomerase with weak superhelical relaxing activity and a strict requirement for substrates containing single-stranded DNA (ssDNA) for strand-passing activity (28,29). The biological function of Top3 is unclear, but in addition to its relaxing activity E. coli topoisomerase III is notable for its ability to decatenate gapped ssDNA circles (29). The recent demonstration that eukaryotic Top3 and E. coli RecQ helicase functionally interact to catenate fully duplex DNA circles (30) suggested a role for these enzymes at the termination of DNA replication to decatenate daughter chromosomes (31,32). Although it has been suggested that RecQ helicases might function to restart stalled replication forks (7,33–35) a role for Top3 in this process is unclear.
The SGS1 gene of yeast was identified as a mutation that suppressed the slow growth phenotype of top3 mutants (22). Thus, in contrast to top3 strains, top3 sgs1 double mutants exhibit a near wild-type growth rate as well as suppression of other top3 phenotypes (22,36). Compared to wild type cells the sgs1 single mutant displays increased rates of mitotic recombination, both at the ribosomal DNA locus and throughout the genome (22,37), as well as increased rates of chromosome loss and missegregation (38). Like mutations in BLM, SGS1 mutations result in a hypersensitivity to methylmethanesulfonate (MMS) (16) and hydroxyurea (HU) (39).
SGS1 was cloned in a two-hybrid screen with TOP3, suggesting that Top3 interacted with the first 550 amino acids of Sgs1 (22). Because two-hybrid results do not provide evidence for direct binding, we set out to biochemically confirm this result, refine the domain of interaction and determine if binding was through direct protein-protein interaction. We identified an Sgs1-Top3 complex by co-immunoprecipitating and co-fractionating these proteins from yeast extracts. The results indicate that Sgs1 and Top3 are present in a stable complex and that the N-terminal 158 amino acids of Sgs1 are sufficient for complex formation. The proteins do not appear to form a simple heterodimer however, as the full length proteins co-fractionate at a large native molecular weight. We determined that only the N-terminal 158 amino acids of Sgs1 were required to bind Top3 based on an enzyme-linked immunosorbent assay (ELISA) using purified proteins. These biochemical results are consistent with our observation that phenotypes caused by mutations in the first 158 amino acids of Sgs1 can be suppressed by overexpressing Top3, while larger deletions cannot.
EXPERIMENTAL PROCEDURES
Yeast Strains and Plasmids
Strain construction, growth and transformation followed standard protocols (40). S. cerevisiae strain NJY620 expresses epitope-tagged versions of Sgs1 and Top3. This strain was constructed by modifying the chromosomal SGS1 gene of wild type strain CHY125 (41) by integrating BglII linearized plasmid pJM1526 which places three consecutive HA epitopes (YPYDVPDYA) at the C-terminus of Sgs1. This gene and protein are henceforth called SGS1-HA and SGS1-HA, respectively. The chromosomal TOP3 gene was modified by integrating SphI linearized pJM2565 which places a single V5 epitope (GKPIPNPLLGLDSTRTG, Invitrogen) followed by six histidines at the C-terminus of Top3. This gene is henceforth referred to as TOP3-V5 and its encoded protein as Top3-V5. Strain WFY822 was created by integrating pJM2565 into strain NJY531 (sgs1::loxP) (16). Strain NJY560 was constructed by deleting the SGS1 and SLX4 genes of CHY125 (41) with loxP-KAN-loxP cassettes (42) and maintaining the strain with plasmid pJM500 (SGS1/URA3). SGS1 and sgs1-34 were integrated at the LEU2 locus of NJY560 to create strains BSY1228 and BSY1229, respectively. SGS1 mutant phenotypes were assayed as described (16).
Plasmid pJM1526, which expresses the epitope tagged truncation Sgs1645-1447-HA, contains the insert from pSM105-HA (16) in the vector pRS405 (43). Plasmid pJM2565 contains a fragment of the TOP3 gene encoding a C-terminal in-frame fusion to the V5-His6 epitope (Invitrogen) in pRS404. To overexpress Top3 in yeast, TOP3 was subcloned downstream of the GAL1 promoter in pRS424 to make pJM2566. Plasmids expressing SGS1-HA truncations were described (16), except for pKR1554 and pKR1555 which express epitope tagged proteins Sgs11-158-HA and Sgs11-322-HA, respectively. To create these plasmids the first 474 and 966 base pairs of SGS1 were amplified by polymerase chain reaction (PCR) so as to place an NdeI site in the context of the initiating ATG and a NotI site at the end of the coding region. These fragments were subcloned into NdeI/ NotI digested pSM100-HA (16). For expression of recombinant yeast proteins in E. coli, TOP3-V5 was subcloned into the T7 inducible vector pET11a (44), yielding plasmid pSAS402. Glutathione S-transferase (GST) fusion proteins were expressed by subcloning NdeI/ BamHI fragments from pKR1554 and pKR1555 into pET11GTK-WF to create pKR1564 and pKR1565. Plasmid pET11GTK-WF was created by destroying the NdeI site of pET11GTK (45) and placing an in-frame NdeI downstream of the GST target coding region by PCR.
Yeast Extracts, Immunoprecipitations, and Immunoblotting
Extract preparation and chromatography were performed at 4°C. To prepare large scale extracts, yeast cells were grown in 12 liters yeast extract-peptone-dextrose (YPD) at 30°C to OD600= 1.5, the media was supplemented with an additional 2% dextrose and growth continued to OD600= 2.8 which yielded 90 g of wet-weight cells. Cells were washed once with H2O and resuspended in Buffer A [25 mM Tris-HCl (pH 7.5), 1 mM EDTA, 0.01% (v/v) NP40, 10% (v/v) glycerol, 0.1 mM phenylmethylsulfonyl flouride (PMSF), 1 mM DTT] plus 200 mM NaCl and the following protease inhibitors: pepstatin, 10 µg/ml; leupeptin, 5 µg/ml; benzamidine, 10 mM; bacitracin, 100 µg/ml. The cells were broken in a bead-beater (Biospec Products) with 50% volume of glass beads in 30 sec bursts (separated by 90 sec pauses) for a total of 5 min of breakage. The lysate was centrifuged at 16,000 × g for 10 min and the resulting supernatant cleared at 235,000 × g for 90 min in a Beckman Ti45 rotor. This centrifugation was observed to pellet a significant portion of the chromatin as reported (46). The cleared lysate was precipitated by stirring 350 mg of (NH4)2SO4 per ml of lysate for 60 min followed by centrifugation at 188,000 × g for 15 min. The pellet was resuspended in 84 ml Buffer A and dialyzed to a conductivity of Buffer A plus 250 mM NaCl. Small scale extracts for immunoprecipitations were prepared as described (16). Protein concentrations were determined by the Bio-Rad protein assay using BSA as a standard. Superose 6 chromatography was performed in Buffer B [25 mM Hepes-HCl (pH 7.5), 1 mM EDTA, 0.01% (v/v) NP40, 0.1 mM PMSF, 1 mM DTT] containing 150 mM NaCl at 0.4 ml/min. Fractions were collected, precipitated with trichloroacetic acid and resolved by 10% SDS-PAGE.
Immunoprecipitations (IPs) were performed at 4°C essentially as described (16). Unless otherwise indicated, all IPs were performed by incubating extract with 1 µl of anti-HA (Boehringer Mannheim, 5 µg/µl) or anti-V5 (Invitrogen, 1 µg/µl) antibodies for one hour in RIPA buffer [150 mM NaCl, 50 mM Tris-HCl (pH 7.5), 1% (v/v) NP40, 0.5% (w/v) deoxycholate, 0.1% (w/v) SDS] (47). Twenty microliters of Protein-A sepharose beads (Amersham-Pharmacia) were added to each sample, followed by rocking for one hour. The immune complexes were then washed three times with 1 ml of RIPA buffer. Following SDS-PAGE the gels were transferred to nitrocellulose membranes (48) and treated with either anti-V5-HRP or anti-V5 as the primary antibody (1:10,000). Blots were treated with anti-mouse HRP conjugate secondary antibody as required (1:10,000; Gibco-BRL) and developed with chemiluminescence reagents (Gibco-BRL) to detect Top3-V5. Blots were reprobed with anti-HA (1:10,000) as the primary antibody and treated as above to detect SGS1-HA. For phosphate labeling experiments, yeast cells were grown and labeled with 32P(PO4) as described (49). Extract preparation and immunoprecipitations were then performed as described above.
Purification of Recombinant Yeast Proteins
Plasmids pET11GTK (expressing GST alone), pKR1564 (GST-Sgs11-158-HA), pKR1565 (GST-Sgs11-322-HA), and pSAS402 (Top3-V5) were transformed into E. coli BL21-RIL cells (Gibco-BRL). Cells were grown by shaking in LB media containing 0.1 mg/ml ampicillin at 37°C to an OD600 of 0.4. To induce the expression of the recombinant protein cultures were treated with isopropyl-1-thio-D-galactopyranoside at a final concentration of 0.1 mM for 2 hrs at 37°C, except for cells expressing Top3-V5 which were induced for 6 hrs at 20°C. Induced cells were pelleted and resuspended in Buffer A plus protease inhibitors (above) containing 250 mM NaCl for GST and GST fusions, and 150 mM KCl for Top3. Extractions and chromatography were performed at 4°C, except where noted. Cell suspensions were incubated with 0.1 mg/ml lysozyme for 30 min and then sonicated three times for 1 min using a Branson sonifier 450 microtip at setting 4, 60% duty cycle. Lysed cells were clarified by centrifugation at 32,500 × g and the supernatant collected as extract.
GST and GST-Sgs11-158-HA proteins were purified by batch binding the extract from one liter of cells to 1 ml glutathione sepharose 4B resin (Pharmacia) for 2 hrs. The resin was washed with three column volumes of Buffer A plus 250 mM NaCl, then half column volume fractions were eluted at room temperature with Buffer A (pH 8.0) plus 150 mM NaCl and 10 mM glutathione. The peak fraction was determined by Bradford assay and SDS-PAGE, then 200 µl was fractionated on a Superdex 75 (Pharmacia) gel filtration column in Buffer B plus 150 mM NaCl to achieve greater purity. GST-Sgs11-322-HA extract from 2 liters of cells was diluted in Buffer A to a conductivity of Buffer A plus 50 mM NaCl and bound to a SP sepharose (Pharmacia) column at 20 mg extract/ml resin. SP sepharose was washed with three column volumes of Buffer A plus 200 mM NaCl, then GST-Sgs11-322-HA was eluted in Buffer A plus 500 mM NaCl. The resulting SP 500 mM pool was diluted in half with Buffer A and affinity purified by glutathione sepharose 4B and Superdex 75 chromatography as above.
Top3-V5 containing extract from 3 liters of cells was bound to P11 phosphocellulose (Whatman) at a ratio of 10 mg extract/ml of resin in Buffer A plus 150 mM KCl. The column was washed with three column volumes of Buffer A plus 400 mM KCl, then Top3-V5-containing fractions were eluted from the column in Buffer A plus 600 mM KCl. Top3-V5-containing fractions were precipitated with 400 mg/ml (NH4)2SO4 for 1 hr and then pelleted at 32,500 × g. The resulting Top3-V5-containing pellet was resuspended in Buffer N [25 mM Tris-HCl (pH 8.0), 0.01% (v/v) NP40, 10% (v/v) glycerol, 0.1 mM PMSF, 250 mM NaCl] plus 20 mM imidazole and batch bound to 1.5 ml Probond Ni resin (Invitrogen) for 4 hrs. Resin was poured into a column and washed with three column volumes of Buffer N plus 20 mM imidazole and ten column volumes Buffer N plus 50 mM imidazole. Top3-V5 protein was then eluted in six half column volume fractions of Buffer N plus 250 mM imidazole.
ELISA Assays
In order to detect a direct interaction between Top3 protein and the N-terminus of Sgs1, 15 pmol of purified GST and GST-SGS1-HA fragments were first immobilized in DYNEX imulon 2 HB 0.4 ml wells. Immobilization of GST and GST-SGS1-HA fragments was carried out in 75 µl of PBS (10.1 mM Na2HP04, 2.4 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl), pH 7.2, containing 0.1% Tween-20 (PBST) and 1 mM DTT by shaking at 60 rpm for 1 hour at room temperature. After immobilization, wells were washed once with 0.4 ml PBST plus 1 mM DTT, then blocked with 0.4 ml of 5% dried milk (w/v) in PBST plus 1 mM DTT for 1 hour at room temperature. After blocking, cells were washed three times with 0.4 ml PBST plus 1 mM DTT. A titration of 0 to 40 pmol of Top3 protein was added to each set of coated wells in PBST plus 1 mM DTT in a volume of 75 µl and incubated for 30 minutes at room temperature. After incubation with Top3-V5 protein the wells were washed three times with 0.4 ml of PBST. In order to detect the Top3-V5 protein 100 µl of anti-V5 antibody (diluted 1:5000 in PBST plus 0.5% dried milk) was added to each well for 1 hour at room temperature. Wells were then washed three times with PBST and 100 µl of anti-mouse HRP conjugate secondary antibody (diluted 1:5000 in PBST plus 0.75% (w/v) dried milk) was added to each well and incubated for 1 hour at room temperature. After the secondary antibody incubation, wells were washed three times with 0.4 ml PBST and then 200 µl of 3,3’, 5,5’ -tetramethylbenzidine liquid substrate system for ELISA (Sigma) was added to each well and incubated for 30 min at room temperature. After incubation, 100 µl of 0.5 N H2SO4 was added to each well and OD450 of each solution read to determine the amount of Top3-V5 protein present.
RESULTS
Functional Complementation of Epitope-tagged SGS1 and TOP3
In order to characterize the interaction between Top3 and Sgs1, we constructed yeast strains whose chromosomal copies of the SGS1 and TOP3 genes were modified to express the C-terminally tagged proteins SGS1-HA and Top3-V5 (see Experimental Procedures). These strains allowed us to immunoprecipitate and immunoblot the products of stable single-copy genes expressed under their native promoters. To verify that the epitope-tagged alleles behaved like wild type, we tested their ability to complement various sgs1 and top3 phenotypes. Two very sensitive measures of SGS1 and TOP3 activity are resistance to the DNA damaging agent MMS and resistance to the DNA synthesis inhibitor HU (16). The strains expressing the tagged proteins were serially diluted and replica-plated to media containing MMS or HU. As shown in Figure 1, the epitope-tagged strains grew as well as wild type on YPD plates and did not show the HU or MMS hypersensitivity characteristic of sgs1 or top3 strains. For example, top3 mutants grow very slowly on YPD; SGS1 TOP3-V5 cells do not display the slow growth of SGS1 top3 cells and in fact grow at the wild type rate (data not shown). Similarly, sgs1 strains grow somewhat slower than wild type and SGS1-HA TOP3 cells grow noticeably faster than sgs1 cells. While sgs1 and top3 single mutants are hypersensitive to MMS and HU (Fig. 1), the SGS1-HA and TOP3-V5 strains do not display either of these sensitivities; these strains grow like wild type in the presence of these drugs as does the SGS1-HA TOP3-V5 doubly-tagged strain. Based on these growth phenotypes we conclude that the epitope-tagged alleles SGS1-HA and TOP3-V5 function exactly like wild type.
Fig. 1. Epitope tagged alleles of SGS1 and TOP3 function like wild type.
S. cerevisiae strains with the indicated genotypes were scraped from freshly grown plates, resuspended in H2O to equal OD600 and serially diluted 1 to 10 in a microtiter plate. Five µl of each dilution was then replica-plated to YPD plates containing 100 mM HU, 0.012% MMS, or no drug as shown. Cells were grown at 30°C for 24 hrs (YPD) or 48 hrs (HU, MMS) and photographed. The top row contains the wild type parent strain CHY125.
Co-immunoprecipitation and Co-fractionation of Sgs1 and Top3
To identify an interaction between Sgs1 and Top3, extracts were prepared from a wild type strain and from strain NJY620 expressing SGS1-HA and Top3-V5. Following incubation of the extracts with anti-HA or anti-V5 antibodies, the immune complexes were precipitated with protein-A beads and analyzed by immunoblot. Using extracts from cells expressing the tagged proteins, we observed that anti-V5 precipitated Top3-V5, as expected, and co-precipitated SGS1-HA (Fig. 2A, lane 6). Similarly, anti-HA precipitated SGS1-HA, as expected, and co-precipitated Top3-V5 (Fig. 2A, lane 4). These signals are specific to the epitope-tagged proteins as extract from the untagged wild type strain showed no bands of corresponding size. We note that under optimal conditions Top3-V5 co-precipitated SGS1-HA more efficiently than SGS1-HA co-precipitated Top3-V5 (Fig. 2A, compare lanes 2 and 4 with 6 and 8). The simplest explanation for this effect is that there is an excess of Top3 over Sgs1 protein in the extract. Such a result is consistent with the genetics of this system; lowering the Top3:Sgs1 ratio either by mutating TOP3 (22) or by overexpressing SGS1 (16) results in a profound growth defect.
Fig 2. Co-Immunoprecipitation of SGS1-HA and Top3-V5.
A, Extracts were prepared from strain NJY620 (SGS1-HA TOP3-V5) expressing Sgs1 and Top3 epitope-tagged proteins (even numbered lanes) and a wild type strain (CHY125) expressing no epitope-tagged proteins (odd numbered lanes). One mg of each extract was immunoprecipitated with anti-HA or anti-V5 antibodies under RIPA conditions and the products resolved by SDS-PAGE. Following transfer to nitrocellulose the membrane was probed with anti-V5-HRP to detect Top3 (left) or anti-HA to detect Sgs1 (right). B, IPs were performed as above except that the NJY620 extract was prepared and the immune complexes were washed under the following conditions: Buffer A plus 150 mM NaCl, RIPA buffer, or RIPA buffer plus 50 µg/ml ethidium bromide, as indicated. The upper blot was probed with anti-HA to detect Sgs1 and the bottom blot was probed with anti-V5-HRP to detect Top3.
The previous experiment indicates that Sgs1 and Top3 interact in cell extracts but does not address the strength of the interaction or whether these proteins require DNA to interact. We addressed these questions by varying the conditions of the immunoprecipitation from non-stringent (Buffer A plus 150 mM NaCl) to very stringent (RIPA buffer plus 50 µg/ml ethidium bromide). As shown in Figure 2B, the intensity of the SGS1-HA signal that co-precipitated with Top3-V5 was unaffected by changing these conditions. Likewise, the efficiency with which Top3-V5 was co-precipitated with SGS1-HA was unaffected by changing these conditions (Fig. 2B, lower panel). Both proteins were found to co-precipitate even under the most harsh conditions. We conclude that Sgs1 and Top3 are stably bound and that their interaction is not mediated by DNA.
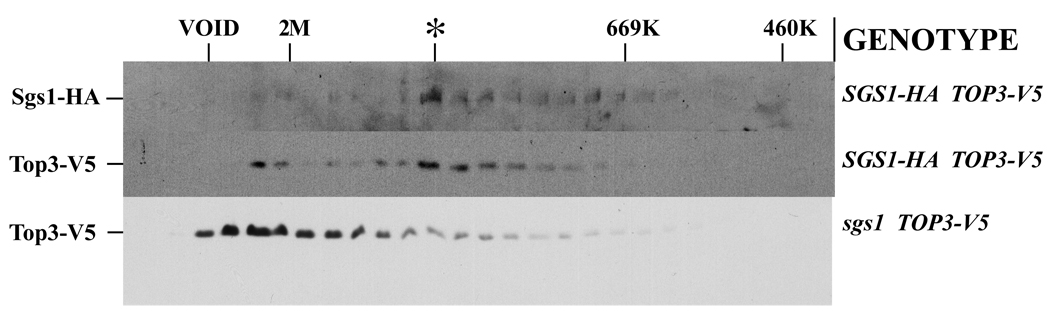
If Sgs1 and Top3 are present in a complex then they would be expected to co-fractionate over a gel filtration column. An extract from NJY620 cells was fractionated over a Superose 6 gel filtration column and the fractions immunoblotted to determine the elution volumes of SGS1-HA and Top3-V5 (Fig. 3). A portion of the SGS1-HA and Top3-V5 proteins were found to elute with a similar profile, the peak of which corresponds to a native molecular weight of approximately 1.3 MDa (Fig. 3, upper and middle panels). Additional Top3-V5 signal was detected in a second peak close to the void volume, although this signal was not associated with SGS1-HA (Fig. 3, middle blot). When WFY822 (sgs1 TOP3-V5) extract was fractionated on a Superose 6 column only the Top3-V5 signal eluting near the void volume was detected (Fig. 3, bottom). We conclude that the 1.3 MDa peak of Top3 is Sgs1-dependent and the Top3-V5 signal near the void is likely to represent aggregated Top3-V5 protein that is in excess of SGS1-HA. If a single polypeptide of Sgs1 were to interact with a single polypeptide of Top3 the expected size would be 240 kDa. The larger size of 1.3 MDa suggests that these proteins have a different stoichiometry or are complexed with additional proteins.
Fig 3. Co-fractionation of SGS1-HA and Top3-V5 by gel filtration chromatography.
Extracts from strains NJY620 (SGS1-HA TOP3-V5) and WFY822 (sgs1 TOP3-V5) were fractionated by Superose 6 chromatography as indicated. The fractions were TCA precipitated, resolved by SDS-PAGE and immunoblotted to detect SGS1-HA (upper panel) or Top3-V5 (middle and lower panels). The relative position of the molecular weight standards blue dextran (2M), thyroglobulin (669K) and beta-galactosidase (460K) are indicated along with the peak of Sgs1-HA and Top3-V5 elution (*).
TOP3 and SGS1 Interact Through the N-terminus of SGS1
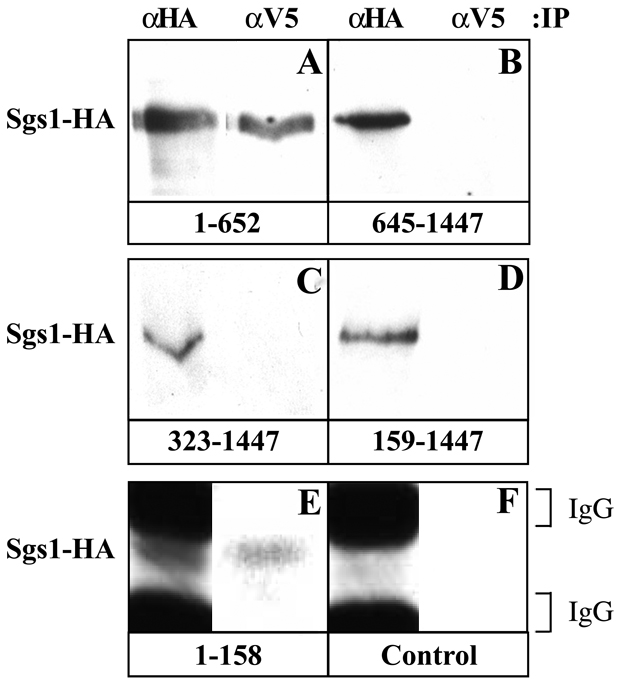
To determine the domain(s) of Sgs1 responsible for interaction with Top3, strain WFY822 (TOP3-V5 sgs1::loxP) was transformed with a series of plasmids expressing SGS1-HA truncations under the control of the native SGS1 promoter (16). Extracts were prepared and IPs performed under RIPA conditions. A fragment of Sgs1 consisting of amino acids 1 - 652 (Sgs11-652-HA) co-precipitated with Top3-V5 (Fig. 4A), consistent with a Top3-interaction domain in the N-terminal 550 amino acids as determined by the two-hybrid assay (22). In contrast, no interaction was detected between Top3-V5 and the DNA helicase domain of Sgs1 (Sgs1645-1447-HA) (Fig. 4B). These results indicate that the interaction between Sgs1 and Top3 is mediated through the N-terminus of Sgs1. To more accurately map the interaction domain, fragments of SGS1-HA containing all but the N-terminal 158 or 322 amino acids were expressed in the presence of Top3-V5. Neither Sgs1159-1447-HA nor Sgs1323-1447-HA was successfully co-precipitated with Top3-V5 (Fig. 4C and D). This result indicates that the first 158 amino acids are necessary to detect an interaction with Top3-V5 under these conditions. We tested whether the first 158 amino acids were sufficient for this interaction and observed that Sgs11-158-HA was indeed co-precipitated with Top3-V5 (Fig. 4E). When Sgs11-158-HA was immunoprecipitated with anti-HA it migrated in between a doublet of small IgG chains on SDS-PAGE. Comparing this signal to a control IP from an untagged strain confirms the identity of this band as Sgs11-158-HA (Fig. 4F). We were unable to test whether Sgs11-322-HA could be co-precipitated with Top3-V5 as this protein was insoluble when expressed in yeast (data not shown). We conclude that the first 158 amino acids of Sgs1 are necessary and sufficient to interact with Top3-V5 in vivo.
Fig 4. Co-immunoprecipitation of Top3 and Sgs1 deletions.
Strain WFY822 (sgs1Δ TOP3-V5) was transformed with plasmids expressing the indicated SGS1-HA protein fragments under the control of the natural SGS1 promoter. Cells were grown under selective conditions, whole cell extracts were prepared and the indicated IPs were performed under RIPA conditions. The precipitated SGS1-HA proteins were then detected by immunoblot using anti-HA antibody. The 50 kDa Sgs11-158-HA protein (E) migrates between two small immunoglobulin proteins present in the anti-HA antibody. A control extract from the untransformed parent strain (F) was used to indicate the position of the immunoglobulin bands that are detected by the secondary antibody. The following SGS1-HA expression plasmids were used: A: pJM1541; B: pSM105-HA; C: pSM103-HA; D: pSM102-HA; E: pKR1554.
After determining that the first 158 amino acids of Sgs1 are sufficient for interacting with Top3 in yeast extracts, we wanted to find out if this was due to a direct protein-protein interaction. We initially tried to express Top3-V5 and SGS1-HA fragments in rabbit reticulocyte lysates and immunoprecipitate them under mild conditions (Buffer A plus 150 mM NaCl) but these assays revealed no interaction (data not shown). We then turned to the more sensitive ELISA assay to identify a direct interaction. GST fusions of Sgs11-158-HA and Sgs11-322-HA were expressed in bacteria and purified on glutathione beads. As shown in Figure 5A, unfused GST protein and GST-Sgs11-322-HA were highly purified, while GSTS-gs11-158-HA contained several smaller bands that are likely to be breakdown products as their abundance varied between preparations. Recombinant full length Top3-V5 was highly purified using Ni-affinity chromatography (Fig. 5A). ELISA wells were coated with 15 pmol of purified GST, GST-Sgs11-158-HA, or GST-Sgs11-322-HA and non-specific sites blocked with 5% dried milk. Increasing amounts of purified Top3-V5 protein were then incubated in a series of well prior to washing and detecting bound Top3-V5 with anti-V5 antibody and a chromogenic substrate. This assay revealed weak background binding of Top3-V5 to unfused GST protein that saturated at 30 pmol of input Top3-V5 (Fig. 5B). In contrast, both GST-Sgs11-158-HA and GST-Sgs11-322-HA bound increasing amounts of Top3-V5 protein. At the highest input level of Top3-V5, these Sgs1 domains bound three times more Top3-V5 than GST alone. This result demonstrates a direct protein-protein interaction between the amino terminus of Sgs1 and Top3. Little difference between GST-Sgs11-158-HA and GST-Sgs11-322-HA was detected, confirming that the first 158 amino acids contains a significant portion of the interacting domain.
Fig. 5. Purified Top3-V5 and GST-Sgs1 protein fragments bind in vitro.
A, Recombinant Top3-V5, GST-Sgs11-158-HA, GST-Sgs11-322-HA, and GST were purified from bacterial extracts and analyzed by SDS-PAGE and Coomassie blue staining. The low molecular-weight bands in the GST-Sgs11-158-HA and Top3 preparations are most likely breakdown products. Proteins loaded: marker proteins, 2 µg/band; GST-Sgs11-322-HA, 1.15 µg total; GST-Sgs11-158-HA, 1.25 µg total; GST, 1.3 µg total; Top3-V5, 1.0 µg total. B, Fifteen pmol of purified GST-Sgs11-158-HA, GST-Sgs11-322-HA or GST were immobilized in ELISA wells, blocked with 5% milk protein and challenged with the increasing amounts of Top3-V5. The wells were washed and the bound Top3-V5 was detected by treatment with anti-V5, HRP-conjugated secondary antibodies and a chromogenic reagent. The reaction was stopped and the absorbance measured at 450 nm.
Top3 Overexpression Complements Mutations in the N-terminus of Sgs1
We previously used a synthetic-lethal screen to identify several novel SLX mutants that require SGS1 for viability (41). Phenotypically, sgs1Δ and slx4Δ single mutants are viable but the sgs1Δ slx4Δ double mutant is dead. Since Sgs1 activity is essential for viability in this background, slx4Δ mutants provide a genetic system to identify functional domains of SGS1. Structure-function analysis previously revealed that small N-terminal deletions or mutations in the DNA helicase domain of Sgs1 were lethal (16).
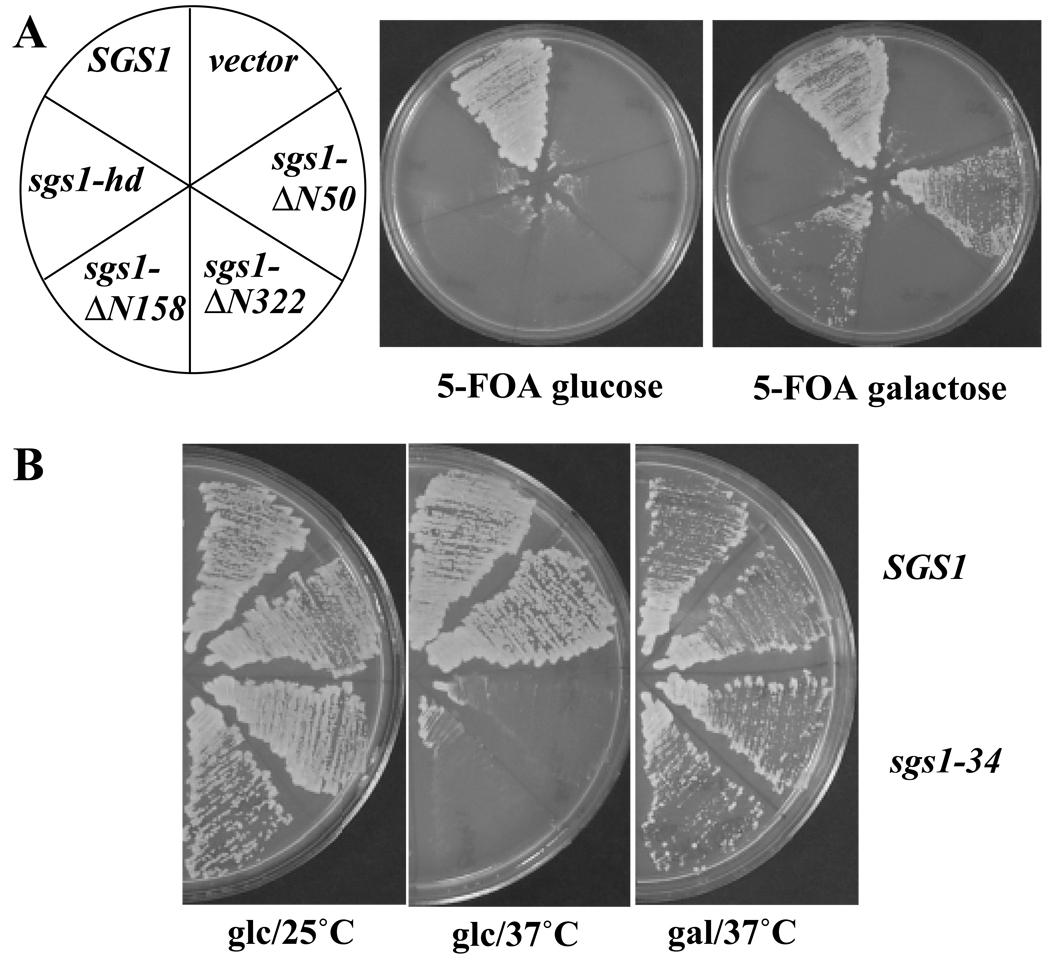
To address the question of why N-terminal deletions of Sgs1 were defective in this assay, we tested whether overexpression of Top3 could rescue the synthetic-lethal phenotype. The starting strain, NJY560 (slx4Δ sgs1Δ pJM500, SGS1/URA3), is inviable on media containing the drug 5-FOA as it selects against the SGS1/URA3 plasmid which is essential for viability in this background. NJY560 was first transformed with a plasmid expressing the TOP3 gene under control of the inducible GAL1 promoter (pJM2566, GAL1p-TOP3), and then with a series of SGS1 deletions in a LEU2 vector. In contrast to the LEU2 vector alone, wild type SGS1 allowed these cells to grow on 5-FOA (Fig. 6A). Complementation of the synthetic-lethal phenotype by SGS1 is independent of Top3 overexpression as growth is observed under both repressed (glucose) and induced (galactose) conditions. As expected, a helicase-defective allele of SGS1 (sgs1-hd) and all N-terminal truncations of Sgs1 were lethal when streaked onto 5-FOA plates containing glucose (Fig. 6A). In contrast, when these strains were streaked on 5-FOA galactose, the sgs1-Δ N50 and sgs1-Δ N158 alleles displayed complementing activity. Neither sgs1-hd nor sgs1-Δ N322 complemented, even when Top3 was overexpressed. These results indicate that while DNA helicase activity is required under all conditions, the first 158 amino acids of Sgs1 are not required if Top3 is overexpressed. The suppression of the sgs1-Δ N158 allele is not specific to the synthetic-lethal phenotype as Top3 overexpression also suppressed the MMS hypersensitivity of this allele (data not shown). The simplest explanation for these results is that deletion of amino acids 1-158 significantly impairs the interaction between Top3 and Sgs1 and increasing the Top3 concentration restores this interaction. Based on this assay we conclude that the size of the interaction domain in vivo must be larger than amino acids 1-158 and smaller than 1-322.
Fig. 6. Overexpression of Top3 suppresses N-terminal mutations in Sgs1.
A, Strain NJY560 (sgs1Δ slx4Δ pJM500, SGS1/URA3) was transformed with plasmid pNJ2566 (GAL1p-TOP3, TRP1) and the following LEU2 plasmids: pSM100 (SGS1), pJM531 (sgs1-Δ N50), pSM109 (sgs1-Δ N158), pSM110 (sgs1-Δ N322), pSM100-hd (sgs1-hd), and pRS415 (vector). Transformants were streaked onto media lacking tryptophane and leucine but containing 5-FOA and the indicated sugar to select stains growing in the absence of the URA3 plasmid pJM500. B, Strains BSY1228 (SGS1 slx4Δ) and BSY1229 (sgs1-34 slx4Δ) were transformed with plasmid pNJ2566 and streaked in duplicate onto selective plates lacking tryptophane but containing either glucose (glc) or galactose (gal) at 25°C or 37°C.
The sgs1-34 mutation was isolated as a temperature-sensitive allele of SGS1 caused by the amino acid change Q31P. At the restrictive temperature (37°C) sgs1-34 behaves like sgs1-ΔN158, suggesting that it lacks Sgs1 N-terminal function (VK and SJB manuscript in preparation). As a result of this mutation, strain BSY1229 (sgs1-34 slx4) is viable at 25°C, but not at 37°C. We tested whether Top3 overexpression could suppress this N-terminal point mutation. Strain BSY1229 was transformed with pJM2566 (GAL1p-TOP3) and the transformants streaked on selective plates containing glucose or galactose at 25°C or 37°C (Fig. 6B). When Top3 expression was repressed by growth on glucose plates, the strain grew at 25°C, but not at 37°C. However, when Top3 was overexpressed by growth in the presence of galactose the strain was able to grow at 37°C (Fig. 6B). The suppression of sgs1-34 by Top3 overexpression is allele-specific as two other SGS1 temperature-sensitive alleles whose mutations map to the DNA helicase domain could not be suppressed (data not shown). As above, we conclude that the sgs1-34 mutation impairs the binding of Top3 to Sgs1 at the restrictive temperature and that increasing Top3 concentration restores the interaction.
Post-translational Modification of Sgs1
We suspected that the failure to identify a strong Sgs1/Top3 interaction by in vitro translation might be due to the requirement for an in vivo modification to Sgs1. We addressed this question by asking whether Sgs1 is phosphorylated in vivo. Yeast cells expressing SGS1-HA or a contol HA-tagged protein were grown in the presence of 32P orthophosphate. Extracts were prepared from these strains and proteins were immunoprecipitated and analyzed by SDS-PAGE and autoradiography. As shown in Figure 7, immunoprecipitation of SGS1-HA results in a 32P-labelled band migrating at 220 kDa, as expected for SGS1-HA. The 220 kDa band is specific to SGS1-HA as only the expected 100 kDa protein is precipitated with anti-HA from a control extract. An additional control revealed that only the expected 69 kDa RPA1 protein was immunoprecipitated from the SGS1-HA extract with an anti-serum to RPA1. Taken together, these data indicate that phosphorylated forms of Sgs1 and RPA1 do not interact under these conditions. We conclude that Sgs1 is phosphorylated during exponential growth in vivo.
Fig 7. Sgs1 is a phosphoprotein.
Extracts were prepared from 32P-P04-labeled yeast cells expressing either SGS1-HA or a 100 kDa HA-tagged control protein (Mms4-HA). The control extract was immunoprecipitated with anti-HA while the SGS1-HA extract was immunoprecipitated with anti-HA or antiserum to RPA1 (RPA1).
DISCUSSION
Although a DNA fragment encoding the amino-terminal 550 amino acids of Sgs1 was isolated in a two-hybrid screen using Top3 as bait (22), there has been no biochemical confirmation of this interaction or any evidence of a direct interaction between these two proteins. To address the in vivo association of these proteins we created epitope-tagged alleles of SGS1 and TOP3 that were stably integrated at their chromosomal locations and expressed under their native promoters. These alleles were active in all the biological assays we examined, indicating that the tagged proteins retain wild type function. Our data show that Sgs1 and Top3 can be co-immunoprecipitated under stringent buffer conditions including 0.1% SDS and ethidium bromide. These results are consistent with the idea that these proteins are present in a stable complex in vivo.
As summarized in Figure 8, our deletion analysis indicates that a domain as small as the N-terminal 158 amino acids of Sgs1 is able to bind to Top3 in vivo. ELISA assays using highly purified proteins provide biochemical evidence that Top3 binds Sgs11-158 through direct protein-protein interactions. As expected for such a physical interaction, Top3 overexpression suppressed phenotypes associated with mutations in the Sgs1 amino terminus. The fact that overexpressed Top3 suppressed Sgs1 with N-terminal deletions as large as 158 amino acids suggests that the Top3 interaction domain extends further than residues 1-158 in vivo (Fig. 8). Because overexpressed Top3 failed to suppress a deletion of 322 amino acids, we conclude that the Top3-interaction domain in vivo is larger than amino acids 1-158 and smaller than 1-322.
Fig 8. Summary of Sgs1-Top3 interactions.
The full length Sgs1 protein and deletion derivatives are drawn schematically as indicated on the left. The results of Co-IP, ELISA, and genetic suppression studies are indicated on the right: +, Sgs1/Top3 interaction; −, no Sgs1/Top3 interaction; ND, not done. The fragment consisting of residues 1-158 of Sgs1 (gray bar) is consistent with the minimal Top3 binding domain based on Co-IP and ELISA results. The full interaction domain as determined by genetic suppression is likely to extend somewhat further as indicated the black bar.
Recent evidence suggests that DNA topoisomerase III interacts with some, but not all, RecQ family members. Bacterial topoisomerase III and RecQ were shown to functionally interact in vitro to catenate double-stranded DNA circles (30). In S. pombe, the top3+ gene has been identified as an essential gene whose lethal phenotype is suppressed by mutations in rqh1+, the S. pombe RecQ homolog (21). In human cells, immunolocalization studies indicate that BLM is present in promyelocytic leukemia (PML) nuclear bodies together with Top3α (19,50). The human RecQ5β protein was also shown to co-localize and co-immunoprecipitate with topoisomerase III (4). In contrast, there is as yet no evidence that WRN interacts with topoisomerase III, although it is associated with a large complex of replication proteins including topoisomerase I (51).
Recently, Wu et al. used a far-western blotting technique to show that human topoisomerase IIIα bound BLM by direct protein-protein interactions (20). These experiments demonstrated that the N-terminal 212 amino acids of BLM was sufficient for binding topoisomerase III. Our observation that Top3 binds a corresponding region of Sgs1 (1-158) suggests that the N-termini of BLM and Sgs1 are functionally conserved. A comparison of the Sgs1 and BLM amino acid sequences, however, reveals no obvious similarities or motifs that might mediate the Top3 interaction. Our studies identified a single Top3-binding domain of Sgs1, whereas human DNA topoisomerase III was found to bind a second region of BLM (residues 1266-1417) (20). This difference may be due to the fact that the far-western method included chemical cross-linking of the two proteins, which was not used in our studies. We conclude that this interaction is either not conserved in yeast or is not sufficiently stable to be detected by the methods used here.
While this work was in progress additional evidence of an interaction between Sgs1 and Top3 was reported. Bennett and Wang showed that Sgs1 fragments bind Top3 in yeast extracts and inhibit Top3 activity in vitro (52). Maximal inhibition was obtained with a fragment of Sgs1 spanning residues 1-283. In addition, recent two-hybrid mapping studies identified a weak Top3-interaction domain between residues 1-116 of Sgs1 and a stronger interaction domain between residues1-282 (53). Consistent with our genetic results (Fig. 6), these investigators proposed that a specific interaction between Sgs1 and Top3 is required for certain Sgs1 functions. The Top3-interacting domains identified in these studies agree closely with the Top3-binding domain identified in our experiments, as well as the N-terminal topoisomerase III binding domain of BLM (20). Taken together with earlier data, it now appears that Sgs1 is more closely related to BLM than to WRN. Amino acid sequence analysis had initially shown that Sgs1 and BLM lack the exonuclease domain found in the N-terminus of WRN (17). Genetic complementation experiments have also shown that BLM is capable of complementing the HU hypersensitivity, top3 slow-growth suppression and premature aging phenotypes of sgs1 mutants that WRN could not (39,54). Given that an interaction between Sgs1 and Top3 appears to be essential for Sgs1 activity, it will be of interest to determine if the ability of human BLM to complement yeast sgs1 phenotypes depends on its ability to bind yeast Top3.
The mapping of the Top3 interaction to the region of amino acids 1-158 is significant in that small N-terminal truncations of Sgs1, such as expressed by the sgs1-ΔN158 allele, produce hypermorphic phenotypes that are more extreme than the null phenotypes (16). Considering that the phenotypes of top3 mutants are more extreme than sgs1 mutants one might hypothesize that the full N-terminal domain of Sgs1 is required for complete Top3 activity in vivo. Although this model is consistent with the ability of overexpressed Top3 to suppress the sgs1-ΔN158 phenotype, it is inconsistent with the fact that Sgs1 fragments inhibit Top3 activity in vitro (52) and that sgs1-ΔN158 produces growth defects even in a top3 background (16). An alternative model to explain these results is that a third factor interacts with Top3 and Sgs1. Deletions of the Sgs1 N-terminal 158 amino acids might result in growth defects by reducing binding to Top3 as well as this yet to be identified third factor.
The Top3/Sgs1 complex isolated from yeast is resistant to RIPA buffer suggesting that the interaction is very stable. This affinity is retained even in a complex between Top3 and the relatively small Sgs11-158-HA fragment (Fig. 4). Given this apparent high affinity, it is surprising that multiple studies have required very sensitive methods to detect interactions between Top3 and RecQ helicases in vitro. We required a very sensitive ELISA assay and it was reported that a Top3/Sgs1 complex formed in vitro was dissociated by the relatively gentle conditions of 140–250 mM NaCl (52). As mentioned above, chemical cross-linking was used to detect an interaction between DNA topoisomerase III and BLM (20). This suggests that the Top3/helicase complexes formed in vitro are different from those formed in vivo. This difference in affinity may simply reflect sub-optimal binding conditions in vitro; higher protein concentrations and/or co-translation might be required to form a stable complex. Alternatively, the correct interaction between Top3 and Sgs1 might depend on other cellular factors or modifications. As a test of this we found that Sgs1 is phosphorylated. The biological function of the phosphorylation is unknown but it might regulate Sgs1 DNA helicase activity or the interaction of Sgs1 with Top3 or other proteins.
The Top3/Sgs1 complex eluted from a Superose 6 column at an approximate size of 1.3 MDa, indicating that it exists in a multimeric complex. Although we cannot rule out the possibility that a small amount of DNA mediates this complex, we feel it is unlikely for the following reasons. First, high speed extracts were used in order to remove bulk chromatin. Second, Top3 and Sgs1 co-immunoprecipitated despite treatment with EtBr which has been found to disrupt protein-DNA interactions. Third, we have examined the elution of double-stranded DNA by Superose 6 chromatography and found that DNA larger than 4 kb elutes in the void while1 kb DNA fragments elute at approximately 2 MDa. Thus, if the complex at 1.3 MDa were mediated by DNA, the fragments would have to be very small and discreet in size.
If the 1.3 MDa complex is a multimer, it might consist of a hexamer of Top3 with a hexamer of Sgs1 which would be expected to run at that size. Other helicases have been shown to exist as hexamers, such as E. coli DnaB (55) and, more significantly, human BLM (56). Alternatively, other proteins might be present in the complex as recently reported for BLM. In addition to its presence in PML bodies, BLM is associated with a number of human DNA repair proteins in the BASC complex including some that are conserved in yeast (50,57,58). Based on the interactions of BLM in human cells, it is possible that Sgs1 and Top3 are associated with additional proteins that contribute to its large native molecular weight and stable association. Purification of the Sgs1/Top3 complex will be required to determine which of these models is correct.
Acknowledgements
The authors thank Suzanne Shanower for plasmid DNA and Hee-Sook Kim, Janet Mullen and Marty Nemeroff for comments on the manuscript.
The abreviations used are
- Top3
DNA topoisomerase III
- GST
glutathione S-transferase
- ELISA
enzyme-linked immunosorbent assay
- RIPA
radio-immuno-precipitation assay
- HU
hydroxyurea
- MMS
methylmethanesulfonic acid
- DTT
dithiothreitol
- PAGE
polyacrylamide gel electrophoresis
- PMSF
phenylmethylsulfonyl fluoride
- RPA
replication protein A
- IP
immunoprecipitation
- PML
promyelocytic leukemia
- PCR
polymerase chain reaction
Footnotes
This work was supported by National Institutes of Health Grants GM55583 and AG16637.
REFERENCES
- 1.Ellis NA, Groden J, Ye T-Z, Straughen J, Lennon DJ, Ciocci S, Proytcheva M, German J. Cell. 1995;83:655–666. doi: 10.1016/0092-8674(95)90105-1. [DOI] [PubMed] [Google Scholar]
- 2.Yu CE, Oshima J, Fu YH, Wijsman EM, Hisama F, Alisch R, Matthews S, Nakura J, Miki T, Ouais S, Martin GM, Mulligan J, Schellenberg GD. Science. 1996;272:258–262. doi: 10.1126/science.272.5259.258. [DOI] [PubMed] [Google Scholar]
- 3.Umezu K, Nakayama H. J. Mol. Biol. 1993;230:1145–1150. doi: 10.1006/jmbi.1993.1231. [DOI] [PubMed] [Google Scholar]
- 4.Shimamoto A, Nishikawa K, Kitao S, Furuichi Y. Nucleic Acids Res. 2000;28:1647–1655. doi: 10.1093/nar/28.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitao S, Shimamoto A, Goto M, Miller RW, Smithson WA, Lindor NM, Furuichi Y. Nat. Genet. 1999;22:82–84. doi: 10.1038/8788. [DOI] [PubMed] [Google Scholar]
- 6.Murray JM, Lindsay HD, Munday CA, Carr AM. Mol. Cell Biol. 1997;17:6868–6875. doi: 10.1128/mcb.17.12.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart E, Chapman CR, Al-Khodairy F, Carr AM, Enoch T. EMBO J. 1997;16:2682–2692. doi: 10.1093/emboj/16.10.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salk D, Au K, Hoehn H, Martin GM. Cytogenet. Cell Genet. 1981;30:92–107. doi: 10.1159/000131596. [DOI] [PubMed] [Google Scholar]
- 9.Chaganti RS, Schonberg S, German J. Proc. Natl. Acad. Sci. USA. 1974;71:4508–4512. doi: 10.1073/pnas.71.11.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miozzo M, Castorina P, Riva P, Dalpra L, Fuhrman Conti AM, Volpi L, Hoe TS, Khoo A, Wiegant J, Rosenberg C, Larizza L. Int. J. Cancer. 1998;77:504–510. doi: 10.1002/(sici)1097-0215(19980812)77:4<504::aid-ijc5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 11.Umezu K, Nakayama K, Nakayama H. Proc. Natl. Acad. Sci. USA. 1990;87:5363–5367. doi: 10.1073/pnas.87.14.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu J, Mullen JR, Brill SJ, Kleff S, Romeo A, Sternglanz R. Nature. 1996;383:678–679. doi: 10.1038/383678a0. [DOI] [PubMed] [Google Scholar]
- 13.Bennett RJ, Sharp JA, Wang JC. J. Biol. Chem. 1998;273:9644–9650. doi: 10.1074/jbc.273.16.9644. [DOI] [PubMed] [Google Scholar]
- 14.Karow JK, Chakraverty RK, Hickson ID. J. Biol. Chem. 1997;272:30611–30614. doi: 10.1074/jbc.272.49.30611. [DOI] [PubMed] [Google Scholar]
- 15.Gray MD, Shen JC, Kamath-Loeb AS, Blank A, Sopher BL, Martin GM, Oshima J, Loeb LA. Nat. Genet. 1997;17:100–103. doi: 10.1038/ng0997-100. [DOI] [PubMed] [Google Scholar]
- 16.Mullen JR, Kaliraman V, Brill SJ. Genetics. 2000;154:1101–1114. doi: 10.1093/genetics/154.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mushegian AR, Bassett DE, Jr., Boguski MS, Bork P, Koonin EV. Proc. Natl. Acad. Sci. USA. 1997;94:5831–5836. doi: 10.1073/pnas.94.11.5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang S, Li B, Gray MD, Oshima J, Mian IS, Campisi J. Nat. Genet. 1998;20:114–116. doi: 10.1038/2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson FB, Lombard DB, Neff NF, Mastrangelo MA, Dewolf W, Ellis NA, Marciniak RA, Yin Y, Jaenisch R, Guarente L. Cancer Res. 2000;60:1162–1167. [PubMed] [Google Scholar]
- 20.Wu L, Davies SL, North PS, Goulaouic H, Riou JF, Turley H, Gatter KC, Hickson ID. J. Biol. Chem. 2000;275:9636–9644. doi: 10.1074/jbc.275.13.9636. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin A, Wang SW, Toda T, Norbury C, Hickson ID. Nucleic Acids Res. 1999;27:4050–4058. doi: 10.1093/nar/27.20.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gangloff S, McDonald JP, Bendixen C, Arthur L, Rothstein R. Mol. Cell Biol. 1994;14:8391–8398. doi: 10.1128/mcb.14.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallis JW, Chrebet G, Brodsky G, Rolfe M, Rothstein R. Cell. 1989;58:409–419. doi: 10.1016/0092-8674(89)90855-6. [DOI] [PubMed] [Google Scholar]
- 24.Maftahi M, Han CS, Langston LD, Hope JC, Zigouras N, Freyer GA. Nucleic Acids Res. 1999;27:4715–4724. doi: 10.1093/nar/27.24.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim YC, Lee J, Koo HS. Nucleic Acids Res. 2000;28:2012–2017. doi: 10.1093/nar/28.9.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanai R, Caron PR, Wang JC. Proc. Natl. Acad. Sci. USA. 1996;93:3653–3657. doi: 10.1073/pnas.93.8.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng SW, Liu Y, Hasselblatt KT, Mok SC, Berkowitz RS. Nucleic Acids Res. 1999;27:993–1000. doi: 10.1093/nar/27.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim RA, Wang JC. J. Biol. Chem. 1992;267:17178–17185. [PubMed] [Google Scholar]
- 29.DiGate RJ, Marians KJ. J. Biol. Chem. 1988;263:13366–13373. [PubMed] [Google Scholar]
- 30.Harmon FG, DiGate RJ, Kowalczykowski SC. Mol. Cell. 1999;3:611–620. doi: 10.1016/s1097-2765(00)80354-8. [DOI] [PubMed] [Google Scholar]
- 31.Wang JC. J. Biol. Chem. 1991;266:6659–6662. [PubMed] [Google Scholar]
- 32.Rothstein R, Gangloff S. Genome Res. 1995;5:421–426. doi: 10.1101/gr.5.5.421. [DOI] [PubMed] [Google Scholar]
- 33.Courcelle J, Hanawalt PC. Mol. Gen. Genet. 1999;262:543–551. doi: 10.1007/s004380051116. [DOI] [PubMed] [Google Scholar]
- 34.Karow JK, Constantinou A, Li JL, West SC, Hickson ID. Proc. Natl. Acad. Sci. USA. 2000;97:6504–6508. doi: 10.1073/pnas.100448097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chakraverty RK, Hickson ID. Bioessays. 1999;21:286–294. doi: 10.1002/(SICI)1521-1878(199904)21:4<286::AID-BIES4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 36.Gangloff S, de Massy B, Arthur L, Rothstein R, Fabre F. EMBO J. 1999;18:1701–1711. doi: 10.1093/emboj/18.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watt PM, Hickson ID, Borts RH, Louis EJ. Genetics. 1996;144:935–945. doi: 10.1093/genetics/144.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watt PM, Louis EJ, Borts RH, Hickson ID. Cell. 1995;81:253–260. doi: 10.1016/0092-8674(95)90335-6. [DOI] [PubMed] [Google Scholar]
- 39.Yamagata K, Kato J, Shimamoto A, Goto M, Furuichi Y, Ikeda H. Proc. Natl. Acad. Sci. USA. 1998;95:8733–8738. doi: 10.1073/pnas.95.15.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rose MD, Winston F, Hieter P. NY: Cold Spring Harbor Laboratory Press. Cold Spring Harbor; 1990. [Google Scholar]
- 41.Mullen JR, Kaliraman V, Ibrahim SS, Brill SJ. Genetics. doi: 10.1093/genetics/157.1.103. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wach A, Brachat A, Pohlmann R, Philippsen P. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 43.Sikorski RS, Hieter P. Genetics. 1989;12:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Studier FW, Rosenberg AH, Dunn JJ, Dubendorff JW. Meth. Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 45.Ruppert JM, Stillman B. Mol. Cell Biol. 1993;13:3811–3820. doi: 10.1128/mcb.13.6.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frei C, Gasser SM. Genes Dev. 2000;14:81–96. [PMC free article] [PubMed] [Google Scholar]
- 47.Harlow E, Lane D. Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 48.Towbin H, Staehelin T, Gordon J. Proc. Natl. Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Din S, Brill SJ, Fairman MP, Stillman B. Genes Dev. 1990;4:968–977. doi: 10.1101/gad.4.6.968. [DOI] [PubMed] [Google Scholar]
- 50.Ishov AM, Sotnikov AG, Negorev D, Vladimirova OV, Neff N, Kamitani T, Yeh ET, Strauss JF, 3rd, Maul GG. J. Cell. Biol. 1999;147:221–234. doi: 10.1083/jcb.147.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lebel M, Spillare EA, Harris CC, Leder P. J. Biol. Chem. 1999;274:37795–37799. doi: 10.1074/jbc.274.53.37795. [DOI] [PubMed] [Google Scholar]
- 52.Bennett RJ, Noirot-Gros MF, Wang JC. J. Biol. Chem. 2000;275:26898–26905. doi: 10.1074/jbc.M003137200. [DOI] [PubMed] [Google Scholar]
- 53.Duno M, Thomsen B, Westergaard O, Krejci L, Bendixen C. Mol. Gen. Genet. 2000;264:89–97. doi: 10.1007/s004380000286. [DOI] [PubMed] [Google Scholar]
- 54.Heo SJ, Tatebayashi K, Ohsugi I, Shimamoto A, Furuichi Y, Ikeda H. Genes Cells. 1999;4:619–625. doi: 10.1046/j.1365-2443.1999.00288.x. [DOI] [PubMed] [Google Scholar]
- 55.Reha-Krantz LJ, Hurwitz J. J. Biol. Chem. 1978;253:4043–4050. [PubMed] [Google Scholar]
- 56.Karow JK, Newman RH, Freemont PS, Hickson ID. Curr Biol. 1999;9:597–600. doi: 10.1016/s0960-9822(99)80264-4. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, Cortez D, Yazdi P, Neff N, Elledge SJ, Qin J. Genes Dev. 2000;14:927–939. [PMC free article] [PubMed] [Google Scholar]
- 58.Zhong S, Hu P, Ye TZ, Stan R, Ellis NA, Pandolfi PP. Oncogene. 1999;18:7941–7947. doi: 10.1038/sj.onc.1203367. [DOI] [PubMed] [Google Scholar]