Abstract
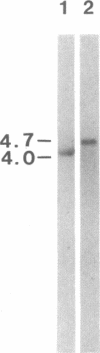
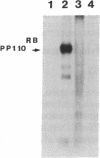
Formation of retinoblastoma, a cancer arising in the retinas of young children, is determined by mutational inactivation of an autosomal gene (RB), which has been molecularly cloned. Whereas all normal tissues and many tumor cells express an RB mRNA of 4.7 kilobases, six of six retinoblastomas were previously found either to lack RB gene expression or to have RB transcripts of abnormal (reduced) length. To further characterize the latter type of mutation, we chose to examine retinoblastoma cell line Y79, which expressed a shortened RB mRNA of about 4.0 kilobases. RB cDNA clones isolated from a library constructed with Y79 mRNA demonstrated an internal loss of 470 nucleotides near the 5' end, which corresponded to a deletion of exons 2-6. Genomic clones containing the deletion junction were isolated from a library made with Y79 DNA, which allowed precise localization and sequencing of deletion endpoints in introns 1 and 6. These regions had no apparent homology to each other or to the Alu family of repetitive sequences, implying that the deletion must have occurred by a mechanism other than recombination of homologous sequences. Deletion of exons 2-6 would interrupt the open reading frame in RB mRNA and would result in premature termination of translation. Since no normal RB protein was detected by immunoprecipitation with specific antibody, the other, apparently normal RB allele in Y79 cells was necessarily inactivated by a different mutation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Bookstein R., Lee E. Y., To H., Young L. J., Sery T. W., Hayes R. C., Friedmann T., Lee W. H. Human retinoblastoma susceptibility gene: genomic organization and analysis of heterozygous intragenic deletion mutants. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2210–2214. doi: 10.1073/pnas.85.7.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Comings D. E. A general theory of carcinogenesis. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3324–3328. doi: 10.1073/pnas.70.12.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby M. K., Herrera R. E., Vosberg H. P., Nordheim A. DNA topoisomerase II cleaves at specific sites in the 5' flanking region of c-fos proto-oncogenes in vitro. EMBO J. 1986 Sep;5(9):2257–2265. doi: 10.1002/j.1460-2075.1986.tb04493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darras B. T., Francke U. A partial deletion of the muscular dystrophy gene transmitted twice by an unaffected male. Nature. 1987 Oct 8;329(6139):556–558. doi: 10.1038/329556a0. [DOI] [PubMed] [Google Scholar]
- DiLella A. G., Marvit J., Lidsky A. S., Güttler F., Woo S. L. Tight linkage between a splicing mutation and a specific DNA haplotype in phenylketonuria. 1986 Aug 28-Sep 3Nature. 322(6082):799–803. doi: 10.1038/322799a0. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Rapaport J. M., Joyce J. M., Petersen R. A. Molecular detection of deletions involving band q14 of chromosome 13 in retinoblastomas. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7391–7394. doi: 10.1073/pnas.83.19.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Francke U. Retinoblastoma and chromosome 13. Birth Defects Orig Artic Ser. 1976;12(7):131–134. [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Horowitz J. M., Gerber M. R., Wang X. F., Bogenmann E., Li F. P., Weinberg R. A. Deletions of a DNA sequence in retinoblastomas and mesenchymal tumors: organization of the sequence and its encoded protein. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9059–9063. doi: 10.1073/pnas.84.24.9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Murphree A. L., T'Ang A., Qian J., Hinrichs S. H., Benedict W. F. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987 Jun 26;236(4809):1657–1661. doi: 10.1126/science.2885916. [DOI] [PubMed] [Google Scholar]
- Gasser S. M., Laemmli U. K. Cohabitation of scaffold binding regions with upstream/enhancer elements of three developmentally regulated genes of D. melanogaster. Cell. 1986 Aug 15;46(4):521–530. doi: 10.1016/0092-8674(86)90877-9. [DOI] [PubMed] [Google Scholar]
- Klein G., Klein E. Evolution of tumours and the impact of molecular oncology. Nature. 1985 May 16;315(6016):190–195. doi: 10.1038/315190a0. [DOI] [PubMed] [Google Scholar]
- Klein G. The approaching era of the tumor suppressor genes. Science. 1987 Dec 11;238(4833):1539–1545. doi: 10.1126/science.3317834. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G., Jr The genetics of childhood cancer. Cancer. 1975 Mar;35(3 Suppl):1022–1026. doi: 10.1002/1097-0142(197503)35:3+<1022::aid-cncr2820350726>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Shew J. Y., Hong F. D., Sery T. W., Donoso L. A., Young L. J., Bookstein R., Lee E. Y. The retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activity. Nature. 1987 Oct 15;329(6140):642–645. doi: 10.1038/329642a0. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Wheatley W., Benedict W. F., Huang C. M., Lee E. Y. Purification, biochemical characterization, and biological function of human esterase D. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6790–6794. doi: 10.1073/pnas.83.18.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff S. E., Rosenfeld M. G., Evans R. M. Complex transcriptional units: diversity in gene expression by alternative RNA processing. Annu Rev Biochem. 1986;55:1091–1117. doi: 10.1146/annurev.bi.55.070186.005303. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Goldstein J. L., Russell D. W., Brown M. S. Duplication of seven exons in LDL receptor gene caused by Alu-Alu recombination in a subject with familial hypercholesterolemia. Cell. 1987 Mar 13;48(5):827–835. doi: 10.1016/0092-8674(87)90079-1. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Russell D. W., Goldstein J. L., Brown M. S. Alu-Alu recombination deletes splice acceptor sites and produces secreted low density lipoprotein receptor in a subject with familial hypercholesterolemia. J Biol Chem. 1987 Mar 5;262(7):3354–3361. [PubMed] [Google Scholar]
- Lin C. R., Kapiloff M. S., Durgerian S., Tatemoto K., Russo A. F., Hanson P., Schulman H., Rosenfeld M. G. Molecular cloning of a brain-specific calcium/calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5962–5966. doi: 10.1073/pnas.84.16.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga E. Hereditary retinoblastoma: delayed mutation or host resistance? Am J Hum Genet. 1978 Jul;30(4):406–424. [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McFall R. C., Sery T. W., Makadon M. Characterization of a new continuous cell line derived from a human retinoblastoma. Cancer Res. 1977 Apr;37(4):1003–1010. [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr The mutation and polymorphism of the human beta-globin gene and its surrounding DNA. Annu Rev Genet. 1984;18:131–171. doi: 10.1146/annurev.ge.18.120184.001023. [DOI] [PubMed] [Google Scholar]
- Reid T. W., Albert D. M., Rabson A. S., Russell P., Craft J., Chu E. W., Tralka T. S., Wilcox J. L. Characteristics of an established cell line of retinoblastoma. J Natl Cancer Inst. 1974 Aug;53(2):347–360. doi: 10.1093/jnci/53.2.347. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes R. S., Murphree A. L., Lingua R. W., Sparkes M. C., Field L. L., Funderburk S. J., Benedict W. F. Gene for hereditary retinoblastoma assigned to human chromosome 13 by linkage to esterase D. Science. 1983 Feb 25;219(4587):971–973. doi: 10.1126/science.6823558. [DOI] [PubMed] [Google Scholar]
- Sparkes R. S., Sparkes M. C., Wilson M. G., Towner J. W., Benedict W., Murphree A. L., Yunis J. J. Regional assignment of genes for human esterase D and retinoblastoma to chromosome band 13q14. Science. 1980 May 30;208(4447):1042–1044. doi: 10.1126/science.7375916. [DOI] [PubMed] [Google Scholar]
- Squire J., Gallie B. L., Phillips R. A. A detailed analysis of chromosomal changes in heritable and non-heritable retinoblastoma. Hum Genet. 1985;70(4):291–301. doi: 10.1007/BF00295364. [DOI] [PubMed] [Google Scholar]
- Vanin E. F., Henthorn P. S., Kioussis D., Grosveld F., Smithies O. Unexpected relationships between four large deletions in the human beta-globin gene cluster. Cell. 1983 Dec;35(3 Pt 2):701–709. doi: 10.1016/0092-8674(83)90103-4. [DOI] [PubMed] [Google Scholar]
- Vogel F. Genetics of retinoblastoma. Hum Genet. 1979 Nov 1;52(1):1–54. doi: 10.1007/BF00284597. [DOI] [PubMed] [Google Scholar]
- Yunis J. J., Ramsay N. Retinoblastoma and subband deletion of chromosome 13. Am J Dis Child. 1978 Feb;132(2):161–163. doi: 10.1001/archpedi.1978.02120270059012. [DOI] [PubMed] [Google Scholar]