Abstract
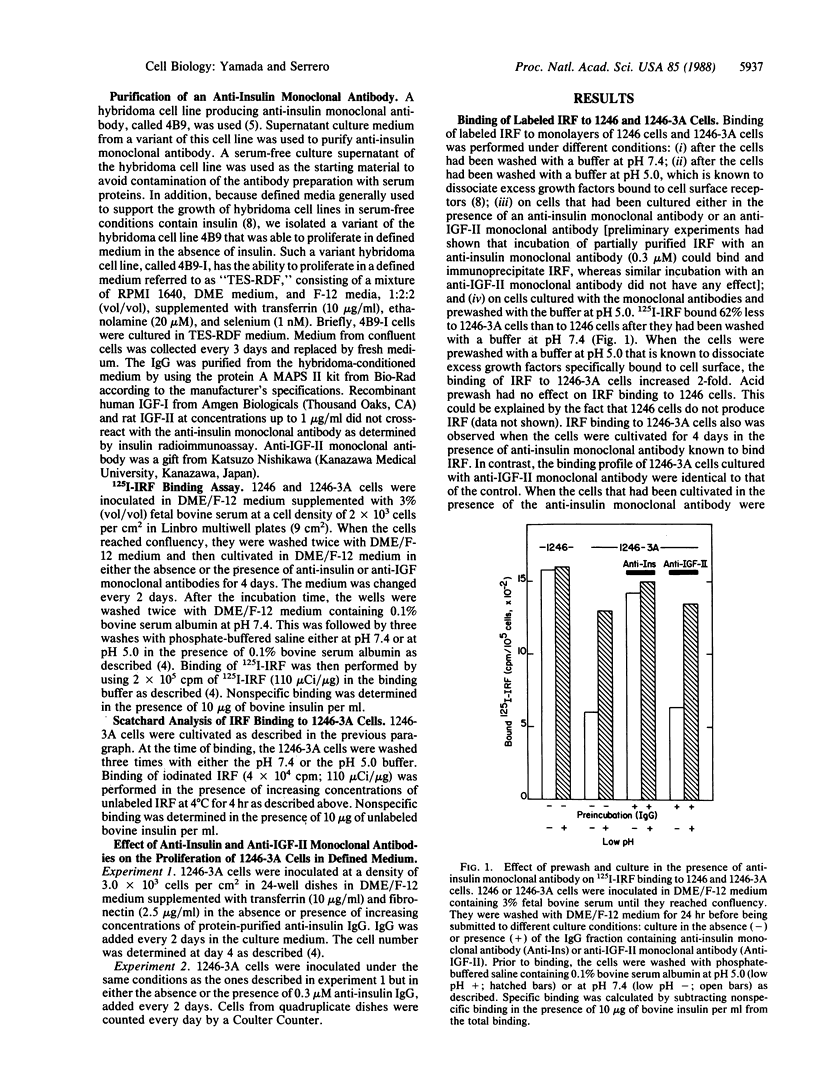
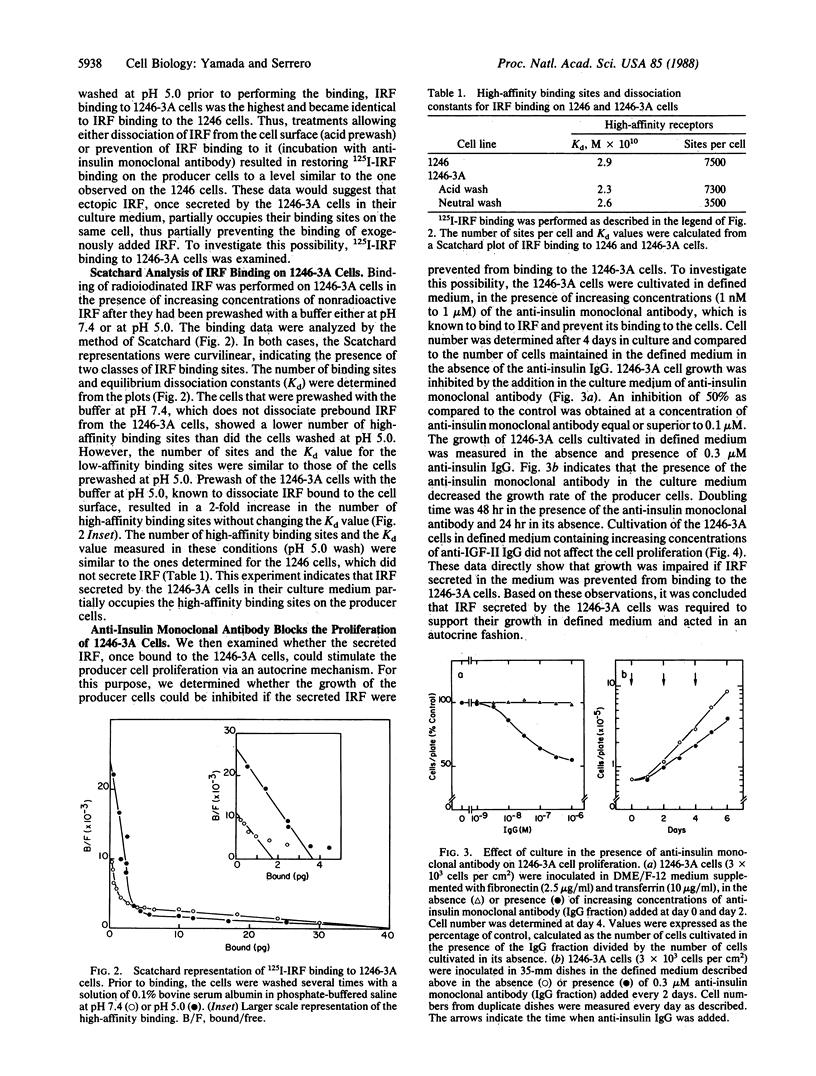
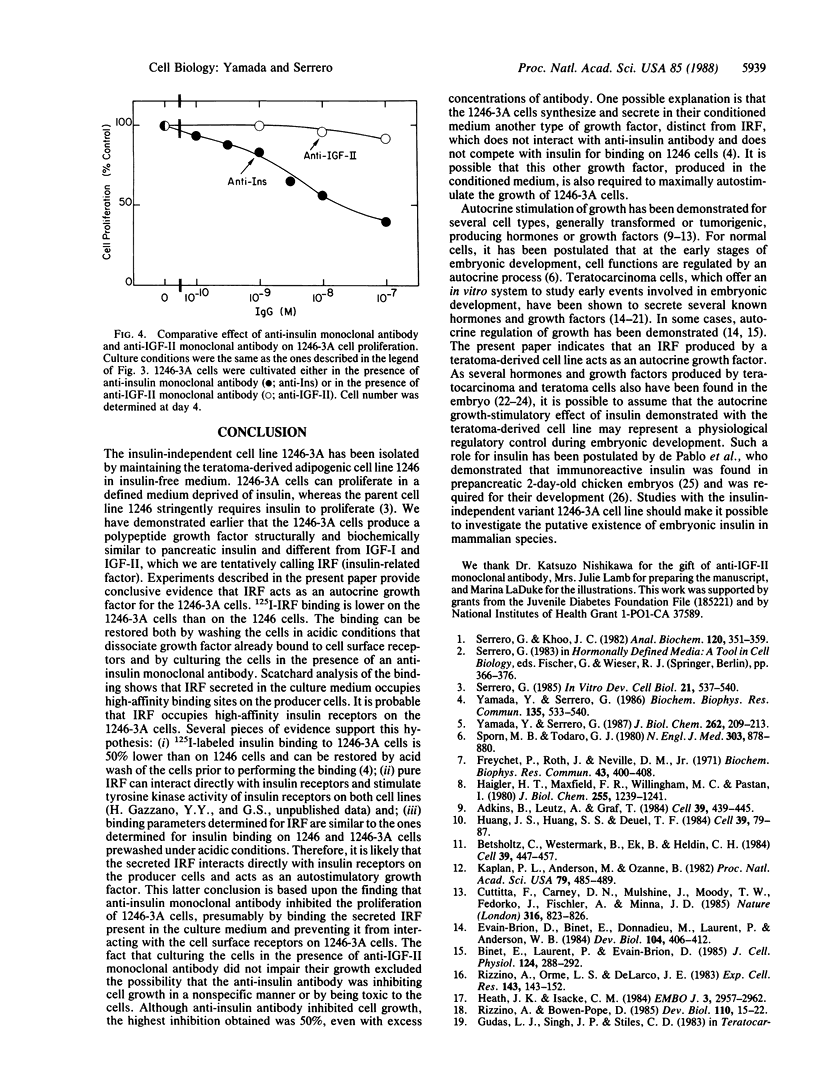
An insulin-independent teratoma-derived cell line, called 1246-3A, has been isolated from the adipogenic cell line 1246, which stringently requires insulin for proliferation. The 1246-3A cell line, which can proliferate in the absence of exogenous insulin, produces in its conditioned medium a growth factor similar to pancreatic insulin by its biological and immunological properties. This factor, called "insulin-related factor" (IRF), was purified and iodinated to study its binding to cell surface receptors. 125I-labeled IRF binding to intact 1246-3A cells is lower than to 1246 cells. Cell surface binding can be restored by culturing the 1246-3A cells in the presence of an anti-porcine insulin monoclonal antibody or by acid prewash of the cells prior to performing the binding. Scatchard analysis of binding indicates that IRF secreted by the 1246-3A cells partially occupies high-affinity binding sites on the producer cells. Moreover, insulin monoclonal antibody inhibits the proliferation of the IRF-producing 1246-3A cells, suggesting that these cells are dependent on the secreted IRF for growth in culture. We conclude that the insulin-related factor secreted by the insulin-independent 1246-3A cells stimulates their proliferation in an autocrine fashion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson E. D. The location and synthesis of transferrin in mouse embryos and teratocarcinoma cells. Dev Biol. 1982 Jun;91(2):227–234. doi: 10.1016/0012-1606(82)90029-x. [DOI] [PubMed] [Google Scholar]
- Adkins B., Leutz A., Graf T. Autocrine growth induced by src-related oncogenes in transformed chicken myeloid cells. Cell. 1984 Dec;39(3 Pt 2):439–445. doi: 10.1016/0092-8674(84)90451-3. [DOI] [PubMed] [Google Scholar]
- Betsholtz C., Westermark B., Ek B., Heldin C. H. Coexpression of a PDGF-like growth factor and PDGF receptors in a human osteosarcoma cell line: implications for autocrine receptor activation. Cell. 1984 Dec;39(3 Pt 2):447–457. doi: 10.1016/0092-8674(84)90452-5. [DOI] [PubMed] [Google Scholar]
- Binet E., Laurent P., Evain-Brion D. F.9 embryonal carcinoma cell calcitonin autocrine system: correlation between immunoreactive calcitonin secretion and calcitonin receptor number. J Cell Physiol. 1985 Aug;124(2):288–292. doi: 10.1002/jcp.1041240218. [DOI] [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. 1985 Aug 29-Sep 4Nature. 316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- D'Ercole A. J., Applewhite G. T., Underwood L. E. Evidence that somatomedin is synthesized by multiple tissues in the fetus. Dev Biol. 1980 Mar 15;75(2):315–328. doi: 10.1016/0012-1606(80)90166-9. [DOI] [PubMed] [Google Scholar]
- De Pablo F., Roth J., Hernandez E., Pruss R. M. Insulin is present in chicken eggs and early chick embryos. Endocrinology. 1982 Dec;111(6):1909–1916. doi: 10.1210/endo-111-6-1909. [DOI] [PubMed] [Google Scholar]
- Evain-Brion D., Binet E., Donnadieu M., Laurent P., Anderson W. B. Production of immunoreactive calcitonin and parathyroid hormone by embryonal carcinoma cells: alteration with retinoic acid-induced differentiation. Dev Biol. 1984 Aug;104(2):406–412. doi: 10.1016/0012-1606(84)90095-2. [DOI] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., Maxfield F. R., Willingham M. C., Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980 Feb 25;255(4):1239–1241. [PubMed] [Google Scholar]
- Heath J. K., Isacke C. M. PC13 embryonal carcinoma-derived growth factor. EMBO J. 1984 Dec 1;3(12):2957–2962. doi: 10.1002/j.1460-2075.1984.tb02240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Deuel T. F. Transforming protein of simian sarcoma virus stimulates autocrine growth of SSV-transformed cells through PDGF cell-surface receptors. Cell. 1984 Nov;39(1):79–87. doi: 10.1016/0092-8674(84)90193-4. [DOI] [PubMed] [Google Scholar]
- Kaplan P. L., Anderson M., Ozanne B. Transforming growth factor(s) production enables cells to grow in the absence of serum: an autocrine system. Proc Natl Acad Sci U S A. 1982 Jan;79(2):485–489. doi: 10.1073/pnas.79.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzino A., Bowen-Pope D. F. Production of PDGF-like growth factors by embryonal carcinoma cells and binding of PDGF to their endoderm-like differentiated cells. Dev Biol. 1985 Jul;110(1):15–22. doi: 10.1016/0012-1606(85)90058-2. [DOI] [PubMed] [Google Scholar]
- Rizzino A. Early mouse embryos produce and release factors with transforming growth factor activity. In Vitro Cell Dev Biol. 1985 Sep;21(9):531–536. doi: 10.1007/BF02620847. [DOI] [PubMed] [Google Scholar]
- Rizzino A., Orme L. S., De Larco J. E. Embryonal carcinoma cell growth and differentiation. Production of and response to molecules with transforming growth factor activity. Exp Cell Res. 1983 Jan;143(1):143–152. doi: 10.1016/0014-4827(83)90116-7. [DOI] [PubMed] [Google Scholar]
- Serrero G., Khoo J. C. An in vitro model to study adipose differentiation in serum-free medium. Anal Biochem. 1982 Mar 1;120(2):351–359. doi: 10.1016/0003-2697(82)90357-8. [DOI] [PubMed] [Google Scholar]
- Serrero G. Tumorigenicity associated with loss of differentiation and of response to insulin in the adipogenic cell line 1246. In Vitro Cell Dev Biol. 1985 Sep;21(9):537–540. doi: 10.1007/BF02620848. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Todaro G. J. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980 Oct 9;303(15):878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Serrero G. Characterization of an insulin-related factor secreted by a teratoma cell line. Biochem Biophys Res Commun. 1986 Mar 13;135(2):533–540. doi: 10.1016/0006-291x(86)90027-6. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Serrero G. Purification of an insulin-related factor secreted by a teratoma-derived mesodermal cell line. J Biol Chem. 1987 Jan 5;262(1):209–213. [PubMed] [Google Scholar]
- de Pablo F., Girbau M., Gomez J. A., Hernandez E., Roth J. Insulin antibodies retard and insulin accelerates growth and differentiation in early embryos. Diabetes. 1985 Oct;34(10):1063–1067. doi: 10.2337/diab.34.10.1063. [DOI] [PubMed] [Google Scholar]


