Abstract
The emergence and global impact of the novel influenza A(H1N1)v highlights the continuous threat to public health posed by a steady stream of new and unexpected infectious disease outbreaks in animals and humans. Once an emerging epidemic is detected, public health authorities will attempt to mitigate the epidemic by, among other measures, reducing further spread as much as possible. Scarce and/or costly control measures such as vaccines, anti-infective drugs, and social distancing must be allocated while epidemiological characteristics of the disease remain uncertain. Here we present first principles for allocating scarce resources with limited data. We show that under a broad class of assumptions, the simple rule of targeting intervention measures at the group with the highest risk of infection per individual will achieve the largest reduction in the transmission potential of a novel infection. For vaccination of susceptible persons, the appropriate risk measure is force of infection; for social distancing, the appropriate risk measure is incidence of infection. Unlike existing methods that rely on detailed knowledge of group-specific transmission rates, the method described here can be implemented using only data that are readily available during an epidemic, and allows ready adaptation as the epidemic progresses. The need to observe risk of infection helps to focus the ongoing planning and design of new infectious disease surveillance programs; from the presented first principles for allocating scarce resources, we can adjust the prioritization of groups for intervention when new observations on an emerging epidemic become available.
Keywords: human influenza, pandemic, immunization, school closure, mathematical model
The need to plan for countering new emerging diseases is highlighted by the worldwide rise of HIV infections since its discovery in 1981 (1, 2), by the spread of the foot-and-mouth epidemic in the United Kingdom in 2002 (3), by the global impact of several outbreaks of severe acute respiratory syndrome (SARS) in 2003 (4), by the projected impact of a possible new influenza pandemic (5–7), and recently by the actual spread of a novel influenza A(H1N1)v pandemic (8, 9). Available control measures for new infections will be scarce because of supply (e.g., vaccines, drugs, masks) and logistical (e.g., distribution) constraints, and their use will be costly. As a consequence, public health bodies face the questions of how to deploy limited control measures to minimize transmission, and of which groups in the population should be targeted for infectious disease control (10, 11). The general problem is how to choose groups of the population that should receive priority in getting the intervention. Typically groups are defined by age, but for some diseases sex, occupation, or other demographic characteristics are more salient. Hereafter we refer to age groups, for brevity.
Existing approaches to allocating infection control rely either on detailed knowledge of transmission parameters (12–15) that may remain ambiguous, as standard methods for estimating transmission rates among groups suffer from indeterminacy (16–18), or on predictions for the eventual number of infections that occur in each group during the entire epidemic (16, 19–21), which are unlikely to be available at the start of an emerging epidemic. Here we provide a robust solution to the allocation problem that applies to a general class of infectious diseases for which the objective is to minimize transmission and at-risk contacts are reciprocal, that is, for which spread of infection requires the proximity of two individuals (17). We show that there exist simple principles to find optimal allocation schemes for scarce control measures that require observation of only a few key risk measures of infection that can be observed in the initial phase of an emerging epidemic.
Results
Vaccination.
Suppose that we wish to target vaccination of susceptible individuals to minimize transmission of an infection. A measure of the transmission potential is the reproduction number R, defined as the number of secondary infections caused by a typical primary case (22–25); this monotonically affects the rate of increase in the number of cases (26). We find that the marginal benefit of allocating a dose of vaccine to a given age group i is approximately proportional to the product of the incidence rate per person, denoted by xi/ni, and the force of infection, denoted by xi/si, where force of infection is defined as incidence rate per susceptible person (22). If contact reciprocity holds and all else is equal, this implies that the greatest reduction in transmission of the infection population-wide can be achieved by vaccinating a person in the group with the highest product of incidence and force of infection. More generally, this reduction depends on the efficacy of the vaccine in each group qi, the per contact probability of becoming infected for each group ai, and the per contact infectiousness of each group ci:
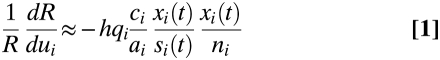 |
[relative change in transmission]≈[constant] [vaccine efficacy] [per contact probability of transmitting infection/ per contact probability of becoming infected] [force of infection] [incidence of infection].
The relative change in transmission depends on the product of two measures for risk infection, which implies that small differences between groups in risk of infection could hint at substantial benefits for targeting specific groups.
Intuitively, the change in transmission by vaccinating one susceptible individual has two components: first, the risk of infection of this individual; and second, the number of resulting infections that this individual will cause once infected. The first component, an individual's risk of being infected, is given by the incidence of infection (xi/ni). The second component, an infected individual's expected number of future infections, is proportional to force of infection (xi/si) when contacts are reciprocal (Fig. 1). Taken together, these two components imply that the change in transmission is proportional to the product of incidence and force of infection. We have tested this approximate equation in simulation experiments and have found that it provided accurate predictions (SI). The largest reduction in transmission from vaccinating a small fraction of the population is achieved by targeting vaccination at the group in which individuals experience the highest risk of infection.
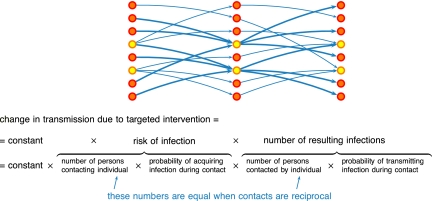
Fig. 1.
A schematic representation of transmission of infection in a population that consists of different groups. Individuals are depicted as nodes; potentially infectious contacts from one individual to another are depicted as arrows; and group membership is indicated by node color. Individuals with a yellow node have a larger number of incoming contacts and therefore have a higher risk of infection. Due to reciprocity of contacts, individuals with a yellow node also have a larger number of outgoing contacts and therefore a higher number of infections that they would cause if they become infected. Therefore targeting interventions at individuals in the group with a higher risk of infection (yellow nodes rather than orange nodes) would result in a larger reduction in transmission potential. The sensitivity to targeted intervention of the transmission potential is determined by two components: the risk of infection of an individual, and the number of infections that would result from this individual if infected. When contacts are reciprocal, the number of resulting infections is proportional to the risk of infection.
Allocation of Vaccines.
This result for the marginal benefit from one dose of vaccine can be exploited to devise allocation schemes for a vaccine stockpile that is large enough to vaccinate a substantial proportion of the population. One promising allocation scheme that we have identified is “importance leveling,” which maintains for doses after the first dose the principle of allocating vaccines to the group with the highest product of incidence and force of infection, after accounting for the reduced risk of infection in a group due to vaccination, but is approximate in that it considers only the first-order effects of reduced risk of infection due to use of prior doses of vaccine. Specifically, the scheme assigns each group an initial score for its importance to transmission; the initial score of group i is equal to the square root of relative change in transmission after one individual in group i is vaccinated: 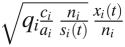 . Vaccinating one individual in group i lowers the importance of this group after vaccination by a factor approximately qi/ni if the individual makes contacts mostly within group i. The scheme distributes vaccine such that the expected importance of all groups is leveled down to a constant value after allocation of the entire vaccine stockpile. This principle defines a unique allocation of a stockpile of a given size (SI Text). In simulated epidemics, we find that for small stockpiles it reduces the reproductive number as well as any allocation that could be found by an optimization algorithm, and for larger stockpile sizes (50% of the population) it is nearly optimal (Fig. 2). Remarkably, the importance leveling scheme provides near-optimal allocation of vaccines using only observations of group-specific risk of infection early in an emerging epidemic.
. Vaccinating one individual in group i lowers the importance of this group after vaccination by a factor approximately qi/ni if the individual makes contacts mostly within group i. The scheme distributes vaccine such that the expected importance of all groups is leveled down to a constant value after allocation of the entire vaccine stockpile. This principle defines a unique allocation of a stockpile of a given size (SI Text). In simulated epidemics, we find that for small stockpiles it reduces the reproductive number as well as any allocation that could be found by an optimization algorithm, and for larger stockpile sizes (50% of the population) it is nearly optimal (Fig. 2). Remarkably, the importance leveling scheme provides near-optimal allocation of vaccines using only observations of group-specific risk of infection early in an emerging epidemic.
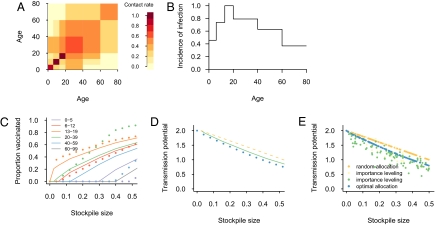
Fig. 2.
A test of the importance-leveling scheme against simulated data. (A) The 21 contact parameters required to describe reciprocal contacts among six age groups, based on self-reported number of social contacts (17). (B) Incidence of infection during the initial phase of the epidemic, as simulated from the contact parameters in panel A. (C) Allocation of a perfect vaccine over six age groups for different stockpile sizes, according to the importance leveling scheme (lines) that uses only information about the incidence of infection as in B; for comparison, we show the optimal allocation that requires knowing the entire contact matrix (dots). (D) Reduction in transmission potential by importance leveling (green line) is indistinguishable from maximal reduction by optimal allocation (blue dots) for stockpile sizes up to 20% of the total population. Both are considerably better than random allocation (orange line). (E) Sensitivity analysis of reduction in transmission potential to age-specific variation in per contact probability of acquiring infection, ai, and per contact probability of transmitting infection, ci. Importance leveling was applied while ignoring the variation in ai and ci (yellow) and while accounting for this variation (green) (Methods). Transmission parameters are scaled such that the largest value equals 1 in A; incidence of infection is scaled such that the largest value equals 1 in B. Size of stockpile is expressed relative to total population size; transmission potential is scaled such that it equals 2.0 when stockpile size is zero in C, D, and E.
School and Workplace Closure.
The approach to calculating the marginal benefit of targeting specific groups can be modified to consider social distancing measures, such as closing schools and workplaces (5–7, 27). Here we define such a measure as one that reduces the extent of contact between individuals within group i at which it is aimed, such as school children or working-age adults. In this case, the marginal effect of reducing contacts within group i is approximately proportional to the squared incidence of infection within group i:
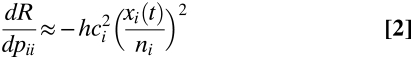 |
[change in transmission]≈[constant][per contact probability of transmitting infection]2[incidence]2
The intuition behind this equation is similar to that of change in transmission due to targeted vaccination, with one minor change. We target links between individuals who transfer infection with a probability ci ai si (t)/ni rather than individuals who respond with probability qi. The largest reduction in the expected contribution to future infections is obtained if social distancing measures are targeted to prevent contacts within the group with the highest risk of infection per contact ci (if this differs between groups) and the highest incidence of infection.
Robustness.
If information is available (for example from contact tracing studies) on the group-specific risk of becoming infected, ai, or infecting others, ci, it can be incorporated directly into Eqs. 1 and 2. If this information is not available and we observe group-specific differences in risk of infection, we may incorrectly attribute these differences to variation in contact patterns between groups, possibly introducing error into the estimates of who should receive priority for interventions. Figure 2E shows a sensitivity analysis in which the importance-leveling scheme is applied under a model with a 100 different parameter sets for the ai and ci, in which—unknown to the vaccine allocator—some of the intergroup variation in risk is due to up to 4-fold variation in these parameters, rather than variation in contacts. Even in this situation, allocation by the importance leveling scheme performs considerably better than random allocation and comparably to the optimal allocation if the group-specific variation in ai and ci were known. Additional sensitivity analyses show that the importance leveling scheme performs nearly identically to optimal allocation for a range of vaccination coverages, reproduction numbers, for different contact patterns, and with uncertain estimates of risk of infection (Methods and SI Text).
Applications to Pandemic Influenza.
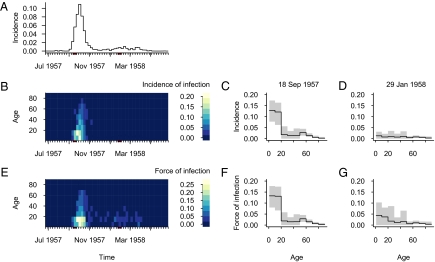
Practical application of this approach depends on the existence of large and detectable differences between groups in risk of infection in a real emerging epidemic. Reconstruction of the time course of the 1957–1958 influenza A(H2N2) pandemic shows (Fig. 3) that both incidence and force of infection were significantly greater in persons less than 20 years of age 2 weeks before the first pandemic peak (the week of 18 September 1957), indicating that interventions in this group would have been most effective early on. After the first peak, the group-specific risks of infection become more similar. Two weeks before the second peak (the week of 29 January 1958), the age groups revealed no measurable differences in incidence, and differences in force of infection were modest and not statistically significant, suggesting that at least for social distancing and possibly for vaccination, there would have been no benefit in targeting the 0- to 19-year-old group during the second pandemic wave. Two results emerge: first, the current best target group can be identified through observing differences between group-specific risk of infection during emerging epidemics; and second, the best target group for intervention may change during the course of an epidemic.
Fig. 3.
Incidence and force of infection during the “Asian” 1957–1958 influenza A(H2N2) pandemic in the Netherlands, as reconstructed from age-specific records of mortality and from serological cross-sectional surveys conducted in June 1957 and June 1958. (A) Time course of overall weekly incidence of infection. (B) Time course of weekly incidence of infection by age group. (C) Age-specific incidence of infection in week of 18 September 1957, 2 weeks before peak of first pandemic wave, and (D) in week of 29 January 1958, 2 weeks before peak of second pandemic wave. (E) Time course of weekly force of infection by age groups. (F) Age-specific force of infection 2 weeks before peak of first pandemic wave, and (G) age-specific force of infection 2 weeks before peak of second pandemic wave. Gray areas in C, D, F, and G indicate 95% bootstrapped confidence intervals (Methods).
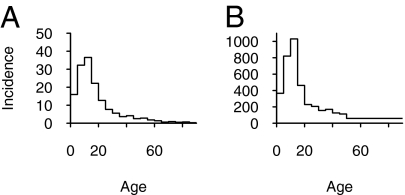
The principles for allocating scarce control measures are immediately applicable in the influenza A(H1N1)v pandemic. Age-specific incidence has been reported for several countries, including the United States and Chile. In these two countries, the highest incidence during the initial phase occurred among the 0- to 19-year-olds, higher by a factor of at least 4 compared with that in 30- to 39-year-olds (Fig. 4) (34). From these observations we would conclude, assuming no difference between groups in per contact probability of transmitting infection, that the highest-priority age groups for social distancing in the initial phase would have been the 0- to 19-year-olds and that differences between groups in expected reduction of transmission after targeting social distancing can be greater than 16-fold.
Fig. 4.
Incidence by age during first phase of outbreak of A(H1N1)v, as cases per million. (A) Incidence in United States up to 13 May 2009. A total of 3,369 confirmed and probable cases have been reported. Incidence was calculated using 3,097 case patients for which age was reported or could be calculated using date of birth and who did not report a recent history of travel from Mexico. (B) Incidence in Chile up to 21 June 2009. A total of 5,186 confirmed cases have been reported. Incidence was calculated using 5,085 confirmed cases for which age was known (34).
The observed age-specific incidence for influenza A(H1N1)v also suggests that in the initial phase the highest priority age groups for vaccination would have been the 0- to 19-year-olds, assuming no difference between groups in proportion of those susceptible to infection. A striking feature of the age-specific incidence is the relative sparing of older age groups. Older age groups are spared because they have lower levels of social contact with infectious age groups, or because of preexisting immunity to symptomatic infection. To refine estimates of the benefits of group-specific vaccination, which depend on the proportion of susceptible individuals in each group, one would need estimates of the proportion of individuals who are immune. This could be provided by serological surveys of the population before vaccination, or by keeping track of the number of infections per group. If a serological survey finds that there is indeed preexisting immunity among older age groups, this leads to a counterintuitive consequence that the score for importance for prioritization of these older age groups would increase: for a given number of cases, a higher level of immunity implies a higher value for force of infection, which in turn implies a larger expected reduction in transmission if the older groups are targeted for vaccination.
Discussion
Ongoing comparisons of incidence by age are an appropriate way to quantify how target groups change as the epidemic progresses and more persons become immune. For the influenza A(H1N1)v pandemic, interventions targeted at school-aged children, such as school closures, should be most effective in the early stages; but, as in 1957, such interventions may be considerably less effective as the epidemic progresses. In particular, if the incidence becomes comparable among children and adults, such measures will be significantly less valuable than they would have been at the start of the pandemic.
Our results suggest that the key risk factors for infection should be used to define a population structure that is relevant for monitoring incidence of infection and for optimal targeting interventions. For newly emerging infections, this is an important result: elucidating the risk factors for infection helps in deciding whether we should partition the population by age, sex, occupation, or other demographic characteristics before we set out to monitor risk of infection per group and identify the best target group for infection control.
The principles for identifying target groups apply immediately when the objective of control is to reduce transmission. Simulations suggest that allocation strategies that reduce transmission also tend to do better than random allocation in minimizing the peak incidence and minimizing the total number of infections (Fig. S1). Therefore, these principles may also apply to a wider range of objectives, such as the reduction of the total number of infections in the population or the reduction of the total number of severe cases. For this wider range of objectives, there is evidence that small supplies of vaccines should target high-risk groups, whereas larger supplies should target groups who are major transmitters (13–15, 19, 21, 28). The contribution of this paper is to define how to identify the major transmitters, given limited data. For the objectives other than reducing transmission, the question remains open at what level of supplies of vaccine there is a switch from targeting high-risk groups to targeting major transmitters.
Before targeting interventions at the groups with the highest risk of infection, the underlying assumptions and conditions of this simple rule should be checked and verified. In absence of data on incidence of infection by location and household size, we have assumed here that transmission occurs according to mass-action–type dynamics, thus ignoring the subtlety of network interactions, such as the intense and repeated nature of contacts within households. The analysis assumes that at-risk contacts for transmission are reciprocal. Reciprocal contacts arise, for example, whenever spatial and temporal proximity of two individuals is required for transmission to occur. This condition is not strongly violated by a broad class of diseases such as influenza. The analysis requires furthermore that the distribution of risk of infection over groups should not change much before or during the observation interval, and the number of infections should be large enough to obtain reliable estimates of risk of infection. Our stochastic simulations have shown that there is only a small risk that stochastic variation in the epidemic will mislead the analyst. A more salient problem is bias in group-specific incidence, when detection may be heavily biased toward severe cases (29). It is important for users of our approach to base estimates of incidence and force of infection on the best possible proxy for infection, and to consider biases introduced by the surveillance system.
In conclusion, we have described a robust strategy to target infectious disease interventions in the face of uncertainty about the precise epidemiological characteristics of a newly emerging infection. If all else is equal, priority for vaccination goes to groups with the highest product of incidence and force of infection, and priority for social distancing goes to groups with the highest incidence of infection. Real-time monitoring of group-specific risk differences during an emerging epidemic will be crucial to determine the optimal targets of an intervention with scarce resources.
Methods
The 1957–1958 “Asian” Influenza A(H2N2) Epidemic in The Netherlands.
We obtained data on mortality with influenza as a primary cause in the Netherlands from 26 June 1957 to 25 June 1958 from Statistics Netherlands. The number of deaths was reported weekly and stratified by 10 year age cohorts (1–9 years, 10–19 years, 20–29 years, …, 70–79 years, 80 years and older). The number of persons in 1957 in these age groups was also obtained from Statistics Netherlands. The age-specific proportions of susceptible individuals were estimated from a cross-sectional serological study conducted in June 1957, before the first pandemic wave arrived (30), and from a follow-up study conducted in June 1958, after the second pandemic wave had passed (31). The age-specific risk of death upon infection is obtained by dividing the number of deaths due to influenza by the number of influenza infections. As the time lag between moment of death and moment of infection was short for all age groups (32), we obtain the number of infections for any week in each age group by dividing the number of deaths in that week by the risk of death upon infection, and we obtain the number of susceptible individuals for any week by subtracting the cumulative number of infections until that week from the initial number of susceptible individuals as observed in June 1957. The weekly incidence is calculated as the number of infections divided by population size, and the weekly force of infection is calculated as the number of infections divided by the number of susceptible individuals. To assess the uncertainty in the outcome, we constructed 1000 bootstrapped data sets for the serological surveys, and repeated the calculations for each data set to obtain 95% bootstrap intervals.
Outline of Derivation of Eqs. 1 and 2.
To specify the precise relationship between the expected change in the reproduction number R and the magnitude of a targeted intervention, we study the transmission of an infection in a host population that is stratified into m groups (key to notation in Table S1). The transmission of infection from group j to group i is quantified by kij, the mean number of individuals in group i that are infected by a single individual in group j during its entire infectious period. The matrix with elements kij is the reproduction matrix K. A targeted intervention, such as vaccination or social distancing, will change the values of one or more elements of this reproduction matrix K; this change in the reproduction matrix is denoted by dK. The reproduction number R is defined as the top eigenvalue of the reproduction matrix K, and it gives the number of secondary infections produced by a “typical” infective in the stratified population (23). In general, it is not possible to compute the change in the top eigenvalue dR from a change in the reproduction matrix dK when the precise values of all matrix elements kij are unknown. However, for a broad class of infectious diseases, the reproduction matrix K has a special structure that does allow for an explicit derivation of the change in the top eigenvalue dR, even when the values of kij are unknown. The derivation proceeds in four steps. First, when contacts are reciprocal, the reproduction matrix K can be factorized into a product of symmetric matrices. Specifically, we can write kij = si ai bij cj. From right to left, cj reflects the per contact probability of transmitting infection; bij = bji reflects the contact parameter (defined here as the proportion of individuals in group i contacted by an individual in group j during its entire infectious period); ai reflects the per contact probability of becoming infected; si reflects the number of susceptible individuals. Second, because of this structure, the normalized top left eigenvector v of the reproduction matrix is directly related to the normalized top right eigenvector w of the reproduction matrix. Specifically, the ith element of the top left eigenvector v can be related to the ith element of the top right eigenvector w as vi ∼ ci wi /si ai. Third, the normalized top right eigenvector can be related to the group-specific number of new infections x(t): the ith element of the top right eigenvector w is proportional to the group-specific number of new infections in group i as observed around time t: wi ∼ xi(t). Fourth, the change in top eigenvalue dR is given by the matrix product of the normalized top left eigenvector v, the change in the reproduction matrix dK, and the normalized top right eigenvector w (33). By multiplying the left eigenvector, the change in the reproduction matrix (expressed in terms of either R qi dui / ni when vaccination is targeted at group i, or ai ci si dpii / ni2 when social distancing is targeted at group i), and the right eigenvector we obtain the Eqs. 1 and 2 for the change in reproduction number R (full derivation and conditions in SI Text).
Sensitivity Analysis.
We explored the sensitivity of reduction in transmission potential R to 4-fold variation in per contact probability of acquiring infection, a, and per contact probability of transmitting infection, c. 100 parameter sets were generated using a contact matrix as shown in Fig. 2A and Table S2. For each parameter set, the per contact probabilities of acquiring infection, ai, and the per contact probabilities of transmitting infection, ci, were drawn independently for each group from a uniform distribution on the interval 0.25–1. In each parameter set, a fixed quantity of vaccine was assumed to be available, randomly chosen between 0 and 0.5 of the population size. The results are shown in Fig. 2E. Initial incidences of infection were simulated, and the importance leveling algorithm was used to calculate the allocation of the vaccines over the age groups, as if we were ignorant of the existing variation in ai and ci (Fig. 2E, yellow dots). Subsequently, the importance leveling algorithm was applied again with knowledge of the age-specific variation in ai and ci (Fig. 2E, green dots). The difference in the reduction of transmission potential between both approaches is very small. For comparison, the outcome is shown for random allocation (Fig. 2E, orange dots) and the optimal allocation that minimizes the transmission potential, using simulated annealing and taking advantage of the full information on the entire transmission matrix and parameter set (Fig. 2E, blue dots). These results show that the performance of the importance leveling scheme is robust to ignoring existing variation in the per contact probability of acquiring infection and the per contact probability of transmitting infection.
In addition, we compared the performance of the importance leveling scheme against random allocation and optimal allocation in a simulation study for a range of vaccine coverages, for different values of reproduction numbers, and for different contact patterns while explicitly allowing for a delay between observing risk of infection and implementing control measures (Fig. S1). We also addressed the uncertainty about group-specific risk of infection (Fig. S2).
Supplementary Material
Acknowledgments
We thank L. Finelli, C. Reed, and colleagues at the US CDC for sharing age distributions of A(H1N1)v confirmed and probable cases, L. van Asten for assistance in acquiring the 1957–1958 influenza-related mortality data, and T. Britton and E. Goldstein for discussion. This work was supported by the US National Institutes of Health cooperative agreement 1U54GM088558 and 5U01GM076497 “Models of Infectious Disease Agent Study.”
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908491107/DCSupplemental.
References
- 1.Woolhouse ME, Haydon DT, Antia R. Emerging pathogens: The epidemiology and evolution of species jumps. Trends Ecol Evol. 2005;20:238–244. doi: 10.1016/j.tree.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones KE, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tildesley MJ, et al. Optimal reactive vaccination strategies for a foot-and-mouth outbreak in the UK. Nature. 2006;440:83–86. doi: 10.1038/nature04324. [DOI] [PubMed] [Google Scholar]
- 4.Donnelly CA, et al. Epidemiological and genetic analysis of severe acute respiratory syndrome. Lancet Infect Dis. 2004;4:672–683. doi: 10.1016/S1473-3099(04)01173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson NM, et al. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–452. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Germann TC, Kadau K, Longini IM, Jr, Macken CA. Mitigation strategies for pandemic influenza in the United States. Proc Natl Acad Sci USA. 2006;103:5935–5940. doi: 10.1073/pnas.0601266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatchett RJ, Mecher CE, Lipsitch M. Public health interventions and epidemic intensity during the 1918 influenza pandemic. Proc Natl Acad Sci USA. 2007;104:7582–7587. doi: 10.1073/pnas.0610941104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Swine influenza A (H1N1) infection in two children—Southern California, March–April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:400–402. [PubMed] [Google Scholar]
- 9.Fraser C, et al. WHO Rapid Pandemic Assessment Collaboration. Pandemic potential of a strain of influenza A (H1N1): Early findings. Science. 2009;324:1557–1561. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uscher-Pines L, Omer SB, Barnett DJ, Burke TA, Balicer RD. Priority setting for pandemic influenza: An analysis of national preparedness plans. PLoS Med. 2006;3:e436. doi: 10.1371/journal.pmed.0030436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. WHO Pandemic influenza preparedness and response: A WHO guidance document ( http://www.who.int/csr/disease/influenza/pipguidance2009/en/index.html)(11 June 2009) [PubMed]
- 12.Cairns AJ. Epidemics in heterogeneous populations: Aspects of optimal vaccination policies. IMA J Math Appl Med Biol. 1989;6:137–159. doi: 10.1093/imammb/6.3.137. [DOI] [PubMed] [Google Scholar]
- 13.Dushoff J, et al. Vaccinating to protect a vulnerable subpopulation. PLoS Med. 2007;4:e174. doi: 10.1371/journal.pmed.0040174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medlock J, Galvani AP. Optimizing influenza vaccine distribution. Science. 2009;325:1705–1708. doi: 10.1126/science.1175570. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein E, et al. Distribution of vaccine/antivirals and the ‘least spread line’ in a stratified population. J R Soc Interface; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Britton T. Epidemics in heterogeneous communities: Estimation of R0 and secure vaccination coverage. J R Stat Soc B. 2001;63:705–715. [Google Scholar]
- 17.Wallinga J, Teunis P, Kretzschmar M. Using data on social contacts to estimate age-specific transmission parameters for respiratory-spread infectious agents. Am J Epidemiol. 2006;164:936–944. doi: 10.1093/aje/kwj317. [DOI] [PubMed] [Google Scholar]
- 18.Mossong J, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5:e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longini IM, Jr, Ackerman E, Elveback LR. An optimization model for influenza A epidemics. Math Biosci. 1978;38:141–157. [Google Scholar]
- 20.Ball F, Britton T, Lyne O. Stochastic multitype epidemics in a community of households: Estimation and form of optimal vaccination schemes. Math Biosci. 2004;191:19–40. doi: 10.1016/j.mbs.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Patel R, Longini IM, Jr, Halloran ME. Finding optimal vaccination strategies for pandemic influenza using genetic algorithms. J Theor Biol. 2005;234:201–212. doi: 10.1016/j.jtbi.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 22.Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford: Oxford University Press; 1991. [Google Scholar]
- 23.Diekmann O, Heesterbeek JAP. Mathematical Epidemiology of Infectious Diseases: Model Building, Analysis and Interpretation. Chichester: Wiley; 2000. [Google Scholar]
- 24.Keeling MJ, Rohani P. Modeling Infectious Diseases in Humans and Animals. Princeton: Princeton University Press; 2008. [Google Scholar]
- 25.Grassly NC, Fraser C. Mathematical models of infectious disease transmission. Nat Rev Microbiol. 2008;6:477–487. doi: 10.1038/nrmicro1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallinga J, Lipsitch M. How generation intervals shape the relationship between growth rates and reproductive numbers. Proc Biol Sci. 2007;274:599–604. doi: 10.1098/rspb.2006.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cauchemez S, Valleron AJ, Boëlle PY, Flahault A, Ferguson NM. Estimating the impact of school closure on influenza transmission from Sentinel data. Nature. 2008;452:750–754. doi: 10.1038/nature06732. [DOI] [PubMed] [Google Scholar]
- 28.Bansal S, Pourbohloul B, Meyers LA. A comparative analysis of influenza vaccination programs. PLoS Med. 2006;3:e387. doi: 10.1371/journal.pmed.0030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipsitch M, Hayden FG, Cowling BJ, Leung GM. How to maintain surveillance for novel influenza A H1N1 when there are too many cases to count. Lancet. 2009;374:1209–1211. doi: 10.1016/S0140-6736(09)61377-5. [DOI] [PubMed] [Google Scholar]
- 30.Mulder J, Masurel N. Pre-epidemic antibody against 1957 strain of Asiatic influenza in serum of older people living in the Netherlands. Lancet. 1958;1:810–814. doi: 10.1016/s0140-6736(58)91738-0. [DOI] [PubMed] [Google Scholar]
- 31.Mulder J, Masurel N. The epidemiology of pandemic A2 influenza in the Netherlands, 1957–58. Bull World Health Organ. 1960;22:399–407. [PMC free article] [PubMed] [Google Scholar]
- 32.Polak MF. Influenza mortality in the fall of 1957. Ned Tijdschr Geneeskd. 1959;103:1098–1109. [PubMed] [Google Scholar]
- 33.Golub GH, Van Loan CF. Matrix Computations. Baltimore: Johns Hopkins University Press; 1996. [Google Scholar]
- 34.Ministry of Health of Chile Reporte Semanal. 27 June 2009.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.