Abstract
We have previously demonstrated that pregnant ovine endometrium expresses the gastrin releasing peptide (GRP) gene at a high level following conceptus implantation. Here we report the isolation, characterization and biological activity of ovine GRP1-46, the primary product of this gene in the pregnant endometrium. Full thickness 125–140 day pregnant sheep uterus (term is 145 day) was homogenized in 80% acetonitrile/2% trifluoroacetic acid (1:7 ACN/TFA), concentrated on reverse-phase C18 cartridges and chromatographed successively on gel filtration (Sephadex G-50) and reverse-phase HPLC (C18 μBondapak). Purification was monitored by RIA. Purified GRP peptide was analysed by mass spectrometry giving a major mass ion at 4963 which corresponds exactly to GRP1-46. Other mass ions from pro-GRP did not contain a biologically active N-terminus or antigenic determinant. Proteolytic cleavage of pro-GRP to give rise to GRP1-46 would require preferential cleavage at the Glu-Glu bond by a Glu-C2-like enzyme, rather than the trypsin-like and C-terminal amidation enzymes (PAM) that produce GRP18-27 and GRP 1-27 in other tissues. GRP1-46 was synthesized and receptor binding and biological activity tested on a range of rodent and human cell lines that express GRP-related receptors GRPR, NMBR and BRS3. GRP1-46 bound GRPR and NMBR with low affinity, and mobilized inositol phosphate in cell lines expressing the GRPR and NMBR, but not BRS-3. This study describes a new processed product of the GRP gene, GRP1-46, which is highly expressed in the pregnant sheep endometrium and which acts as a weak agonist at the GRPR and NMBR.
Keywords: Ovine, gastrin-releasing peptide, pregnancy, endometrium
1. Introduction
Gastrin releasing peptides (GRPs) are the mammalian homologs of the frog skin peptide bombesin, and have a broad spectrum of regulatory functions in tissues as diverse as the central nervous system, pituitary and gastrointestinal tract [17]. The major recognised bioactive forms of GRP are the amidated GRP1-27 and 18-27. GRPs mediate these functions primarily via neurotransmission and also locally by paracrine or autocrine means. No hormonal role for this peptide family has yet been described, except for the presence of an immunoreactive GRP peptide product which circulates at high levels in the fetal and maternal circulation of the pregnant sheep [8].
We and others have previously shown that the pregnant ovine and bovine endometrium expresses the GRP gene, producing very large amounts of a translated and processed product which is different to the well characterized amidated bioactive peptides GRP1-27 and 18-27, as well as the C-terminally Gly extended forms. [1, 2, 6, 7, 9, 24, 26]. Indeed this protein was by far the major stored and secreted form of proGRP processing in the pregnant sheep [26]. Using antisera directed against the the amidated C-terminus of GRP 1-27 (common to all mammalian species), we showed that the primary gene product synthesised by the ovine endometrium during pregnancy is a 5–6.5 kD protein that cross-reacts weakly with our antiserum [26]. The peptide cannot be an N-terminally extended form of GRP, as the known processing products occur immediately C-terminal to the pro-GRP signal sequence, and cross-reactivity with other related gene products with homology to GRP such as NMB have been excluded [26]. This suggests that since an alternate GRP transcript has not been detected [27], the protein in question is most likely to be a C-terminally extended form of GRP, and that it is bound with low affinity by the detection antiserum. This observation, combined with difficulties in determining the molecular mass ion by mass spectroscopy have previously precluded unambiguous identification of this GRP product.
Recently we have tested a new GRP antiserum which was raised to the C-terminal region of GRP18-27 extended by glycine residue (GRP18-27gly). Unexpectedly, this antiserum bound avidly to the ovine pregnant endometrial GRP peptide, substantially reducing the detection threshold for monitoring purification by radioimmunoassay (RIA). Here we report the successful isolation, characterization and biological activity of the principal pro-GRP-derived processing product of the pregnant ovine endometrium, which corresponds to GRP1-46 (oGRP1-46).
2. Materials and Methods
2.1 Isolation of oGRP1-46
2.1.1 Tissue extraction and initial purification
Late pregnant ovine endometrium was obtained from 125–140 day pregnant ewes after ethical culling. Protocols were assessed and passed by the appropriate institutional eanimal ethics committee. Three different extraction procedures were evaluated in terms of extraction efficacy of immunoreactive GRP eluting prior to the GRP1-27 standard at 5–6.5kDal. The extraction conditions were 3% acetic acid, ice-cold acetonitrile/trifluoacetic acid (TFA), and ice-cold formic acid/TFA. Ice-cold 80% acetonitrile/2%TFA extraction was found to be the most effective extraction medium.
Briefly, frozen endometrium was pulverised in liquid nitrogen using a mortar and pestle to produce a fine frozen powder. 2g was immediately added to 10vol 80% acetonitrile/2% TFA and homogenized. After centrifugation (10,000g for 15min) the supernatant was collected and the pellet re-extracted in the same solution. Supernatants were combined and acetonitrile evaporated under a continuous stream of air. The liquid phase was cooled on ice, then passed 3 times through a C18 Sep-pak reverse-phase cartridge which had been activated and washed according to the manufacturer’s instructions (Millipore/Waters Rydalmere, Australia). GRP was eluted with 6ml of 75% acetonitrile/0.05% TFA.
2.1.2 Gel filtration of semi-purified GRP
Sep-pak eluates were freeze dried and concentrated to < 100 ul, reconstituted in 3% glacial acetic acid and chromatographed on Sephadex G-50 (1.5 × 90cm) with 3% glacial acetic acid as the eluant. Fractions (100 × 1.4ml) were collected, lyophilised and assayed for GRP immunoreactivity by RIA. The gel filtration column was calibrated with BSA (void volume), ovine GRP1-46, human GRP1-27 and human GRP18-27.
2.1.3 Reverse-phase high pressure liquid chromatography (RP-HPLC)
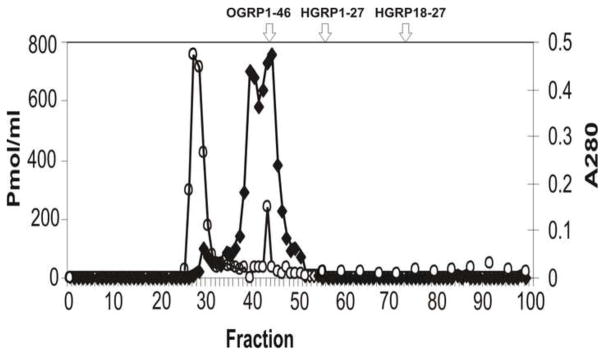
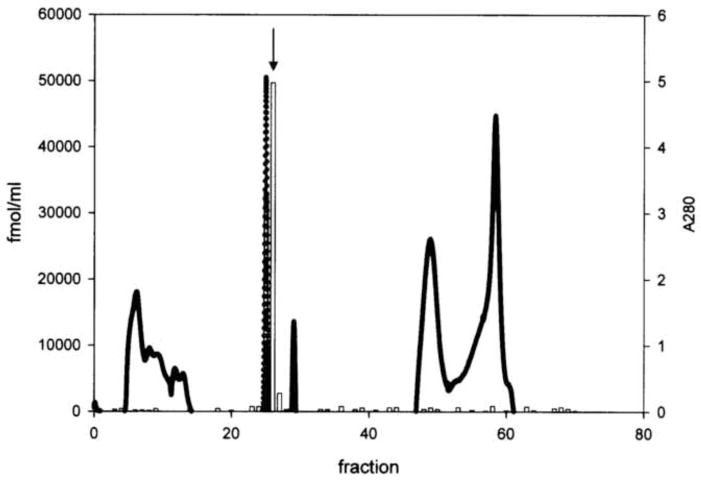
The GRP immunoreactive profile on gel filtration gave an overlapping immunoreactive doublet at fractions 40/41 and 44/45 (see Fig. 1A). This peak eluted between the void volume and human (h) GRP1-27. Each peak (2.8ml) was freeze dried to <100ul and separately chromatographed by RP-HPLC on a C18 μ Bondapak column (Millipore-Waters) eluted with a gradient of 0–70% acetonitrile/0/05% TFA. Peak fractions 40/41 and 44/45 each gave a single sharp Gaussian peak with identical elution position (~ 30% acetonitrile) containing 82 and 95 pmol immunoreactivity respectively (Fig 1B). These were used for subsequent Matrix Assisted Laser Desorption Ionization Time-of-flight (Maldi-TOF) analysis.
Fig. 1.
(A) Gel filtration chromatography of protein extract of pregnant ovine endometrium. Open circles indicate the total protein elution profile (A280nM) while filled rectangles show the immunoreactive GRP profile. The elution positions of human (h) GRP18-27 and hGRP1-27 as well as oGRP1-46 are indicated. (B) Reverse-phase C18 chromatography (μBondpak) of pooled fractions 40 and 41 from gel filtration step. The immunoreactive profile is indicated by the open bars with the peak fraction used subsequently for Maldi-TOF indicated with an arrow. Total protein is shown by the solid line.
2.1.4 Mass spectroscopy
The HPLC fraction was concentrated using a vacuum centrifugal concentrator and then 1μl was mixed with 1μl of matrix (α-cyano-4-hydroxy-cinnamic acid) and dried onto a sample plate followed by mass spectrometric analysis using the Maldi-TOF method on an Qstar mass spectrometer (Applied Biosystems, Foster City, CA).
2.2 RIA of GRP
Two RIAs were used to detect oGRP1-46; one used initially for primary extraction monitoring and directed to the amidated C-terminus of GRP18-27 and 1-27 (#R40), and the other used for final purification monitoring, and directed against gly-extended GRP18-27 (#8684; GRP18-27gly).
2.2.1 GRP #R40 RIA
The details of this assay has been previously published [4]. Briefly this antiserum detects amidated GRP of all mammalian species tested. 125I-labelled Tyr4-bombesin was used as the tracer and was prepared using iodogen (Pierce Chemical, Rockford, IL) followed by reduction with dithiothreitol to reverse methionine oxidation and purified by RP-HPLC. Assay cocktail was 100μl tracer (3000–5000cpm), 100μl antibody (1:75,000 final dilution), sample and standard (2–2000 fmol/ml bombesin) made up to 1ml with 0.02M veronal buffer containing 0.1% BSA and 2μM NaN3. Bound from free counts were separated using either activated charcoal or SacCel (100ul/tube). ID50 was 100 fmol/tube, intra-assay and inter-assay variation 4% and 14% respectively.
2.2.2 GRP18-27gly #8684 RIA
Antiserum 8684 was produced by immunization of rabbits with GRP18-27gly conjugated to keyhole limpet hemocyanin. GRP18-27gly (Auspep, Melbourne, Vic., Australia) was used for the standard curve, and the tracer was 125I-labeled GRP-gly which was prepared using the iodogen method and purified by reverse-phase HPLC. The ID50 was 40 fmol/tube. The antiserum cross reacts (60%) with amidated forms of GRP1-27 and GRP18-27 and less than 1% with amidated bombesin. GRP18-27 differs from bombesin by a Gln to His substitution at position 20.
2.3 Western blotting
Proteins for immunoblotting were taken after reverse-phase C18 chromatography. 50 μL aliquots containing 20μg protein were taken, and subjected to electrophoresis on a 15% SDS PAGE gel under reducing conditions. After electrophoresis and protein transfer, membranes were incubated with C-terminal GRP antiserum #R40 at 1:5000 (#8684 has not been validated for immunoblotting), peroxide-conjugated swine anti-rabbit IgG-HRP secondary antibody (1:1000) and visualized by enhanced chemiluminescence (Amersham, Buckinghamshire, UK). The blot was calibrated using GRP1-27 and low range molecular weight markers (2.35–46 kDal; Amersham).
2.5 Activity of oGRP1-46
2.5.1 Synthesis of oGRP1-46
oGRP1-46 (APVTAGRAGALAKMYTRGNHWAVGHLMGKKSVAESPQLREEES LKE) was synthesized by solid phase means (Peptide Solutions, Bundoora, Australia) and supplied lyophilized at 70% purity. It was stored dried at −80°C until use.
2.5.2 Measurement of [3H]IP
Non-adherent, NCI-H69, NCI-H209, NCI-N417 or NCI-N592 (1 × 106 cells/ml) were sub-cultured into a 75-cm2 tissue culture flask containing 15 ml of RPMI-160 supplemented with 2% (v/v) FBS and 3mCi/ml myo-[2-3H]inositol and incubated for 24 hr at 37C. The cells were then washed and incubated for 10 min at 37C with equivalent volume of PBS (pH 7.0) containing 20 mM lithium chloride. The cell were then re-suspended in IP assay buffer containing 135 mM sodium chloride, 20 mM lithium chloride, 20 mM HEPES (pH 7.4), 2 mM calcium chloride, 2 mM magnesium sulfate, 1 mM EGTA, 11.1 mM glucose and 0.05% BSA. 300μl of the cell suspension was added to test tubes containing the peptides to be tested and incubated at 37C for 60 min after which the incubation was terminated with 1 ml ice-cold HCl/methanol (1% v/v). [3H]IP generation was determined as described previously [11,21]. Briefly, the samples were loaded on AG1-X8 anion exchange resin columns, washed with 4 ml of water to remove [3H]inositol, and then washed with 2 ml of 5 mM disodium tetraborate/60 mM sodium formate to remove [3H]glycerophosphorylinositol. The columns were the eluted with 2 ml of 1 mM ammonium formate/100 mM formic acid solution to elute total [3H]IP. 10 ml of scintillation cocktail was added to the eluates and radioactivity was measured in a beta scintillation counter. For hNMBR-transfected BALB 3T3 cells, hNMBR-transfected H1299 cells, hGRPR-transfected BALB cells and HuTu80 (Gonzalez etal. 2009) which grow as monolayers on plates and flasks, cells were subcultured in 24-well plates (5 × 104 cell/well) in their growing media and incubated at 37C for 24 hr. [3H]IP generation was determined as described previously [11,21]. Briefly, the cells were then loaded with 3μCi/ml myo-[2-3H]inositol in growth media supplemented with 2% FBS and incubated for another 24 hr. The 24-well plates were then incubated with 1 ml/well of wash buffer (PBS (pH 7.0) containing 20 mM lithium chloride) for 30 min. The wash buffer was aspirated and replaced with 500 μl IP assay buffer with or without the peptides studied and incubated at 37C for 60 min after which the incubation was terminated with 1 ml ice-cold HCl/methanol (1% v/v). The samples were applied to columns and total [3H]IP determined as stated above.
2.5.3 Binding of the BRS3 preferring ligand 125I-[D-Tyr6, β-Ala11, Phe13, Nle14]Bn(6-14) to various cells
125I-[D-Tyr6, β-Ala11, Phe13, Nle14]Bn(6-14) (2200 Ci/mmol) was prepared as described previously [14]. The standard binding buffer contained 24.5 mM HEPES (pH 7.4), 98 mM NaCl, 6 mM KCl, 5 mM MgCl2, 2.5 mM NaH2PO4, 5 mM sodium pyruvate, 5 mM sodium fumarate, 0.01% (w/v) soybean trypsin inhibitor, 1% amino acid mixture, 0.2% (w/v) bovine serum albumin and 0.05% (w/v) bacitracin. For H1299 cells stably transfected with hNMBR(0.8 × 106); HuTu 80 cell(1 × 106); BALB 3T3 cells stably expressing hGRPR (0.3 × 106), hNMBR (0.03 × 106), rNMBR(0.3 × 106), mNMBR(1.5 × 106) or hBRS-3 (0.3 × 106) binding was determined as described previously [11] with an incubation with 50 pM 125I-[D-Tyr6, β-Ala11, Phe13, Nle14]Bn(6-14) at 22°C for 60 min. Aliquots (100 μl) were removed and centrifuged through 300 μl of incubation buffer in 400 μl microfuge tubes at 10,000 × g for 1 min using a Beckman Micro-centrifuge B. The pellets were washed twice with buffer and counted for radioactivity in a gamma counter. The nonsaturable binding was the amount of radioactivity associated with cells in incubations containing 50 pM 125I-[D-Tyr6, β-Ala11, Phe13, Nle14]Bn(6-14) and 1 μM unlabeled ligand. Nonsaturable binding was <10% of total binding in all the experiments.
3. Results
3.1 Optimization of initial extraction conditions for ovine endometrial GRP
3 different extraction conditions coupled with the R40 antiserum-based RIA were compared as detailed in Materials and Methods. Organic extraction with ice-cold 80% acetonitrile/2% TFA yielded higher concentrations of GRP-IR (7.2 ± 2.6 pmol/g, n = 4) than either 3% acetic acid extraction (1.3 ± 0.6 pmol/g, n = 6) or 5% formic acid/1% TFA (2.2 pmol/g, n=1). Chromatography of extracts using different methodologies showed that unlike the other extraction methods, acidified acetonitrile gave a single major peak of immunoreactivity without evidence of proteolysis (data not shown).
3.2 Isolation of oGRP1-46
Yield from acidified acetonitrile extraction was 6–8 pmol immunoreactivity/g tissue and ~ 3pmol/g after batchwise sep-pak (reverse-phase C18) chromatography. This was further purified by gel filtration on Sephadex G-50 and RP-HPLC prior to mass spectroscopy. Purification during chromatography steps was monitored by RIA using # 8684 anti-GRP18-27gly antiserum which gave a very similar elution profile to #R40, but was much more sensitive in detecting ovine endometrial GRP.
Gel filtration chromatography of the initial semi-purified extract showed two closely overlapping immunoreactive peaks which eluted together with baseline separation and much later than the void volume and main protein peak (Fig. 1A). Peak fractions from the gel filtration doublet were further purified by RP-HPLC on a C18 μ-bondapak column eluted with 0–70% ACN/0.05% TFA. Each peak eluted with identical hydrophobicity (~30% ACN/TFA; Fig. 1B) as a sharp Gaussian peak, and these were pooled and subjected to mass spectroscopy (Fig. 2).
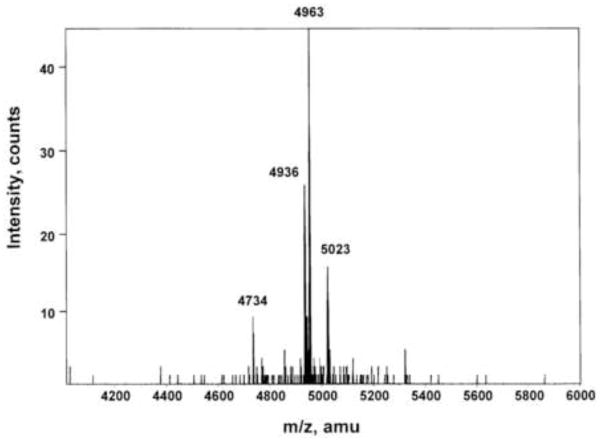
Fig. 2.
Mass spectroscopy of the RP-HPLC purified peptide on the Maldi MS spectrum with major mass ion of 4963 and minor ions at 4934, 4737 and 5023 Da The most abundant mass ion corresponds to GRP1-46 (APVTAGRAGALAKMYTRGNHWA VGHLMGKKSVAESPQLREEESLKE).
Mass spectroscopy of the RP-HPLC purified peptide on the Maldi MS spectrum gave a major mass ion of 4963 and minor ions at 4733, 4934, 4937 and 5022 Da (Fig. 2; Table 3). The most abundant mass ion corresponds to GRP1-46 which has a theoretical mass of 4963.67 and contains both the antigenic determinant required for binding by either antiserum R40 (GHLM-NH2) or #8684 (GHLMG) as well as an intact N-terminus which is shared by all established GRP peptides with in vivo bioactivity, derived from pro-GRP processing. Proteolytic cleavage of pro-GRP to give rise to GRP1-46 would require preferential cleavage at the Glu-Glu bond by a Glu-C2-like enzyme, rather than the tryptic and C-terminal amidation enzymes (PAM) that produce GRP18-27 and 1-27 in other tissues.
Table 3.
Ovine pre-pro-GRP and major mass ions derived from Maldi-TOF analysis of purified pregnant ovine endometrial immunoreactive proteins. The antigenic determinant for antiserum R40 is shown in light gray (GHLM-NH2) and for #8684 (GHLMG-OH). Only GRP1-46, which is the major mass ion detected has an intact N-terminus common to all bioactive GRP peptides and the antigenic determinant.
| Pre-pro-GRP |
| MRSREVSLVLLALVLCPAPRGSAAPVTAGRAGALAKMYTRGNHWAVGHLMGKKSVAESPQLREEESLKEQLREYAQWEEATRNLLSLLQAKVAQGHQPPRWEPLSIHQPAWDSKDVSNFKDSGSQREGGNPQLY |
| 4733 GRP52-98 |
| AQWEEATRNLLSLLQAKVAQGHQPPRWEPLSIHQPAWDSKDVSNFKD |
| 4934 GRP34-75 |
| ESPQLREEESLKEQLREYAQWEEATRNLLSLLQAKVAQGHQP |
| 4937 GRP3-47 |
| TAGRAGALAKMYTRGNHWAVGHLMGKKSVAESPQLREEESLKEQL |
| 4963 GRP1-46 |
| APVTAGRAGALAKMYTRGNHWAVGHLMGKKSVAESPQLREEESLKE |
| 5023 GRP18-60 |
| GNHWAVGHLMGKKSVAESPQLREEESLKEQLREYAQWEEATRN |
The minor mass ions at 4734 and 4934 correspond to GRP52-98 and GRP34-75, and since they do not contain the antigenic determinant required for antibody binding nor an intact N-terminus are unlikely to be bioactive. Mass ions at 4937 and 5023 correspond to GRP3-47 and GRP18-60. These contain the antibody binding epitope but lack an intact N-terminus, so are unlikely to be bioactive.
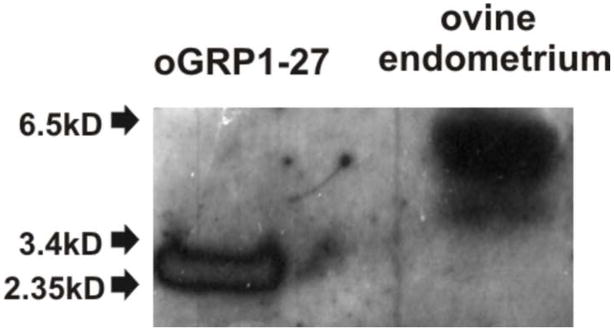
Fig. 3 shows a representative Western blot of the partially purified material after reverse-phase chromatography of pregnant ovine endometrial extracts. Immunoreactive GRP in the pregnant endometrium has a mass of 5–6 kDal with a slightly smaller and faster migrating uncharacterised form also being apparent. Ovine endometrial GRP migrates more slowly than the oGRP1-27 standard.
Fig. 3.
Western blot of the partially purified material after reverse-phase chromatograpy of pregnant ovine endometrium extract. Immunoreactive GRP has a mass of 5–6 kDal with a slightly smaller and faster migrating form also being apparent. Elution position of the oGRP1-27 standard (1 μg) is shown for comparison.
3.3 Biological activity of oGRP1-46
oGRP1-46 was produced commercially by solid-phase synthesis. Lyophilised oGRP 1-46 was reconstituted in binding buffer and its receptor affinity quantified in binding assays using a series of cell lines that express the rat, mouse or human GRPR, the human or rat NMBR, or BRS3, and activity compared to GRP or NMB. In addition, the ability of oGRP 1-46 to activate phospholipase C quantified by 3H-inositol release from the same cell lines as used for receptor binding was quantified. The data is summarised in Table 1. oGRP 1-46 interacted with GRPR from human rat and mouse as well as human and rat NMBR. For hGRPR, oGRP1-46 had a 300–400 fold lower affinity than GRP, and was similar to NMB. For rat and mouse GRPR, oGRP1-46 had a 150-fold lower affinity than GRP and 6-fold less active than NMB. For hNMBR, GRP had a 4000 fold lower affinity than NMB and oGRP1-46 was approximately 10-fold less potent than GRP, with a similar outcome for the rNMBR. For hBRS-3, similar to GRP and NMB, oGRP1-46 had a very low affinity (i.e. >10,000 nM). As shown in Table 1, oGRP1-46 could also activate the human GRPR as well as the human and rat NMBR. For the hGRPR and similar to the binding results, oGRP1-46 was 40–100 fold less potent than GRP at releasing 3H-inositol, and had similar potency to NMB. Likewise, with the human NMBR, oGRP1-46 was 15-fold less potent than GRP, which in turn was 35–50 fold less potent than NMB(Table 1). This indicates that oGRP1-46 is a weak agonist at the GRPR and NMBR, and was without activity at the BRS3 receptor.
Table 1.
Affinity and potency of sheep GRP(1-46), GRP and NMB for human, mouse and rat bombesin receptor members.
| Cell ( receptor subtype) | Binding (IC50, nM) | [3H]IP stimulation (EC50, nM) | ||||
|---|---|---|---|---|---|---|
| GRP | oGRP1-46 | NMB | GRP | oGRP1-46 | NMB | |
| hGRPR BALB cells 1 | 0.17 ± 0.01 | 52 ± 2 | 41 ± 1 | 4.8 ± 0.2 | 339 ± 99 | 257 ± 8 |
| HuTu cancer cells 1 | 0.19 ± 0.01 | 79 ± 4 | 56 ± 1 | 3.2 ± 0.1 | 112 ± 1 | 354 ± 5 |
| AR42J pancreatic cancer 2 | 1.8 ± 0.1 | 309 ± 6 | 52 ± 2 | |||
| mGRPR BALB cells 3 | 3.2 ± 0.2 | 371 ± 9 | 52 ± 2 | |||
| hNMBR BALB cells 4 | 123 ± 9 | 1175 ± 38 | 0.03 ± 0.01 | 76 ± 3 | 1175 ± 49 | 2.1 ± 0.2 |
| hNMBR 1299 NSCLC cells 4 | 148 ± 11 | 1032 ± 23 | 0.17 ± 0.01 | 56 ± 2 | 758 ± 26 | 1.0 ± 0.1 |
| rNMBR BALB cells 5 | 430 ± 38 | 1032 ± 42 | 3.0 ± 0.2 | |||
| BRS-3 BALB cells 6 | >10,000 | >10,000 | >10,000 | |||
To determine the binding affinity the indicated cells were incubated with 50 pM I-[D-Tyr, β Ala11, Phe13, Nle14]Bn(6-14) with increasing concentrations (0.01 nM-1 uM) of GRP, NMR or sheep GRP(1-46). The affinity is expressed as the concentration causing half-maximal inhibition of saturable binding and is the mean ± SEM from at least 4 experiments. To assess the ability of the agonists to activate phospholipase C, the ability of each agonist to stimulate generation of [3H]IP generation was determined as described in Methods. The concentration causing half-maximal stimulation (EC50) for [3H]IP are listed. The results are expressed as means ± SEM and are the results from at least 4 experiments. Abbreviations: h, human; GRPR, gastrin-releasing peptide receptor; r, rat; m, mouse; NMBR, neuromedin B receptor; s, sheep; BRS-3, bombesin receptor subtype 3.
hGRPR;
rGRPR;
mGRPR;
hNMBR;
rNMBR;
hBRS-3
To explore the ability of oGRP1-46 to activate known human bombesin receptors on human cancer cells, and possibly to bind a unique receptor, we studied its ability to activate phospholipase C and stimulate the generation of [3H]IP in 4 human SCLC cell lines that are known to frequently ectopically express bombesin receptors [11, 13, 14, 15, 21). This was quantified and data are shown in Table 2. For 3 of the SCLC cell lines (H69, N417, H209) but not N592, at least one of the know bombesin receptor agonists (GRP for GRPR, NMB for NMBR, or [D-Phe6, β-Ala11, Phe13, Nle14] Bn(6-14) for hBRS-3 [5,15] stimulated phospholipase C activity. oGRP1-46 also stimulated phospholipase C activity in two of these cell lines (H69, H209), however, this was inhibited by either a specific GRPR or NMBR antagonist, demonstrating that the actions of oGRP1-46 were mediated through interaction with either a GRPR or NMBR in these non-ovine cells, and not through a unique receptor.
Table 2.
Comparison of the ability of oGRP(1-46) and other activators of human bombesin receptors to stimulate phospholipase C activity in different human small cell lung cancer cell lines (SCLC).
| Experimental/control | |||||
|---|---|---|---|---|---|
| SCLC CELL | NMB | [D-Phe6, β-Ala11, Phe13, Nle14] Bn(6-14) | GRP | oGRP(1-46) | oGRP(1-46) + GRPR or NMBR antagonist |
| (0.1 uM) | (0.1 uM) | (0.1 uM) | (0.1 uM) | (0.1 uM) | |
| NCI-H69 | 1.03±0.06 | 1.31±0.04** | 1.23±0.07* | 1.10±0.02** | 0.99±0.06 |
| NCI-N417 | 1.01±0.06 | 3.14±0.34** | 1.03±0.01 | 0.99±0.05 | 0.96±0.08 |
| NCI-H209 | 1.25±0.11* | 1.17±0.03** | 1.11±0.04 | 1.17±0.04** | 1.01±0.06 |
| NCI-N592 | 1.08±0.06 | 1.05±0.04 | 1.03±0.04 | 1.01±0.04 | 1.05±0.02 |
The ability to activate phospholipase C and stimulate [3H]IP breakdown was determined for each of the Bn related peptides and oGRP(1-46), either alone or in the presence of a GRPR receptor antagonist ([(3-Ph-Pr6), His7, D-Ala11, D-Pro13, ψ (13–14), Phe14]Bn(6-14)NH2 [1 uM]) or a NMBR receptor antagonist (PD 168368)[1 uM]. Results are expressed as the experimental value divided by the control value. Results are means±SEM and are the average of at least 4 experiments.
p<0.05,
p<0.01 compared to no additions. Abbreviations (Jensen etal. 2008); SCLC, small cell lung cancer cells; β-Ala, β-alanine; Nle, norleucine; others -see Table 1 footnote.
4. Discussion
We have previously shown that the main processing product of the GRP gene precursor in the pregnant ovine uterus is a peptide which is considerably larger than GRP18-27 and 1-27 [8, 10], the best characterised biologically active products of pro-GRP processing in tissues outside the reproductive tract [12]. This ovine peptide has been shown to be produced by uterine gland cells in large amounts during pregnancy, beginning soon after implantation and continuing until parturition, and is secreted into the uterine lumen (“uterine milk”), and also via the utero-placental circulation into fetal and maternal plasma, where it may act as a hormone of pregnancy [8]. A peptide of similar size has also been detected in the pregnant, but not non-pregnant human endometrium, suggesting a phylogenetically conserved role for GRP-related peptides in mammalian fetal development [25].
Here we have isolated the main GRP peptide from the pregnant ovine endometrium and shown it to be GRP1-46 by mass spectroscopy. Although correlative sequencing of this peptide was unable to be completed, previous isolation attempts gave partial sequence of 42 amino acids including an intact N-terminus and antigenic determinant, but was incomplete due to limiting sample abundance. The isolation of oGRP1-46 has important implications for our understanding of the proteolytic processing of the GRP precursor, and for regulation of post-translational processing by proteases, as a mechanism for producing different peptide products from the same precursor at different times, and in the same tissue. The non-pregnant ovine uterus prior to luteal regression and oestrus, has been shown to produce only GRP1-27 and a smaller tryptic peptide GRP18-27; the same peptide products elaborated by other mammalian tissues that express the GRP gene [26, 27]. No evidence for larger processing products has been convincingly demonstrated in any of these tissues, and GRP1-27 is produced as the result of sequential trypsin-like, carboxypeptidase and peptide amide monoamine oxygenase (PAM) processing to give the carboxyl terminus alpha-amidated peptide. During luteal regression and oestrus in the non-pregnant uterus, both GRP1-27 and 18-27 are detected, as well as small amounts of a larger molecular weight species of about 5–6 kDal, and which is likely to be oGRP1-46. In the pregnant uterus however, little or no GRP1-27 or 18-27 are produced, but the expression of a larger molecular weight form is dramatically increased, and we now show that this main processing product is oGRP1-46.
Several conclusions derive from these results. First, the proteolytic cascade that involves at least 3 enzymes and results in C-terminally amidated products, is likely inhibited in the ovine uterus during the luteal regression phase of the oestrus cycle and in pregnancy. Whether this is also true for other tissues that express the GRP gene needs to be assessed. There are many precedents for the production from a single precursor of multiple peptides, with independent receptors, different bioactivities and different expression profiles. For example glucagon, glucagon-like peptide 1 and glucagon-like peptide 2 are all cleaved from the 160 amino acid precursor proglucagon. Each peptide has its own distinct receptor, biological role and tissue specific expression.[3].
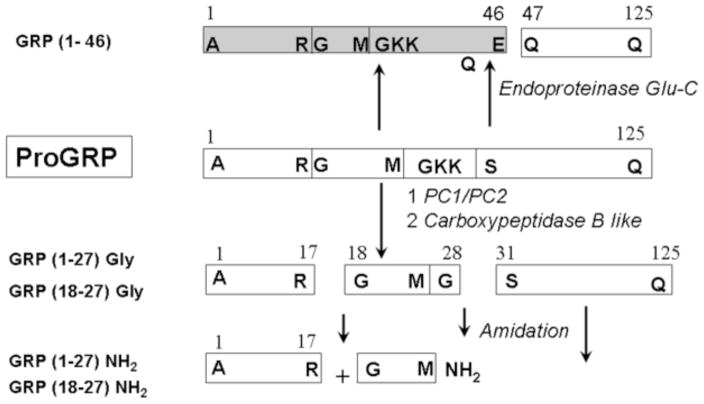
Second, we predict that the expression of an enzyme which has Glu-C2-like specificity, that is with Glu or Asp at P1 and any amino acid at P1′ and P2′ (http://prolysis.phys.univ-tours.fr; nomenclature of Schecter and Berger [22, 23], is transiently induced at the end of oestrus, and again during pregnancy, and then is down-regulated following parturition. Presumably this occurs in order to produce peptide products with bioactivity specifically related to preparing and maintaining the uterus for implantation of the conceptus. Although such an endogenous enzyme has not yet been described to our knowledge in the pregnant endometrium, there are numerous examples of processing events with similar specificity, such as that for gastrin 10 (Glu-Ala, P1-P1′) [20] and the 3C-like protease of rabbit hemorrhagic disease virus which is self-cleaved from its precursor (N-terminal cleavage, Glu-Gly) [29]. Thus we would predict that in the pregnant endometrium, endogenous PAM activity would be reduced and that of endogenous Glu-C2 protease increased. A proteolytic scheme for how this might be accomplished is given in Figure 4.
Fig. 4.
Comparison of processing events required to produce GRP1-46 or GRP18-27, 1-27 or GRP18-27gly, GRP1-27gly from the proGRP precursor.
Third, C-terminally extended peptide (that is peptides containing the Gly-Lys-Arg cleavage sequence) products of GRP gene processing, are likely to have biological activity in their own right. At present the endogenous cellular targets for ovine GRP1-46 is unknown, but roles in uterine remodelling or fetal maturation are likely. This is especially true given the fact that we have shown that oGRP1-46 is an agonist at the GRPR which mediates GRP functions such as proliferation and local cellular regulatory events. Likewise, whether or not oGRP1-46 binds only the GRPR (and NMBR weakly), or mediates its hormonal or local tissue regulatory outcomes via a unique receptor is unknown. This possibility is made more plausible since GRPR, NMB and BRS-3 are undetectable or found only at very low levels in the sheep placenta, endometrium and conceptus during fetal development [24]. Competitive binding studies performed here on human small cell lung carcinoma cell lines do not support the existence of an alternative receptor mediating the effects of oGRP1-46 in this tissue, however the sheep peptide is yet to be tested in suitable homologous ovine assay systems.
There are obvious parallels between the actions of oGRP1-46 and other biologically active, non-amidated and C-terminally extended products of the GRP gene [ 4, 16] including GRP-gly (GRP1-28), and pro-GRP(GRP1-125). Both of these peptides stimulate proliferation and migration of colorectal cancer cell lines, with the former activating GRPR, while the latter shows no activity at either GRPR or BRS-3, suggesting the existence of a novel receptor subtype, at least on some cancer cells [18, 19]. The present data further supports the existence of multiple, biologically active forms being generated from the proGRP precursor.
5. Conclusion
We have isolated and characterized a processing product of the abundant GRP gene precursor in the pregnant ovine endometrium, which corresponds exactly in molecular weight to ovine GRP1-46. This peptide, which co-elutes and is similarly bound by detection antisera to a GRP peptide that circulates in both the pregnant ewe and fetus, and that is strongly induced at the time of implantation, we now show acts as a weak agonist at both the GRPR and NMBR. The expression and disposition of oGRP1-46 suggests that it may act as a hormone of pregnancy, possible regulating aspects of conceptus development, or maintaining receptivity of the endometrium during fetal maturation.
Acknowledgments
We thank the late Professor John Walsh and the late Ms Helen Wong (Department of Medicine, UCLA, Los Angeles, CA) for donating antisera 8684.
This work was supported by projects grants from the National Health and Medical Research Council of Australia and intramural funds of NIDDK, NIH.
Nomenclature
- BSA
bovine serum albumin
- C-terminus
Carboxyl terminus
- CNS
central nervous system
- BRS-3
bombesin receptor subtype 3
- GRP
gastrin releasing peptide
- GRPR
gastrin-releasing peptide receptor
- HRP
Horseredish peroxidase
- SCLC
small cell lung cancer cells
- MALDI-TOF
Matrix Assisted Laser Desorption Ionization Time-of-flight
- NaN3
Sodium azide
- NMBR
neuromedin B receptor
- RIA
Radioimmunoassay
- RP-HPLC
reverse-phase high pressure liquid chromatography
- SacCel
Silica beads with cellulose
- TFA
trifluoroacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Budipitojo T, Matsuzaki S, Cruzana MBC, Baltazar ET, Hondo E, Sunaryo S, Kitamura N, Yamada J. Immunolocalization of gastrin-releasing peptide in the bovine uterus and placenta. J Vet Med Sci. 2001;63:11–15. doi: 10.1292/jvms.63.11. [DOI] [PubMed] [Google Scholar]
- 2.Budipitojo T, Sasaki M, Matsuzaki S, Cruzana MBC, Iwanaga T, Kitamura N, Yamada J. Localization and expression of gastrin-releasing peptide (GRP) in the bovine cervix. J Reprod Dev. 2004;50:119–129. doi: 10.1262/jrd.50.119. [DOI] [PubMed] [Google Scholar]
- 3.Drucker DJ. Biological actions and therapeutic potential of the glucagon-like peptides. Gastroenterology. 2002;122:531–44. doi: 10.1053/gast.2002.31068. [DOI] [PubMed] [Google Scholar]
- 4.Dumesny C, Patel O, Lachal S, Giraud AS, Baldwin GS, Shulkes A. Synthesis, expression and biological activity of the prohormone for gastrin releasing peptide (ProGRP) Endocrinology. 2006;147:502–9. doi: 10.1210/en.2005-0574. [DOI] [PubMed] [Google Scholar]
- 5.Fathi Z, Corjay MH, Shapira H, Wada E, Benya R, Jensen R, et al. BRS-3: novel bombesin receptor subtype selectively expressed in testis and lung carcinoma cells. J Biol Chem. 1993;268(8):5979–84. [PubMed] [Google Scholar]
- 6.Fraser M, Carter AM, Challis JRG, McDonald TJ. Gastrin releasing peptide immunoreactivity is present in ovine amniotic fluid and fetal and maternal circulations. Endocrinology. 1992;131:2033–2035. doi: 10.1210/endo.131.4.1396347. [DOI] [PubMed] [Google Scholar]
- 7.Fraser M, McDonald TJ, Spindel ER, Fahy M, Hill D, Challis JRG. Gastrin-releasing peptide is produced in the pregnant ovine uterus. Endocrinology. 1994;135:2440–2445. doi: 10.1210/endo.135.6.7988429. [DOI] [PubMed] [Google Scholar]
- 8.Giraud AS, Parker LM, Taupin D, Hardy K, Shulkes A. Mammalian bombesin as a hormone in ovine pregnancy: ontogeny, origin, and molecular forms. Am J Physiol. 1993;265:E866–873. doi: 10.1152/ajpendo.1993.265.6.E866. [DOI] [PubMed] [Google Scholar]
- 9.Giraud AS, Salamonsen L, Whitley J, Shulkes A. Gastrin releasing peptide is synthesized and secreted by the ovine endometrium in early pregnancy. Endocrinology. 1994;135:2806–2809. doi: 10.1210/endo.135.6.7988475. [DOI] [PubMed] [Google Scholar]
- 10.Giraud AS, Whitley J, Shulkes A, Parker LM. The pregnant ovine endometrium constitutively expresses and secretes a highly stable bombesin-like peptide, which shares C-terminal sequence, but differs structurally from gastrin-releasing peptide. Biochim Biophys Acta. 1996;1296:189–197. doi: 10.1016/0167-4838(96)00070-2. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez N, Mantey SA, Pradhan TK, Sancho V, Moody TW, Coy DH, et al. Characterization of putative GRP and NMB-receptor antagonist’s interaction with human receptors. Peptides. 2009 doi: 10.1016/j.peptides.2009.05.007. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ischia J, Patel O, Shulkes A, Baldwin GS. Gastrin-releasing peptide: different forms, different functions. Biofactors. 2009;35:69–75. doi: 10.1002/biof.10. [DOI] [PubMed] [Google Scholar]
- 13.Jensen RT, Battey JF, Spindel ER, Benya RV. International Union of Pharmacology. LVIII. Mammalian Bombesin Receptors: Nomenclature, distribution, pharmacology, signaling and functions in normal and disease states. Pharmacol Rev. 2008;60(1):1–42. doi: 10.1124/pr.107.07108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mantey SA, Weber HC, Sainz E, Akeson M, Ryan RR, Pradhan TK, et al. Discovery of a high affinity radioligand for the human orphan receptor, bombesin receptor subtype 3, which demonstrates it has a unique pharmacology compared to other mammalian bombesin receptors. J Biol Chem. 1997;272(41):26062–71. doi: 10.1074/jbc.272.41.26062. [DOI] [PubMed] [Google Scholar]
- 15.Moody TW, Staley J, Zia F, Coy DH, Jensen RT. Neuromedin B binds with high affinity, elevates cytosolic calcium and stimulates the growth of small cell lung cancer cell lines. J Pharmacol Exp Ther. 1992;263:311–7. [PubMed] [Google Scholar]
- 16.Patel O, Dumesny C, Giraud AS, Baldwin GS, Shulkes A. Stimulation of proliferation and migration of a colorectal cancer cell line by amidated and glycine-extended gastrin-releasing peptide via the same receptor. Biochem Pharmacol. 2004;68:2129–42. doi: 10.1016/j.bcp.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Patel O, Shulkes A, Baldwin GS. Gastrin-releasing peptide and cancer. Biochim Biophys Acta. 2006;1766:23–41. doi: 10.1016/j.bbcan.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Patel O, Dumesny C, Shulkes A, Baldwin GS. Recombinant C-terminal fragments of the gastrin-releasing peptide precursor are bioactive. Cancer Lett. 2007;254:87–93. doi: 10.1016/j.canlet.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Patel O, Dumesny C, Shulkes A, Baldwin GS. C-terminal fragments of the gastrin-releasing peptide precursor stimulate cell proliferation via a novel receptor. Endocrinology. 2007;148:1330–9. doi: 10.1210/en.2006-0466. [DOI] [PubMed] [Google Scholar]
- 20.Rehfeld JF, Hansen CP, Johnsen AH. Post-poly(Glu) cleavage and degradation modified by O-sulfated tyrosine: a novel post-translational processing mechanism. EMBO J. 1995;14(2):389–96. doi: 10.1002/j.1460-2075.1995.tb07013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowley WH, Sato S, Huang SC, Collado-Escobar DM, Beaven MA, Wang LH, et al. Cholecystokinin-induced formation of inositol phosphates in pancreatic acini. Am J Physiol. 1990;259:G655–G665. doi: 10.1152/ajpgi.1990.259.4.G655. [DOI] [PubMed] [Google Scholar]
- 22.Schechter I, Berger A. On the active site of proteases. 3. Mapping the active site of papain; specific peptide inhibitors of papain. Biochem Biophys Res Comm. 1968;32:898–902. doi: 10.1016/0006-291x(68)90326-4. [DOI] [PubMed] [Google Scholar]
- 23.Schechter I, Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Comm. 1967;27:157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- 24.Song G, Satterfield C, Kim J, Bazer FW, Spencer TE. Gastrin-releasing peptide (GRP) in the ovine uterus: regulation by interferon tau and progesterone. Biol Reprod. 2008;79:376–386. doi: 10.1095/biolreprod.108.068403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitley J, Giraud AS, Shulkes A. Expression of gastrin-releasing peptide (GRP) and GRP receptors in the pregnant human uterus at term. J Clin End Metab. 1996;81:11. doi: 10.1210/jcem.81.11.8923842. [DOI] [PubMed] [Google Scholar]
- 26.Whitley J, Shulkes A, Salamonsen L, Vogiagis D, Familari M, Giraud AS. Temporal expression and cellular localization of a gastrin-releasing peptide-related gene in ovine uterus during the oestrus cycle pregnancy. J Endocrinology. 1998;157:139–148. doi: 10.1677/joe.0.1570139. [DOI] [PubMed] [Google Scholar]
- 27.Whitley JM, Giraud AS, Mahoney AO, Clarke I, Shulkes A. Tissue-specific regulation of gastrin releasing peptide synthesis, storage and secretion by oestrogen and progesterone in the uterus. J Endocrinology. 2000;166:649–658. doi: 10.1677/joe.0.1660649. [DOI] [PubMed] [Google Scholar]
- 28.Whitley JC, Moore C, Giraud AS, Shulkes A. Isolation and characterization of the ovine gastrin-releasing peptide gene; abundant expression in the pregnant endometrium and myometrium, and selective expression in fetal tissues. J Endocrinol. 2002;175:447–457. doi: 10.1677/joe.0.1750447. [DOI] [PubMed] [Google Scholar]
- 29.Wirblich C, Sibilia M, Boniotti MB, Rossi C, Thiel HJ, Meyers G. 3C-like protease of rabbit hemorrhagic disease virus: identification of cleavage sites in the ORF1 polyprotein and analysis of cleavage specificity. J Virol. 1995;69:7159–68. doi: 10.1128/jvi.69.11.7159-7168.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]