Abstract
Many insects are dependent on bacterial symbionts that provide essential nutrients (ex. aphid–Buchnera and tsetse–Wiglesworthia associations), wherein the symbionts are harbored in specific cells called bacteriocytes that constitute a symbiotic organ bacteriome. Facultative and parasitic bacterial symbionts like Wolbachia have been regarded as evolutionarily distinct from such obligate nutritional mutualists. However, we discovered that, in the bedbug Cimex lectularius, Wolbachia resides in a bacteriome and appears to be an obligate nutritional mutualist. Two bacterial symbionts, a Wolbachia strain and an unnamed γ-proteobacterium, were identified from different strains of the bedbug. The Wolbachia symbiont was detected from all of the insects examined whereas the γ-proteobacterium was found in a part of them. The Wolbachia symbiont was specifically localized in the bacteriomes and vertically transmitted via the somatic stem cell niche of germalia to oocytes, infecting the incipient symbiotic organ at an early stage of the embryogenesis. Elimination of the Wolbachia symbiont resulted in retarded growth and sterility of the host insect. These deficiencies were rescued by oral supplementation of B vitamins, confirming the essential nutritional role of the symbiont for the host. The estimated genome size of the Wolbachia symbiont was around 1.3 Mb, which was almost equivalent to the genome sizes of parasitic Wolbachia strains of other insects. These results indicate that bacteriocyte-associated nutritional mutualism can evolve from facultative and prevalent microbial associates like Wolbachia, highlighting a previously unknown aspect of the parasitism-mutualism evolutionary continuum.
Keywords: B vitamins, bacteriome, Cimex lectularius, nutritional mutualism
The rise of sophisticated mutualism from less-intimate biological associations is of evolutionary interest. Obligate insect-bacterium endosymbioses are among the most impressive cases of such mutualism, wherein the partners are integrated into a coherent symbiotic entity and can no longer survive independently. For example, aphids harbor Buchnera aphidicola, which provide essential amino acids that are deficient in their plant sap diet, in specialized cells called bacteriocytes (1). Tsetse flies possess Wigglesworthia glossinidia, which synthesize B vitamins that are devoid in their blood meal, in bacteriocytes (2). Acquisition of novel biological properties via endosymbiosis seems to have played important roles in adaptation, evolution, and diversification of insects (3, 4). However, it is poorly understood how sophisticated endocellular symbionts such as Buchnera and Wigglesworthia have evolved from less-specialized bacterial ancestors.
Wolbachia represents the most prevalent endosymbiotic bacterial group, associated with over 60% of insect species (5). In infected insects, Wolbachia symbionts are localized in diverse cells and tissues (6) and usually affect host fitness negatively, often manipulating host reproduction to enhance their own transmission (7). Unlike Buchnera in aphids and Wigglesworthia in tsetse flies, Wolbachia is generally not regarded as obligate bacteriocyte-associated nutritional symbiont.
The common bedbug Cimex lectularius (Fig. 1A) is notorious as a pest that feeds on human blood, whose recent resurgence is causing hygienic, economical, and social problems (8). Since the early 1920s, the presence of bacteriocyte-associated symbionts has been recognized in C. lectularius. A pair of symbiotic organs consisting of many bacteriocytes, called bacteriomes, are located adjacent to the gonads (Fig. 1 B and C), wherein numerous bacteria were observed endocellularly (9–12). Early experimental works suggested a role for the symbiont in the reproduction of the bedbug (13, 14), but its microbiological nature has been elusive to date. Previous works detected 16S rRNA gene sequences of a Wolbachia strain and an unnamed γ-proteobacterium from the bedbug (15, 16). A recent review of the issue suggested that either the γ-proteobacterium or other undescribed symbionts may be required for reproduction of the bedbug (17).
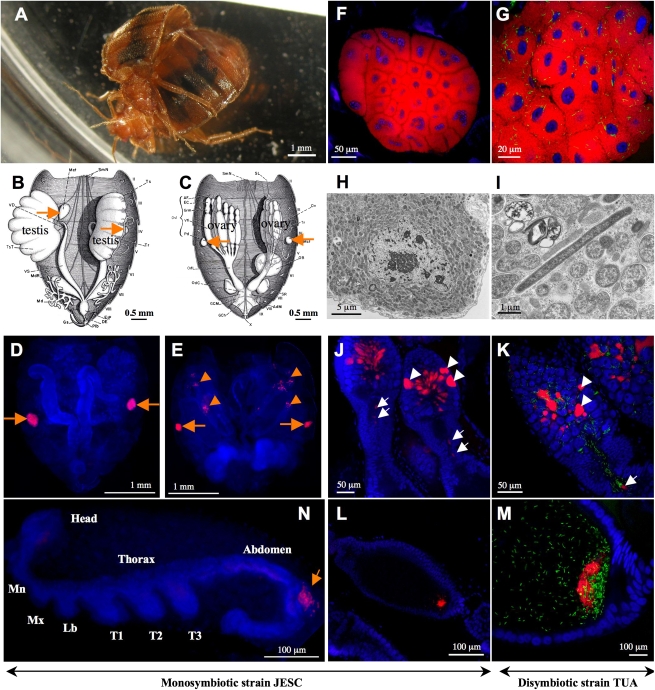
Fig. 1.
The common bedbug Cimex lectularius and localization of its symbiotic bacteria. (A) A mating pair of adult bedbugs. (B and C) Gonad-associated bacteriomes in adult male (B) and female (C) highlighted by orange arrows, from Davis (10). (D and E) FISH detection of Wolbachia in adult male (D) and female (E); orange arrows and arrowheads indicate bacteriome infections and ovarial infections, respectively. (F and G) FISH detection of Wolbachia and γ-proteobacteria in bacteriomes of monosymbiotic bedbug strain JESC (F) and disymbiotic bedbug strain TUA (G). (H and I) Transmission electron microscopy of rod-shaped Wolbachia cells in a bacteriocyte of JESC insect (H) and a tubular γ-proteobacterial cell in addition to Wolbachia cells in a bacteriocyte of TUA insect (I). (J and K) Infection patterns of Wolbachia and γ-proteobacteria in germalia of JESC female (J) and TUA female (K); white arrowheads and arrows indicate Wolbachia infections in somatic stem cell niche and nutritive cord, respectively. (L and M) Localization of Wolbachia and γ-proteobacteria at posterior pole of developing oocytes in JESC female (L) and TUA female (M). (N) Localization of Wolbachia in primordial bacteriome (orange arrow) in developing embryo. Abbreviations: Mn, mandible; Mx, maxilla; Lb, labium; T1–3, first-third thoracic appendages. In D–G and J–N, red, green, and blue signals indicate Wolbachia, γ-proteobacteria and insect nuclei, respectively.
Here we report the discovery that Wolbachia is the bacteriocyte-associated nutritional mutualist essential for survival and reproduction of the bedbug C. lectularius, which unveils an evolutionary pathway toward obligate nutritional mutualist and highlights the parasitism-mutualism evolutionary continuum.
Results and Discussion
Characterization of Bacterial Symbionts from Bacteriome of Bedbugs.
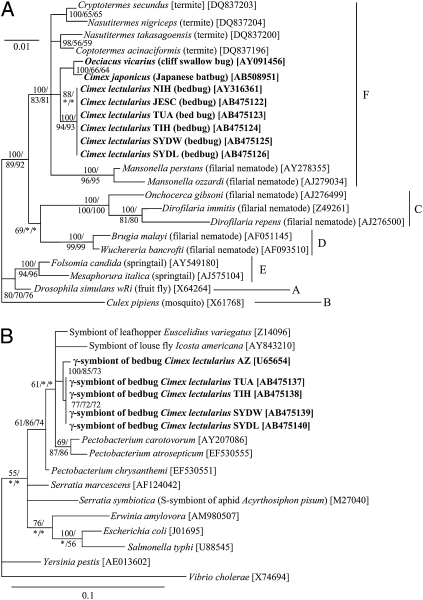
We carefully dissected bacteriomes from individual insects and conducted PCR, cloning, and sequencing of a bacterial 16S rRNA gene fragment. Two types of sequences were reproducibly obtained from two Japanese strains (TUA and TIH) and two Australian strains (SYDW and SYDL). These sequences were completely or nearly identical to the Wolbachia sequence and the γ-proteobacterial sequence that had been identified previously from US strains (15, 16). Meanwhile, in JESC, a Japanese strain, only the Wolbachia sequence was identified (Tables S1 and S2). Molecular phylogenetic analyses of the 16S rRNA gene sequences indicated that the Wolbachia sequences derived from different bedbug populations were phylogenetically coherent, as were the γ-proteobacterial sequences (Fig. 2). Molecular phylogenetic analysis of Wolbachia ftsZ gene sequences confirmed phylogenetic coherence of the Wolbachia sequences (Fig. S1). The ftsZ gene sequences obtained in this study were identical to the ftsZ gene sequence of a Wolbachia strain from C. lectularius that was placed in the F supergroup and genotyped by the multilocus strain typing system in a previous study (18). Sequences from no other bacterial types were detected from the bacteriome. Of 105 bedbugs examined, the Wolbachia sequence was identified from every insect, whereas the γ-proteobacterial sequence was detected from only 53% of them (Table S1). These results indicated that two bacterial symbionts, the Wolbachia and the γ-proteobacterium, are present in the bacteriome of C. lectularius. It seemed unlikely that the γ-proteobacterium is an essential symbiont for the bedbug because it was not present in all samples.
Fig. 2.
Phylogenetic placement of symbiotic bacteria from C. lectularius and allied bugs. (A) A 16S rRNA gene phylogeny of Wolbachia from the bedbugs (1,441 aligned nucleotide sites). (B) A 16S rRNA gene phylogeny of γ-proteobacteria from the bedbugs (1,089 sites). Bayesian (BA) phylogenies are shown. Host insect names are in italic, accession numbers in brackets, and Wolbachia supergroups A–F on the right side are shown in A. Posterior probabilities for BA analyses and bootstrap probabilities for maximum parsimony (MP) and maximum likelihood (ML) analyses greater than 50% are indicated at the nodes in the order of BA/MP/ML.
Localization of Bacterial Symbionts in Bedbugs.
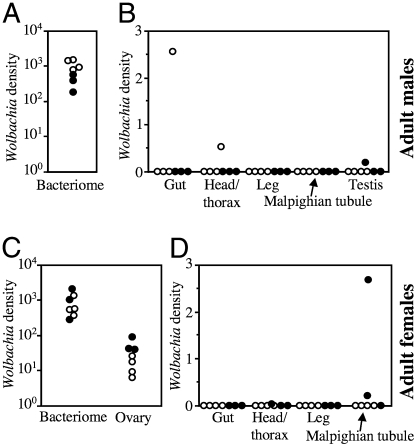
Quantitative PCR assays unveiled a striking tissue tropism of the Wolbachia symbiont in C. lectularius. The Wolbachia densities in the bacteriome were approximately 30 times higher than those in the ovary, and 2,000–900,000 times higher than in other organs (Fig. 3). These results indicated that, except for the bacterial fraction passed to the ovary, the Wolbachia infection is substantially restricted to the bacteriome. This is atypical of Wolbachia symbionts, whose infection is generally observed in multiple types of cells and tissues of their host insects (6). Fluorescent in situ hybridization and electron microscopy unequivocally demonstrated the bacteriome-specific localization of the Wolbachia symbiont: in males, the Wolbachia signals were restricted to the testis-associated bacteriomes (Fig. 1D), whereas in females, the signals were found in the bacteriomes and the ovaries (Fig. 1E). In the monosymbiotic JESC strain, the bacteriocytes were occupied solely by the rod-shaped Wolbachia cells (Fig. 1 F and H), whereas in the disymbiotic TUA strain, tubular γ-proteobacterial cells were found at low densities in addition to the Wolbachia cells (Fig. 1 G and I). The γ-proteobacterial symbiont was sporadically observed in diverse cells and tissues, particularly in Malpighian tubules and ovariole pedicels (Fig. S2).
Fig. 3.
Quantification of Wolbachia density in different organs of C. lectularius. (A and B) Infection densities in male insects; and (C and D) infection densities in female insects in terms of Wolbachia wsp gene copies per insect elongation factor 1α gene copy. Filled and open dots indicate insects of the monosymbiotic strain JESC and the disymbiotic strain TUA, respectively.
Ovarial Transmission of Bacterial Symbionts in Bedbugs.
Fluorescent in situ hybridization using Wolbachia-specific probes revealed characteristic localization patterns of Wolbachia symbiont in the ovary, which were suggestive of the route and mechanism of its vertical transmission. At the tip of the ovarioles, the Wolbachia signals were found inside specific cells in a middle region of the germalia (Fig. 1 J and K, arrowheads), where the somatic stem cell niche is presumably located. In Drosophila melanogaster, the somatic stem cell niche was demonstrated to be the entry point of Wolbachia infection to germline (19). The Wolbachia signals were also found in the nutritive cord connecting nurse cells and developing oocytes (Fig. 1 J and K, arrows), which probably indicate migrating symbiont cells with trophic flow from the germalia to the oocytes. In the oocytes, the Wolbachia signals were concentrated at a posterior pole region (Fig. 1 L and M), the location of germ plasm destined to form germline cells. Such colocalization with the germ plasm has been known from a wide variety of Wolbachia and other insect endosymbionts (11, 20), which may facilitate stable vertical transmission of the maternally inherited microbes. During embryonic development, the Wolbachia signals migrated to a specific region associated with the germ band, participating in the formation of the bacteriome (Fig. 1N). From these results, we concluded that Wolbachia, rather than the γ-proteobacterium, is the major bacteriocyte-associated symbiont in the bedbug, whose localization and infection dynamics are comparable to those of obligate mutualistic bacteriocyte symbionts like Buchnera in aphids and Wigglesworthia in tsetse flies.
Wolbachia as an Essential Symbiont for Bedbugs.
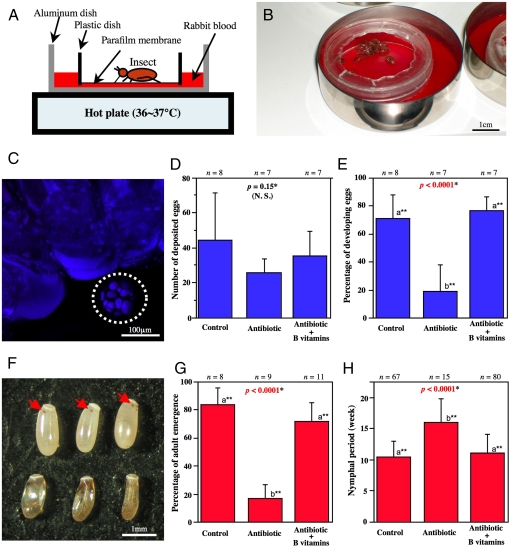
On account of the elaborate symbiotic configuration, we predicted that the Wolbachia symbiont would play a biological role for the bedbug host. By making use of an artificial feeding system (Fig. 4 A and B), we performed antibiotic treatments of the monosymbiotic JESC strain. Continuous rearing of newly-emerged adult insects on a rifampicin-supplemented blood meal resulted in complete elimination or drastic reduction (1/100∼1/10,000) of the Wolbachia infection (Fig. 4C; Fig. S3). The aposymbiotic insects normally survived and laid eggs, as did the control insects (Fig. 4D). However, the rate of normally developing eggs was much reduced compared to the controls (Fig. 4E). Usually red eyespots appeared on the control eggs within five days after oviposition, whereas the antibiotic-affected eggs were darkened and deformed (Fig. 4F). When newly-hatched nymphs were continuously reared on the antibiotic-supplemented diet, the insects suffered a significantly lower adult emergence rate (Fig. 4G) and a prolonged nymphal period (Fig. 4H). These results clearly indicated that Wolbachia is essential for normal growth and reproduction of the bedbug.
Fig. 4.
Biological role of Wolbachia for C. lectularius demonstrated by antibiotic treatment and nutritional supplementation using an artificial feeding system. (A and B) An illustration (A) and a photo (B) of the artificial feeding system for bedbug. (C) FISH of an antibiotic-treated adult insect, wherein Wolbachia signal in the bacteriome disappeared (dotted circle). (D and E) Effects of antibiotic treatment and vitamin B supplementation on adult insects: (D) number of deposited eggs; and (E) percentage of developing eggs. (F) Eggs 5 days after oviposition: (Upper) eggs laid by control insects, wherein arrows indicate red eyespots; (Lower) eggs laid by antibiotic-treated insects, which are darkened, deformed, and dead. (G and H) Effects of antibiotic treatment and vitamin B supplementation on nymphal growth and development: (G) percentage of adult emergence; and (H) nymphal period.
Wolbachia as Supplier of B Vitamins for Bedbugs.
What is the basis of the essentiality of Wolbachia for the bedbug? In other blood-sucking insects like tsetse flies, their bacterial symbionts synthesize B vitamins that are deficient in their blood meal (2). When we supplemented B vitamins to every blood meal for the experimental bedbugs, strikingly, the aposymbiotic insects were almost completely restored to normal egg development, adult emergence rate and nymphal period (Fig. 4 E, G, and H). These results strongly suggested that the bacteriocyte-associated Wolbachia symbiont is the obligate nutritional mutualist for the bedbug, whose major biological role is provisioning of B vitamins.
Wolbachia as Bacteriocyte-Associated Nutritional Mutualist for Bedbug.
In conclusion, Wolbachia has evolved bacteriocyte-specific localization and nutritional mutualism in the bedbug, similar to the ancient bacteriocyte-associated nutritional mutualists Buchnera in aphids and Wigglesworthia in tsetse flies. Wolbachia symbionts are generally regarded as parasitic, affecting the host fitness negatively and often causing cytoplasmic incompatibility or other reproductive aberrations (7). It should be noted, however, that several intermediate forms of Wolbachia that tend toward mutualism have been recognized. Some Wolbachia strains (those in which pathogen resistance, nutritional supplementation, or other mechanisms might be involved) were shown to improve the host fitness conditionally (21–26). In a parasitoid wasp, Wolbachia elimination inhibits the development of ovaries, wherein the symbiont does not necessarily benefit the host but somehow modifies the host to be dependent on the symbiont infection (27, 28). In filarial nematodes, Wolbachia symbionts are regarded as mutualistic, although the exact benefits conferred by the bacteria have been elusive (29). The discovery of Wolbachia as bacteriocyte-associated nutrient supplier in the bedbug provides an unprecedented case of Wolbachia-insect mutualism. Considering that over 60% of insect species are estimated to carry Wolbachia infections (5), we expect that the bedbug case cannot be an exception but many more Buchnera- or Wigglesworthia-like Wolbachia symbionts are to be discovered. In a book louse, a bacteriome-associated Rickettsia symbiont was identified (30).
Evolutionary Origin of Bedbug–Wolbachia Mutualism.
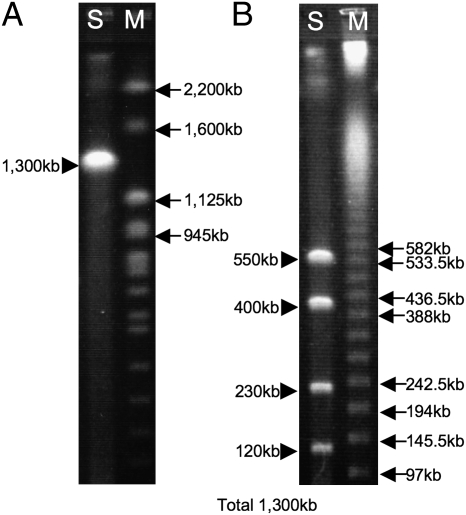
The aphid–Buchnera and tsetse–Wigglesworthia associations are of ancient evolutionary origins dating back to over 100 million years ago, leading to drastically reduced symbiont genomes (1, 2). How old is the bedbug–Wolbachia bacteriocyte-associated nutritional mutualism? Among diverse bedbugs, Wolbachia strains allied to that of C. lectularius have been characterized only from the closely-related genera Cimex and Oeciacus in the subfamily Cimicinae (Fig. 2A and Fig. S1) (16, 17). Pulsed-field gel electrophoresis of the bacteriome of C. lectularius estimated the Wolbachia genome size as 1.3 Mb (Fig. 5), which is closer to the genome sizes of parasitic Wolbachia strains of insects (1.3–1.6 Mb) rather than to those of mutualistic Wolbachia strains of filarial nematodes (1.0–1.1 Mb) (7). Hence, the Wolbachia symbiont of the bedbug may be at an early evolutionary stage of bacteriocyte-associated obligate mutualism. Genome sequencing of the Wolbachia symbiont would lead to insights into how elaborate endocellular mutualists like Buchnera in aphids and Wigglesworthia in tsetse flies have evolved from much less specialized but more prevalent microbial associates like Wolbachia. The mutualistic Wolbachia of C. lectularius belongs to the F supergroup, which also contains Wolbachia strains from termites and filarial nematodes (Fig. 2 and Fig. S1). For the termite strains, their phenotypic aspects are unknown (31), whereas the nematode strains are suspected to be mutualistic (32). No reproductive parasite strains have been known from the F supergroup. Hence it is currently elusive whether the ancestor of the bedbug strains was a reproductive parasite, a commensal, or already a mutualist. Finally, we point out the possibility that the mutualistic Wolbachia symbiont could be a target for controlling this cosmopolitan insect pest.
Fig. 5.
Genome size of Wolbachia from the bacteriome of C. lectularius estimated by pulsed field gel electrophoresis. (A) I-CeuI digestion yielded a single band of 1.3 Mb. (B) AscI digestion produced four bands, which were summed up to 1.3 Mb. Lanes S and M indicate samples and DNA size markers, respectively.
Materials and Methods
Insects.
The samples of C. lectularius used in this study are listed in Table S1. The JESC and TIH strains were maintainable using an artificial feeding system (Fig. 4 A and B). A Parafilm membrane was stretched on a Petri dish, through which the insects were allowed to feed every two weeks on rabbit blood (Kohjin Bio) warmed at 37 °C. Otherwise the insects were kept in Petri dishes with several pieces of filter paper at 25 °C under constant darkness. The TUA insects could not be maintained with the system, plausibly because of adaptation to the avian host, and thus were collected at the quail coop upon necessity. The SYDW and SYDL insects were provided as acetone-preserved specimens. The samples of Cimex japonicus were collected from a bat colony and preserved in acetone.
PCR, Cloning, and Sequencing.
Dissected tissues or whole insects were individually subjected to DNA extraction using NucleoSpin Tissue Kit (Machery-Nagel). A bacterial 16S rRNA gene (1.5 kb) and two Wolbachia genes, wsp (0.56 kb) and ftsZ (0.75 kb), were amplified by PCR as described elsewhere (33, 34). An insect gene, elongation factor 1α (ef1α; 0.54 kb), was amplified with primers efF2 (5′-GCT GGT ACT GGW GAA TTY GAA-3′) and efR1 (5′-CAC DGA CTT DAC TTC AGT GGT-3′). The PCR products were subjected to cloning, restriction fragment length polymorphism genotyping, and sequencing as described (34).
Molecular Phylogenetic Analysis.
Multiple alignments of the nucleotide sequences were generated using Clustal W (35). Phylogenetic analyses were conducted by three methods: maximum parsimony, maximum likelihood and Bayesian using PAUP 4.0b10 (36) and MrBayes v3.0b4 (37).
Diagnostic PCR.
A wsp gene fragment (0.56 kb) of the Wolbachia symbiont was amplified by PCR as described (33). A 16S rRNA gene fragment (1.0 kb) was amplified with primers CLsecF (5′-CGG TAA GGT TAA TAA CCT TGC-3′) and 16SB1 (5′-TAC GGY TAC CTT GTT ACG ACT T-3′).
Quantitative PCR.
Wolbachia wsp gene copies and insect ef1α gene copies were quantified using SYBR green and Mx3000P QPCR system (Stratagene) as described elsewhere (38) with primers CLwspF (5′-CGG CTC TTA TGG CGC TAG C-3′) and CLwspR (5′-CTT TTT GGT TGT ATC GCC AGG A-3′) for wsp and with primers CLefF (5′- GCA AAT GCC TTA TTG AAG CTC TC-3′) and CLefR (5′- GGA AGC CTA AGA GGC TTG TCA G-3′) for ef1α.
Histological Procedures.
For in situ hybridization targeting bacterial 16S rRNA, adult insects were removed of head, thorax, and dorsal or ventral plates, and the dissected abdomens were fixed with Carnoy’s solution (ethanol: chloroform: acetic acid = 6:3:1 [vol/vol]) for several days and were treated with 6% H2O2 in 80% ethanol for a week to eliminate autofluorescence of the tissues (39). The Wolbachia symbiont was detected with two oligonucleotide probes whose 5′ end was labeled with AlexaFluor555, TsWol944R (5′-AAC CGA CCC TAT CCC TTC G-3′) and TsWol1187R (5′-CTC GCG ACT TTG CAG CCC A-3′). The γ-proteobacterial symbiont was visualized with a probe whose 5′ end was labeled with Alexafluor647, CimexSec1229R (5′-TTG CTC TCG CGA GGT CGC TT-3′). The stained samples were observed under an epifluorescent microscope or a laser confocal microscope. For transmission electron microscopy, dissected bacteriomes were fixed with glutaraldehyde and osmium tetroxide, embedded in Spurr resin, processed into ultrathin sections, stained with uranyl acetate and lead citrate, and observed under an electron microscope as described (34).
Feeding Treatments.
For antibiotic treatment, rifampicin solution in methanol was added to the blood meal at a final concentration of 10 μg/mL For nutritional supplementation, aqueous solution of B vitamins was supplied to the blood meal in addition to the antibiotic at final concentrations described in Table S3.
Symbiont Effects on Adult Fecundity and Fertility.
Adult insects of the JESC strain were divided into groups consisting of three males and three females within 2 weeks of emergence. Each of the groups was randomly assigned to one of the following experimental treatments: (i) control treatment, wherein only methanol was added to every blood meal; (ii) antibiotic treatment, wherein rifampicin was added to every blood meal; and (iii) antibiotic + B vitamins treatment, wherein both rifampicin and B vitamins were added to every blood meal. In all groups, the insects were fed for 20 min every week. They were allowed to reproduce for 8 weeks, and the number of deposited eggs and hatching rate of the eggs were recorded for each of the groups.
Symbiont Effects on Nymphal Growth and Survival.
The first instar nymphs of the JESC strain were divided into groups of 10 insects within a week of hatching. Each of the groups was randomly assigned to one of the above experimental treatments. Their growth and survival were monitored until all of the insects either became adult or died.
Pulsed Field Gel Electrophoresis.
Bacteriomes dissected from 25 adult insects were homogenized in 50 μL of a PBS and embedded in 1% low melting point agarose. The agarose plug was incubated in a lysis buffer containing proteinase K at 50°C overnight. After thorough washing, the plug was treated with a restriction enzyme, cut and set on an agarose gel, and subjected to pulsed field gel electrophoresis using CHEF Mapper (BioRad).
Supplementary Material
Acknowledgments
We thank S. L. Doggett, D. Fukui, T. Ishikawa, A. Muto, and T. Yamauchi for insect samples; S. Hanada for help with electron microscopy; and M. T. Siva-Jothy and J. L. Rasgon for comments on the manuscript. This work was supported by a grant from the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN).
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the DNA Data Base in Japan database (accession nos. AB475122-AB475126, AB475132-AB475142, AB508951, and AB508953).
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911476107/DCSupplemental.
References
- 1.Shigenobu S, Watanabe H, Hattori M, Sakaki Y, Ishikawa H. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature. 2000;407:81–86. doi: 10.1038/35024074. [DOI] [PubMed] [Google Scholar]
- 2.Akman L, et al. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat Genet. 2002;32:402–407. doi: 10.1038/ng986. [DOI] [PubMed] [Google Scholar]
- 3.Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet. 2008;42:165–190. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- 4.Moya A, Peretó J, Gil R, Latorre A. Learning how to live together: Genomic insights into prokaryote-animal symbioses. Nat Rev Genet. 2008;9:218–229. doi: 10.1038/nrg2319. [DOI] [PubMed] [Google Scholar]
- 5.Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. How many species are infected with Wolbachia?—A statistical analysis of current data. FEMS Microbiol Lett. 2008;281:215–220. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobson SL, et al. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem Mol Biol. 1999;29:153–160. doi: 10.1016/s0965-1748(98)00119-2. [DOI] [PubMed] [Google Scholar]
- 7.Werren JH, Baldo L, Clark ME. Wolbachia: Master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 8.Reinhardt K, Siva-Jothy MT. Biology of the bed bugs (Cimicidae) Annu Rev Entomol. 2007;52:351–374. doi: 10.1146/annurev.ento.52.040306.133913. [DOI] [PubMed] [Google Scholar]
- 9.Arkwright JA, Atkin EE, Bacot A. An hereditary Rickettsia-like parasite of the bed bug (Cimex lectularius) Parasitology. 1921;13:27–36. [Google Scholar]
- 10.Davis NT. The morphology and functional anatomy of the male and female reproductive systems of Cimex lectularius L. (Heteroptera, Cimicidae) Ann Entomol Soc Am. 1956;49:466–493. [Google Scholar]
- 11.Buchner P. Endosymbiosis of Animals with Plant Microorganisms. New York: Interscience; 1965. p. 909. [Google Scholar]
- 12.Chang KP, Musgrave AJ. Morphology, histochemistry, and ultrastructure of mycetome and its rickettsial symbiotes in Cimex lectularius L. Can J Microbiol. 1973;19:1075–1081. doi: 10.1139/m73-171. [DOI] [PubMed] [Google Scholar]
- 13.De Meillon B, Golberg L. Preliminary studies on the nutritional requirements of the bedbug (Cimex lectularius L.) and the tick Ornithodorus moubata Murray. J Exp Biol. 1946;24:41–63. doi: 10.1242/jeb.24.1-2.41. [DOI] [PubMed] [Google Scholar]
- 14.Chang KP. Effects of elevated temperature on the mycetome and symbiotes of the bed bug Cimex lectularius (Heteroptera) J Invertebr Pathol. 1974;23:333–340. doi: 10.1016/0022-2011(74)90098-6. [DOI] [PubMed] [Google Scholar]
- 15.Hypsa V, Aksoy S. Phylogenetic characterization of two transovarially transmitted endosymbionts of the bedbug Cimex lectularius (Heteroptera:Cimicidae) Insect Mol Biol. 1997;6:301–304. doi: 10.1046/j.1365-2583.1997.00178.x. [DOI] [PubMed] [Google Scholar]
- 16.Rasgon JL, Scott TW. Phylogenetic characterization of Wolbachia symbionts infecting Cimex lectularius L. and Oeciacus vicarius Horvath (Hemiptera: Cimicidae) J Med Entomol. 2004;41:1175–1178. doi: 10.1603/0022-2585-41.6.1175. [DOI] [PubMed] [Google Scholar]
- 17.Sakamoto JM, Rasgon JL. Endosymbiotic bacteria of bed bugs: Evolution, ecology and genetics. Am Entomol. 2006;52:119–122. [Google Scholar]
- 18.Baldo L, et al. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl Environ Microbiol. 2006;72:7098–7110. doi: 10.1128/AEM.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frydman HM, Li JM, Robson DN, Wieschaus E. Somatic stem cell niche tropism in Wolbachia. Nature. 2006;441:509–512. doi: 10.1038/nature04756. [DOI] [PubMed] [Google Scholar]
- 20.Veneti Z, Clark ME, Karr TL, Savakis C, Bourtzis K. Heads or tails: Host-parasite interactions in the Drosophila-Wolbachia system. Appl Environ Microbiol. 2004;70:5366–5372. doi: 10.1128/AEM.70.9.5366-5372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vavre F, Girin C, Boulétreau M. Phylogenetic status of a fecundity-enhancing Wolbachia that does not induce thelytoky in Trichogramma. Insect Mol Biol. 1999;8:67–72. doi: 10.1046/j.1365-2583.1999.810067.x. [DOI] [PubMed] [Google Scholar]
- 22.Dobson SL, Marsland EJ, Rattanadechakul W. Mutualistic Wolbachia infection in Aedes albopictus: accelerating cytoplasmic drive. Genetics. 2002;160:1087–1094. doi: 10.1093/genetics/160.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weeks AR, Turelli M, Harcombe WR, Reynolds KT, Hoffmann AA. From parasite to mutualist: Rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol. 2007;5:e114. doi: 10.1371/journal.pbio.0050114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedges LM, Brownlie JC, O’Neill SL, Johnson KN. Wolbachia and virus protection in insects. Science. 2008;322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- 25.Teixeira L, Ferreira A, Ashburner M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008;6:e2. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brownlie JC, et al. Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog. 2009;5:e1000368. doi: 10.1371/journal.ppat.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dedeine F, et al. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc Natl Acad Sci USA. 2001;98:6247–6252. doi: 10.1073/pnas.101304298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pannebakker BA, Loppin B, Elemans CP, Humblot L, Vavre F. Parasitic inhibition of cell death facilitates symbiosis. Proc Natl Acad Sci USA. 2007;104:213–215. doi: 10.1073/pnas.0607845104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foster J, et al. The Wolbachia genome of Brugia malayi: Endosymbiont evolution within a human pathogenic nematode. PLoS Biol. 2005;3:e121. doi: 10.1371/journal.pbio.0030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perotti MA, Clarke HK, Turner BD, Braig HR. Rickettsia as obligate and mycetomic bacteria. FASEB J. 2006;20:2372–2374. doi: 10.1096/fj.06-5870fje. [DOI] [PubMed] [Google Scholar]
- 31.Lo N, Evans TA. Phylogenetic diversity of the intracellular symbiont Wolbachia in termites. Mol Phylogenet Evol. 2007;44:461–466. doi: 10.1016/j.ympev.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 32.Keiser PB, et al. Molecular identification of Wolbachia from the filarial nematode Mansonella perstans. Mol Biochem Parasitol. 2008;160:123–128. doi: 10.1016/j.molbiopara.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kikuchi Y, Fukatsu T. Diversity of Wolbachia endosymbionts in heteropteran bugs. Appl Environ Microbiol. 2003;69:6082–6090. doi: 10.1128/AEM.69.10.6082-6090.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kikuchi Y, Meng XY, Fukatsu T. Gut symbiotic bacteria of the genus Burkholderia in the broad-headed bugs Riptortus clavatus and Leptocorisa chinensis (Heteroptera: Alydidae) Appl Environ Microbiol. 2005;71:4035–4043. doi: 10.1128/AEM.71.7.4035-4043.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swofford DL. PAUP* Version 4.0b10 [computer program]. In 4.0b10 edition. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- 37.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 38.Narita S, Kageyama D, Nomura M, Fukatsu T. Unexpected mechanism of symbiont-induced reversal of insect sex: feminizing Wolbachia continuously acts on the butterfly Eurema hecabe during larval development. Appl Environ Microbiol. 2007;73:4332–4341. doi: 10.1128/AEM.00145-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koga R, Tsuchida T, Fukatsu T. Quenching autofluorescence of insect tissues for in situ detection of endosymbionts. Appl Entomol Zool (Jpn) 2009;44:281–291. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.