Abstract
A novel classical antifolate N-{4-[(2,4-diamino-5-methyl-furo[2,3-d]pyrimidin-6-yl)thio]-benzoyl}-l-glutamic acid 5 and 11 nonclassical antifolates 6–16 were designed, synthesized, and evaluated as inhibitors of dihydrofolate reductase (DHFR) and thymidylate synthase (TS). The nonclassical compounds 6–16 were synthesized from 20 via oxidative addition of substituted thiophenols using iodine. Peptide coupling of the intermediate acid 21 followed by saponification gave the classical analog 5. Compound 5 is the first example, to our knowledge, of a 2,4-diamino furo[2,3-d]pyrimidine classical antifolate that has inhibitory activity against both human DHFR and human TS. The classical analog 5 was a nanomolar inhibitor and remarkably selective inhibitor of P. carinii DHFR and M. avium DHFR at 263-fold and 2107-fold respectively compared to mammalian DHFR. The nonclassical analogs 6–16 were moderately potent against pathogen DHFR or TS. This study shows that the furo[2,3-d]pyrimidine scaffold is conducive to dual human DHFR-TS inhibitory activity and to high potency and selectivity for pathogen DHFR.
Keywords: Furo[2,3-d]pyrimidines; Thymidylate Synthase; Dihydrofolate Reductase; Dual Inhibitors
Introduction
Thymidylate synthase (TS) catalyzes the de novo synthesis of 2’-deoxythymidine-5’-monophosphate (dTMP) from 2’-deoxyuridine-5’-monophosphate (dUMP) by transferring a methyl group from 5,10-methylenetetrahydrofolate polyglutamates (5,10-CH2-H4PteGlun) to the C-5 position of the pyrimidine ring of the substrate dUMP. During the TS catalyzed reaction, 5,10-CH2-H4PteGlun species are converted to the corresponding 7,8-dihydrofolate (7,8-H2PteGlun). Dihydrofolate reductase (DHFR) carries out the first step in the regeneration of 5,10-CH2-H4PteGlun, i.e., formation of 5,6,7,8-tetrahydrofolate utilizing NADPH as the reductant.2 dTMP is the product of the TS catalyzed reaction and is subsequently phosphorylated to 2’-deoxythymidine-5’-diphosphate (dTDP) and 2’-deoxythymidine-5’-triphosphate (dTTP) and finally incorporated into DNA via DNA polymerase. Thus, both TS and DHFR play a crucial role in DNA biosynthesis and cell proliferation and hence both enzymes have been extensively targeted for developing cancer chemotherapeutic and antiopportunistic infection agents.3 Raltitrexed4 (Figure 1) and pemetrexed5 are folate based inhibitors of TS and represent important examples of clinically used antitumor agents, while methotrexate (MTX) (Figure 1) a DHFR inhibitor is a mainstay in single agent and combination cancer chemotherapy.6
Figure 1.
Many cultured tumor cell lines show synergistic cytotoxicity when grown in a medium containing a combination of a DHFR inhibitor with either a TS or glycinamide ribonucleotide formyltransferase (GARFT) antifolate.7 This effect is termed the “Kisliuk effect.”8 In addition, synergistic growth inhibition has also been observed by combining a DHFR inhibitor with a TS inhibitor, against cell cultures of Lactobacillus casei,9,10 rat hepatoma cells,11,12 and human lymphoma cells.9,13,14 Numerous examples of antifolates have been reported that possess dual TS and DHFR activity.5,15–18 It has also been suggested15 that the chemotherapeutic utility of such analogs would be viable if the inhibitory activity against DHFR is similar to that against TS. Pemetrexed5 represents an important example of a classical antifolate that has reported dual TS and DHFR inhibitory activity. In addition, pemetrexed and its polyglutamylated metabolites are also reported as inhibitors of several other important folate-dependent enzymes including GARFT and 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase (AICARFT).19 The clinical success of pemetrexed has generated a renewed interest in designing single agents that could function as multitargeted inhibitors of folate metabolizing enzymes. Such inhibitors could also circumvent the pharmacokinetic disadvantages of administering separate agents in combination chemotherapy protocols.
Antifolate inhibitors of TS are characterized by the presence of a 2-substituted-4-oxo moiety in the pyrimidine ring as exemplified by pemetrexed and raltitrexed.20,21 In contrast, inhibitors of DHFR are characterized by the presence of a 2,4-diamino substitution in the pyrimidine ring20,21 as represented by MTX.
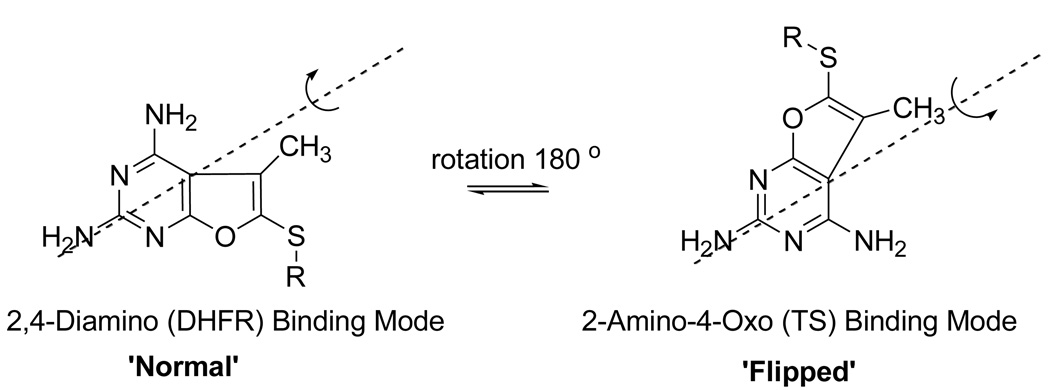
The 2,4-diaminofuro[2,3-d]pyrimidine ring system represents an ideal scaffold that could function as both a 2,4-diamino and 2-amino-4-oxo substituted system. To test this hypothesis we 22,23 reported compounds 1–3 (Figure 1) as potential dual DHFR-TS inhibitors. Compounds 1–3 inhibited human DHFR with an IC50 in the 0.22–1.0 µM range however they did not possess any (IC50 >200 µM) human TS inhibitory activity. One probable reason for this is that when these 5-substituted analogs binds to human TS in the proposed flipped mode22,23 (Figure 2) the 5-position side chain substituent lies between the 6- and 7-carbon atoms of a 6-6 fused ring system and may account for its lower or lack of TS inhibitory activity. Compounds 1–3 were found to be substrates for folyl poly-γ-glutamate synthetase (FPGS) and demonstrated significant tumor cell growth inhibitory activity. As an extension of our ongoing program compound 5 (Figure 1) was synthesized as a potential dual inhibitor of TS and DHFR. Compound 5 is a furo[2,3-d]pyrimidine analog of 4 and was designed to bind to human DHFR in the normal 2,4-diamino binding mode (Figure 2) and to human TS in the flipped mode wherein the furo 7-O could mimic the 4-oxo of classical 2-substituted-4-oxo pyrimidine moiety containing antifolate.
Figure 2.
We17 recently reported the design and synthesis of the 2,4-diamino 6-substituted pyrrolo[2,3-d]pyrimidine 4 (Figure 1) as a dual inhibitor of human DHFR and human TS. The 5-methyl group was incorporated to provide hydrophobic interaction with Val115 in human DHFR. Compound 4 was initially designed as a potential nonpolyglutamylatable DHFR inhibitor. However, unexpectedly 4 was found to be a dual inhibitor of human DHFR (IC50 = 210 nM) and human TS (IC50 = 540 nM) with reasonable FPGS substrate activity. Molecular modeling using SYBYL 6.824 indicated that 4 binds to human DHFR in the normal 2,4-diamino mode while it could bind to human TS in the flipped mode similar to its furo[2,3-d]pyrimidine counterpart 5. In addition, molecular modeling also indicated that the 5-CH3 group in 4 could have a hydrophobic interaction with Trp109 in human TS.
Thus, compound 5 was synthesized as an oxygen (furo) isostere of 4, and to also determine the importance of the pyrrole 7-NH in 4 for binding to both human TS and human DHFR. The replacement of an “NH” of a pyrrolo[2,3-d]pyrimidine with an “O” to afford the furo[2,3-d]pyrimidine was also anticipated to evaluate the importance of a “hydrogen donor” (NH) versus a “hydrogen acceptor” (O). Molecular modeling (see below) allows a prediction of activity depending on the mode of binding of the molecule at the enzyme active site. Thus N-[4-[(2,4-diamino-5-methylfuro[2,3-d]pyrimidin-6-yl)thio]benzoyl]-l-glutamic acid 5 (Figure 1) was designed and synthesized as a potential dual DHFR-TS inhibitor and as a potential antitumor agent. Since the 6-substitution of 4 afforded dual inhibitory (TS and DHFR) activity and the 5-substitution of furo[2,3-d]pyrimidines 1–3 did not afford dual TS/DHFR inhibitory activity, it was anticipated, on the basis of molecular modeling, that the transposed side chain from the 5-position (1–3) to the 6-position in 5 would afford dual TS/DHFR inhibitory activity akin to 4. Compound 5 was expected to possess FPGS substrate activity similar to its pyrrolo[2,3-d]pyrimidine counterpart 4.
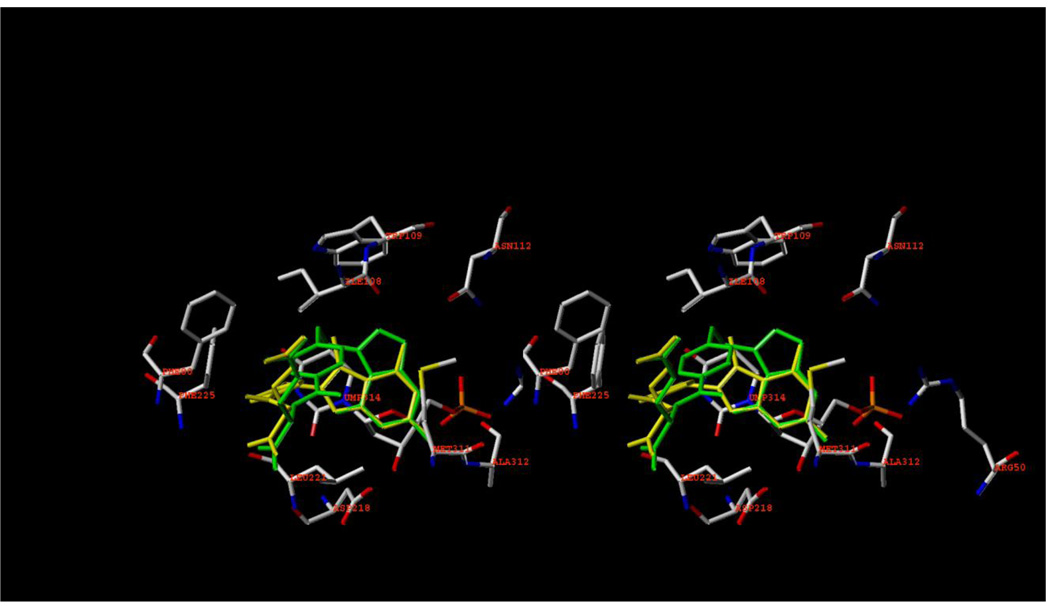
Molecular modeling of 5 (SYBYL 8.1) predicted dual human TS/DHFR inhibitory activity for 5. Compound 5 (yellow in Figure 3) in the flipped orientation was minimized and superimposed onto pemetrexed (green) in the X-ray crystal structure of pemetrexed in human TS.25
Figure 3.
Stereoview 5 (yellow) superimposed on pemetrexed (green) in the X-ray crystal structure of pemetrexed in human TS (PDB: 1JUJ)25 using SYBYL 8.1.
Like pemetrexed, compound 5 is anchored in the active site of human TS by aromatic stacking between the furo[2,3-d]pyrimidine ring of 5 and the dUMP (314 in Figure 3) pyrimidine ring. In addition, the N1 and 2NH2 of 5 are probably protonated much like the 2,4-diNH2 substitution of MTX that allows a protonation of the N1 and 2NH2. 26 This protonated N1 and 2NH2 of 5 could interact with Asp218 of human TS in a salt bridge interaction much like that seen with the N1 and 2NH2 of MTX with Glu30 in human DHFR.26 The 2NH2 could also interact with the backbone CO of Ala312 and the 4NH2 of 5 could H-bond with Arg50 and/or Asn112 mimicking the N1 and N7 of pemetrexed.25 The 5-CH3 of 5 interacts with Trp109 and with Ile108. The phenyl ring of 5 makes hydrophobic contact with Phe225 much like the phenyl ring of pemetrexed.25 There is no direct H-bonding with the 4-oxo moiety of pemetrexed and none with the O7 of 5 in its “flipped’ mode (Figure 2). Thus molecular modeling (Figure 3) predicts that 5 would bind to human TS and afford inhibition of human TS.
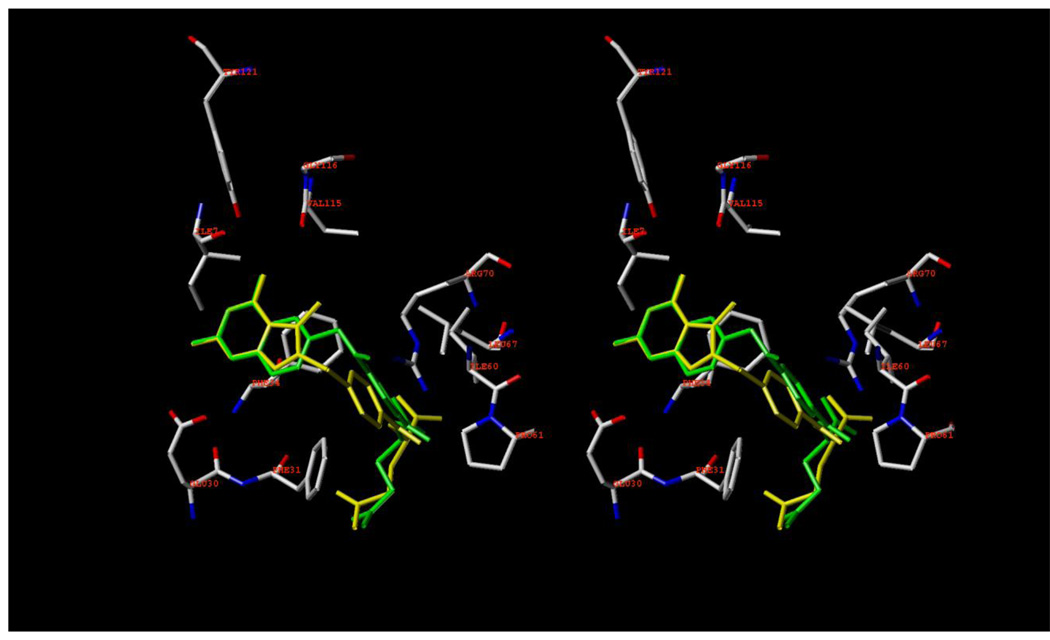
Energy minimized (SYBYL 8.1) 5 (yellow) (Figure 4) was superimposed with its pyrimidine ring on the pyrimidine ring of MTX (green) in the X-ray crystal structure of MTX in human DHFR.26 Compound 5 superimposes very well onto MTX in Figure 4. The N1 and 2NH2 of 5 are protonated, much like MTX26 and form a salt bridge with Glu30. In addition, the 4NH2 moiety is hydrogen bonded to the backbone CO of Ile7 and Val115. The phenyl ring of 5 makes hydrophobic contacts with Phe34, Phe31, Ile60, Pro61 and Leu67. The α-COOH of 5 makes a salt bridge with Arg70. The N8 of MTX forms H-bonding via a conserved active-site water molecule (H2O257, not shown in Figure4) to the indole ring of Trp24 and the COOH of Glu30. In Figure 4 the O7 of the furo ring of 5 mimics the N8 of MTX and could also H-bond via H2O257 to Trp84 and Glu30. Thus the interaction of 5 with human DHFR mimic those of MTX and 5 was expected to provide human DHFR inhibitory activity. Hence on the basis of molecular modeling, 5 was anticipated to possess dual human DHFR and TS inhibitory activity.
Figure 4.
Stereoview of minimized 5 (yellow) superimposed onto MTX (green) in the X-ray crystal structure of MTX in human DHFR. (PDB: 1U72).26
As mentioned above, inhibition of either DHFR or TS can result in cytotoxicity and TS and DHFR inhibitors can be combined in cancer chemotherapy to achieve synergistic effect.27, 28 Nonclassical (compounds lacking a l-glutamate side-chain) DHFR inhibitor such as piritrexim (PTX)29 and TS inhibitors such as N6-[4-(morpholinosulfonyl)benzyl]-N6-methyl-2,6-diaminobenz-[c–d]-indole glucuronate30 and nolatrexed31 (Figure 1) are also potent antitumor agents. Hence, nonclassical antifolates that display dual TS-DHFR inhibition are attractive antitumor agents and like their classical counterparts could afford “combination therapy” in a single agent. Thus in addition to the antitumor activity of 5, we were also interested in the antitumor activity of the nonclassical analogs 6–16, which could perhaps function as dual TS-DHFR inhibitors.
Another aspect of our interest in nonclassical dual TS-DHFR inhibitors lies in the treatment of opportunistic infections in immune-compromised patients, especially those with AIDS.32, 33 Some of the most prevalent opportunistic infections which are the major cause of morbidity and mortality in patients with AIDS and in other immune compromised patients, such as heart and other transplant patients, are caused by Pneumocystis jirovecii (P. jirovecii) formerly Pneumocystis carinii (P. carinii)32, 33 and Toxoplasma gondii (T. gondii).32, 33 A third infection, though not usually fatal, is Mycobacterium avium (M. avium) complex (MAC) a group of organisms responsible for disseminated infections in AIDS patients, that is responsible for a decrease in the quality of life of the patients.32, 33 Several nonclassical pyrrolo[2,3-d]pyrimidines, and furo[2,3-d]pyrimidines have been evaluated as inhibitors of P. carinii, T. gondii, and M. avium DHFR.21,34 Some of these have been found to be selective for DHFR from these pathogens. Thus, we were also interested in evaluating the nonclassical compounds 6–16 as inhibitors of P. carinii DHFR, T. gondii DHFR, and M. avium DHFR. The phenyl ring substitutions in 6–16 are based on similar substitutions that have provided potent and/or selective agents.21,34 The nonclassical analogs 6–16 were anticipated to inhibit DHFR and/or TS from P. carinii and/or T. gondii, and/or M. avium and perhaps provide selective inhibitors against these pathogens. P. carinii, T. gondii and M. avium lack the transport system(s) required for classical antifolates; however, the lipophilic nonclassical compounds 6–16 were anticipated to gain access to the pathogenic cells by passive diffusion.
Chemistry
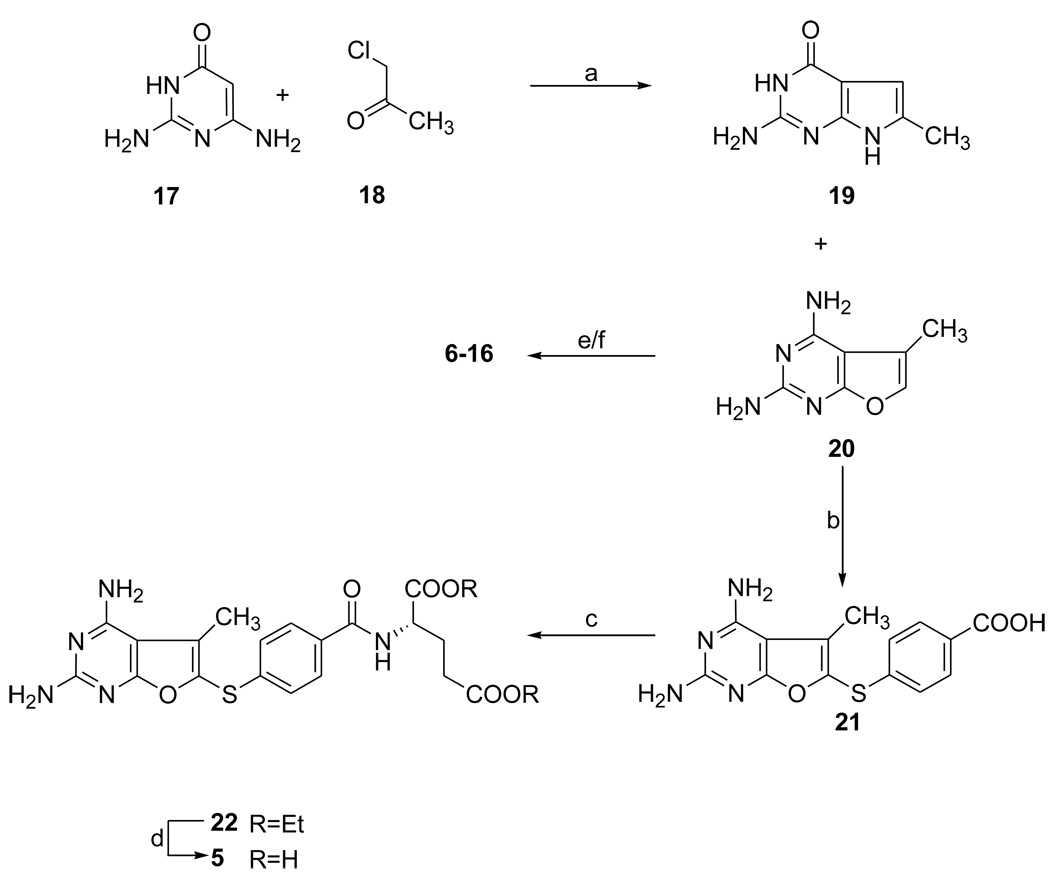
The synthesis of nonclassical 2,4-diamino-5-methyl-6-substituted arylthio-furo[2,3-d]pyrimidines (6–16) is shown in Scheme 1. 2,6-Diamino-4-hydroxypyrimidine (17) was condensed with chloroacetone (18) to afford the known 2,4-diamino-5-methylfuro[2-3-d]pyrimidine (20)35 which was reacted with appropriately substituted thiophenols in the presence of iodine to afford 6–16.
Scheme 1a.
aConditions: (a) DMF, 50–60 °C, 72 h; (b) 4-mercaptobenzoic acid, EtOH/H2O (2:1), 100–110 °C, I2; (c) 2-chloro-4,6-dimethoxy-1,3,5-triazine, N-methylmorpholine, diethyl-l-glutamate hydrochloride, 0 °C to r.t.; (d) 1 N NaOH, 0 °C to r.t.; (e) ArSH, I2, 80–90 °C, 4 h (6 and 7); (f) ArSH, 100–110 °C, I2 (8–16).
Compound 20 was prepared by a modified procedure reported by Secrist and Liu.35 As previously reported,35 the cycloaddition of 2,6-diamino-4-hydroxypyrimidine (17) and chloroacetone (18) in DMF at 50–60 °C gave two products i.e., 2-amino-4-oxo-6-methylpyrrolo[2,3-d]pyrimidine (19) and 2,4-diamino-5-methylfuro[2,3-d]pyrimidine (20) in a 2:1 ratio, the former being the major product. Chromatographic separation afforded 20. The yield of the furo[2,3-d]pyrimidine 20 by this reported method was 20%. In our hands with careful protection of the reaction from light and moisture, the yield of 20 was increased to 25%. The 1H NMR spectrum of 20 was identical to that reported in the literature35 except that both the 5-CH3 peak and the 6-CH peak were doublets with a coupling constant of J = 2.0 Hz rather than two singlets as reported in the literature.35 This was due to the higher resolution of the current 1H NMR spectrometer (300 MHz compared to 70 MHz in the literature),35 which readily detected the allylic coupling between the two sets of protons.
With 20 in hand the key step was to add appropriately substituted aryl thiols to the 6-position of the furo[2-3-d]pyrimidine. In 1971, Beveridge and Harris36 reported the oxidative coupling of thiols to pyrroles and indoles in the presence of I2. The proposed mechanism of this oxidative coupling was that first the I2 reacts with the thiol to form a sulphenyl iodide species, which then couples with the pyrroles or indoles via an electrophilic substitution reaction. To the best of our knowledge, no oxidative thiolation has been reported for the substitution of furans. Since furans and pyrroles are both five-membered aromatic ring systems, with a heteroatom contributing one lone pair of electrons to its aromaticity, it was envisioned that the oxidative coupling reaction could also be adopted for substitution at the 6- position of 20 to afford target molecules 6–16. We17, 18, 37, 38 have extensively utilized the oxidative thiolation procedure in their synthesis of both 5- and 6-arylthio substituted pyrrolo[2,3-d]pyrimidine containing antifolates.
For the synthesis of 6 and 7, 20 in EtOH/water was treated with iodine followed by the appropriate 1-naphthalene thiol or 2-naphthalene thiol to afford on workup and chromatographic purification 6 and 7 respectively. The 1H NMR of the products showed the disappearance of the 6-CH (δ 7.09) of the starting material and new peaks corresponding to the protons of the aromatic side chains at δ 7.10–8.30. The structure of the products was confirmed by elemental analysis. Recently, we39, 40 reported an improved oxidative thiolation procedure, which involved the addition of I2 after the thiophenols rather than before, resulting in significant improvement in the yields. Thus, compounds 8–16 were prepared via the modified method in 10–25% yields. The yields did not correlate with the extent of furo[2,3-d]pyrimidine substitution or the electron donating or withdrawing effects of substituents in the thiophenol.
For the synthesis of the classical analog 5, 4-mercaptobenzoic acid was oxidatively added to the 6-position of 20 in the presence of I2 in H2O/EtOH mixture at reflux (Scheme 1). The desired intermediate 21 was contaminated with the disulfide of 4-mercaptobenzoic acid as indicated by its 1H NMR. This crude product was purified by washing with a mixture of ethyl acetate and ethyl alcohol (v/v 2:1) to afford pure 21 in 87% yield.
This method gave 21 in one step with high yield, which significantly simplified the procedure for classical compounds compared to the previously reported methods18,37 (Scheme 1), where the pteroate esters were first synthesized followed by saponification to afford the free acid.
Peptide coupling41 of the acid 21 with diethyl l-glutamate using 2,6-dimethoxy-4-chlorotriazine and N-methyl morpholine, followed by chromatographic purification gave the coupled product 22 in 60% yield. Hydrolysis18,37 of the diester 22 at room temperature, followed by acidification gave 5 in 86% yield.
Biological Evaluation and Discussion
Compound 5 was evaluated as an inhibitor of human, Escherichia coli (E. coli), and T. gondii TS and DHFR. The inhibitory potency (IC50) values are listed in Table 1 and compared with 4, raltitrexed, pemetrexed and MTX. Compound 5 was about 2-fold more potent as a human TS inhibitor than pemetrexed and about 14-fold less potent than raltitrexed. Against human DHFR 5 was 1.5-fold more potent than pemetrexed and 5-fold more potent than raltitrexed. Thus, compound 5 is a novel dual DHFR-TS inhibitor. To the best of our knowledge this is the first example of a classical 2,4-diamino furo[2,3-d]pyrimidine antifolate that possess dual DHFR-TS inhibitory activity that is equal to or better than the clinically used pemetrexed.
Table 1.
Inhibitory concentrations (IC50, µM) against isolated TS and DHFRa
| TS | DHFR | |||||
|---|---|---|---|---|---|---|
| compd | Humanb | E. colib | T.gondiic | Humand | E.colie | T. gondiic |
| 4 | 0.54 | >180 | 1.8 | 0.21 | 0.016 | 0.17 |
| 5 | 5.4 | 230 | 27 | 4.0 | 0.020 | 2.2 |
| Raltitrexedf | 0.38 | 5.7 | 0.9 | 21 | 23 | 2.3 |
| Pemetrexedg | 9.5 | 76 | 2.8 | 6.6 | 230 | 0.46 |
| MTX | 29 | 90 | 18 | 0.022 | 0.0066 | 0.011 |
The percent inhibition was determined at a minimum of four inhibitor concentrations within 20% of the 50% point. The standard deviations for determination of 50% points are within ±10% of value given.
Kindly provided by Dr. Frank Maley, New York State Department of Health, Albany, NY.
Kindly provided by Dr. K. Anderson, Yale University.
Kindly provided by Dr. J. H. Freisheim, Medical College of Ohio, Toledo, OH.
Kindly provided by Dr. R. L. Blakley, Jude Children’s hospital, Memphis TN.
Kindly provided by Dr. Ann Jackman, Institute of Cancer Research, Sutton, Surrey, UK.
Kindly provided by Dr. Chuan Shih, Eli Lilly and Co.
Dihydrofolate Reductase (DHFR) Assay.42 All enzymes were assayed spetrophotometrically in a solution containing 50 µM dihydrofolate, 80 µM NADPH, 0.05 M Tris-HCl, 0.001 M 2-mercaptoethanol, and 0.001 M EDTA at pH 7.4 and 30 °C. The reaction was initiated with an amount of enzyme yielding a change in OD at 340 nm of 0.015/min.
Thymidylate Synthase (TS) Assay. TS was assayed spectrophotometrically at 30 °C and pH 7.4 in a mixture containing 0.1 M 2-mercaptoethanol, 0.0003 M (6R,S)-tetrahydrofolate, 0.012 M formaldehyde, 0.02 M MgCl2, 0.001 M dUMP, 0.04 M Tris-HCl, and 0.00075 M NaEDTA. This was the assay described by Wahba and Friedkin,43 except that the dUMP concentration was increased 25-fold according to the method of Davisson et al.44 The reaction was initiated by the addition of an amount of enzyme yielding a change in absorbance at 340 nm of 0.016/min in the absence of inhibitor.
Compound 5 was 10-fold and 19-fold less potent than its pyrrolo[2,3-d]pyrimidine counterpart 4, against human TS and human DHFR, respectively. These results indicate that the pyrrole 7-NH in 4 plays an important role in binding and that a hydrogen donor 7-NH as in the pyrrolo[2,3-d]pyrimidine 4 is more conducive for binding to both human DHFR and human TS than the hydrogen acceptor 7-O in the furo[2,3-d]pyrimidine 5. Surprisingly, the nonclassical analogs 6–16 (data not included) were inactive against both human and E. coli TS and DHFR with IC50 values > 2 × 10−5 M. A possible reason for the inactivity of the nonclassical analogs 6–16 could be that the 6-substituted single atom sulfur bridge is perhaps too short to allow appropriate interactions with the enzymes in the absence of the glutamate side substituent present in 5. Studies are currently underway to increase the bridge length and to provide other substitutions on the side chain phenyl ring to afford better inhibitors of TS and/or DHFR.
Compounds 5–16 were also evaluated as inhibitors of P. carinii, T. gondii, M. avium, and rat liver DHFR in a slightly different assay system than that reported in Table 1. The inhibitory potencies (IC50) and selectivity ratios are listed in Table 2 along with that for trimetrexate (TMQ) and trimethoprim (TMP). Compound 5 displayed a two digit nanomolar potency against P. carinii DHFR and single digit nanomolar potency against M. avium DHFR. In addition compound 5 displayed a 263-fold selectivity for P. carinii DHFR and a remarkable 2100-fold selectivity for M. avium DHFR, compared with the mammalian standard rat liver DHFR. Compound 5 would not be expected to be useful against P. carinii and M. avium infections in immunocompromized patients because these organisms lack the transport mechanisms necessary for classical antifolates like 5. However the potent inhibitory activity along with the remarkable selectivity of 5 against these pathogen DHFRs provides useful information on structural features that afford both high potency and high selectivity and served as a template for the design of lipophilic nonclassical analogs 6–16 containing various lipophilic substituents in the side chains and lacking the polar l-glutamate moiety.
Table 2.
| Compound | P. carinii | rat liver | rl/pcb | T. gondii | rl/tgb | M. avium | rl/mab |
|---|---|---|---|---|---|---|---|
| 5 | 0.078 | 20.5 | 262.8 | 12 | 1.7 | 0.00973 | 2106.9 |
| 6 | 8.6 | >83 | >10 | >83 | NDc | ||
| 7 | >12 | >12 | ND | >12 | ND | ||
| 8 | 20(27%)d | 196(41%) | ND | 19.7 | ND | 196(20%) | ND |
| 9 | 60 | 14.4 | 0.2 | 25.5 | 0.6 | 56.2 | 0.26 |
| 10 | 24 | 42.2 | 1.8 | 38.4 | 1.1 | 61.9 | 0.68 |
| 11 | 34.7 | 61.9 | 1.8 | 69.4 | 0.9 | 25.5 | 2.43 |
| 12 | 31(39%) | 39.1 | ND | 81.8 | 0.5 | 31(8%) | ND |
| 13 | 20.3 | 55.6 | 2.7 | 44.2 | 1.3 | 23.9 | 2.33 |
| 14 | 25.2 | 51.3 | 2.0 | 45.9 | 1.1 | 28.4 | 1.81 |
| 15 | 36.4 | 66.6 | 1.8 | 51.7 | 1.3 | 16.3 | 4.09 |
| 16 | 36.5 | 24.3 | 0.7 | 16.2 | 1.5 | 23.4 | 1.04 |
| TMQ | 0.042 | 0.003 | 0.07 | 0.010 | 0.3 | 0.0015 | 2.0 |
| TMP | 12 | 180 | 14 | 2.8 | 65 | 0.3 | 610 |
These assays were carried out at 37 °C under conditions of saturation with substrate (90 µM dihydrofolic acid) and cofactor (119 µM NADPH) in the presence of 150 mM KCl.
Selectivity ratios, rl/pc = IC50 rat liver dihydrofolate reductase/ IC50 P. carinii dihydrofolate reductase; rl/tg = IC50 rat liver dihydrofolate reductase/ IC50 T. gondii dihydrofolate reductase; rl/ma = IC50 rat liver dihydrofolate reductase/ IC50 M. avium dihydrofolate reductase.
ND = not determined.
Number in parenthesis indicate the percentage inhibition at the given concentration
Lipophilic nonclassical analogs containing pyrrolo[2,3-d]pyrimidine or furo[2,3-d]pyrimidine scaffolds have been extensively synthesized as potential antibacterial, antiprotozoan and antifungal agents.21,34 Biological evaluation of compounds 6–16 indicated that they were moderately potent inhibitors of P. carinii, T. gondii and M. avium DHFR. The most potent and selective compound against P. carinii DHFR was 6 containing a 1-naphthyl side chain. The most potent and selective compound against T. gondii DHFR was 16 containing a 2-isopropyl-6-methyl phenyl side chain. Against M. avium DHFR compound 15 was the most potent and selective and contained an 3,4-dimethoxy phenyl side chain. Most of the compounds tested against all the three pathogen DHFR lacked the high potency and selectivity of compound 5. A possible explanation for the lack of DHFR inhibitory activity of the nonclassical analogs is that the single atom 6-S bridge between the furo[2,3-d]pyrimidine and the substituted phenyl side chain is too short and lack a α-COOH and does not permit sufficient interaction of the substituted phenyl ring with appropriate hydrophobic (Phe31, Phe34, Ile60, Pro61, Leu67) and/or ionic (Arg70) portions of the DHFR enzyme. Alternatively the side chain could be held in a conformation that is not conducive to optimum binding in the absence of the α-COOH of the L-glutamate as in 5. Why then was the classical analog a potent inhibitor of P. carinii DHFR and M. avium DHFR? The answer probably lies in the α-carboxylic acid moiety of the l-glutamate of 5 which should make ionic (salt bridge) interactions with an invariant Arg in DHFR. Thus, the classical analog 5 has additional binding sites which appropriately orients the molecule for optimum hydrophobic and ionic interactions with DHFR and translates into better inhibitory activity.
In summary we have reported the synthesis and biological evaluation of a classical 2,4-diamino-5-methyl-6-substituted furo[2,3-d]pyrimidine 5 along with eleven nonclassical antifolate analogs 6–16. The classical compound 5 is the first analog with the furo[2,3-d]pyrimidine scaffold to demonstrate dual human TS and DHFR inhibitory activity. In addition against P. carinii DHFR and M. avium DHFR compound 5 demonstrated high potency (IC50 = 78 nM; P. carinii DHFR and IC50 = 9.73 nM; M. avium DHFR) and selectivity (263-fold for P. carinii DHFR and 2100-fold for M. avium DHFR). Since P. carinii and M. avium lack the transport system(s) for classical folates compound 5 would not enter these pathogen cells.
The nonclassical analogs 6–16 had moderate potency and lacked appreciable selectivity for pathogen DHFR. This study provides the first example of a classical 2,4-diamino furo[2,3-d]pyrimidine scaffold with dual human TS-DHFR activity and affords a scaffold, the furo[2,3-d]pyrimidine, that with appropriate bridge length variation and substitution(s) on the phenyl ring could afford nonclassical antifolate inhibitors of TS and/or DHFR.
Experimental Section
All evaporations were carried out in vacuum with a rotary evaporator. Analytical samples were dried in vacuum (0.2 mmHg) in an Abderhalden drying apparatus over P2O5 at 70 °C. Thin-layer chromatography (TLC) was performed on silica gel plates (Whatman 250 µM PE SiLG/UV) with fluorescent indicator. Spots were visualized by UV light (254 and 365 nm). All analytical samples were homogeneous on TLC in at least two different solvent systems. Purification by column and flash chromatography was carried out using Merck silica gel 60 (200–400 mesh). The amount (weight) of silica gel for column chromatography was in the range of 50–100 times the amount (weight) of the crude compounds being separated. Columns were dry-packed unless specified otherwise. Solvent systems are reported as volume percent of mixture. Melting points were determined on a Mel-Temp II melting point apparatus and are uncorrected. Proton nuclear magnetic resonance (1H NMR) spectra were recorded on a Bruker WH-300 (300 MHz) spectrometer. The chemical shift (δ) values are reported as parts per million (ppm) relative to tetramethylsilane as internal standard; s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad singlet. Elemental analyses were performed by Atlantic Microlab, Inc., Norcross, GA. Elemental compositions were within ±0.4% of the calculated values. Fractional moles of water or organic solvents frequently found in some analytical samples of antifolates could not be removed despite 24 h of drying in vacuum and were confirmed, where possible, by their presence in the 1H NMR spectrum. High-resolution mass spectra (HRMS), using Electron impact (EI), were recorded on a VG Autospec (Fisons Instruments) micromass (EBE Geometry) double focusing mass spectrometer. All solvents and chemicals were used as received.
2,4-Diamino-5-methyl-furo[2,3-d]pyrimidine (20)
To a suspension of 2,6-diamino-4-hydroxypyrimidine (17) (1.0 g, 8 mmol) in anhydrous DMF (12 mL) was added chloroacetone (18) (0.74 g, 8 mmol). The mixture was heated at 50–60 °C for 40 h under N2 and protected from light with aluminium foil. Two major spots at Rf = 0.36 (corresponding to 19) and Rf = 0.56 (corresponding to 20) were visualized on TLC (CHCl3/MeOH, 4/l). To the suspension was added silica gel (3 g) followed by MeOH (100 mL) and the solvent evaporated under reduced pressure to dryness to afford a dry plug. The silica gel plug was loaded on top of a dry silica gel column and eluted with 10% MeOH in CHCl3. Fractions containing the desired spot (TLC) were pooled and evaporated to dryness under vacuum to give 290 mg (26%) of 20 as a white solid: Mp 264–266 °C (lit. >260 °C); 1H NMR (DMSO-d6); δ2.18 (d, 3 H, 5-CH3, J =1.1 Hz), 5.96 (bs, 2 H, 2/4-NH2), 6.42 (bs, 2 H, 2/4-NH2), 7.09 (s, 1 H, 6-CH, J = 1.1 Hz).
4-[(2,4-Diamino-5-methyl-furo[2,3-d]pyrimidin-6-yl)thio]benzoic Acid (21)
To a suspension of 20 (500 mg, 3 mmol) and 4-mercaptobenzoic acid (940 mg, 6.0 mmol) in a 250 mL round-bottom flask was added a solution of EtOH/water (v/v 2:1, 40 mL) and heated to reflux. Iodine (1.5 g, 6.0 mmol) was then added to the solution at reflux. The reaction mixture was monitored for completion at reflux (3 h) and then allowed to cool to room temperature. The precipitate formed was filtered, and to the filtrate was added excess Na2S2O3, and the resulting precipitate formed was filtered. The residue was washed first with EtOAc and then with EtOH to afford 21 (838 mg, 87%) as a white solid.
Diethyl N-[4-[(2,4-diamino-5-methyl-furo[2,3-d]pyrimidin-6-yl)thio]-benzoyl]-l-glutamate (22)
To a suspension of the acid 21 (500 mg, 1.57 mmol) in anhydrous DMF (40 mL) under N2 was added N-methylmorpholine (217 µL, 2.00 mmol) and the resulting suspension was cooled to 0 °C. At this point 2-chloro-4,6-dimethoxy-1,3,5-triazine (350 mg, 2.00 mmol) was added and the suspension was stirred for 2 h during this time it formed a solution. The reaction mixture was again cooled to 0 °C and diethyl-l-glutamate (475 mg, 2.00 mmol) was added followed by N-methylmorpholine (217 µL, 2.00 mmol). The solution was slowly allowed to warm to room temperature with stirring and left at room temperature for a total of 24 h. To the resulting solution was added silica gel (5 g) and the solvent was evaporated under reduced pressure using an oil pump. The silica gel plug was loaded on top of a wet (CHCl3) silica gel column and eluted with a gradient of 1–3% MeOH in CHCl3. Fractions containing the desired spot (TLC) were pooled and evaporated to dryness under vacuum to give 475 mg (60%) of 22 as a white solid: Mp 210–217 °C; TLC Rf = 0.61 (CHCl3/MeOH, 5/1); 1H NMR (DMSO-d6) δ1.13–1.20 (m, 6 H, CH2CH3), 1.98–2.10 (m, 2 H, Glu β-CH2), 2.33 (s, 3 H, 5-CH3), 2.39–2.44 (t, 2 H, Glu γ-CH2), 4.00–4.13 (q, 4 H, CH2CH3), 4.39–4.44 (m, 1 H, Glu α-CH), 6.26 (bs, 2 H, 2/4-NH2), 6.72 (bs, 2 H, 2/4-NH2), 7.16–7.19 (d, 2 H, C6H4), 7.78–7.81 (d, 2 H, C6H4), 8.68–8.70 (d, 1 H, CONH). Anal. Calcd for (C23H27N5O6S) C, H, N, S.
N-[4-[(2,4-Diamino-5-methyl-furo[2,3-d]pyrimidin-6-yl)thio]-benzoyl]-l-glutamic Acid (5)
To a suspension of 22 (200 mg, 0.4 mmol) in EtOH (15 mL) was added 1 N NaOH (6 mL) and the suspension stirred at 0 °C (4 h) and then at room temperature for 24 h. The solvent was evaporated under reduced pressure, the yellow oil was dissolved in water (5 mL) and the solution was cooled in an ice-bath and acidified carefully to pH 4.0 with drop wise addition of 3 N HCl. This suspension was left at 5 °C for 24 h and filtered. The residue was washed well with water and Et2O and then dried over P2O5 /vacuum to afford 159 mg (86%) of 5 as a white solid: Mp 217–222 °C; 1H NMR (DMSO-d6) δ1.98–2.10 (m, 2 H, Glu β-CH2), 2.33 (s, 3 H, 5-CH3), 2.39–2.44 (t, 2 H, Glu γ-CH2), 4.39–4.44 (m, 1 H, Glu α-CH), 6.26 (bs, 2 H, 2/4-NH2), 6.72 (bs, 2 H, 2/4-NH2), 7.16–7.19 (d, 2 H, C6H4), 7.78–7.81 (d, 2 H, C6H4), 8.68–8.70 (d, 1 H, CONH). Anal. Calcd for (C19H19N5SO6•1.0H2O) C, H, N, S.
2,4-Diamino-5-methyl-6-(1-naphthylthio)furo[2,3-d]pyrimidine (6)
A suspension of 20 (0.1 g, 0.61 mmol) in EtOH/water (5:4, 4.5 mL) was heated to 80 °C. Iodine (0.31 g, 1.22 mmol) was added, followed by dropwise addition of a solution of 1-naphthalenethiol (0.15 g, 0.91 mmol) in EtOH (3 mL). The reaction mixture was then heated to 90 °C for 3 h after which a dark colored solution formed. The reaction mixture was then allowed to cool to room temperature and the solvent was evaporated to dryness under reduced pressure. The resulting residue was washed with a minimum amount of EtOAc until the color of the residue became light yellow. The solid was then suspended in cold water (3 mL) and the pH adjusted to 7.0 with conc. NH4OH. The suspension was then sonicated, cooled to 5 °C and filtered. The residue was washed twice with cold water (3 mL) and then stirred in MeOH (3 mL) for 12 h and filtered. The solid was washed twice with MeOH (2 mL) to afford 0.10 g (52.5%) of pure 6: Mp 300–305 °C (dec.); TLC Rf = 0.48 (CHCl3/MeOH, 7:1 with one drop of NH4OH, silica gel); 1H NMR DMSO-d6 2.38 (s, 3 H, 5-CH3), 6.23 (s, 2 H, 2/4-NH2), 6.70 (s, 2 H, 2/4-NH2), 7.16 (d, 1 H, 5’-CH, J = 7.0 Hz), 7.44 (m, 1 H, 3’-CH), 7.60–7.70 (m, 2 H, 6’,7’-CH), 7.84 (d, 1 H, 4’-CH, J = 8.0 Hz), 7.99 (d, 1 H, 2’-CH, J = 7.6 Hz), 8.29 (d, 1 H, 8’-CH, J = 8.2 Hz). Anal Calcd for (C17H14N4OS•0.5 H2O) C, H, N, S.
2,4-Diamino-5-methyl-6-(2-naphthylthio)furo[2,3-d]pyrimidine (7)
A suspension of 20 (0.1 g, 0.61 mmol) in EtOH/water (5:4, 4.5 mL) was heated to 80 °C. Iodine (0.31 g, 1.22 mmol) was added, followed by the dropwise addition of a solution of 2-naphthalenethiol (0.15 g, 0.91 mmol) in EtOH (3 mL). The reaction mixture was heated to 90 °C for 4 h and then allowed to cool to room temperature. The solvent was evaporated to dryness under reduced pressure. The resulting residue was washed with EtOAc (1 mL) and then suspended in cold water (3 mL) and the pH was adjusted to 7.0 with conc. NH4OH. The suspension was sonicated, cooled to 5 °C, filtered and the residue was washed twice with cold water (3 mL) and dissolved in MeOH (100 mL). The solution was mixed with silica gel (0.7 g) and evaporated under reduced pressure. The residue was dried in vacuo, powdered and poured on top of a wet silica column and eluted with 3% MeOH in CHCl3. Fractions corresponding to a single spot of the product were pooled and evaporated to dryness to afford 0.025 g (12.7%) of 7: Mp 225–230 °C; TLC Rf = 0.45 (CHCl3/MeOH, 7:1 with one drop of NH4OH, silica gel); 1H NMR (DMSO-d6)δ 2.38 (s, 3 H, 5-CH3), 6.25 (s, 2 H, 2/4-NH2), 6.72 (s, 2H, 2/4-NH2), 7.25 (dd, 1 H, 3’-CH, 7.40–7.51 (m, 2 H, C10H6), 7.68 (d, 1 H, 1’-CH), 7.83–7.89 (m, 2 H, C10H6). Anal. Calcd for (C17H14N4OS•H2O) C, H, N, S.
2,4-Diamino-5-methyl-6-(2’,6’-dimethylphenylsulfanyl)-furo[2,3-d]pyrimidine (8)
To a 250 mL flask containing 20 (0.2 g, 1.22 mmol) and 2,6-dimethylphenylthiol (0.337 g , 2.44 mmol) was added a mixture of EtOH/water (2:1, 25 mL) and the reaction mixture was heated to 100–110 °C. Upon reflux I2 (0.619 g, 2.44 mmol) was added and the heating continued with stirring for a total of 3 h. To this mixture was added excess Na2S2O3, and the mixture was concentrated under reduced pressure. To the resulting residue was added silica gel (5 g) and MeOH (50 mL), and the solution was evaporated to dryness under reduced pressure to afford a dry silica gel plug which was loaded on top of a wet (CHCl3) silica gel column and eluted with a gradient of 0–3% MeOH in CHCl3. Fractions containing the desired spot (TLC) were pooled and evaporated to dryness. The resulting residue was washed with MeOH, filtered, and dried to yield 0.055 g (15%) of 8 as a pale white solid; Mp 305–311 °C; TLC Rf = 0.60 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH); 1H NMR (DMSO-d6) δ 2.33 (s, 3 H, 5-CH3), 2.50 (s, 6 H, 2’,6’-diCH3), 6.05 (s, 2 H, 2/4–NH2), 6.54 (s, 2 H, 2/4–NH2), 7.15 (s, 3 H, C6H3). Anal. Calcd for (C15H16N4OS) C, H, N, S.
2,4-Diamino-5-methyl-6-(2’,6’-dichlorophenylsulfanyl)-furo[2,3-d]pyrimidine (9)
Compound 9 was synthesized as described for 8: yield 12%; Mp >305 °C (dec.); TLC Rf = 0.55 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH); 1H NMR (DMSO-d6) δ 2.34 (s, 3 H, 5-CH3), 6.14 (s, 2 H, 2/4–NH2), 6.62 (s, 2 H, 2/4–NH2), 7.38–7.44 (t, 1 H, C6H3), 7.56–7.59 (d, 2 H, C6H3). Anal. Calcd for (C13H10N4OSCl2) C, H, N, S, Cl.
2,4-Diamino-5-methyl-6-(2’,5’-dimethoxyphenylsulfanyl)-furo[2,3-d]pyrimidine (10)
Compound 10 was synthesized as described for 8: yield 19%; Mp 292–295 °C; TLC Rf = 0.58 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH); 1H NMR (DMSO-d6) δ 2.30 (s, 3 H, 5-CH3), 3.58 (s, 3 H, 2’-OCH3), 3.80 (s, 3 H, 5’-OCH3), 6.08 (s, 1 H, C6H3), 6.24 (s, 2 H, 2/4–NH2), 6.69 (s, 2 H, 2/4–NH2), 6.72–6.75 (d, 1 H, C6H3), 6.95–6.98 (d, 1 H, C6H3). Anal. Calcd for (C15H16N4O3S) C, H, N, S.
2,4-Diamino-5-methyl-6-(4’-methoxyphenylsulfanyl)-furo[2,3-d]pyrimidine (11)
Compound 11 was synthesized as described for 8: yield 16%; Mp 273.5–276 °C; TLC Rf = 0.54 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH); 1H NMR (DMSO-d6) δ 2.34 (s, 3 H, 5-CH3), 3.71 (s, 3 H, 4’-OCH3), 6.17 (s, 2 H, 2/4–NH2), 6.63 (s, 2 H, 2/4–NH2), 6.89–6.92 (d, 2 H, C6H4), 7.15–7.18 (d, 2 H, C6H4). Anal. Calcd for (C14H14N4O2S•0.2H2O) C, H, N, S.
2,4-Diamino-5-methyl-6-(2’,3’,5’,6’-tetrafluoro-4’-trifluoromethylphenylsulfanyl)-furo[2,3-d]pyrimidine (12)
Compound 12 was synthesized as described for 8: yield 10%; Mp 305–307 °C; TLC Rf = 0.52 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH); 1H NMR (DMSO-d6) δ 2.37 (s, 3 H, 5-CH3), 6.27 (s, 2 H, 2/4–NH2), 6.72 (s, 2 H, 2/4–NH2). Anal. Calcd for (C14H7OSN4F7) C, H, N, S, F.
2,4-Diamino-5-methyl-6-(2’-methoxyphenylsulfanyl)-furo[2,3-d]pyrimidine (13)
Compound 13 was synthesized as described for 8: yield 21%; Mp >280 °C (dec.); TLC Rf 0.63 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH); 1H NMR (DMSO-d6) δ 2.30 (s, 3 H, 5-CH3), 3.86 (s, 3 H, 2’-OCH3), 6.22 (s, 2 H, 2/4–NH2), 6.59–6.62 (d, 1 H, C6H4), 6.67 (s, 2 H, 2/4–NH2), 6.84–6.88 (t, 1 H, C6H4), 7.01–7.04 (d, 1 H, C6H4), 7.14–7.19 (t, 1 H, C6H4). Anal. Calcd for (C14H14N4O2S•0.1H2O) C, H, N, S.
2,4-Diamino-5-methyl-6-(3’-methoxyphenylsulfanyl)-furo[2,3-d]pyrimidine (14)
Compound 14 was synthesized as described for 8: yield 25%; Mp 288–295 °C; TLC Rf = 0.58 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH); 1H NMR (DMSO-d6) δ 2.33 (s, 3 H, 5-CH3), 3.71 (s, 3 H, 3’-OCH3), 6.23 (s, 2 H, 2/4–NH2), 6.63–6.68 (m, 4 H, 2/4–NH2 and C6H4), 6.77–6.80 (d, 2 H, C6H4), 7.21–7.26 (t, 1 H, C6H4). Anal. Calcd for (C14H14N4O2S•0.2H2O) C, H, N, S.
2,4-Diamino-5-methyl-6-(3’,4’-dimethoxyphenylsulfanyl)-furo[2,3-d]pyrimidine (15)
Compound 15 was synthesized as described for 8: yield 22%; Mp 291–293 °C; TLC Rf = 0.60 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH); 1H NMR (DMSO-d6) δ 2.36 (s, 3 H, 5-CH3), 3.72 (s, 6 H, 3’,4’-diOCH3), 6.19 (s, 2 H, 2/4–NH2), 6.64 (s, 2 H, 2/4–NH2), 6.68–6.70 (d, 1 H, C6H3), 6.85 (s, 1 H, C6H3), 6.90–6.93 (d, 1 H, C6H3). Anal. Calcd for (C15H16N4O3S•0.3H2O) C, H, N, S.
2,4-Diamino-5-methyl-6-(2’-isopropyl-6’-methylphenylsulfanyl)-furo[2,3-d]pyrimidine (16)
Compound 16 was synthesized as described for 8: yield 12%; Mp 275–277 °C; TLC Rf = 0.59 (CHCl3/MeOH, 5:1, with 2 drops of concentrated NH4OH); 1H NMR (DMSO-d6) δ 1.18–1.20 (d, 6 H, CH(CH3)2), 2.35 (s, 3 H, 5-CH3), 2.50 (s, 3 H, 6’-CH3), 4.03–4.07 (m, 1 H, CH(CH3)2 ), 6.07 (s, 2 H, 2/4–NH2), 6.53 (s, 2 H, 2/4–NH2), 7.13–7.29 (m, 3 H, C6H3). Anal. Calcd for (C17H20N4OS•0.2H2O) C, H, N, S.
Supplementary Material
Acknowledgment
This work was supported in part by the National Institutes of Health, Grants AI41743 (A.G.); AI44661 (A.G.), CA89300 (A.G.), and Core Grants CA16056 (R.L.K.), CA10914 (R.L.K.) and AI47759 (A.G.) from the National Institute of Allergy and Infections Diseases.
Abbreviations
- TS
Thymidylate synthase
- dTMP
2’-deoxythymidine-5’-monophosphate
- dUMP
2’-deoxyuridine-5’-monophosphate
- DHFR
dihydrofolate reductase
- dTDP
2’-deoxythymidine-5’-diphosphate
- dTTP
2’-deoxythymidine-5’-triphosphate
- MTX
methotrexate
- GARFT
glycinamide ribonucleotide formyltransferase
- AICARFT
5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase
- FPGS
folyl poly-y-glutamate synthetase
- PTX
piritrexim; P. carinii, Pneumocystis carinii
- T. gondii
Toxoplasma gondii
- M. avium
Mycobacterium avium
- MAC
Mycobacterium avium complex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Material Available: Results from elemental analyses. This material is available free of charge via the Internet.
References
- 1.(a) Taken in part from the dissertation submitted by H.J. to the Graduate School of Pharmaceutical Sciences, Duquesne University, in partial fulfillment of the requirements for the degree of Doctor of Philosophy, July 2006. (b) Presented in part at the 228th American Chemical Society National Meeting, Philadelphia, PA, United States, August 22–26, 2004; Abstr. MEDI-101.
- 2.Schnell JR, Dyson HJ, Wright PE. Annu. Rev. Biophys. Biomol. Struct. 2004;33:119–140. doi: 10.1146/annurev.biophys.33.110502.133613. [DOI] [PubMed] [Google Scholar]
- 3.Berman EM, Werbel LM. J. Med. Chem. 1991;34:479–485. doi: 10.1021/jm00106a001. [DOI] [PubMed] [Google Scholar]
- 4.Jackman AL, Taylor GA, Gibson W, Kimbell R, Brown M, Calvert AH, Judson IR, Hughes LR. Cancer Res. 1991;51:5579–5586. [PubMed] [Google Scholar]
- 5.Taylor EC, Kuhnt D, Shih C, Rinzel SM, Grindey GB, Barredo J, Jannatipour M, Moran R. J. Med. Chem. 1992;35:4450–4454. doi: 10.1021/jm00101a023. [DOI] [PubMed] [Google Scholar]
- 6.Bertino JR, Kamen B, Romanini A. In: Cancer Medicine. Holland JF, Frei E, Bast RC, Kufe DW, Morton DL, Weichselbaum RR, editors. Vol. 1. Baltimore, MD: Williams & Wilkins; 1997. pp. 907–921. [Google Scholar]
- 7.Kisliuk RL. Pharmacol. Ther. 2000;85:183–190. doi: 10.1016/s0163-7258(99)00056-x. [DOI] [PubMed] [Google Scholar]
- 8.Boritzki TJ, Zhang C, Bartlett CA, Jackson RC. In: Antifolate Drugs in Cancer Therapy. Jackman AL, editor. Totowa, New Jersey: Humana Press; 1999. pp. 281–292. [Google Scholar]
- 9.Kisliuk RL, Gaumont Y, Kumar P, Coutts M, Nair MG, Nanavati NT, Kalman TI. In: Proceedings of the Second Workshop on Folyl and Antifolyl Polyglutamates. Goldman ID, editor. New York: Praeger; 1985. pp. 319–328. [Google Scholar]
- 10.Kisliuk RL, Gaumont Y, Powers JF, Thorndike J, Nair MG, Piper JR. In: Folic Acid Metabolism in Health and Disease. Picciano MF, Stokstad ELR, Gregory JF III, editors. New York: Wiley-Liss; 1990. pp. 79–89. [Google Scholar]
- 11.Galivan J, Nimec Z, Rhee M. Cancer Res. 1987;47:5256–5260. [PubMed] [Google Scholar]
- 12.Galivan J, Rhee MS, Johnson TB, Dilwith R, Nair MG, Bunni M, Priest DG. J. Biol. Chem. 1989;264:10685–10692. [PubMed] [Google Scholar]
- 13.Gaumont Y, Kisliuk RL, Emkey R, Piper JR, Nair MG. In: Chemistry and Biology of Pteridines. Curtius H-C, Blau N, Ghisla S, editors. Berlin: de Gruyter; 1990. pp. 1131–1136. [Google Scholar]
- 14.Gaumont Y, Kisliuk RL, Parsons JC, Greco WR. Cancer Res. 1992;52:2228–2235. [PubMed] [Google Scholar]
- 15.Hynes JB, Patil SA, Tomazic A, Kumar A, Pathak A, Tan X, Li X, Ratnam M, Delcamp TJ, Freisheim JH. J. Med. Chem. 1988;31:449–454. doi: 10.1021/jm00397a031. [DOI] [PubMed] [Google Scholar]
- 16.Gangjee A, Yu J, McGuire JJ, Cody V, Galitsky N, Kisliuk RL, Queener SF. J. Med. Chem. 2000;43:3837–3851. doi: 10.1021/jm000200l. [DOI] [PubMed] [Google Scholar]
- 17.Gangjee A, Lin X, Kisliuk RL, McGuire JJ. J. Med. Chem. 2005;48:7215–7222. doi: 10.1021/jm058234m. [DOI] [PubMed] [Google Scholar]
- 18.Gangjee A, Jain HD, Phan J, Lin X, Song X, McGuire JJ, Kisliuk RLDual. J. Med. Chem. 2006;49:1055–1065. doi: 10.1021/jm058276a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendelsohn LG, Shih C, Chen VJ, Habeck LL, Gates SB, Shackelford KA. Sem. Oncol. 1999;26:42–47. [PubMed] [Google Scholar]
- 20.Rosowsky A. In: Progress in Medicinal Chemistry. Ellis GP, West GB, editors. Vol. 26. New York: Elsevier Science; 1989. pp. 1–252. [Google Scholar]
- 21.Gangjee A, Elzein E, Kothare M, Vasudevan A. Curr. Pharm. Des. 1996;2:263–280. [Google Scholar]
- 22.Gangjee A, Devraj R, McGuire JJ, Kisliuk RL, Queener S, Barrows LR. J. Med. Chem. 1994;37:1169–1176. doi: 10.1021/jm00034a015. [DOI] [PubMed] [Google Scholar]
- 23.Gangjee A, Devraj R, McGuire JJ, Kisliuk RL. J. Med. Chem. 1995;38:3798–3805. doi: 10.1021/jm00019a009. [DOI] [PubMed] [Google Scholar]
- 24.Tripos Inc., 1699 South Hanley Road, St. Louis, MO, 63144.
- 25.Sayre PH, Finer-Moore JS, Fritz TA, Biermann D, Gates SB, MacKellar WC, Patel VF, Stroud RM. J. Mol. Biol. 2001;313:813–829. doi: 10.1006/jmbi.2001.5074. [DOI] [PubMed] [Google Scholar]
- 26.Cody V, Luft JR, Pangborn W. Acta Crystallogr. D Biol. Crystallogr. 2005;61:147–155. doi: 10.1107/S0907444904030422. [DOI] [PubMed] [Google Scholar]
- 27.Jackson RC. In: Antifolate Drugs in Cancer Therapy. Jackman AL, editor. Totowa, NJ: Humana Press; 1999. pp. 1–12. [Google Scholar]
- 28.Kisliuk RL. Pharmacol. Ther. 2000;85:183–190. doi: 10.1016/s0163-7258(99)00056-x. [DOI] [PubMed] [Google Scholar]
- 29.Grivsky EM, Lee S, Sigel CW, Duch DS, Nichol CA. J. Med. Chem. 1980;23:327–336. doi: 10.1021/jm00177a025. [DOI] [PubMed] [Google Scholar]
- 30.Varney MD, Marzoni GP, Palmer CL, Deal JG, Webber S, Welsh KM, Bacquet RJ, Bartlett CA, Morse CA, Booth CLJ, Herrmann SM, Howland EF, Ward RW, White J. J. Med. Chem. 1992;35:663–676. doi: 10.1021/jm00082a006. [DOI] [PubMed] [Google Scholar]
- 31.Webber SE, Bleckman TM, Attard J, Deal JG, Katherdekar V, Welsh KM, Webber S, Janson CA, Matthews DA, Smith WW, Freer ST, Jordan SR, Bacquet RJ, Howland EF, Booth CJL, Ward RW, Hermann SM, White J, Morse CA, Hilliard JA, Bartlett CA. J. Med. Chem. 1993;36:733–746. doi: 10.1021/jm00058a010. [DOI] [PubMed] [Google Scholar]
- 32.Willemot P, Klein MB. Expert Rev. Anti-Infect. Ther. 2004;2:521–532. doi: 10.1586/14787210.2.4.521. [DOI] [PubMed] [Google Scholar]
- 33.Klepser ME, Klepser TB. Drugs. 1997;53:40–73. doi: 10.2165/00003495-199753010-00004. [DOI] [PubMed] [Google Scholar]
- 34.Chan DCM, Anderson AC. Curr. Med. Chem. 2006;13:377–398. doi: 10.2174/092986706775527938. [DOI] [PubMed] [Google Scholar]
- 35.Secrist JA, III, Liu PS. J. Org. Chem. 1978;43:3937–3941. [Google Scholar]
- 36.Beveridge S, Harris RLN. Austr. J. Chem. 1971;24:1229–1236. [Google Scholar]
- 37.Gangjee A, Jain HD, McGuire JJ, Kisliuk RL. J. Med. Chem. 2004;47:6730–6739. doi: 10.1021/jm040144e. [DOI] [PubMed] [Google Scholar]
- 38.Gangjee A, Jain HD, Phan J, Kisliuk RL. J. Heterocycl. Chem. 2005;42:165–168. [Google Scholar]
- 39.Gangjee A, Jain HD, Kisliuk RL. Bioorg. Med. Chem. Lett. 2005;15:2225–2230. doi: 10.1016/j.bmcl.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 40.Gangjee A, Jain HD, Queener SF. J. Heterocycl. Chem. 2005;42:589–594. [Google Scholar]
- 41.Taylor EC, Patel HH, Jun J-G. J. Org. Chem. 1995;60:6684–6687. [Google Scholar]
- 42.Kisliuk RL, Strumpf D, Gaumont Y, Leary RP, Plante L. J. Med. Chem. 1977;20:1531–1533. doi: 10.1021/jm00221a038. [DOI] [PubMed] [Google Scholar]
- 43.Wahba AJ, Friedkin M. J. Biol. Chem. 1962;237:3794–3801. [PubMed] [Google Scholar]
- 44.Davisson VJ, Sirawaraporn W, Santi DV. J. Biol. Chem. 1989;264:9145–9148. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.