Abstract
The Nedd4 (neural precursor cell-expressed developmentally downregulated gene 4) family of ubiquitin ligases (E3s) is characterized by a distinct modular domain architecture, with each member consisting of a C2 domain, 2–4 WW domains, and a HECT-type ligase domain. Of the nine mammalian members of this family, Nedd4 and its close relative, Nedd4-2, represent the ancestral ligases with strong similarity to the yeast, Rsp5. In Saccharomyces cerevisiae Rsp5 has a key role in regulating the trafficking, sorting, and degradation of a large number of proteins in multiple cellular compartments. However, in mammals the Nedd4 family members, including Nedd4 and Nedd4-2, appear to have distinct functions, thereby suggesting that these E3s target specific proteins for ubiquitylation. In this article we focus on the biology and emerging functions of Nedd4 and Nedd4-2, and review recent in vivo studies on these E3s.
Keywords: ubiquitylation, endocytosis, trafficking, receptor, signaling
Introduction
Ubiquitylation controls biological signaling in many different ways.1,2 For example, the ubiquitylation of a misfolded or damaged protein leads to its degradation by the 26S proteasome before it can get to its subcellular site where it normally functions. A ligand-activated receptor may undergo ubiquitylation, where the tagging with ubiquitin acts as a signal for the endocytosis of the receptor; thus, removal from the membrane resulting in the termination of the signal generated by the activated receptor. Ubiquitylation of a cell-cycle inhibitor could lead to its rapid degradation, allowing cells to progress through the cycle. Thus, protein ubiquitylation can control signaling by regulating the cellular levels of a protein, by controlling its subcellular localization, by preventing accumulation of defective or damaged proteins, and in some instances, by preventing protein degradation. Clearly, a process that is so critical for cellular homeostasis, is itself tightly regulated by multiple enzymatic steps and many accessory proteins that provide specificity to the system.
The ubiquitylation of a protein needs conjugation between the carboxyl group of the C-terminal Gly residue of ubiquitin, and the ε-amino group of an internal Lys (K) in the substrate.1,2 The transfer of the activated ubiquitin to substrates occurs through a series of enzymes. These enzymes include a ubiquitin activating enzyme (E1), multiple ubiquitin conjugating enzymes (E2), and several hundreds of ubiquitin-protein ligase (E3). A protein can be monoubiquitylated, multi-monoubiquitylated, or polyubiquitylated, and the type of ubiquitylation determines the fate of the protein.1,2 Ubiquitin itself contains seven lysine residues, all of which can participate in the formation of ubiquitin chains. However, in mammals, K48 and K63 linked chains are most common.1,2 Substrates containing K48 linked ubiquitin chains are usually degraded by the 26S proteasome, whereas those containing K63 linked chains may undergo a number of different outcomes, including trafficking and/or lysosomal degradation. Recent work demonstrates that polyubiquitin chains through six of the seven Lys residues in ubiquitin (with the exception of K63), can target proteins for degradation.3 This study also showed that substrates linked through K11 chains are targets of endoplasmic reticulum-associated degradation (ERAD) pathway.3
The HECT ubiquitin-protein ligases
The specificity of ubiquitylation system is critical and is largely regulated by the E3s, which are responsible for mediating the transfer of ubiquitin from E2 to the substrate. Given that many cellular proteins are ubiquitylated, there are over 600 putative E3s in mammals. There are two main classes of E3s; the RING (Really Interesting New Gene) E3s, and the HECT (Homologous to E6-AP C-Terminus) E3s.4 The HECT ligases accept ubiquitin from an E2 and is then transferred to the substrate. On the other hand RING E3s mediate the E2-substrate interaction to facilitate the transfer of ubiquitin directly from an E2 to the substrate. Most human E3s belong to the RING family, whereas HECT family consists of 28 proteins. HECT is an ~350 amino-acid domain that was first described in E6AP (human papilloma virus E6 Associated Protein).5 Although a number of proteins containing HECT domain, including the Sacchromyces cerevisiae protein, Rsp5, and the mouse Nedd4 (neural precursor cell-expressed developmentally downregulated gene 4), were deposited in the public databases around the same time when E6AP was cloned,6,7 the function of the HECT as a ubiquitin ligase only became apparent from studies on E6AP.5,8 E6AP binds human papillomavirus (HPV) E6 oncoprotein and acts as a ubiquitin ligase for p53 in HPV infected cells.9 HECT domains are usually found at the C-termini of proteins. It is a bilobal domain, in which the two lobes are bridged by a flexible hinge loop.10 The E2 binds to the N-terminal lobe, opposite to the C-terminal lobe, which also carries the catalytic Cys residue. Substrate binding is defined by regions outside the HECT, in most cases, towards the N-terminus of the protein. Based on domain architecture, majority of the HECT E3s belong to two families: the Nedd4 family and the HERC family.4
Nedd4 and Nedd4-2
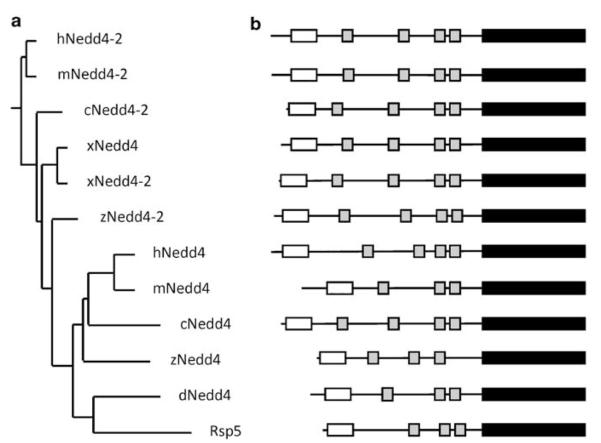
The Nedd4 family contains nine members in human including NEDD4, NEDD4-2 (NEDD4L), ITCH, SMURF1, SMURF2, WWP1, WWP2, NEDL1, and NEDL2.11-13 Nedd4 and Nedd4-2, the subjects of this review, are most closely related to each other. The phylogenetic analysis of these proteins from various species (Figure 1a) indicates that Nedd4 is the likely ancestral member of the family, whereas Nedd4-2 originated later in evolution, perhaps by gene duplication. Thus, Nedd4 is closely related to Rsp5 in S. cerevisiae, which has only a single member of the Nedd4 family. There are four members of the Nedd4 family in Drosophila melanogaster, including dNedd, but there are no Nedd4-2 orthologues (Figure 1). All vertebrates appear to have both Nedd4 and Nedd4-2 genes. Nedd4 and Nedd4-2 have identical specificity for ubiquitin conjugating enzymes (E2).14 The prototypic member of the family, Nedd4, was discovered as a developmentally regulated gene in the central nervous system of mouse.6,15 It contains an N-terminal calcium/lipid and/or protein binding C2 domain, 3 (in mouse and rat) or 4 (in human) WW domains (protein–protein interaction domains), and a C-terminal HECT domain (Figure 1b). This modular domain structure is characteristic of all the Nedd4 family members, the only variable being the number of WW domains, with the mammalian Nedd4 family members containing 2–4 of these domains.12,13,16 WW domains are the main determinants of substrate binding by the Nedd4 E3s, although other regions, such as the C2 domain of Nedd4, can also bind substrates.17 Given the presence of multiple WW domains in Nedd4 and Nedd4-2, these E3 can potentially interact with several substrates (or regulatory proteins). The Nedd4 and Nedd4-2 WW domains can bind PPxY (PY) or LPSY motifs, and in vitro studies suggest that WW3 and WW4 of both proteins bind PY motifs in the key substrates, with WW3 generally exhibiting higher affinity.11,16,18,19 Most Nedd4 family members, especially Nedd4-2, also have multiple splice variants, which might play different roles in regulating their substrates.20,21
Figure 1.
Phylogenetic relationship between the Nedd4 and Nedd4-2 among selected species. (a) Phylogenetic tree of Nedd4 and Nedd4-2 ubiquitin ligases. (b) The modular protein domain structures of Nedd4 and Nedd4-2 from selected species. The protein sequences and domain structure were compared between Nedd4 and Nedd4-2 among different species, including the yeast homolog, Rsp5. The open boxes are the C2 domains, the grey boxes are the WW domains, and the closed boxes are the HECT domains. The length of each box is proportional to the domain size. The domain structures were defined by PROSITE profiles (www.expasy.org/prosite). The protein information was obtained from Ensembl (www.ensembl.org). Protein sequence alignment and phylogenetic tree construction were carried out at Biology WorkBench (http://workbench.sdsc.edu). c: chicken (Gallus gallus); d: fruitfly (Drosophila melanogaster); h: human (Homo sapiens); m: mouse (Mus musculus); x: frog (Xenopus tropicalis); and z: zebrafish (Danio rerio)
Nedd4 targets and function
The first described target of Nedd4 was the epithelial sodium channel (ENaC).22 However, after the discovery of Nedd4-2, it was suggested that Nedd4 and Nedd4-2 probably have redundant functions in ENaC regulation.23-25 Subsequently, a number of potential substrates for Nedd4 have been described and a partial list of these is shown in Table 1. Many of these targets have been identified by the use of heterologous or in vitro systems, thus their functional significance in vivo remains to be fully explored. Here we discuss the main pathways/targets regulated by Nedd4.
Table 1.
Potential substrates and/or binding partners of Nedd4 and Nedd4-2
| Nedd4 | ||
|---|---|---|
| β2-AR | β2-adrenergic receptor | 92 |
| Cbl | Casitas B-lineage lymphoma | 38,40,93 |
| connexin43 | Gap junction protein | 94 |
| EGFR | Endothelial growth factor receptor | 27,95 |
| ENaC | Epithelial sodium channel | 22,96 |
| γ2-Adaptin | Ubiquitin-interacting adaptor | 97 |
| MTMR4 | Inositol phosphatase | 98 |
| Nav | Voltage-gated sodium channels | 52,53 |
| Notch1 | Notch homolog 1 | 99,100 |
| pol II | RNA polymerase II | 101 |
| Smad4 | Mothers against decapentaplegic homolog 4 |
102 |
| Spy1A | Cyclin-dependent kinase activator | 103 |
| VEGF-R2 | Vascular endothelial growth factor receptor-2 |
28 |
| Nedd4-2 | ||
|---|---|---|
| 14-3-3 | Adaptor/regulatory protein | 73,74 |
| ACK1 | Activated Cdc42-associated kinase 1 | 104 |
| ATA2 | Amino acid transporter | 105 |
| ClC-5 | Chloride ion channels | 106 |
| CLC-Ka/barttin | Chloride ion channels | 107 |
| DT | Dopamine transporter | 108 |
| EAAT1/2 | Glutamate transporter | 109,110 |
| ENaC | Epithelial sodium channel | 23,24 |
| KCNQ 1, KCNQ2/3, and KCNQ3/5 |
Voltage-gated K(+)channels | 111,112 |
| Kv1.3 | Voltage gated K+ channel | 113 |
| Kv 4.3 | Cardiac shal-related potassium channel | 114 |
| NaPi IIb | Intestinal phosphate cotransporter | 115 |
| Nav (several) | Voltage-gated sodium channels | 52–54 |
| Nedd4-2 | Ubiquitin ligase | 83 |
| Sgk1 | Serum glucocorticoid-inducible kinase | 56 |
| SGLT1 | Glucose transporter | 84 |
| SP-C | Surfactant protein C | 57,58 |
| Smad4 | Mothers against decapentaplegic homolog 4 |
102 |
| TGFβ | Transforming growth factor-β | 116 |
| Trk | Trk neurotrophin receptors | 117 |
| Tweety | Chloride ion channels | 118 |
Growth factor receptor regulation by Nedd4
Nedd4 has been implicated in the downregulation of growth factor receptors through biochemical analyses of cells transfected with Nedd4.26 Many types of receptors have been studied, including epidermal growth factor receptor (EGFR),27 vascular endothelial growth factor receptor-2 (VEGF-R2),28 insulin receptor, and IGF-1R.29 However, the gene knockout (KO) studies indicate that one of the main in vivo functions of Nedd4 is the regulation of IGF-1R, and perhaps insulin receptor (IR).30,31 The predominant phenotype of Nedd4 KO mice is growth retardation (with a body weight less than 40% of that of wild type littermates), and associated perinatal lethality.30 Consistent with the overall growth retardation, mouse embryonic fibroblasts (MEFs) isolated from Nedd4 KO embryos show slower growth, and respond poorly to serum after serum starvation than cells isolated from wild type embryos.30 Both IGF-1R and IR signaling are affected by Nedd4 deficiency. Three lines of evidence conveyed this point. (i) Both MAPK and PI3K signaling pathways were diminished in the Nedd4-/- cells when stimulated with IGF-1 or insulin, with PI3K pathway affected more severely; (ii) cell surface expression of IGF-1R and IR were reduced in the Nedd4 KO cells; and (iii) Nedd4 action was mediated by Grb10.30 Grb10 has been shown previously to interact with Nedd4.26 However, earlier studies, in contrast to the KO mouse data, suggested that Grb10 acts as an adaptor for Nedd4 to ubiquitylate IGF-1R.32 The mouse KO data indicate that Grb10 levels are elevated in Nedd4-/- MEFs,30 although Grb10 is not a direct target of the Nedd4-mediated ubiquitylation.30 It is important to note that the lethality in Nedd4-/- mice was partially rescued by maternal inheritance of a disrupted Grb10 allele.30
The interactions between Nedd4 and IGF-1R/IR appear to be receptor specific. The signaling cascade propagated from a distinct transmembrane tyrosine kinase receptor, EGFR, was not affected in the Nedd4-/- MEFs.30 Therefore, it is unlikely that Nedd4 serves as a ubiquitous component of the endocytic machinery for all growth factor receptors.
It is clear from these physiological and cellular studies that Nedd4 is a positive regulator of growth and proliferation, especially during embryonic development, through interactions with IGF-1R/IR; however, the details of these interactions are still not clear. As other ubiquitin ligases, such as Mdm2 and c-Cbl,33,34 in addition to Nedd4,32 have been implicated in mediating ubiquitylation of IGF-1R, two models of potential interaction have been proposed and are being tested: sequential ubiquitylation and competing ubiquitylation (Figure 2). In the first model, Nedd4 does not directly ubiquitylate IGF-1R, but controls the activity of another E3 (Mdm2, c-Cbl, or another ubiquitin ligase), which in turn is responsible for IGF-1R ubiquitylation and degradation. In the competing ubiquitylation model, both Nedd4 and the other E3 directly ubiquitylate IGF-1R; however, Nedd4-mediated IGF-1R ubiquitylation does not lead to its degradation through the proteasome. Both models can explain the current result that Nedd4 deficiency results in increased endocytosis of IGF-1R and reduced IGF-1 signaling.
Figure 2.
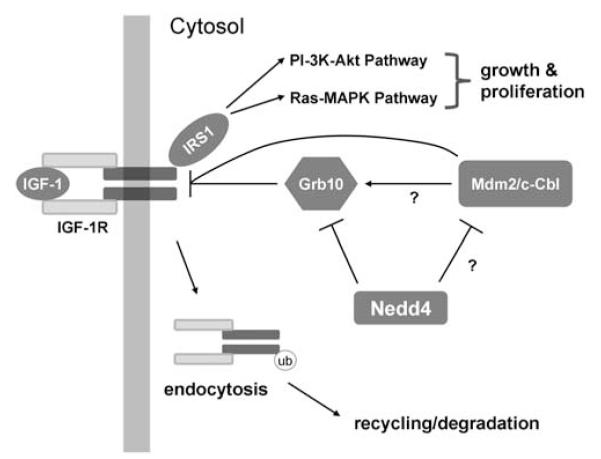
A possible model of Nedd4 function in regulating IGF-1 signaling. Ligand (IGF-1) interaction with IGF-1R results in autophosphorylation of the receptor, which then phosphorylates insulin receptor substrates, such as IRS1. IRS molecules act as docking sites for other proteins, which lead to the activation of both PI3K (phosphoinositide-3 kinase) and MAPK (mitogen-activated protein kinase) signaling pathways, and growth and proliferation of the cells. IGF-1R activity is regulated by ubiquitin-mediated endocytosis, and the endocytosed receptors can be degraded or recycled back to the plasma membrane. Nedd4 might have multiple roles in the regulation of IGF-1R. Nedd4 has been shown to be involved in the downregulation of Grb10, an adaptor protein which binds directly to IGF-1R. Grb10 has been proposed to mediate the interaction between IGF-1R and Nedd4. Other ubiquitin ligases, such as Mdm2 and c-Cbl, have been shown to target IGF-1R for degradation. It is not clear, whether these interactions are mediated by Grb10
Regulation of T-cell function by Nedd4
The potential involvement of Nedd4 in T-cell activation was first suggested by Heissmeyer et al.35 In this study it was concluded that Itch, a closely related family member of Nedd4, and possibly Nedd4, contribute to T cell anergy – a state of T-cell unresponsiveness induced when T cells are stimulated through the T-cell receptor in the absence of co-stimulation. The authors showed that unresponsiveness occurs, in part, because PKCθ and PLCγ1 are degraded by a ubiquitin-dependent process. The role that Nedd4 played in this process was not entirely clear, and because of the high degree of similarity between Itch and Nedd4, it was suggested that they have overlapping functions.36 However, Itch and Nedd4 deficient mice exhibit different phenotypes, suggesting that these two E3-ubiquitin ligases regulate distinct pathways in vivo.37,38
As mentioned earlier, mice lacking Nedd4 are perinatal lethal due to a severe growth retardation.30 To study the role of Nedd4 in T cells, fetal liver chimeras (Nedd4-/-FLCh) in which Nedd4 was only deficient in cells of hematopoietic origin were generated.38 Mice lacking Nedd4 (Nedd4-/-) displayed normal T-cell development in the thymus, but their T cells in the periphery were hypo-responsive. Nedd4-/- T cells proliferate poorly in response to antigen and are less likely to produce IL-2. Furthermore, their B cells undergo class switching with lower frequency.38 In contrast, mice lacking Itch have hyper-responsive T cells 39 that are skewed towards T-helper type 2 (Th2) cytokine production. This is due to the well-described role for Itch in ubiquitylation of JunB, a transcription factor that promotes Th2 cytokine production. From these results, it was reasoned that Nedd4 likely promotes T-cell activation and enhancement of the adaptive immune response, whereas Itch prevents Th2 differentiation. The dissimilarities seen between the immunologic phenotypes of Nedd4-/- and Itch-/- mice suggest that these E3 ligases ubiquitylate unique targets and thus, function quite differently in the regulation of T cell responses. Thus, while the Th2-mediated disease in Itch mutant mice can be explained by the loss of Itch-mediated degradation of JunB, it was found that the diminished activation of Nedd4-/- T cells can be explained by the lack of ubiquitylation and degradation of Cbl-b. Nedd4-/- T cells contained increased levels of Cbl-b protein because Nedd4 is needed for the polyubiquitylation of Cbl-b.38,40 Although these data support that these two E3 ligases act on a unique subset of targets, further studies are needed to address whether this is true for all Nedd4 and Itch targets, or whether these ligases share the responsibility of ubiquitylating some targets.
Nedd4 regulates neuromuscular junctions
In addition to the phenotypes of Nedd4 KO mice described above, a recent study of the Nedd4-/- embryos indicate that this E3 is also needed for the proper formation and functioning of the neuromuscular junctions.41 Nedd4 is highly expressed in skeletal muscle. In Nedd4-/- embryos the size of skeletal muscle fibers and the number of motor neurons were reduced, even though, very interestingly, Nedd4 is not expressed in motor neurons. This suggests that the function of Nedd4 in this system is non-cell autonomous. A detailed analysis indicated that Nedd4 regulates the interaction between the motor neurons and the muscle, especially nerve fasciculation. In the KO embryos, the pre-synaptic nerve terminal branches at the neuromuscular junctions are reduced in diameter but increased in number.41 Although these studies clearly define an important role of Nedd4 in regulating neuromuscular junctions, the mechanism by which this occurs remains unknown. Furthermore, it is unclear whether the motor neuron and skeletal muscle fiber phenotypes are directly due to the defective regulation of the formation and functioning of the neuromuscular junctions. The discovery of the substrate(s) of Nedd4 responsible for these phenotypes in the Nedd4 KO mice will delineate the precise mechanisms by which Nedd4 controls the size of skeletal muscle fibers and the number of motor neurons.
Regulation of VEGF-R2 by Nedd4
Another potential target of Nedd4 is VEGF-R2.28 In this system Grb10 protein is a positive regulator of VEGF signaling; VEGF enhances Grb10 expression, and Grb10 overexpression induces increased Tyr phosphorylation of VEGF-R2 and increased accumulation of this protein.42 A study by Murdaca et al. 28 found that Nedd4 mediates VEGF-R2 degradation and Grb10 inhibits this by binding to Nedd4. It is interesting to note that although VEGF-R2 is ubiquitylated, Nedd4 does not directly mediate this ubiquitylation. Given that Nedd4 KO mice accumulate Grb10,30 it will be interesting to study whether the loss of Nedd4 leads to increased VEGF-R2 protein and a deregulation of vasculogenesis and angiogenesis.
Does Nedd4 play a role in the regulation of PTEN?
It was previously reported that PTEN is ubiquitylated and degraded in the cells, and biochemical purification using HeLa cell lysate showed that Nedd4 was the putative E3 responsible for PTEN ubiquitylation43 and nuclear localization.44 As PTEN is one of the major tumor suppressor genes, these reports suggested an important role for NEDD4 as an oncogene.43 In supporting the role of Nedd4 in regulating PTEN, it was shown that Nedd4 is upreguated in certain type of tumors when compared with nearby normal tissue.44 As the interaction between Nedd4 and PTEN was based largely on studies using heterologous expression system with either Nedd4 or Nedd4 and PTEN both overexpressed in cultured cells, it prompted the testing of this interaction in Nedd4 deficient cells from gene KO mice. Independent studies from a number of Nedd4 KO lines have failed to detect changes in the amount of PTEN in MEFs.30,31,38 In addition the stability and localization of PTEN were not affected by the absence of Nedd4.31,38 Furthermore, the results obtained using siRNA knockdown of Nedd4 presented in the earlier reports 43,44 could not be reproduced.31,38 Overall, these results argue against a general role of Nedd4 in PTEN stability and localization. However, to fully resolve this controversy, additional work may be needed, for example, to establish if Nedd4 has any cell-type specific function (such as in human tumor cells) in PTEN regulation.
Regulation of viral budding by Nedd4
Once mammalian cells are infected with viruses, virus replicates within the cells and exhausts the host cells to make viral progenies. The progenies leave the host cells via several routes, and one of them is called viral budding.45 Nedd4 and Nedd4-like proteins (including Nedd4-2) have been implicated in this process for many viruses including the Epstein–Barr virus,46 retrovirus,47 and Ebola virus.48 These viruses contain motifs that interact with Nedd4 or Nedd4-2 WW domains, leading to the ubiquitylation of viral matrix protein and recognition by the cellular ESCRT machinery. One of the best described viral proteins involved in Nedd4 interaction is the latent membrane protein 2A (LMP2A) of the Epstein–Barr virus.46 Through the PY motifs at its N-terminus LMP2A recruits the Nedd4 family of E3s, leading to the ubiquitylation of LMP2A and LMP2A-associated proteins, such as Lyn.46 Ubiquitylation of viral proteins serves as a signal for trafficking through the host cell’s vesicular transport machinery, however the precise mechanisms of viral egress are not fully understood.
Nedd4-2 targets and function
Regulation of ENaC
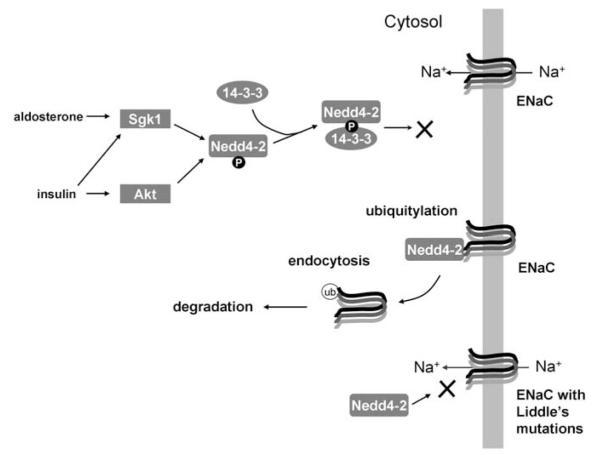
Similar to Nedd4, there is a growing list of proteins that can be targeted by Nedd4-2 (Table 1). Of those the best-characterized target is ENaC (Figure 3). Extensive in vitro evidence existed from heterologous overexpression systems or biochemical analyses that Nedd4-2 targets ENaC for endocytosis and degradation, and it was predicted that the disruption of the interaction between Nedd4-2 and ENaC by mutations at the C-termini of either β- or γ-ENaC subunit was the cause of Liddle’s syndrome, an autosomal dominant disorder with severe sodium retention and hypertension.22,23 However, the in vivo evidence was lacking until recently to support the functional interaction between Nedd4-2 and ENaC. The mice expressing a mutant β-ENaC–PY motif 49 have salt-sensitive hypertension (Liddle’s mouse), and recently published data show that an absence of Nedd4-2 leads to hypertension in animals on a normal diet, and to marked salt-sensitivity of this hypertension.50 However, the analysis of the Nedd4-2 KO mice identified some features that, when contrasted with those of the Liddle’s mouse, raise important questions about the precise mechanistic role of Nedd4-2 in hypertension. Specifically, the Nedd4-2 KO mice are more hypertensive than Liddle’s mice, and they do not display the degree of hypokalemia51 seen in Liddle’s mice. These differences suggest that Nedd4-2 not only regulates ENaC, but may also affect blood pressure control by other means.
Figure 3.
Nedd4-2 function in regulating epithelial sodium channel (ENaC). Nedd4-2 has been shown to bind directly with and ubiquitylate ENaC, and disruption of this interaction leads to increased ENaC surface expression and activity, as seen in Liddle’s syndrome. Nedd4-2 is (in part) the converging point for both aldosterone and insulin signals, mediated through Sgk1 (serum glucocorticoid-inducible kinase) and Akt (also called protein kinase B, PKB), respectively. Phosphorylated Nedd4-2 can bind to 14-3-3, a phospho-protein binding protein, which inhibits the interaction between Nedd4-2 and ENaC
The observed hypertension in Nedd4-2 KO mice was probably mediated by increased ENaC activity because (i) all three ENaC subunits (but not other Na+ transporters in the kidney) were expressed in the kidney at higher levels in Nedd4-2 KO mice than in their wild-type counterparts; (ii) the downregulation of ENaC function in the colon was impaired in Nedd4-2 KO mice; and (iii) salt-sensitive hypertension was substantially reduced in the presence of amiloride, a specific inhibitor of ENaC.50 Nedd4-2 KO mice on a chronic high-salt diet also showed cardiac hypertrophy and markedly depressed cardiac function, symptoms that are also associated with chronic hypertension. Overall, the results from this KO study show that, in vivo, Nedd4-2 is critical regulator of ENaC activity and blood pressure, and that the absence of the encoding gene is sufficient to produce salt-sensitive hypertension.50
An additional Nedd4-2 KO line in a different genetic background has also been generated (N. Boase and S. Kumar, unpublished data). These KO animals show perinatal lethality with some animals surviving up to 3 weeks after birth. The primary cause of perinatal lethality appears to be respiratory distress because of collapsed lungs, whereas the survivors develop severe inflammation of the lungs. These phenotypes are also consistent with increased activity of the ENaC in the Nedd4-2 KO mice (N. Boase and S. Kumar, unpublished data), again suggesting that Nedd4-2 is an essential regulator ENaC.
Regulation of voltage-gated sodium channels (Navs)
In addition to the ENaC, several voltage gated sodium channels (Navs) are potential targets for regulation by Nedd4-2.52-54 In cells that can be electrically excited, Navs has a key role in generating and propagating action potentials. These channels are thus crucial for the functioning of many cells. For example, in the nervous system where many Navs are expressed, these channels are needed for the development and plasticity, and the maintenance of excitability following nerve and tissue injury. The pore forming α-subunits of seven of the nine Navs contain typical PY motifs. Indeed, in vitro the PY motif containing Navs bind Nedd4-2 (and also Nedd4) WW domains.52 Several of these PY motif containing Navs were shown to be ubiquitylated by Nedd4-2.52-54 Furthermore, in Xenopus oocytes, Nedd4-2 strongly inhibited the activities of a number of Navs, including the cardiac Nav (Nav 1.5) 53,54 and the neuronal Navs (Nav1.2, Nav1.7, and Nav1.8).52 In the Xenopus oocyte system, Nedd4 also suppressed the activities of Nav1.2 and Nav1.7, but it was a poor inhibitor of Nav1.7.52 The in vivo validation of these findings awaits further studies using cells derived from the KO mice.
Regulation of Sgk1
A recent study showed that Sgk1 protein levels are regulated by the ubiquitin proteasome pathway, adding another dimension to the mechanism of regulation of this unusual serine–threonine kinase.55,56 That polyubiquitin-modified Sgk1 is predominantly localized to the membrane-associated fraction of the cell, suggests that Sgk1 is ubiquitylated at or near the membrane rather than in the cytosol. Overexpression of Nedd4-2 decreased Sgk1 expression by inducing polyubiquitylation and degradation of Sgk1 in the 26S proteasome. Conversely, Nedd4-2 siRNA stabilized Sgk1.56 This raises the interesting possibility that Sgk1 may both phosphorylate (see below) and act as a substrate of Nedd4-2, thereby achieving a tight negative feedback regulation of Nedd4-2 and Sgk1. Regulation of serine–threonine kinases by ubiquitin modification underscores the potential importance of phosphorylation-independent mechanisms in the regulation of signaling molecules.
Nedd4-2 mediated surfactant protein C (SP-C) trafficking
Two recent reports have suggested that Nedd4-2, and to a lesser extent Nedd4, contributes to the trafficking of surfactant protein C (SP-C).57,58 Pulmonary surfactant, a phospholipid-rich film synthesized and secreted by alveolar type-II epithelial cells, is the mixture of lipids and proteins needed to reduce surface tension. Deficiency of pulmonary surfactant is the principal cause of respiratory distress syndrome in premature infants.59 SP-C is a hydrophobic protein that enhances the surface-tension–lowering properties of surfactant lipids. Mutations in the SP-C gene have been linked to the development of interstitial lung disease.60 A PY motif (PPDY) is present at the N-terminus of SP-C pro-protein, and is conserved across all species. Mutations within the PY motif abrogated binding of SP-C to Nedd4-2 and completely blocked secretion of SP-C.57,58 However, knockdown of Nedd4-2 using siRNA or in Nedd4-2 KO cells, processing of proSP-C to mature peptide was not affected;58 whereas the other study showed that the processing was blocked by siRNA.57 As different cell types were used in these two studies, these observations need further validation.
Regulation of Nedd4 and Nedd4-2
There are a number of ways Nedd4 and Nedd4-2 E3s are regulated. As described below, these ligases can be regulated by binding of proteins that either assist (e.g. Ndfips) or preclude (e.g. 14-3-3) recruitment of the substrates. In addition, both Nedd4 and Nedd4-2 are likely to be regulated by phosphorylation and intramolecular interactions.
Ndfip1 and Ndfip2
Studies during the past few years have shown that in S. cerevisiae, where there is a single Nedd4 family member Rsp5, a number of binding proteins regulate Rsp5p mediated ubiquitylation. One of these is Bsd2p, which acts as an adaptor for Rsp5p to control the trafficking of two vacuolar enzymes Cps1p and Phm5p 61 and the metal-ion transporter Smf1p.62,63 Bsd2p homologues in mammals, Ndfip1 (N4WBP5, Nedd4 WW domain binding protein 5), and Ndfip2 (N4WBP5A) also act as adaptors for a number of the Nedd4 family members, to control ubiquitylation of specific targets.13 Ndfip1 was initially discovered as a Nedd4 WW domain-interacting protein (N4WBP5) in a far-Western screen.64 Ndfip2 was later identified as a closely related protein in the database.65-67 Ndfip1 and Ndfip2, through their three transmembrane domains localize to the golgi, golgi-derived transport vesicles, endosomes and MVBs (multivesicular bodies), the subcellular sites of biosynthetic, secretory, and endocytic trafficking. Through their PY motifs, both Ndfips can interact with the WW domains in a number of the Nedd4 family members, including Nedd4, Nedd4–2, WWP1, WWP2, and Itch.
The gene KO data with Ndfip1 support the prediction from in vitro studies that Ndfips are crucial adaptor proteins. The KO animals die because of inflammatory disease caused by aberrant T-cell activation.68 Further analysis of the KO phenotype indicated that the loss of Ndfip1 leads to JunB accumulation. As JunB is normally targeted for degradation by Itch, the studies show that in T cells Ndfip1 acts as an adaptor for Itch to facilitate its interaction with JunB.68 In addition to Itch, the in vitro and KO studies indicate that Ndfip1 (and perhaps Ndfip2) also serve as physiological adaptors for another Nedd4 family member WWP2 to regulate the mammalian divalent metal-ion transporter DMT1.69 In the context of this review article, Ndfips are also predicted to regulate ubiquitylation by Nedd4 and Nedd4-2 as both proteins interact with these ligases. Ndfip1 expression has been shown to be neuroprotective following traumatic brain injury,70 but it is not known which E3 and what substrates are involved. It is interesting to note that Ndfip1 was recently shown to be involved in exosomal secretion in neurons and overexpression of Ndfip1 results in the recruitment of Nedd4, Nedd4-2, and Itch proteins in the exosomes.71 Ndfip2 over-expression in Xenopus oocytes inhibits the Nedd4-2-mediated regulation of ENaC, presumably by sequestering Nedd4-2 away from ENaC.66
14-3-3
14-3-3s are ubiquitously expressed eukaryotic proteins that bind a phosphoserine or phosphothreonine motif in a large number of proteins.72 These proteins regulate signaling by providing scaffolding, by causing conformation change or by preventing the interaction of the target protein with other proteins.72 A number of studies have shown that 14-3-3 bind at least one discrete phosphoserine motif in Nedd4-2.73,74 A number of Ser/Thr kinases, including Sgk1, Akt, and IKKβ regulate the activity of ENaC, and several studies have shown that ENaC regulation by these kinases, at least to some extent, is mediated through Nedd4-2 and 14-3-3 proteins.74-81 Insulin and aldosterone, two hormones that upregulate ENaC activity, in part do so by inducing Akt and Sgk1 activation (Figure 3). These kinases then phosphorylate specific Ser residue of Nedd4-2 contained in a 14-3-3 binding motif located between the WW domains. This phosphorylation results in the recruitment of 14-3-3 to Nedd4-2. The binding of 14-3-3 and Nedd4-2 prevents Nedd4-2 binding to ENaC and thus, circumventing the negative regulation of ENaC and allowing the channel to stay on the membrane (Figure 3).
Grb10 and ISG15
Recent work shows that Nedd4 is negatively regulated by a ubiquitin-like protein, ISG15.82 ISG15 binds Nedd4 and interferes with Nedd4 interaction with E2, thereby affecting the catalytic activity of Nedd4. This leads to an inhibition of the ability of Nedd4 to ubiquitylate viral matrix proteins and the release of virus particles.82
As discussed above, Grb10 protein binds Nedd4 to regulate IGF-1 and insulin signaling.30 Grb10 also regulates Nedd4-mediated degradation of VEGF-R2.28 In this case Grb10 interacts with Nedd4 to prevent Nedd4-dependent degradation of VEGF-R2. However, in this pathway Nedd4 does not appear to directly ubiquitylate VEGF-R2.28 Nevertheless, in both cases binding of Grb10 to Nedd4 regulates Nedd4 function in specific signaling pathways.
Intramolecular interactions and protein conformation
There is evidence to suggest that intramolecular interaction can inhibit autoubiquitylation of Nedd4-2.83 It was found that the WW domains of Nedd4-2 weakly bind a LPxY motif located within its HECT domain. This intramolecular interaction inhibits autoubiquitylation of Nedd4-2. The mutation of the LPxY motif decreases the stability of Nedd4-2 protein, without affecting its ability to ubiquitylate ENaC. Based on these observations the authors have proposed a model, where the intramolecular interaction between WW domains and HECT stabilizes Nedd4–2 by preventing autoubiquitylation, whereas substrate binding disrupts this interaction, leading to Nedd4-2 downregulation.83
Sgk1 and Akt
As stated above Sgk1, which is an aldosterone- and insulin-dependent, positive regulator of ENaC density at the plasma membrane, has been shown to act through the phosphorylation of Nedd4-2 on one or more Ser/Thr consensus sites.75,76 Akt/ PKB (protein kinase B) can also phosphorylate the same Ser residue in Nedd4-2 as Sgk1, and indeed has a similar effect on ENaC.84 Phosphorylated Nedd4-2, with the phosphorylation sites within the WW domains, could interfere with the interaction with ENaC through its PY motif.75,76 Both ENaC and Sgk1 contain PY motifs that can be used for binding with Nedd4-2 through its WW domains, and it has been shown that the WW3 and WW4 domains of Nedd4-2 are responsible for binding to both Sgk1 and ENaC 85.
Both Nedd4 and Nedd4-2 also undergo phosphorylation at multiple sites during mitosis.86 However, the function consequences of this phosphorylation and the kinases responsible have not been identified.
Future directions
From the apparent non-overlapping phenotypes of the Nedd4 and Nedd4-2 gene KO mice it is clear that the main substrates of these E3s are distinct, despite the very close similarity between these two ubiquitin ligases. Although Nedd4 and Nedd4-2 show very similar binding affinities for the same substrates in vitro (e.g., both bind all three ENaC subunits in vitro with high affinity), mouse data suggest that in vivo their substrate binding may be more restricted than envisaged. Many of the channels, transporters, and other potential targets of Nedd4 and Nedd4-2 (Table 1) would need further validation, perhaps by the use of cells and tissues derived from the KO mice. Given the perinatal lethality of Nedd4 KO animals, it may be necessary to generate conditional KO mice to enable the study of physiological targets of Nedd4. It is possible that in some cases the functions of Nedd4 and Nedd4-2 are redundant. Thus, it may be informative to generate double KO mice.
It has been shown that accessory and adaptor proteins contribute to the specificity, diversity, and overall function of both Nedd4 and Nedd4-2.13 Identification and biochemical characterization of the interactomes for both Nedd4 and Nedd4-2 would facilite our understanding of these E3s and their substrates. To that end, systemic approaches using the current proteomic and bioinformatic techniques will be needed to generate the much desired information. The challenges for these approaches will include the low abundance of endogenous Nedd4 or Nedd4-2 and the potential false interactions sometimes associated with overexpression systems. It may be advantageous to tag the endogenous Nedd4 or Nedd4-2 using techniques, such as the TAP (tamdem affinity purification) tag.87
Nedd4 protein is known to be cleaved by caspases during apoptosis.88 The cleavage of Nedd4 removes the N-terminal C2 domain from the rest of the protein without disrupting the WW domains and the HECT. The removal of the N-terminal region of Nedd4 was reported to make the protein unstable, leading to the degradation of the WW–HECT cleavage product.88 As the functional significance of Nedd4 cleavage and degradation during apoptosis remains unknown, it may be interesting to study the apoptotic response of Nedd4-deficient cells from the KO animal.
Despite some recent advances, the regulation of Nedd4 and Nedd4-2 is still not fully understood. For example, many kinases seem to phosphorylate Nedd4 and Nedd4-2, but in most cases physiological relevance and link to cellular signaling seem unclear. Several proteins, such as Ndfip1 and Ndfip2, can bind both Nedd4 and Nedd4-2. However, it is not known whether the binding occurs in vivo and whether the adaptors are used by Nedd4 and/or Nedd4-2 to facilitate substrate recruitment and protein trafficking. In yeast S. cerevisiae Rsp5, the only Nedd4-like protein, uses many adaptor proteins, in addition to the Ndfip-like protein, Bsd2.13,89 One recently identified group of proteins, arrestin-related proteins (ARTS), facilitates Rsp5 recruitment to plasma membrane proteins.90,91 Rsp5 then ubiquitylates specific targets at the membrane leading to their endocytosis. Further cellular and biochemical studies will be necessary to establish whether Nedd4 and Nedd4-2 also use ARTS in mammalian cells to recognize and bind their targets at the plasma membrane.
Acknowledgements
The work in the Yang laboratory is supported by the NIH (DK52617, AR052647, and DE16215 to BY), and in the Kumar Laboratory by the National Health and Medical Research Council (508086), and the Australian Research Council (DP0880571). We thank the members of our respective laboratories and Dr. Paula Oliver (Children’s Hospital of Philadelphia) for helpful comments.
Abbreviations
- Nedd4
neural precursor cell-expressed developmentally downregulated gene 4
- HECT
homologous to E6-AP C-terminus
- RING
really interesting new gene
- E6AP
human papilloma virus E6 associated protein
- MAPK
mitogen-activated protein kinase
- PI3K
phosphoinositide-3 kinase
- ENaC
epithelial sodium channel
- Grb10
growth factor receptor-bound protein 10
- MEF
mouse embryonic fibroblast
- PTEN
phosphatase and tensin homolog
- ESCRT
endosomal sorting complex required for transport
- LMP2A
latent membrane protein 2A
- Sgk1
serum glucocorticoid-inducible kinase 1
- Ndfip
Nedd4 family interacting protein
- DMT1
divalent metal ion transporter 1
References
- 1.Ciechanover A. Proteolysis: From the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6:79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- 2.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 3.Xu P, Duong DM, Seyfried NT, Cheng D, Xie Y, Robert J, et al. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell. 2009;137:133–145. doi: 10.1016/j.cell.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rotin D, Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol. 2009;10:398–409. doi: 10.1038/nrm2690. [DOI] [PubMed] [Google Scholar]
- 5.Huibregtse JM, Scheffner M, Beaudenon S, Howley PM. A family of proteins structurally and functionally related to the E6-AP ubiquitin-protein ligase. Proc Natl Acad Sci USA. 1995;92:2563–2567. doi: 10.1073/pnas.92.7.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S, Tomooka Y, Noda M. Identification of a set of genes with developmentally down-regulated expression in the mouse brain. Biochem Biophys Res Commun. 1992;185:1155–1161. doi: 10.1016/0006-291x(92)91747-e. [DOI] [PubMed] [Google Scholar]
- 7.Hein C, Springael JY, Volland C, Haguenauer-Tsapis R, Andre B. NPl1, an essential yeast gene involved in induced degradation of Gap1 and Fur4 permeases, encodes the Rsp5 ubiquitin-protein ligase. Mol Microbiol. 1995;18:77–87. doi: 10.1111/j.1365-2958.1995.mmi_18010077.x. [DOI] [PubMed] [Google Scholar]
- 8.Matentzoglu K, Scheffner M. Ubiquitin ligase E6-AP and its role in human disease. Biochem Soc Trans. 2008;36(Pt 5):797–801. doi: 10.1042/BST0360797. [DOI] [PubMed] [Google Scholar]
- 9.Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 10.Huang L, Kinnucan E, Wang G, Beaudenon S, Howley PM, Huibregtse JM, et al. Structure of an E6AP-UbcH7 complex: Insights into ubiquitination by the E2-E3 enzyme cascade. Science. 1999;286:1321–1326. doi: 10.1126/science.286.5443.1321. [DOI] [PubMed] [Google Scholar]
- 11.Harvey KF, Kumar S. Nedd4-like proteins: An emerging family of ubiquitin-protein ligases implicated in diverse cellular functions. Trends Cell Biol. 1999;9:166–169. doi: 10.1016/s0962-8924(99)01541-x. [DOI] [PubMed] [Google Scholar]
- 12.Ingham RJ, Gish G, Pawson T. The Nedd4 family of E3 ubiquitin ligases: Functional diversity within a common modular architecture. Oncogene. 2004;23:1972–1984. doi: 10.1038/sj.onc.1207436. [DOI] [PubMed] [Google Scholar]
- 13.Shearwin-Whyatt L, Dalton HE, Foot N, Kumar S. Regulation of functional diversity within the Nedd4 family by accessory and adaptor proteins. Bioessays. 2006;28:617–628. doi: 10.1002/bies.20422. [DOI] [PubMed] [Google Scholar]
- 14.Fotia AB, Cook DI, Kumar S. The ubiquitin-protein ligases Nedd4 and Nedd4–2 show similar ubiquitin-conjugating enzyme specificities. Int J Biochem Cell Biol. 2006;38:472–479. doi: 10.1016/j.biocel.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S, Harvey KF, Kinoshita M, Copeland NG, Noda M, Jenkins NA. cDNA cloning, expression analysis, and mapping of the mouse Nedd4 gene. Genomics. 1997;40:435–443. doi: 10.1006/geno.1996.4582. [DOI] [PubMed] [Google Scholar]
- 16.Harvey KF, Dinudom A, Komwatana P, Jolliffe CN, Day ML, Parasivam G, et al. All three WW domains of murine Nedd4 are involved in the regulation of epithelial sodium channels by intracellular Na+ J Biol Chem. 1999;274:12525–12530. doi: 10.1074/jbc.274.18.12525. [DOI] [PubMed] [Google Scholar]
- 17.Plant PJ, Lafont F, Lecat S, Verkade P, Simons K, Rotin D. Apical membrane targeting of Nedd4 is mediated by an association of its C2 domain with annexin XIIIb. J Cell Biol. 2000;149:1473–1484. doi: 10.1083/jcb.149.7.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fotia AB, Dinudom A, Shearwin KE, Koch JP, Korbmacher C, Cook DI, et al. The role of individual Nedd4–2 (KIAA0439) WW domains in binding and regulating epithelial sodium channels. FASEB J. 2003;17:70–72. doi: 10.1096/fj.02-0497fje. [DOI] [PubMed] [Google Scholar]
- 19.Kanelis V, Bruce MC, Skrynnikov NR, Rotin D, Forman-Kay JD. Structural determinants for high-affinity binding in a Nedd4 WW3* domain-Comm PY motif complex. Structure. 2006;14:543–553. doi: 10.1016/j.str.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Itani OA, Campbell JR, Herrero J, Snyder PM, Thomas CP. Alternate promoters and variable splicing lead to hNedd4–2 isoforms with a C2 domain and varying number of WW domains. Am J Physiol Renal Physiol. 2003;285:F916–F929. doi: 10.1152/ajprenal.00203.2003. [DOI] [PubMed] [Google Scholar]
- 21.Itani OA, Stokes JB, Thomas CP. Nedd4–2 isoforms differentially associate with ENaC and regulate its activity. Am J Physiol Renal Physiol. 2005;289:F334–F346. doi: 10.1152/ajprenal.00394.2004. [DOI] [PubMed] [Google Scholar]
- 22.Staub O, Dho S, Henry P, Correa J, Ishikawa T, McGlade J, et al. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle’s syndrome. EMBO J. 1996;15:2371–2380. [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey KF, Dinudom A, Cook DI, Kumar S. The Nedd4-like protein KIAA0439 is a potential regulator of the epithelial sodium channel. J Biol Chem. 2001;276:8597–8601. doi: 10.1074/jbc.C000906200. [DOI] [PubMed] [Google Scholar]
- 24.Kamynina E, Debonneville C, Bens M, Vandewalle A, Staub O. A novel mouse Nedd4 protein suppresses the activity of the epithelial Na+ channel. FASEB J. 2001;15:204–214. doi: 10.1096/fj.00-0191com. [DOI] [PubMed] [Google Scholar]
- 25.Snyder PM, Steines JC, Olson DR. Relative contribution of Nedd4 and Nedd4–2 to ENaC regulation in epithelia determined by RNA interference. J Biol Chem. 2004;279:5042–5046. doi: 10.1074/jbc.M312477200. [DOI] [PubMed] [Google Scholar]
- 26.Morrione A, Plant P, Valentinis B, Staub O, Kumar S, Rotin D, et al. mGrb10 Interacts with Nedd4. J Biol Chem. 1999;274:24094–24099. doi: 10.1074/jbc.274.34.24094. [DOI] [PubMed] [Google Scholar]
- 27.Katz M, Shtiegman K, Tal-Or P, Yakir L, Mosesson Y, Harari D, et al. Ligand-independent degradation of epidermal growth factor receptor involves receptor ubiquitylation and Hgs, an adaptor whose ubiquitin-interacting motif targets ubiquitylation by Nedd4. Traffic. 2002;3:740–751. doi: 10.1034/j.1600-0854.2002.31006.x. [DOI] [PubMed] [Google Scholar]
- 28.Murdaca J, Treins C, Monthouel-Kartmann MN, Pontier-Bres R, Kumar S, Van Obberghen E, et al. Grb10 prevents Nedd4-mediated vascular endothelial growth factor receptor-2 degradation. J Biol Chem. 2004;279:26754–26761. doi: 10.1074/jbc.M311802200. [DOI] [PubMed] [Google Scholar]
- 29.Peruzzi F, Prisco M, Morrione A, Valentinis B, Baserga R. Anti-apoptotic signaling of the insulin-like growth factor-I receptor through mitochondrial translocation of c-Raf and Nedd4. J Biol Chem. 2001;276:25990–25996. doi: 10.1074/jbc.M103188200. [DOI] [PubMed] [Google Scholar]
- 30.Cao XR, Lill NL, Boase N, Shi PP, Croucher DR, Shan H, et al. Nedd4 controls animal growth by regulating IGF-1 signaling. Sci Signal. 2008;1:ra5. doi: 10.1126/scisignal.1160940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fouladkou F, Landry T, Kawabe H, Neeb A, Lu C, Brose N, et al. The ubiquitin ligase Nedd4–1 is dispensable for the regulation of PTEN stability and localization. Proc Natl Acad Sci USA. 2008;105:8585–8590. doi: 10.1073/pnas.0803233105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vecchione A, Marchese A, Henry P, Rotin D, Morrione A. The Grb10/Nedd4 complex regulates ligand-induced ubiquitination and stability of the insulin-like growth factor I receptor. Mol Cell Biol. 2003;23:3363–3372. doi: 10.1128/MCB.23.9.3363-3372.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Girnita L, Girnita A, Larsson O. Mdm2-dependent ubiquitination and degradation of the insulin-like growth factor 1 receptor. Proc Natl Acad Sci USA. 2003;100:8247–8252. doi: 10.1073/pnas.1431613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sehat B, Andersson S, Girnita L, Larsson O. Identification of c-Cbl as a new ligase for insulin-like growth factor-I receptor with distinct roles from Mdm2 in receptor ubiquitination and endocytosis. Cancer Res. 2008;68:5669–5677. doi: 10.1158/0008-5472.CAN-07-6364. [DOI] [PubMed] [Google Scholar]
- 35.Heissmeyer V, Macian F, Im SH, Varma R, Feske S, Venuprasad K, et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 36.Mueller DL. E3 ubiquitin ligases as T cell anergy factors. Nat Immunol. 2004;5:883–890. doi: 10.1038/ni1106. [DOI] [PubMed] [Google Scholar]
- 37.Fang D, Elly C, Gao B, Fang N, Altman Y, Joazeiro C, et al. Dysregulation of T lymphocyte function in itchy mice: A role for Itch in TH2 differentiation. Nat Immunol. 2002;3:281–287. doi: 10.1038/ni763. [DOI] [PubMed] [Google Scholar]
- 38.Yang B, Gay DL, MacLeod MKL, Cao X, Sweezer EM, Kappler J, et al. Nedd4 augments the adaptive immune response by promoting ubiquitin mediated degradation of Cbl-b in activated T cells. Nat Immunol. 2008;9:1356–1363. doi: 10.1038/ni.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perry WL, Hustad CM, Swing DA, O’Sullivan TN, Jenkins NA, Copeland NG. The itchy locus encodes a novel ubiquitin protein ligase that is disrupted in a18 H mice. Nat Genet. 1998;18:143–146. doi: 10.1038/ng0298-143. [DOI] [PubMed] [Google Scholar]
- 40.Magnifico A, Ettenberg S, Yang C, Mariano J, Tiwari S, Fang S, et al. WW domain HECT E3s target Cbl RING finger E3s for proteasomal degradation. J Biol Chem. 2003;278:43169–43177. doi: 10.1074/jbc.M308009200. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Oppenheim RW, Sugiura Y, Lin W. Abnormal development of the neuromuscular junction in Nedd4-deficient mice. Dev Biol. 2009;330:153–166. doi: 10.1016/j.ydbio.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giorgetti-Peraldi S, Murdaca J, Mas JC, Van Obberghen E. The adapter protein, Grb10, is a positive regulator of vascular endothelial growth factor signaling. Oncogene. 2001;20:3959–3968. doi: 10.1038/sj.onc.1204520. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, et al. NEDD4–1 is a protooncogenic ubiquitin ligase for PTEN. Cell. 2007;128:129–139. doi: 10.1016/j.cell.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trotman LC, Wang X, Alimonti A, Chen Z, Teruya-Feldstein J, Yang H, et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128:141–156. doi: 10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dimmock NJ. Introduction to Modern Virology. 6th edn Blackwell Science; Oxford, Cambridge, MA, USA: 2007. [Google Scholar]
- 46.Ikeda M, Ikeda A, Longan LC, Longnecker R. The Epstein-Barr virus latent membrane protein 2A PY motif recruits WW domain-containing ubiquitin-protein ligases. Virology. 2000;268:178–191. doi: 10.1006/viro.1999.0166. [DOI] [PubMed] [Google Scholar]
- 47.Strack B, Calistri A, Accola MA, Palu G, Gottlinger HG. A role for ubiquitin ligase recruitment in retrovirus release. Proc Natl Acad Sci USA. 2000;97:13063–13068. doi: 10.1073/pnas.97.24.13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harty RN, Brown ME, Wang G, Huibregtse J, Hayes FP. A PPxY motif within the VP40 protein of Ebola virus interacts physically and functionally with a ubiquitin ligase: Implications for filovirus budding. Proc Natl Acad Sci USA. 2000;97:13871–13876. doi: 10.1073/pnas.250277297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pradervand S, Vandewalle A, Bens M, Gautschi I, Loffing J, Hummler E, et al. Dysfunction of the epithelial sodium channel expressed in the kidney of a mouse model for Liddle syndrome. J Am Soc Nephrol. 2003;14:2219–2228. doi: 10.1097/01.asn.0000080204.65527.e6. [DOI] [PubMed] [Google Scholar]
- 50.Shi PP, Cao XR, Sweezer EM, Kinney TS, Williams NR, Husted RF, et al. Salt-sensitive hypertension and cardiac hypertrophy in mice deficient in the ubiquitin ligase Nedd4–2. Am J Physiol Renal Physiol. 2008;295:F462–F470. doi: 10.1152/ajprenal.90300.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pradervand S, Wang Q, Burnier M, Beermann F, Horisberger JD, Hummler E, et al. A mouse model for Liddle’s syndrome. J Am Soc Nephrol. 1999;10:2527–2533. doi: 10.1681/ASN.V10122527. [DOI] [PubMed] [Google Scholar]
- 52.Fotia AB, Ekberg J, Adams DJ, Cook DI, Poronnik P, Kumar S. Regulation of neuronal voltage-gated sodium channels by the ubiquitin-protein ligases Nedd4 and Nedd4–2. J Biol Chem. 2004;279:28930–28935. doi: 10.1074/jbc.M402820200. [DOI] [PubMed] [Google Scholar]
- 53.Rougier JS, van Bemmelen MX, Bruce MC, Jespersen T, Gavillet B, Apotheloz F, et al. Molecular determinants of voltage-gated sodium channel regulation by the Nedd4/Nedd4-like proteins. Am J Physiol Cell Physiol. 2005;288:C692–C701. doi: 10.1152/ajpcell.00460.2004. [DOI] [PubMed] [Google Scholar]
- 54.van Bemmelen MX, Rougier JS, Gavillet B, Apotheloz F, Daidie D, Tateyama M, et al. Cardiac voltage-gated sodium channel Nav1.5 is regulated by Nedd4–2 mediated ubiquitination. Circ Res. 2004;95:284–291. doi: 10.1161/01.RES.0000136816.05109.89. [DOI] [PubMed] [Google Scholar]
- 55.Brickley DR, Mikosz CA, Hagan CR, Conzen SD. Ubiquitin modification of serum and glucocorticoid-induced protein kinase-1 (SGK-1) J Biol Chem. 2002;277:43064–43070. doi: 10.1074/jbc.M207604200. [DOI] [PubMed] [Google Scholar]
- 56.Zhou R, Snyder PM. Nedd4–2 phosphorylation induces serum and glucocorticoid-regulated kinase (SGK) ubiquitination and degradation. J Biol Chem. 2005;280:4518–4523. doi: 10.1074/jbc.M411053200. [DOI] [PubMed] [Google Scholar]
- 57.Kotorashvili A, Russo SJ, Mulugeta S, Guttentag SH, Beers MF. Anterograde transport of surfactant protein C proprotein to distal processing compartments requires PPDY mediated association with NEDD4 ubiquitin ligases. J Biol Chem. 2009;284:16667–16678. doi: 10.1074/jbc.M109.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Conkright J, Apsley KS, Martin E, Ridsdale R, Rice WR, Cheng-Lun Na C-L, et al. Nedd4–2 mediated ubiquitination facilitates processing of surfactant protein C. Am J Resp Cell Mol. 2009 doi: 10.1165/rcmb.2009-0058OC. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farrell PM, Avery ME. Hyaline membrane disease. Am Rev Respir Dis. 1975;111:657–688. doi: 10.1164/arrd.1975.111.5.657. [DOI] [PubMed] [Google Scholar]
- 60.Nogee LM, Dunbar AE, 3rd, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344:573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 61.Hettema EH, Valdez-Taubas J, Pelham HR. Bsd2 binds the ubiquitin ligase Rsp5 and mediates the ubiquitination of transmembrane proteins. EMBO J. 2004;23:1279–1288. doi: 10.1038/sj.emboj.7600137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu XF, Supek F, Nelson N, Culotta VC. Negative control of heavy metal uptake by the Saccharomyces cerevisiae BSD2 gene. J Biol Chem. 1997;272:11763–11769. doi: 10.1074/jbc.272.18.11763. [DOI] [PubMed] [Google Scholar]
- 63.Stimpson HE, Lewis MJ, Pelham HR. Transferrin receptor-like proteins control the degradation of a yeast metal transporter. EMBO J. 2006;25:662–672. doi: 10.1038/sj.emboj.7600984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jolliffe CN, Harvey KF, Haines BP, Parasivam G, Kumar S. Identification of multiple proteins expressed in murine embryos as binding partners for the WW domains of the ubiquitin-protein ligase nedd4. Biochem J. 2000;351(Pt 3):557–565. [PMC free article] [PubMed] [Google Scholar]
- 65.Harvey KF, Shearwin-Whyatt LM, Fotia A, Parton RG, Kumar S. N4WBP5, a potential target for ubiquitination by the Nedd4 family of proteins, is a novel Golgi-associated protein. J Biol Chem. 2002;277:9307–9317. doi: 10.1074/jbc.M110443200. [DOI] [PubMed] [Google Scholar]
- 66.Konstas AA, Shearwin-Whyatt LM, Fotia AB, Degger B, Riccardi D, Cook DI, et al. Regulation of the epithelial sodium channel by N4WBP5A, a novel Nedd4/Nedd4–2–interacting protein. J Biol Chem. 2002;277:29406–29416. doi: 10.1074/jbc.M203018200. [DOI] [PubMed] [Google Scholar]
- 67.Shearwin-Whyatt LM, Brown DL, Wylie FG, Stow JL, Kumar S. N4WBP5A (Ndfip2), a Nedd4-interacting protein, localizes to multivesicular bodies and the golgi, and has a potential role in protein trafficking. J Cell Sci. 2004;117(Pt 16):3679–3689. doi: 10.1242/jcs.01212. [DOI] [PubMed] [Google Scholar]
- 68.Oliver PM, Cao X, Worthen GS, Shi P, Briones N, MacLeod M, et al. Ndfip1 protein promotes the function of itch ubiquitin ligase to prevent T cell activation and T helper 2 cell-mediated inflammation. Immunity. 2006;25:929–940. doi: 10.1016/j.immuni.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Foot NJ, Dalton HE, Shearwin-Whyatt LM, Dorstyn L, Tan SS, Yang B, et al. Regulation of the divalent metal ion transporter DMT1 and iron homeostasis by a ubiquitin-dependent mechanism involving Ndfips and WWP2. Blood. 2008;112:4268–4275. doi: 10.1182/blood-2008-04-150953. [DOI] [PubMed] [Google Scholar]
- 70.Sang Q, Kim MH, Kumar S, Bye N, Morganti-Kossman MC, Gunnersen J, et al. Nedd4-WW domain-binding protein 5 (Ndfip1) is associated with neuronal survival after acute cortical brain injury. J Neurosci. 2006;26:7234–7244. doi: 10.1523/JNEUROSCI.1398-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Putz U, Howitt J, Lackovic J, Foot N, Kumar S, Silke J, et al. Nedd4 family-interacting protein 1 (Ndfip1) is required for the exosomal secretion of Nedd4 family proteins. J Biol Chem. 2008;283:32621–32627. doi: 10.1074/jbc.M804120200. [DOI] [PubMed] [Google Scholar]
- 72.Bridges D, Moorhead GB. 14–3–3 proteins: A number of functions for a numbered protein. Sci STKE. 2005;2005:re10. doi: 10.1126/stke.2962005re10. [DOI] [PubMed] [Google Scholar]
- 73.Bhalla V, Daidie D, Li H, Pao AC, LaGrange LP, Wang J, et al. Serum- and glucocorticoid-regulated kinase 1 regulates ubiquitin ligase neural precursor cell-expressed, developmentally down-regulated protein 4–2 by inducing interaction with 14–3–3. Mol Endocrinol. 2005;19:3073–3084. doi: 10.1210/me.2005-0193. [DOI] [PubMed] [Google Scholar]
- 74.Ichimura T, Yamamura H, Sasamoto K, Tominaga Y, Taoka M, Kakiuchi K, et al. 14–3–3 proteins modulate the expression of epithelial Na+ channels by phosphorylation-dependent interaction with Nedd4–2 ubiquitin ligase. J Biol Chem. 2005;280:13187–13194. doi: 10.1074/jbc.M412884200. [DOI] [PubMed] [Google Scholar]
- 75.Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, et al. Phosphorylation of Nedd4–2 by Sgk1 regulates epithelial Na(+) channel cell surface expression. EMBO J. 2001;20:7052–7059. doi: 10.1093/emboj/20.24.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Snyder PM, Olson DR, Thomas BC. Serum and glucocorticoid-regulated kinase modulates Nedd4–2–mediated inhibition of the epithelial Na+ channel. J Biol Chem. 2002;277:5–8. doi: 10.1074/jbc.C100623200. [DOI] [PubMed] [Google Scholar]
- 77.Liang X, Peters KW, Butterworth MB, Frizzell RA. 14–3–3 isoforms are induced by aldosterone and participate in its regulation of epithelial sodium channels. J Biol Chem. 2006;281:16323–16332. doi: 10.1074/jbc.M601360200. [DOI] [PubMed] [Google Scholar]
- 78.Nagaki K, Yamamura H, Shimada S, Saito T, Hisanaga SI, Taoka M, et al. 14–3–3 mediates phosphorylation-dependent inhibition of the interaction between the ubiquitin E3 ligase Nedd4–2 and epithelial Na(+) channels. Biochemistry. 2006;45:6733–6740. doi: 10.1021/bi052640q. [DOI] [PubMed] [Google Scholar]
- 79.Lee IH, Dinudom A, Sanchez-Perez A, Kumar S, Cook DI. Akt mediates the effect of insulin on epithelial sodium channels by inhibiting Nedd4–2. J Biol Chem. 2007;282:29866–29873. doi: 10.1074/jbc.M701923200. [DOI] [PubMed] [Google Scholar]
- 80.Liang X, Butterworth MB, Peters KW, Walker WH, Frizzell RA. An obligatory heterodimer of 14–3–3beta and 14–3–3epsilon is required for aldosterone regulation of the epithelial sodium channel. J Biol Chem. 2008;283:27418–27425. doi: 10.1074/jbc.M803687200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Edinger RS, Lebowitz J, Li H, Alzamora R, Wang H, Johnson JP, et al. Functional regulation of the epithelial Na+ channel by I{kappa}B kinase-{beta} occurs via phosphorylation of the ubiquitin ligase Nedd4–2. J Biol Chem. 2009;284:150–157. doi: 10.1074/jbc.M807358200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Malakhova OA, Zhang DE. ISG15 inhibits Nedd4 ubiquitin E3 activity and enhances the innate antiviral response. J Biol Chem. 2008;283:8783–8787. doi: 10.1074/jbc.C800030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bruce C, Kanelis V, Fouladkou F, Debonneville A, Staub O, Rotin D. Regulation of Nedd4–2 self-ubiquitylation and stability by a PY motif located within its HECT-domain. Biochem J. 2008;415:155–163. doi: 10.1042/BJ20071708. [DOI] [PubMed] [Google Scholar]
- 84.Dieter M, Palmada M, Rajamanickam J, Aydin A, Busjahn A, Boehmer C, et al. Regulation of glucose transporter SGLT1 by ubiquitin ligase Nedd4–2 and kinases SGK1, SGK3, and PKB. Obes Res. 2004;12:862–870. doi: 10.1038/oby.2004.104. [DOI] [PubMed] [Google Scholar]
- 85.Asher C, Sinha I, Garty H. Characterization of the interactions between Nedd4–2, ENaC, and sgk-1 using surface plasmon resonance. Biochim Biophys Acta. 2003;1612:59–64. doi: 10.1016/s0005-2736(03)00083-x. [DOI] [PubMed] [Google Scholar]
- 86.Dephoure N, Zhou C, Villen J, Beausoleil SA, Bakalarski CE, Elledge SJ, et al. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci USA. 2008;105:10762–10767. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Angrand PO, Segura I, Volkel P, Ghidelli S, Terry R, Brajenovic M, et al. Transgenic mouse proteomics identifies new 14–3–3–associated proteins involved in cytoskeletal rearrangements and cell signaling. Mol Cell Proteomics. 2006;5:2211–2227. doi: 10.1074/mcp.M600147-MCP200. [DOI] [PubMed] [Google Scholar]
- 88.Harvey KF, Harvey NL, Michael JM, Parasivam G, Waterhouse N, Alnemri ES, et al. Caspase-mediated cleavage of the ubiquitin-protein ligase Nedd4 during apoptosis. J Biol Chem. 1998;273:13524–13530. doi: 10.1074/jbc.273.22.13524. [DOI] [PubMed] [Google Scholar]
- 89.Leon S, Erpapazoglou Z, Haguenauer-Tsapis R. Ear1p and Ssh4p are new adaptors of the ubiquitin ligase Rsp5p for cargo ubiquitylation and sorting at multivesicular bodies. Mol Biol Cell. 2008;19:2379–2388. doi: 10.1091/mbc.E08-01-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin CH, MacGurn JA, Chu T, Stefan CJ, Emr SD. Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell. 2008;135:714–725. doi: 10.1016/j.cell.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 91.Nikko E, Sullivan JA, Pelham HR. Arrestin-like proteins mediate ubiquitination and endocytosis of the yeast metal transporter Smf1. EMBO Rep. 2008;9:1216–1221. doi: 10.1038/embor.2008.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shenoy SK, Xiao K, Venkataramanan V, Snyder PM, Freedman NJ, Weissman AM. Nedd4 mediates agonist-dependent ubiquitination, lysosomal targeting, and degradation of the beta2-adrenergic receptor. J Biol Chem. 2008;283:22166–22176. doi: 10.1074/jbc.M709668200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lafont F, Simons K. Raft-partitioning of the ubiquitin ligases Cbl and Nedd4 upon IgE-triggered cell signaling. Proc Natl Acad Sci USA. 2001;98:3180–3184. doi: 10.1073/pnas.051003498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leykauf K, Salek M, Bomke J, Frech M, Lehmann WD, Durst M, et al. Ubiquitin protein ligase Nedd4 binds to connexin43 by a phosphorylation-modulated process. J Cell Sci. 2006;119(Pt 17):3634–3642. doi: 10.1242/jcs.03149. [DOI] [PubMed] [Google Scholar]
- 95.Aoh QL, Castle AM, Hubbard CH, Katsumata O, Castle JD. SCAMP3 negatively regulates EGFR degradation and promotes receptor recycling. Mol Biol Cell. 2009;20:1816–1832. doi: 10.1091/mbc.E08-09-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dinudom A, Harvey KF, Komwatana P, Young JA, Kumar S, Cook DI. Nedd4 mediates control of an epithelial Na+ channel in salivary duct cells by cytosolic Na+ Proc Natl Acad Sci USA. 1998;95:7169–7173. doi: 10.1073/pnas.95.12.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rost M, Doring T, Prange R. Gamma 2-adaptin, a ubiquitin-interacting adaptor, is a substrate to coupled ubiquitination by the ubiquitin ligase Nedd4 and functions in the endosomal pathway. J Biol Chem. 2008;283:32119–32130. doi: 10.1074/jbc.M802632200. [DOI] [PubMed] [Google Scholar]
- 98.Plant PJ, Correa J, Goldenberg NM, Bain J, Batt JA. The inositol phosphatase MTMR4 is a novel target of the ubiquitin ligase Nedd4. Biochem J. 2009;419:57–63. doi: 10.1042/BJ20081866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koncarevic A, Jackman RW, Kandarian SC. The ubiquitin-protein ligase Nedd4 targets Notch1 in skeletal muscle and distinguishes the subset of atrophies caused by reduced muscle tension. FASEB J. 2007;21:427–437. doi: 10.1096/fj.06-6665com. [DOI] [PubMed] [Google Scholar]
- 100.Fedoroff OY, Townson SA, Golovanov AP, Baron M, Avis JM. The structure and dynamics of tandem WW domains in a negative regulator of notch signaling, Suppressor of deltex. J Biol Chem. 2004;279:34991–35000. doi: 10.1074/jbc.M404987200. [DOI] [PubMed] [Google Scholar]
- 101.Anindya R, Aygun O, Svejstrup JQ. Damage-induced ubiquitylation of human RNA polymerase II by the ubiquitin ligase Nedd4, but not Cockayne syndrome proteins or BRCA1. Mol Cell. 2007;28:386–397. doi: 10.1016/j.molcel.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 102.Moren A, Imamura T, Miyazono K, Heldin CH, Moustakas A. Degradation of the tumor suppressor Smad4 by WW and HECT-domain ubiquitin ligases. J Biol Chem. 2005;280:22115–22123. doi: 10.1074/jbc.M414027200. [DOI] [PubMed] [Google Scholar]
- 103.Al Sorkhy M, Craig R, Market B, Ard R, Porter LA. The cyclin-dependent kinase activator, Spy1A, is targeted for degradation by the ubiquitin ligase NEDD4. J Biol Chem. 2009;284:2617–2627. doi: 10.1074/jbc.M804847200. [DOI] [PubMed] [Google Scholar]
- 104.Chan W, Tian R, Lee YF, Sit ST, Lim L, Manser E. Down-regulation of active ACK1 is mediated by association with the E3 ubiquitin ligase Nedd4–2. J Biol Chem. 2009;284:8185–8194. doi: 10.1074/jbc.M806877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hatanaka T, Hatanaka Y, Setou M. Regulation of amino acid transporter ATA2 by ubiquitin ligase Nedd4–2. J Biol Chem. 2006;281:35922–35930. doi: 10.1074/jbc.M606577200. [DOI] [PubMed] [Google Scholar]
- 106.Hryciw DH, Ekberg J, Lee A, Lensink IL, Kumar S, Guggino WB, et al. Nedd4–2 functionally interacts with ClC-5: Involvement in constitutive albumin endocytosis in proximal tubule cells. J Biol Chem. 2004;279:54996–55007. doi: 10.1074/jbc.M411491200. [DOI] [PubMed] [Google Scholar]
- 107.Embark HM, Bohmer C, Palmada M, Rajamanickam J, Wyatt AW, Wallisch S, et al. Regulation of CLC-Ka/barttin by the ubiquitin ligase Nedd4–2 and the serum- and glucocorticoid-dependent kinases. Kidney Int. 2004;66:1918–1925. doi: 10.1111/j.1523-1755.2004.00966.x. [DOI] [PubMed] [Google Scholar]
- 108.Sorkina T, Miranda M, Dionne KR, Hoover BR, Zahniser NR, Sorkin A. RNA interference screen reveals an essential role of Nedd4–2 in dopamine transporter ubiquitination and endocytosis. J Neurosci. 2006;26:8195–8205. doi: 10.1523/JNEUROSCI.1301-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boehmer C, Palmada M, Rajamanickam J, Schniepp R, Amara S, Lang F. Post-translational regulation of EAAT2 function by co-expressed ubiquitin ligase Nedd4–2 is impacted by SGK kinases. J Neurochem. 2006;97:911–921. doi: 10.1111/j.1471-4159.2006.03629.x. [DOI] [PubMed] [Google Scholar]
- 110.Boehmer C, Henke G, Schniepp R, Palmada M, Rothstein JD, Broer S, et al. Regulation of the glutamate transporter EAAT1 by the ubiquitin ligase Nedd4–2 and the serum and glucocorticoid-inducible kinase isoforms SGK1/3 and protein kinase B. J Neurochem. 2003;86:1181–1188. doi: 10.1046/j.1471-4159.2003.01937.x. [DOI] [PubMed] [Google Scholar]
- 111.Ekberg J, Schuetz F, Boase NA, Conroy SJ, Manning J, Kumar S, et al. Regulation of the voltage-gated K(+) channels KCNQ2/3 and KCNQ3/5 by ubiquitination. Novel role for Nedd4–2. J Biol Chem. 2007;282:12135–12142. doi: 10.1074/jbc.M609385200. [DOI] [PubMed] [Google Scholar]
- 112.Jespersen T, Membrez M, Nicolas CS, Pitard B, Staub O, Olesen SP, et al. The KCNQ1 potassium channel is down-regulated by ubiquitylating enzymes of the Nedd4/Nedd4-like family. Cardiovasc Res. 2007;74:64–74. doi: 10.1016/j.cardiores.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 113.Henke G, Maier G, Wallisch S, Boehmer C, Lang F. Regulation of the voltage gated K+ channel Kv1.3 by the ubiquitin ligase Nedd4–2 and the serum and glucocorticoid inducible kinase SGK1. J Cell Physiol. 2004;199:194–199. doi: 10.1002/jcp.10430. [DOI] [PubMed] [Google Scholar]
- 114.Baltaev R, Strutz-Seebohm N, Korniychuk G, Myssina S, Lang F, Seebohm G. Regulation of cardiac shal-related potassium channel Kv 4.3 by serum- and glucocorticoid-inducible kinase isoforms in Xenopus oocytes. Pflugers Arch. 2005;450:26–33. doi: 10.1007/s00424-004-1369-z. [DOI] [PubMed] [Google Scholar]
- 115.Palmada M, Dieter M, Speil A, Bohmer C, Mack AF, Wagner HJ, et al. Regulation of intestinal phosphate cotransporter NaPi IIb by ubiquitin ligase Nedd4–2 and by serum-and glucocorticoid-dependent kinase 1. Am J Physiol Gastrointest Liver Physiol. 2004;287:G143–G150. doi: 10.1152/ajpgi.00121.2003. [DOI] [PubMed] [Google Scholar]
- 116.Kuratomi G, Komuro A, Goto K, Shinozaki M, Miyazawa K, Miyazono K, et al. NEDD4–2 (neural precursor cell expressed, developmentally down-regulated 4–2) negatively regulates TGF-beta (transforming growth factor-beta) signalling by inducing ubiquitin-mediated degradation of Smad2 and TGF-beta type I receptor. Biochem J. 2005;386(Pt 3):461–470. doi: 10.1042/BJ20040738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Arevalo JC, Waite J, Rajagopal R, Beyna M, Chen ZY, Lee FS, et al. Cell survival through Trk neurotrophin receptors is differentially regulated by ubiquitination. Neuron. 2006;50:549–559. doi: 10.1016/j.neuron.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 118.He Y, Hryciw DH, Carroll ML, Myers SA, Whitbread AK, Kumar S, et al. The ubiquitin-protein ligase Nedd4–2 differentially interacts with and regulates members of the Tweety family of chloride ion channels. J Biol Chem. 2008;283:24000–24010. doi: 10.1074/jbc.M803361200. [DOI] [PMC free article] [PubMed] [Google Scholar]