Abstract
Dendritic cells (DCs) are critical for adaptive immunity and tolerance. Most DCs are strategically positioned as immune sentinels in tissues throughout the body poised to respond to invading pathogens. Differentiated DCs and their precursors also circulate in blood and can get rapidly recruited to sites of challenge. Within peripheral tissues DCs collect antigenic material and then traffic to secondary lymphoid organs where they communicate with lymphocytes to orchestrate adaptive immune responses. Hence, the migration and accurate positioning of DCs is indispensable for immune surveillance. Here, we review the molecular traffic signals that govern DC migration throughout their life cycle.
Introduction
Dendritic cells (DCs) are specialized antigen presenting cells that play a dual role in inducing adaptive immune responses to foreign antigens (Ags) and in maintaining T cell tolerance to self (Steinman and Banchereau, 2007). DCs consist of several distinct subsets distinguishable by surface and intracellular phenotypic markers, immunological function and anatomic distribution (Table I). In mice, all DCs express (to varying amounts) the CD11c integrin and MHC class-II (MHC-II) molecules, and are further phenotypically distinguished by their differential expression of CD8α, CD4, CD11b, Langerin, PDCA-1 as well as a growing list of other markers (Shortman and Naik, 2007). These markers have been used in various combinations to define several subpopulations some of which are highly restricted to specific organs whereas others occur at characteristic frequencies among a mixture of DC subsets, especially in secondary lymphoid organs (SLOs). Arguably the clearest phenotypic and functional distinction can be made between the bulk of CD11chi MHC-II+ DCs (conventional DCs) and the type I interferon producing plasmacytoid DCs (pDCs), which are CD11clo MHC-II+/lo and express unique differentiation markers in both mice and humans. Irrespective of their phenotypic idiosyncracies or immunological role, DCs exert their activity in discrete locations remote from their place of origin, which implies that DCs possess advanced migratory skills to navigate through the body.
| DC Subset and Phenotype | Migratory Route | Chemokine Receptor Expression (Ligand) |
Other Traffic Molecules |
References |
|---|---|---|---|---|
| Precursor DCs | ||||
| • Hematopoeitic Stem and Progenitor Cell (HSPC) CD45+ Lin− c-Kit+ Sca-1+ |
BM ↓ blood ↰(via TD) tissue ↲ ↳ SLO ↳ afferent lymphatics 
|
CXCR4 (CXCL12) | VLA-4, LFA-1, CD44, PSGL-1, S1P1–4, α4- integrin, α5- integrin |
(Wright et al., 2002) (Massberg et al., 2007) |
| • Macrophage DC precursor (MDP) CD34+ Lin− c-Kitint CD11b− |
BM ↓ blood tissue ↲ ↳ spleen |
CX3CR1 (fractalkine) | n.d. | (Fogg et al., 2006) |
| • Common dendritic progenitor (CDP) or Clonal DC precursor (pro-DC) CD34+ Lin− c-Kitint Flt-3+ |
BM ↓ blood tissue ↲ ↳ SLO |
n.d. | CD44 L-selectin |
(Onai et al., 2007) (Naik et al., 2007) |
| • Monocyte subsets CD115+ CD11b+ Ly6Clo/int/hi F4/80lo CD62L+/− |
BM ↓ blood tissue ↲ ↳ SLO |
CX3CR1 (fractalkine), CXCR4 (CXCL12), CCR2 (MCPs), CCR8(CCL1) CCR5 (MIP/RANTES) |
CD99, VLA-4, PSGL-1 |
(Geissmann et al., 2003) (Ley et al., 2007) |
| Differentiated DCs | ||||
| • Langerhans cell Langerin+ MHC II+ Dectin-1+ CD1a+ CD11b++ CD11c+ CD24a+ CD205+ CD45lo CD8α+/− CD103− |
epidermis ↓ dermis ↓ afferent lymphatics ↓ LN |
Immature CCR2 (MCPs), CCR6 (CCL20) CX3CR1(fractalkine) Mature CCR7 (CCL19/21), CXCR4 (CXCL12) |
S1P/S1PR, LFA-1, VLA-4, |
(Bursch et al., 2007) (Stutte et al., 2008) (Poulin et al., 2007) |
| • Dermal DC: langerin+ subset Langerin+ MHCII+ CD103+ CD11blo CD11cint CD45hi CD8α− |
dermis ↓ afferent lymphatics ↓ LN |
Immature CCR2 (MCPs), CX3CR1(fractalkine) Mature CCR7 (CCL19/21) |
P/E selectin ligands |
(Bursch et al., 2007) (Ginhoux et al., 2007) (Poulin et al., 2007) |
| • Dermal DC: langerin- subset Langerin− MHCII+ CD11c+ DEC205+ CD24a− |
dermis ↓ afferent lymphatics ↓ LN |
Immature CCR2(MCPs), Mature CCR7 (CCL19/21), CXCR4 (CXCL12) |
S1P/S1PR, LFA-1, VLA-4 |
(Stutte et al., 2008) |
| • CD8α+ DC CD8α+ MHCII+ CD11c+ CD4− CD205+ SIRP-α− |
blood SLO ↲ ↓ ↳ BM thymus |
Immature CX3CR1 (fractalkine) Mature CCR7 (CCL19/21) |
LFA-1, VLA-4 | (Bonasio et al., 2006) (Cavanagh et al., 2005) (Jung et al., 2000) |
| • CD8α− DC CD8α − MHCII+ CD11c+ CD11b+ CD4− SIRP-α+ DCIR2+ |
blood SLO ↲ ↓ ↳ BM thymus |
Immature CX3CR1 (fractalkine) Mature CCR7(CCL19/21) |
LFA-1, VLA-4 | (Jung et al., 2000) (Bonasio et al., 2006) (Cavanagh et al., 2005) |
| • CD8α− CD4+ DC CD8α− CD4+ CD11b+ MHCII+ DCIR2+ |
blood ↓ spleen |
Immature CX3CR1 (fractalkine) Mature CCR7 (CCL19/21), CCR5 (CXCL13) |
LFA-1, VLA-4 | (Vremec et al., 2000) |
| • pDC B220+ CD11clo Ly6C+ MHCIIlo CD4−/+ CD8a−/+ PDCA-1+ (human: CD123+ BCDA-2+, -4+) |
blood ↙ ↙ ↘ ↘ tissue thymus SLO BM |
Immature CCR2 (MCPs), CCR9 (CCL25) CXCR3 (CCL9/10/11), CXCR4 (SDF-1), CCR5 (MIP/RANTES) Mature CCR7 (CCL19/21) |
L-selectin, ChemR23 |
(Cella et al., 1999) (Diacovo et al., 2005) (Penna et al., 2001) (Vermi et al., 2005) (Zabel et al., 2005) |
| • Lung DCs (2 subsets) (conducting airways) 1. CD11bhi CD11c+ CD103− (lung interstitium) 2. CD11blo CD11c+ CD103+ |
lung ↓ afferent lymphatics ↓ LN |
Immature CCR7lo (CCL19/21) Mature CCR7hi (CCL19/21), CCR8 (CCL1) |
S1P/S1PR, CD38 | (Hammad and Lambrecht, 2007) |
| • Lamina propria DCs (4 subsets) 1. CD11chi CD11b− CD205+ CD103+ 2. CD11chi CD11b+ CD205+ CD103+ 3. CD11cint CD11bint CD205− CD103− 4. CD11cint CD11b+ CD205− CD103− |
lamina propria ↓ afferent lymphatics ↓ LN |
Immature CCR6 (CCL20), CX3CR1 (fractalkine) Mature CCR7(CCL19/21), CCR8(CCL1) |
n.d. | (Iwasaki, 2007) |
| • Peyer’s patch DCs (3 subsets) 1. CD11c+ CD8α+ CD11b− 2. CD11c+ CD8α− CD11b+ 3. CD11c+ CD8α− CD11b− |
Peyer’s patches |
Immature CCR6(CCL20), CCR1(CCL9) CX3CR1 (fractalkine) Mature CCR7 (CCL19/21) |
n.d. | (Iwasaki, 2007) |
All DCs ultimately derive from hematopoietic stem and progenitor cells (HSPCs) in the bone marrow (BM), which give rise to several distinct progenitors that can differentiate into one or more DC subsets (Fogg et al., 2006; Naik et al., 2007; Onai et al., 2007). Facultative DC progenitors are not restricted to the BM (although some fully differentiated DCs are generated there) but can be found in multiple locations, including the thymus, blood, lymph, and most visceral organs (Liu et al., 2007; Massberg et al., 2007; Onai et al., 2007). These progenitors can differentiate into DCs upon challenge in peripheral tissues (Massberg et al., 2007). Substantial numbers of DCs are also physiologically generated in the thymus (Wu and Shortman, 2005).
Fully differentiated DCs are found in healthy tissues as immunologically immature cells, i.e. they are equipped with highly-active endocytic machinery for the sampling of foreign Ags but have not acquired the capacity for full-fledged priming of naive T cells (Banchereau et al., 2000). Some tissues are notably enriched for DCs, such as the skin and mucosal surfaces, the most common sites of entry for microbial pathogens, and the SLOs where adaptive immune responses to such pathogens are initiated. Indeed, a central function of DCs in non-lymphoid tissues is the transport and presentation of antigenic cargo into and within SLOs. This is owed to the DCs’ ability to enter small lymph vessels in peripheral tissues and migrate to local draining lymph nodes (LNs). Somewhere en route to the LN these Ag-bearing DCs mature, i.e. they assume an immunostimulatory phenotype concurrent with increased expression of MHC complexes and upregulation of the co-stimulatory molecules and cytokines needed for efficient T cell priming. A small fraction of DCs that enter lymphatics are not retained in LNs, but travel along the lymphatic tree to the venous circulation. These blood-borne DCs can deliver their antigenic cargo to the spleen (Mullins et al., 2003) and to primary lymphoid tissues, i.e. the BM (Cavanagh et al., 2005) and thymus (Bonasio et al., 2006).
Given this complex life cycle, the ability of DCs and their progenitors to migrate throughout the body is a critical aspect of their immunological function. The term “migration”, as discussed here, encompasses several discrete events that occur in different environments under different biophysical conditions, and invoke numerous context-specific cellular and molecular mechanisms (Figure 1). Specifically, DC migration entails: (1) the ability of newly formed DCs or their progenitors to exit their place of birth (i.e. the BM and possibly also the thymus) and enter the blood; (2) the recruitment of the circulating cells into target tissues; (3) the extravascular lodging and interstitial motility needed to sample Ags; (4) the capacity to access lymph vessels to travel either to LNs or back to the blood; and (5) the ability to interact with migrating lymphocytes and other immune cells in a manner that allows the exchange of critical information regarding the nature and context of presented Ags. Here, we will discuss our current understanding of each of these migration events and provide a programmatic overview of the life cycle of DCs with particular attention on the mechanisms and consequences of DC migration. We will primarily focus on data derived from mouse models and, where appropriate, highlight parallels and differences between mice and humans.
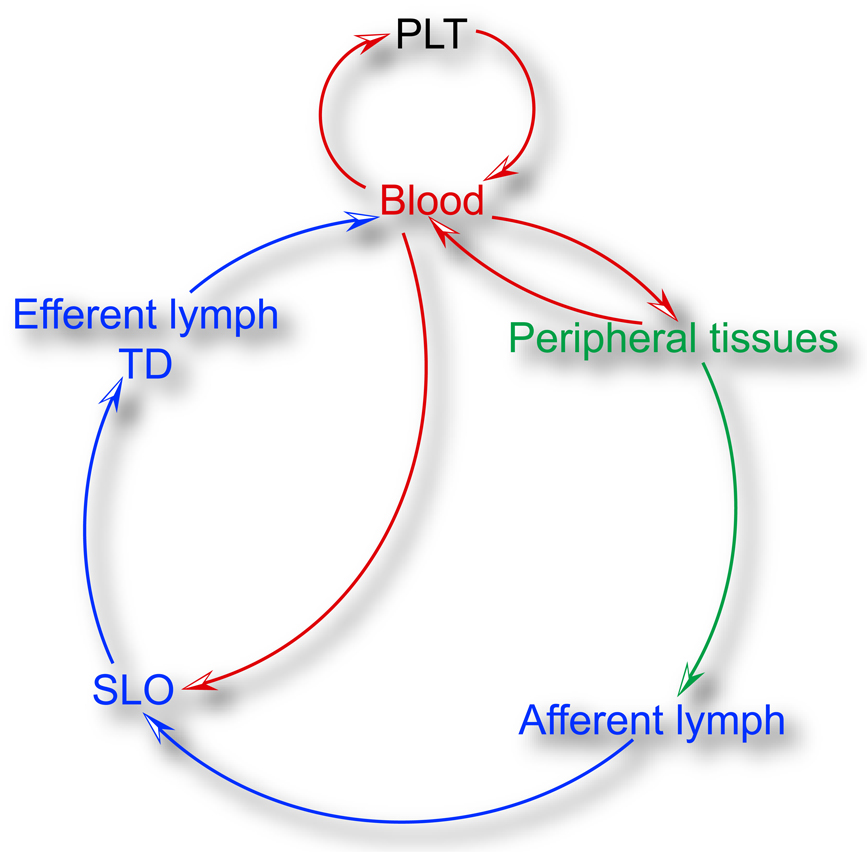
Fig. 1.
Programmatic outline of DC and DC precursor trafficking routes. DCs develop from precursors that originate from primary lymphoid tissues (PLT) such as the BM and the thymus. Precursors and committed DCs enter the circulation and seed peripheral tissues and SLOs (see also Figure 2 for an overview of the hematogenous route). From peripheral tissues, they can access afferent lymph upon receiving a mobilization signal and travel to the draining LN (see also Figure 3 for migration to the draining LN and Figure 4 for migration within the LN). Leukocytes leave LNs via the efferent lymph and are collected in the TD, which eventually guides DCs and their precursors back into the circulation. For individual migratory routes for specific DCs and their precursors refer to table 1.
Methods to study DC migration
Many DCs begin their journey with their release from the BM into the blood and subsequent traffic into peripheral lymphoid and non-lymphoid tissues. In non-lymphoid tissues, DCs eventually proceed into LNs through afferent lymphatics and, in some instances, return back to the blood via the thoracic duct (TD). Throughout this voyage migrating DCs must apply specialized skills to reach their target destination, including the capacity to: traverse vessel walls and other anatomic barriers; recognize and adhere to specific microvascular endothelial cells in the presence of shear stress in the blood-stream; sense and follow soluble and surface-bound chemoattractant cues through the interstitium; and scan and interact productively with a vast number of lymphocytes in SLOs. Although no single method in the immunologist’s toolbox sufficiently covers all the diverse steps that constitute a DC’s longwinded voyage, the combined application of the techniques discussed below has been instrumental to flesh out an ever more detailed picture of how DC migration impacts physiological and pathological immune responses.
A number of techniques have been particularly useful in studying DC trafficking from blood into peripheral tissues or SLOs, such as intravital videomicroscopy (IVM) (Cavanagh et al., 2005; Robert et al., 1999) and flow cytometric or histology-based DC homing assays (Ingulli et al., 1997), which provide useful information at the single-cell and population level, respectively. A detailed analysis of DC interactions with endothelial ligands under precisely controlled biophysical conditions is also afforded by the use of flow chamber devices (Geijtenbeek et al., 2000). Profound insights into DC traffic signals involved in transvascular diapedesis and interstitial navigation have been achieved using in vitro chemotaxis assays (Lin et al., 1998).
Trafficking of DCs from tissues to SLOs via the lymph has been exhaustively studied, in particular, by classic assays that mobilize DCs from peripheral tissues with fluorescent tracers, followed by enumerating the emigration and immigration of fluorescent DCs from the periphery and into the draining LN, respectively (Bonasio and von Andrian, 2006). This approach relies on the assumption that the number and phenotype of fluorescent DCs recovered from a LN are indicative of their migration and not due to acquisition of fluorescent tracer by LN-resident cells. A more recent approach involves genetic manipulations that permanently or conditionally label DCs through fluorescent proteins driven, for example, by promoters for langerin (Kissenpfennig et al., 2005) or CD11c (Lindquist et al., 2004). This offers the advantage that unperturbed endogenous DCs can be studied in situ, but has the potential drawback that reporter expression levels may change during maturation or differentiation. The development of a photoconvertible fluorescent protein, Kaede, which upon exposure to UV light shifts its excitation and emission spectrum, has emerged as another useful system to monitor the cellular trafficking patterns, including those of DCs, in transgenic mice (Tomura et al., 2008). Purified or in vitro differentiated DCs have also been genetically or chemically labeled and injected into tissues to study their trafficking to and function within draining LNs (Ingulli et al., 1997; Smith and Fazekas de St Groth, 1999). This allows for more quantitative and time-resolved analyses of the molecular mechanisms, kinetics, and immunological sequelae of DC migration. However, several caveats of these approaches must be kept in mind, including the large, non-physiological numbers of DCs that must be transferred, the need for ex vivo manipulation, and that transferred DCs are not native to the tissue being studied. In this regard, parabiotic or competitive BM chimeric mouse models offer advantages for studying physiological recruitment and turnover of DCs and, in conjunction with adoptive transfer strategies, have been useful in addressing the migration and differentiation of rare DC precursors (Liu et al., 2007; Massberg et al., 2007; Naik et al., 2007; Onai et al., 2007).
Technological advances in IVM and multi-photon (MP) imaging have recently enabled researchers to directly visualize DC migration and DC interactions in their native environment (Bousso and Robey, 2003; Mempel et al., 2004; Miller et al., 2004a). Conventional IVM uses brightfield transillumination or epifluorescence microscopy that permits two-dimensional imaging of intravascular adhesion events in surgically exposed tissues in real time (Halin et al., 2005). This approach has helped to pinpoint the precise role of trafficking molecules during DC-endothelium interactions as part of the intravascular multistep adhesion cascade (discussed below). MP-IVM uses infrared-pulsed laser excitation to generate high-resolution optical sections of living tissue containing fluorescently-labeled cells, such as DCs, migrating and engaging in various cell-cell interactions (Cahalan et al., 2002). Additional novel imaging modalities have recently been introduced such as bioluminescence imaging, magnetic resonance imaging, and positron emission tomography which provide non-invasive tracking of leukocyte populations, including DCs, throughout the entire body, although with considerable less spatial and/or temporal resolution to visualize single-cell dynamics (Baumjohann and Lutz, 2006).
Traffic Molecules in DC Migration
Circulating DCs and their precursors exit the blood in response to tissue-specific recruitment signals that are displayed on the vascular wall. These include signals that emanate from sites of inflammation (like the pro-inflammatory chemokines (chemotactic cytokines)) or from normal tissues that recruit DC precursors during the initial seeding and subsequent physiological turnover of tissue-resident DCs (Elbe et al., 1989). Circulating leukocytes can only follow these recruitment signals by engaging adhesion molecules, which allow them to withstand the shear stress exerted by microvascular blood flow and to commence transvascular movement into the target tissue. DCs express specific adhesion molecules and maturation-dependent chemoattractant receptors that allow them to respond to a variety of ligands (Sozzani et al., 1997; Sozzani et al., 1995), which control their trafficking. For example, to access non-lymphoid peripheral tissues and navigate within them, immature DCs (and some of their precursors, particularly monocytes) utilize specific chemokine receptor-ligand pathways, such as CCR2-CCL2 (Geissmann et al., 2003; Merad et al., 2002), CCR5-CCL5 (Stumbles et al., 2001; Yamagami et al., 2005), and CCR6-CCL20 (Merad et al., 2004). When DCs become mature they downregulate their responsiveness to these inflammatory chemokine pathways and traffic to the draining LNs by upregulating CCR7, which responds to two ligands, CCL19 and CCL21 (Dieu et al., 1998; Sallusto et al., 1998; Sozzani et al., 1998). These chemokines are expressed by peripheral lymphatic endothelial cells as well as LN stroma cells and guide DCs to downstream LNs (Martin-Fontecha et al., 2003; Saeki et al., 1999; Vassileva et al., 1999).
DCs and their precursors are recruited from blood into tissues (except the spleen) following a cascade of sequential molecular and cellular interactions, analogous to what has been shown for the extravasation of other circulating leukocytes. According to this paradigm, leukocyte extravasation occurs in a series of distinct steps including tethering, rolling, activation by a chemoattractant, firm adhesion, and diapedesis (von Andrian and Mackay, 2000). On most leukocytes, including circulating DCs, tethering and rolling are primarily mediated by one or more of the three members of the selectin family and occasionally by α4 integrins. Two selectins, P- and E-selectin, are expressed on activated endothelium, whereas L-selectin is found on leukocytes. Selectins bind sialyl-Lewis X-like carbohydrates presented by sialomucins, such as P-selectin glycoprotein ligand 1 (PSGL-1) (Vestweber and Blanks, 1999).
Rolling cells must next encounter a chemoattractant stimulus, often (but not always) in the form of a chemokine presented on venular endothelial cells (Rot and von Andrian, 2004). Most chemoattractants signal through pertussis toxin (PTX)-sensitive G protein-coupled receptors (GPCRs), causing clustering and conformational activation of integrins. Activated integrins, in particular LFA-1 (αLβ2), VLA-4 (α4β1), Mac-1 (αMβ2) and α4β7 mediate firm arrest of the rolling cells by binding to members of the immunoglobulin superfamily (IgSF), including ICAM-1 (ligand for LFA-1 and Mac-1), ICAM-2 (ligand for LFA-1), VCAM-1 (ligand for VLA-4 and weakly for α4β7) and MAdCAM-1 (ligand for α4β7) (Springer, 1994).
Upon firm arrest, leukocytes respond to localized chemoattractant and/or adhesion molecule gradients, which provide guidance cues for diapedesis and directed leukocyte migration. The essential molecular determinants involved in tethering, rolling, firm adhesion and diapedesis are expressed by both circulating DCs and precursors that can give rise to DCs, such as monocytes (Imhof and Aurrand-Lions, 2004) and HSPCs (Laird et al., 2008). Indeed, IVM experiments have determined that both conventional DCs and pDCs tether and roll efficiently along venular endothelium in an E- and P-selectin dependent fashion and, like other inflammatory cells, can be recruited from the blood to sites of inflammation (Diacovo et al., 2005; Robert et al., 1999).
The combinatorial use of selectins, chemoattractant receptors, integrins and their respective ligands provides for a great deal of diversity and selectivity in regulating leukocyte migration to distinct tissues (Springer, 1994). Individual leukocyte subsets, including DCs, express only a small selection of the broad palette of traffic molecules and, therefore, can only successfully participate in one or a few specific multi-step cascades. Conversely, many specialized microvascular endothelial cells present a highly tissue-specific assortment of adhesion molecules and chemoattractants, thus providing a unique tissue- and situation-specific molecular “area code”. In the subsequent section, we highlight specific examples of how multi-step adhesion cascades control the movement of intravascular DCs and their precursors into different target organs.
DC migration from blood to tissues
Blood contains both DC precursors and differentiated DC subsets, including pDCs and conventional DCs, which are a mixture of newly generated cells from the BM and of experienced DCs that have re-entered the circulation from peripheral tissues (Bonasio and von Andrian, 2006). There are also pluripotent HSPCs, which recirculate continuously between the blood, peripheral organs and draining lymphatics and can give rise to DCs upon TLR ligation (Massberg et al., 2007). Blood contains also lineage-committed BM-derived DC precursors that can differentiate into any DC subset found in SLOs (Fogg et al., 2006; Naik et al., 2007; Onai et al., 2007). An additional source of DCs are circulating monocytes (Gordon and Taylor, 2005). Two monocyte subsets have been identified in mice and humans that are distinguished by the differential expression of Ly-6C (in mice) and three traffic molecules, CX3CR1, CCR2 and L-selectin (Geissmann et al., 2003; Palframan et al., 2001). Ly-6Chi CX3CR1lo CCR2hi (“inflammatory”) monocytes are preferentially recruited to distressed tissues in a CCR2–CCL2 (MCP-1) dependent manner. They can give rise to a variety of conventional DCs under both inflammatory and steady-state conditions. The second subset, Ly-6C− CX3CR1hi CCR2lo/− (“resident”) monocytes, interacts with fractalkine (CX3CL1), a transmembrane chemokine on resting endothelium. These cells patrol along the lumenal surface of microvessels, enter tissues upon inflammation and differentiate into macrophages (Auffray et al., 2007; Geissmann et al., 2003).
An abrupt increase in circulating DC numbers occurs when DCs are injected intravenously (i.v.). Although non-physiological, such events are clinically relevant because antigen-pulsed autologous DCs have been given to patients by various routes as anti-cancer vaccines (Steinman and Banchereau, 2007). This clinical context highlights the importance of understanding the target organs of circulating DCs and the molecular mechanisms that govern their migration to those sites. Irrespective of their origin and differentiation state, circulating DCs and their precursors gain access to lymphoid and non-lymphoid tissues through multi-step adhesion cascades. The molecules involved in discrete adhesion steps vary depending on the DC subset and the target tissue, thus providing specificity and selectivity in recruitment (Figure 2).
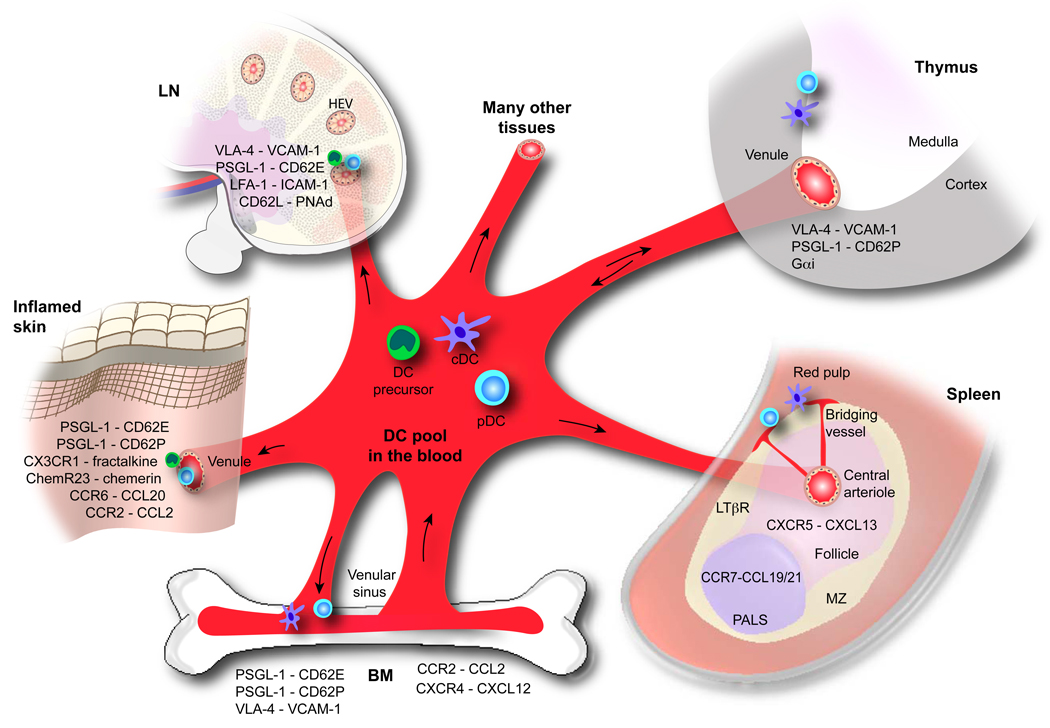
Fig. 2.
Hematogenous DC routes. This schematic outline illustrates various routes that DCs can take to and from the blood into various lymphoid and non-lymphoid tissues. DC precursors are released from the BM and enter the blood pool, which consists of: conventional DCs (or cDC), pDCs, and DC precursors (encompassing monocytes, HSPCs, and other committed DC precursors). Potential destinations of blood-borne DCs as well as the major trafficking molecules implicated in their migration are highlighted, including (from left to right) the skin, LN, thymus, and spleen, as well as their re-entry into the BM.
DC traffic to non-lymphoid tissues
The recruitment mechanisms that guide fully-committed immature or mature DCs from blood into non-lymphoid tissues are only partly characterized. Our current knowledge is mainly based on adoptive transfers of labeled DCs by i.v. injection. A major fraction of injected mature and immature DCs accumulate in the liver and lungs in mice and humans (Cavanagh et al., 2005; de Vries et al., 2005; Morse et al., 1999). Though the underlying mechanisms governing this distribution are poorly understood, the retention of DCs in the lungs is probably due, at least in part, to mechanical trapping in pulmonary capillaries, rather than active adhesion (Cavanagh et al., 2005).
IVM experiments have shown that conventional DCs can efficiently tether and roll in normal murine skin venules, which constitutively express E- and P-selectin (Robert et al., 1999). Because sLeX-decorated PSGL-1, the principal ligand for the vascular selectins, is not only highly expressed on DCs (Robert et al., 1999), but also on monocytes and HSCs (Laird et al., 2008; Lim et al., 1998), it is likely that all DCs and DC precursors can engage in rolling interactions in microvascular beds that express P-and/or E-selectin either constitutively (e.g. in skin, BM and thymus) or in response to inflammation. Interestingly, DC extravasation into inflamed skin depends on selectins but not on PSGL-1, suggesting a contribution by other selectin ligands on DCs (Pendl et al., 2002).
Although selectins are clearly important, they mediate only the first step in the multistep adhesion cascade and do not by themselves support DC arrest or accumulation in normal skin. Additional signals are required to trigger integrin activation, arrest and subsequent diapedesis (Pendl et al., 2002; Robert et al., 1999). However, little is known about the inflammation-induced or constitutive chemoattractants that trigger these steps. Direct experimental observations of steady-state DC recruitment to normal tissues are particularly challenging, because these are very rare events that may only become prominent after long-term adoptive transfers or in parabiosis settings. Some information may be gleaned from in vitro experiments. For example, a recent study found a role for the IgSF molecule ICAM-2 in the transmigration of immature DCs across endothelial monolayers (Wethmar et al., 2006).
Lineage-committed DC precursors (other than monocytes) have not been examined so far for their capacity to home to normal tissues other than SLOs. Reconstitution experiments in irradiated mice with wildtype and mutant BM have shown that circulating LC precursors repopulate severely inflamed skin in a CCR2- and CCR6-dependent manner, but these pathways are apparently not operational in normal skin (Merad et al., 2004; Merad et al., 2002). Accordingly, the CCR2 ligands, CCL2 and CCL7, and the CCR6 ligand, CCL20, are poorly expressed in resting tissues, but markedly increased in inflamed skin (Dieu-Nosjean et al., 2000; Merad et al., 2004; Merad et al., 2002).
The so-called inflammatory CX3CR1lo CCR2+ Ly6Chi monocytes can enter diverse inflamed tissues, including the skin, lung, and intestinal lamina propria, where they give rise to various DC subsets (Ginhoux et al., 2006; Landsman et al., 2007; Varol et al., 2007). The CX3CR1hi CCR2− Ly6Clo monocytes can also give rise to DCs (or at least CD11c+ cells) in the lung (Landsman et al., 2007) and atherosclerotic plaques (Tacke et al., 2007). However, the role of specific traffic molecules in each case is largely unclear. Arguably the best evidence exists for CCR2, since Ly6Chi monocytes fail to accumulate at sites of inflammation in Ccr2−/− mice (Merad et al., 2002). However, it must be noted that CCR2 controls the inflammation-induced release of monocytes from the BM (Serbina and Pamer, 2006). Thus,the observed migration defect could either reflect poor monocyte mobilization or defective peripheral recruitment, or both.
Circulating pDCs can also access inflamed tissues, but their homing properties are thought to differ substantially from those of conventional DCs. Compared to the latter, human pDCs exhibit only a weak capacity in vitro to migrate towards pro-inflammatory chemokines (e.g. CCL2, CCL5, and CCL20) despite expressing a similar chemokine receptor profile (e.g. CCR2, CCR5, CXCR3, CXCR4, and CCR7) (Penna et al., 2001). On the other hand, pDCs migrate effectively towards CCL19 and CCL21, two homeostatic chemokines that act on CCR7 and are constitutively expressed in SLOs (Penna et al., 2001). Indeed, substantial numbers of pDCs are found in SLOs but they are relatively infrequent in most non-lymphoid tissues (for a detailed discussion of pDC distribution, the reader may refer to the article by Villadangos and Young in this issue (2008)). In humans, pDCs are enriched in certain inflamed non-lymphoid tissues, such as lupus erythematosus lesions (Farkas et al., 2001), psoriatic skin (Nestle et al., 2005) and the nasal mucosa of allergic rhinitis patients (Jahnsen et al., 2000), but it has not been determined if their presence at those sites reflects recruitment of differentiated circulating pDCs or local differentiation from progenitors. Direct recruitment of blood-borne pDCs has been documented in normal and inflamed small intestine in mice; pDCs require CCR9 to access the intestinal wall, which physiologically generates CCL25, the ligand for CCR9 (Wendland et al., 2007). Another chemoattractant for circulating pDCs is chemerin (Vermi et al., 2005; Zabel et al., 2005). This non-chemokine molecule is generated by serine proteases that are activated during coagulation, fibrinolysis, and inflammation. Chemerin is absent from normal skin but is markedly upregulated curing cutaneous inflammation and recruits pDCs via the serpentine chemokine-like receptor 1 (CMKLR1 or ChemR23).
DC traffic to primary lymphoid tissue
Some fully committed DCs recirculate from peripheral tissues via the draining lymphatics and blood into primary lymphoid tissues. Through this tortuous route, DCs can deliver Ag from all over the body to both the BM and the thymus. However, immunological consequences in each tissue are markedly different. The BM shares a number of features with bona fide SLOs and serves as a major reservoir for memory T cells (Di Rosa and Pabst, 2005). Ag-laden circulating DCs that home to the BM evoke a vigorous memory response that leads to rapid proliferation and peripheralization of responsive T cells (Cavanagh et al., 2005). Immature and mature DCs enter the BM equally well by employing a multi-step adhesion cascade. IVM in mouse skull BM has shown that rolling is mediated by interactions of PSGL-1 with P- and E-selectin in BM venules and sinusoids, whereas VLA-4–VCAM-1 is required for sticking. Although DCs express β2 integrins, homing to the BM is independent of these molecules. DC homing to BM is also not affected by PTX treatment, suggesting that DCs may use an as yet unidentified chemoattractant receptor(s) that does not signal through the conventional Gαi pathway to activate VLA-4 (Cavanagh et al., 2005).
The thymus harbors two distinct populations of DCs; one is derived from intrathymic early lymphoid progenitors, whereas the second population originates from the periphery (Donskoy and Goldschneider, 2003; Kamath et al., 2000). Ag presentation by either DC subset in the thymus shapes the developing T cell repertoire and results in central tolerance, rather than immunity (Steinman et al., 2003). Monocytes or DC precursors have not been observed to home to the thymus (Geissmann et al., 2003; Naik et al., 2007). By contrast, parabiosis and adoptive transfer experiments have established that small numbers of fully differentiated DCs constantly enter the thymus from the blood (Bonasio et al., 2006; Donskoy and Goldschneider, 2003). This ability to home to the thymus is shared by all immature DC subsets. Inflammation-induced maturation selectively blocks the capacity of DCs to home to the thymus, but does not compromise DC trafficking to other organs (Bonasio et al., 2006). This suggests a mechanism to safeguard against inadvertent deletion of T cells that recognize pathogen-associated Ags, which are much more likely to be presented by mature than immature DCs. The differential capacity of immature DCs to access the thymus is probably regulated by an organ-specific multistep adhesion cascade, whereby rolling and sticking are mediated by PSGL-1–P-selectin and VLA-4–VCAM-1, respectively. Unlike in the BM, DC entry into the thymus is PTX sensitive, but the specific Gαi-coupled chemoattractant receptor(s) remain(s) to be identified (Bonasio et al., 2006).
DC traffic from blood to SLOs
Parabiosis studies and adoptive transfer experiments indicate that committed DC precursors have a very short half-life in the circulation (Liu et al., 2007). In these experiments, the degree of chimerism of DC precursors in the blood closely reflected the degree of chimerism among fully differentiated DCs in SLOs. This suggests that SLO-resident DCs are constantly replenished by circulating DC precursors, although the mechanisms of precursor recruitment are unclear.
The rules that govern the entry of DCs and their precursors into LNs are complex. Intravenously administered, fully differentiated conventional DCs fail to egress across high endothelial venules (HEVs) into normal LNs and Peyer’s patches (PPs) (Cavanagh et al., 2005; Robert et al., 1999). This is consistent with the fact that the multi-step adhesion cascades that recruit leukocytes to both SLOs, depends on L-selectin (and in PP on α4β7 integrin) and CCR7 (von Andrian and Mempel, 2003). In contrast to conventional DCs, pDCs can enter reactive LNs from the circulation via HEVs (Cella et al., 1999; Diacovo et al., 2005). IVM experiments have shown that BM-derived pDCs roll in activated HEVs by employing L-selectin–peripheral node addressin (PNAd) and PSGL-1–E-selectin interactions. Moreover, firm arrest occurred via β1 and β2 integrins and CCR5, but not CXCR3 (Diacovo et al., 2005). However, BM-derived pDCs express elevated amounts of L-selectin, PSGL-1, LFA-1 and VLA-4 compared to their endogenous counterparts (Diacovo et al., 2005). Indeed, ex vivo isolated, adoptively transferred pDC precursors reportedly do not employ L-selectin to enter inflamed LNs (Yoneyama et al., 2004).
Uncommitted DC precursors, particularly monocytes, can also take a hematogenous route to access LNs that drain inflamed tissues. This pathway involves a remote control mechanism, whereby chemokines are produced at a peripheral site of inflammation, enter afferent lymph conduits and are then transported to the luminal surface of HEVs (Palframan et al., 2001). This mechanism has been documented for the inflammatory chemokine CCL2 whose de novo presence in HEVs combined with other endothelial traffic molecules, such as PNAd and E-selectin, enables the recruitment of CCR2+ monocytes (Palframan et al., 2001). This “inflammatory” monocyte population is a known source of DCs (Geissmann et al., 2003). Some monocytes may also be recruited to LN HEVs through another inflammatory chemokine, CXCL9, a ligand for CXCR3, although this receptor is only found on a small subset of monocytes (Janatpour et al., 2001).
Like most blood-borne cells, circulating DCs and their various progenitors can be retained in the spleen (Bonasio et al., 2006; Cavanagh et al., 2005; Liu et al., 2007). However, it is unclear to what extent newly homed cells contribute to the various resident DC subsets and their precursors in the spleen. Adoptive transfer experiments have shown that DCs enter the spleen from the circulation in the marginal zone (MZ) sinus at the border between white and red pulp (Austyn et al., 1988). The traffic signals that recruit (or release) DCs to (or from) the spleen are largely unknown. One molecule that has been implicated in this process is the IgSF member CD47, a ligand for SIRP-α (Van et al., 2006). CD47-deficient mice have reduced numbers of DCs in the MZ, however, this finding is complicated by the fact that CD47-deficient cells are rapidly cleared by splenic macrophages (Blazar et al., 2001).
DC entry into afferent lymphatics and migration to draining LNs
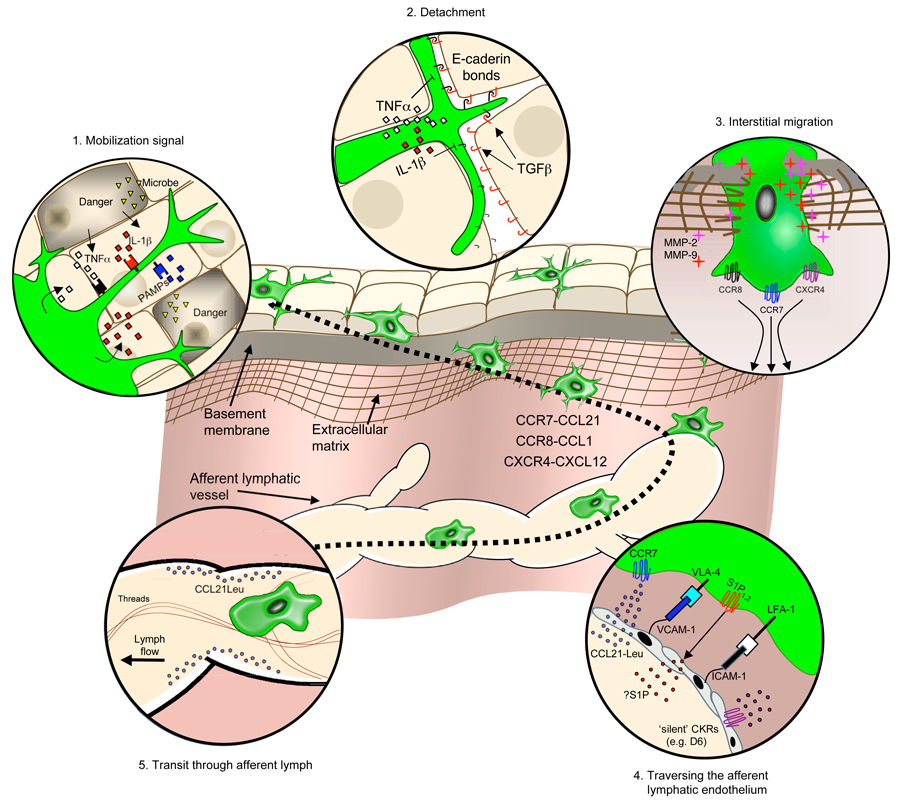
A small but continuous stream of DCs leave non-lymphoid tissues, access the lymphatics and migrate to downstream LNs at steady state (Brand et al., 1992). This physiological trickle can turn into a veritable flood when tissues are exposed to inflammation. Irrespective of the inflammatory state, lymph vessels provide a crucial information conduit by relaying DC-bound and unbound antigenic material from the periphery to the local LNs. DC migration into and along this conduit occurs through a series of steps, including: (1) mobilization; (2) detachment; (3) interstitial migration; (4) entry into the afferent lymphatics, and (5) transit via lymph (Figure 3). Though DCs in all tissues may follow similar migratory cues, our understanding of this process arises predominantly from experimental data obtained in the skin.
Fig. 3.
DC trafficking in peripheral tissues. This schematic illustrates a proposed model for the interstitial migration for skin DCs from the cutaneous microenvironment to the afferent lymphatics en route to the LN. The migratory cascade is divided into five discrete steps (clockwise from top left), starting with recognition of a mobilizing signal (inset 1), detachment from structural tissue elements (inset 2), trafficking through interstitial space (inset 3), traversing the afferent lymphatic endothelium (inset 4), and transit through the afferent lymph vessels (inset 5). Major chemokine-chemokine receptor (CKRs) pathways and other trafficking molecules controlling DC migration are highlighted.
Mobilization Signals
That DCs migrate from peripheral tissues to LNs was first noted in the 1970s when changes in LC density and distribution between epidermal and dermal skin layers and lymph vessels were observed upon contact sensitization (Silberberg-Sinakin et al., 1976). Today, it is firmly established that DCs are mobilized in response to a large variety of pro-inflammatory stimuli that can be chemical (e.g. contact sensitizers and irritants), physical (e.g. UV radiation or trauma) or biological (e.g. microbial or tissue necrosis). The initial exposure to such inflammatory stimuli first induces a brief interval of immobility and enhanced endocytic Ag uptake followed within hours by a period of maturation whereby DCs regain their motility, re-arrange their chemokine receptor repertoire, upregulate their Ag presentation machinery and eventually migrate to LNs (Granucci et al., 1999). Despite their diverse nature, most DC mobilization signals appear to exert their activity through a pair of intermediate messengers, IL-1β and TNF-α. This was first demonstrated for contact sensitizers, which rapidly induce a marked increase in intra-epidermal mRNA levels for these two cytokines (Enk and Katz, 1992). The importance of IL-1β and TNF-α in contact hypersensitivity and DC migration was subsequently validated by experiments using cytokine neutralization (Cumberbatch and Kimber, 1995; Enk et al., 1993) and genetic targeting of the TNF-α type II receptors (Wang et al., 1997), the IL-1 type I receptor (Cumberbatch et al., 1999), and caspase-1, a protease required for release of active IL-1β (Antonopoulos et al., 2001). IL-1β or TNF-α are not only required but also sufficient for DC mobilization, since subcutaneous administration of either cytokine alone promotes rapid DC migration to LNs in the absence of other stimuli (Cumberbatch et al., 1997). The mechanism(s) by which these cytokines prompt DC migration likely include altered expression in adhesion molecules and chemokine receptors on DCs accompanied by a differential responsiveness to the relevant chemotactic ligands in the local microenvironment which pave the way for DCs to traffic to downstream LNs.
Small numbers of DCs also traffic to LNs in the absence of inflammatory stimuli as evidenced by the presence of DCs in afferent lymph (Brand et al., 1992; Bujdoso et al., 1989). Additional evidence for steady-state DC migration stems from recent experiments in Kaede transgenic mice, which provided quantitative estimates on the number of DCs that reach a cutaneous LN from the skin (5% of total LN DCs per day) (Tomura et al., 2008). The signals that prompt this ‘spontaneous’ DC migration have proven difficult to pinpoint, in part, because most experimental migration assays induce some form of inflammation. Despite this, evidence exists in support of a role for CCR7 (Forster et al., 1999) and S1P receptors (Maeda et al., 2007), though the precise step(s) where these pathways are required is unclear. Murine immature BM-derived DCs express S1P receptors S1P2–4, yet they are unresponsive to S1P, unlike their mature counterparts (Czeloth et al., 2005). By contrast, human immature, but not mature, blood-derived DCs are responsive to S1P (Idzko et al., 2002). CCR7 is required for DC entry into dermal afferent lymphatics. Consequently, fewer putative migratory DCs are found in LNs of CCR7-deficient mice compared to wildtype controls when both are kept under specific pathogen- or germ-free conditions (Ohl et al., 2004). However, immature DCs are not thought to express CCR7 nor do they chemotax towards its ligands (Sallusto et al., 1998). Indeed, CCR7 is generally only induced when DCs encounter pro-inflammatory maturation signals (Yanagihara et al., 1998). A potential solution to this conundrum may be that some steady-state DCs upregulate CCR7, but mature only partially.
In support of a dissociated induction of CCR7 are results showing that the activation of the surface Ig-like receptor TREM-2 (‘triggering receptors expressed on myeloid cells-2’) on human monocyte-derived DCs leads to marked upregulation of functional CCR7 despite low expression of costimulatory molecules and other markers of DC maturation (Bouchon et al., 2001). Mice deficient in DAP-12, a signaling adaptor for TREM-2, show an accumulation of DCs in normal skin and gut (Tomasello et al., 2000). Although TREM-2 ligands remain poorly defined (Klesney-Tait and Colonna, 2007), it is worthwhile noting that TREM-2/DAP12 can regulate some aspects of DC function by interacting in cis with plexin-A1/pemaphorin D, a receptor/ligand pair that regulates cytoskeleton and integrin-mediated adhesion pathways (Takegahara et al., 2006; Wong et al., 2003). Another member of the plexin family, plexin-C1, has been shown to mediate the retraction of membrane processes and detachment of adherent DCs. Consequently, plexin-C1-deficient mice show impaired DC chemotaxis in vitro, a partial defect in DC migration to LNs and retention of LCs in the epidermis (Walzer et al., 2005).
Detachment
The relatively long persistence and presumably slow turnover of DCs in some tissues, like the skin (t1/2 ~14–30d), suggests that the tissue microenvironment may provide specific retention signals (Holt et al., 1994; Ruedl et al., 2000). For example, LCs are anchored in the epidermis through E-cadherin, a homophilic adhesion molecule that facilitates intercellular adhesion and tissue integrity in many organ systems, including the skin where it mediates cell-contact junctions with neighboring keratinocytes (Takeichi, 1990; Tang et al., 1993). Selective disruption of E-cadherin junctions prompts DC migration and maturation leading to upregulation of CCR7 without a concomitant increase in pro-inflammatory cytokine production (Jiang et al., 2007). TGF-β may also play a role in retaining LCs because TGF-β1-deficient mice lack LCs (but not other DC subsets) and their migratory counterparts in skin-draining LNs despite the presence of LC precursors (Borkowski et al., 1996). Interestingly, TGF-β upregulates E-cadherin expression on DC precursors and inhibits their maturation (Geissmann et al., 1999) and CCR7 expression (Sato et al., 2000). Conversely, IL-1β, TNF-α, and lipopolysaccharide (LPS) favor DC detachment by decreasing E-cadherin mRNA and protein expression in DCs (Jakob and Udey, 1998). Therefore, it appears that DC retention signals are overruled by mobilizing signals, which bring about detachment from neighboring cells and matrix components to allow DCs to migrate.
Interstitial Migration
Once a mobilization signal has triggered DC detachment, the cells must migrate through tissues rich in extracellular matrix (ECM) proteins, such as collagen types I–IV, fibronectin and laminin. Some DCs (e.g. LCs leaving the epidermis) must additionally traverse a basement membrane before gaining access to afferent lymphatics. To deal with these obstacles, maturing DCs upregulate proteolytic enzymes including membrane-bound and secreted forms of matrix metalloproteinases (MMPs), particularly MMP-2 and MMP-9 (Ratzinger et al., 2002; Yen et al., 2008). Pharmacological inhibition (Lebre et al., 1999) or antibody neutralization (Kobayashi et al., 1999) of MMP activity inhibit DC migration in Matrigel assays as well as LC emigration from skin explants. Likewise, MMP-9-deficient DCs are markedly defective in transepithelial migration in vitro (Ichiyasu et al., 2004) and they also migrate poorly to LNs in vivo (Ratzinger et al., 2002; Yen et al., 2008). Interstitial DC migration is controlled, in part, by tissue inhibitors of metalloproteinases (TIMPs), which function as endogenous regulators of MMP activity and block DC emigration from skin explants (Ratzinger et al., 2002). DCs downregulate TIMP expression upon maturation, thus tipping the balance of MMP-TIMP activity in favor of ECM degradation (Darmanin et al., 2007). Interestingly, the MMP-TIMP system may exert additional control over leukocyte trafficking via their action on chemokines. MMP-mediated proteolysis can inactivate chemokines or generate antagonistic or agonistic ‘cryptic’ chemokine derivatives (Van Lint and Libert, 2007), but the specific consequences of chemokine-MMP interactions for DC biology are still poorly understood.
Besides acquiring the capacity to overcome ECM barriers, maturing DCs must also develop the means to find local lymph vessels by switching their chemokine receptor repertoire to one that favors responsiveness towards LN-tropic chemokines (Dieu et al., 1998; Sallusto et al., 1998; Sozzani et al., 1998). The best-validated chemokine pathway for DC migration to LNs is CCR7 and its ligands, CCL19 and CCL21. Initial studies in mice homozygous for the paucity of lymph node T cell (plt) mutation – later described as a defect in the production of two of the three CCR7 ligands found in mice, CCL19 and CCL21-Ser (Luther et al., 2000; Nakano and Gunn, 2001; Vassileva et al., 1999) – demonstrated defective DC migration to LNs at steady state and following contact sensitization (Gunn et al., 1999). These findings were later substantiated by studies in Ccr7−/− mice (Forster et al., 1999; Martin-Fontecha et al., 2003; Ohl et al., 2004). Interestingly, CCR7 ligands are differentially expressed in mice with both CCL19 and CCL21-Ser localized to the LN paracortex and subcapsular sinus (SCS), whereas afferent lymphatics express both CCL21 isoforms, CCL21-Ser and CCL21-Leu (Vassileva et al., 1999). Exposure to TNF-α substantially increases CCL21 expression by lymphatic endothelial cells, thus making the reactive vessels even more attractive to migrating DCs (Martin-Fontecha et al., 2003). In plt/plt mutant mice, which express CCL21-Leu in peripheral lymphatics, but not in LNs, DCs still migrate out of the epidermis and collect in dermal lymph vessels (Gunn et al., 1999). Consistent with this, neutralizing antibodies to CCL21 inhibit the migration of skin-derived DCs into skin-draining LNs (Saeki et al., 1999), suggesting that the DC migration defect in plt/plt mice occurred at the level of entry from lymph into LNs and not into afferent lymphatics, while CCR7 deficient DCs are compromised at both steps.
Despite the indispensible role of CCR7 for DC migration to draining LNs, CCR7 expression is not an obligatory predictor for this migration event. The capacity to migrate to draining LNs via lymphatics is much greater for CD8α−/lo DCs as compared to CD8αhi DCs (Mempel et al., 2004; Smith and Fazekas de St Groth, 1999). Mature CD8α+ DCs express uniformly high surface amounts of CCR7, whereas only ~60% of mature CD8α− DCs are CCR7+ (Colvin et al., 2004). This discrepancy is consistent with a requirement for functional priming of CCR7 activity and suggests that additional signals, such as lipid mediators (Del Prete et al., 2007) or other trafficking molecules, may be involved. For instance, epicutaneous sensitization increases CXCR4 expression on migratory skin DCs, while the CXCR4 ligand, CXCL12, is concomitantly upregulated in dermal lymphatics (Kabashima et al., 2007). Moreover, CXCR4 inhibition impairs LC and dermal DC migration to draining LNs following FITC painting, indicating that both CCR7 and CXCR4 make independent contributions. Interestingly, LCs chemotax more efficiently to CXCL12 than CCL21, however, when simultaneously exposed to both chemokines they migrate preferentially to CCL21.
Although LCs represent arguably the most prominent DC population in the skin, a number of other DC subsets reside there including dermal DCs, small numbers of pDCs, monocyte-derived DCs, and the recently described dermal Langerin+ DCs (Bursch et al., 2007; Ginhoux et al., 2007; Poulin et al., 2007). The latter subset represents a novel population of DCs that are recruited to the skin via the blood in an E-/P-selectin and CCR2-dependent manner (Ginhoux et al., 2007). These cells constitutively patrol the dermis and migrate in a CCR7-dependent fashion to draining LNs where they present skin-derived Ags (Bursch et al., 2007; Ginhoux et al., 2007). Besides fully differentiated DCs, HSPCs that can give rise to DCs also traffic from the blood through extramedullary tissues into the draining lymphatics and then recirculate back into the blood via the TD (Massberg et al., 2007). HSPCs do not express CCR7, but their egress from tissues into the draining lymphatics is strictly dependent on S1P1 and its sphingolipid ligand, S1P (Massberg et al., 2007). S1P is abundant in lymph fluid, whereas its interstitial concentration is very low due to rapid degradation by sphingosine lyase (Cyster, 2005). S1P and its receptors also play a role in the interstitial migration of fully differentiated DCs, which express mRNA for all five known S1P receptors and migrate toward gradients of S1P in vitro (Czeloth et al., 2005; Maeda et al., 2007). Indeed, small molecule antagonists of S1P receptors block DC migration to the LNs from the skin (Czeloth et al., 2005; Gollmann et al., 2008) or lung (Idzko et al., 2006).
Traversing the Afferent Lymphatic endothelium
DC entry into afferent lymphatics remains poorly understood. It was initially assumed that entry was an indolent process, though there is now accumulating evidence that a number of traffic molecules play a role. For example, skin DCs are thought to enter the afferent lymphatics between overlapping junctions of oak leaf-shaped lymphatic endothelial cells in the initial dermal lymphatics, which are tethered to several molecules forming tight and adherens juntions, including the junctional adhesion molecules (JAMs) (Baluk et al., 2007). JAM-A-deficient mice show an increase in DC trafficking within the afferent lymphatics (Cera et al., 2004), suggesting that lymphatic endothelium normally restricts DC access.
Human and mouse primary lymphatic endothelial cells upregulate E-selectin, chemokines (CCL5, CCL20, and CXCL5), and adhesion molecules (ICAM-1 and VCAM-1) following cytokine stimulation in vitro or in vivo (Johnson et al., 2006). Though this inducible expression pattern is reminiscent of the molecular determinants that participate in multistep adhesion cascades in blood vessels, it is not clear what role selectins or integrins play in lymph vessels where shear stress is comparatively low. Nevertheless, neutralizing antibodies to ICAM-1 and VCAM-1 block DC adhesion to and transmigration across lymphatic endothelium in vitro and in vivo (Johnson et al., 2006), and ICAM-1 deficient mice show impaired LC migration to LNs (Xu et al., 2001). More recently, however, the contribution of integrins was evaluated in DCs that were deficient in all integrin heterodimers (Lämmermann et al., 2008). When mutant DCs were injected subcutaneously, they were able to migrate into the afferent lymphatics and enter LNs, arguing that at least some DCs can travel from peripheral tissues into LNs without requiring integrins. Similarly, LC migration into LNs after contact sensitization is not impaired in FucTVII-deficient mice, which cannot synthesize selectin ligands, suggesting that selectins are also not required (Erdmann et al., 2002).
Although DC traffic into lymphatics is markedly facilitated by inflammatory signals (Martin-Fontecha et al., 2003), this process eventually must be turned off to allow restoration of the steady-state. In this regard, D6, a promiscuous chemokine-scavenging receptor expressed on lymphatic endothelium, plays an important role by controlling the levels of inflammatory chemokines in tissues (Mantovani et al., 2006). Immune-inflammatory responses that are self-limiting in wildtype mice, go unchecked in D6-deficient animals leading to massive inflammatory cell infiltration due to inadequate clearance of pro-inflammatory chemokines (Martinez de la Torre et al., 2005). It seems likely that this process also plays a role in the trafficking of DCs into and/or out of inflamed and healing tissues, but the precise impact of D6 on local DC dynamics remains to be defined. In addition to D6, which intercepts inflammatory chemokines, another non-signaling chemokine receptor, CCX-CKR1 expressed by stromal cells in the epidermis and LNs, has been described; this receptor binds constitutively expressed chemokines (i.e. CCL19 and CCL21) and in doing so is thought to regulate the steady-state migration of CD11c+ MHC-IIhi DCs from the skin to draining LNs via the afferent lymphatics (Heinzel et al., 2007).
Transit through Afferent Lymphatics
The parameters that control leukocyte movement along the lymphatic tree are unclear. The simplest scenario would be that lymph-borne leukocytes are passively swept into LNs along the lymph vessels, which drain interstitial fluid in a uni-directional manner. If so, cellular transit is determined by lymph flow, which depends on peripheral microvascular permeability, interstitial, hydrostatic and oncotic pressure gradients, pulsation of nearby blood vessels, contraction of skeletal musculature, and the intrinsic contractile properties of larger collecting lymph vessels (Swartz, 2001). Indeed, studies cannulating afferent lymph vessels documented a direct association between changes in lymph flow and cell yield (Smith et al., 1970). Although biophysical forces are clearly important for DC transport in lymphatics, it is unclear whether other factors, such as lymph-borne chemokines or S1P could play a modulating role. It is also unknown whether DCs interact with cellular or structural elements inside lymph vessels, such as the thread-like structures that have recently been visualized in collecting lymphatics (Johng et al., 2007), or whether lymph-borne DCs can actually emigrate from lymph vessels in regions other than LNs. In support of the latter, ICAM-1 and VCAM-1 have been shown to be expressed on both luminal and abluminal sides of the lymphatic endothelium and support bi-directional DC migration in vitro (Johnson et al., 2006). While it is unknown if this occurs in vivo, leukocyte movement out of lymphatics could bear important consequences for immune surveillance patterns in peripheral tissues.
DC networks and DC migration and motility within the SLOs
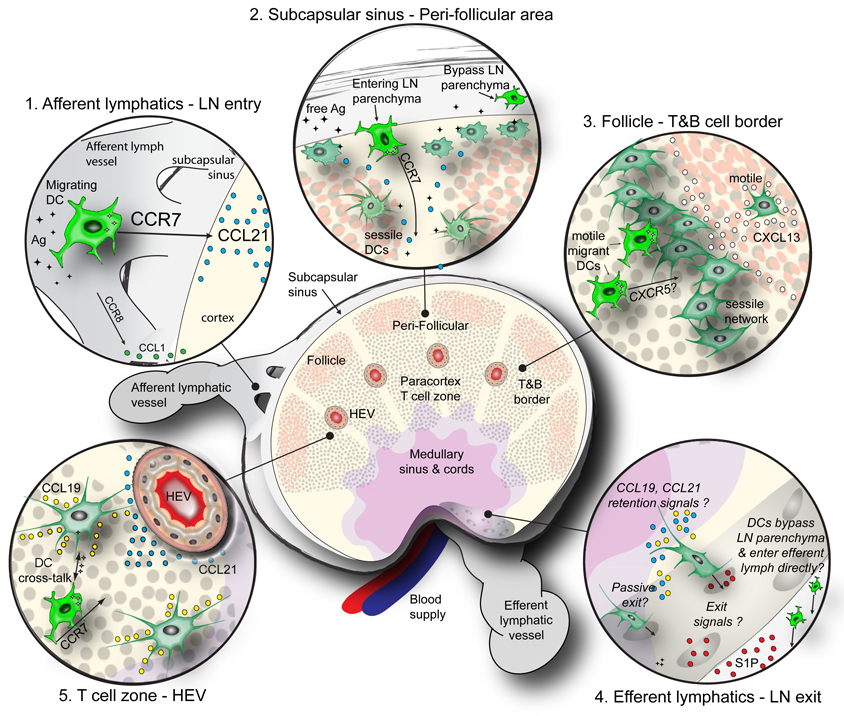
Tissue-derived migratory DCs reach the SCS in draining LNs via afferent lymphatics, but where and how they enter into the underlying LN parenchyma and what determines their further trajectory is not completely understood. For example, although several integrins are highly expressed on DCs, peripheral DCs do not require these molecules to travel into and within the LN cortex (Lämmermann et al., 2008). Thus, it is unclear how DCs gain sufficient traction to maneuver through the densely packed LN parenchyma, which is subdivided into several anatomic compartments (Figure 4). Afferent lymphatic vessels, which attach to the outer surface of the LN capsule and also form continuous connections with the underlying SCS (Kowala and Schoefl, 1986), discharge lymph-borne DCs into the SCS where they are retained by unknown mechanisms. To get into the parenchyma, the newly arrived DCs must overcome a triple barrier imposed by the SCS floor, consisting of sinus-lining cells, a layer of collagenous ECM, and a network of reticular stroma cells that are sandwiched together and encase the superficial cortex. Evidence suggests that DCs overcome this barrier by following a gradient of CCL21, which is abundantly expressed in the paracortex (Nakano and Gunn, 2001). By contrast, in LNs of plt/plt mice that lack CCL21, DCs accumulate in the SCS rather than migrating to the LN paracortex and, consequently, these mutant mice show increased susceptibility to infection, and dysregulated T cell responses (Gunn et al., 1999; Mori et al., 2001). Further confirmation came from studies in Ccr7−/− mice, which have fewer and morphologically altered DCs in the paracortex than wildtype mice (Forster et al., 1999). Besides the CCR7 pathway, it has also been suggested that the CCR8-CCL1 axis may control DC access to the LN parenchyma. CCL1 is expressed near the outer regions of the SCS and the cortex, and monocyte-derived CCR8-deficient DCs migrate poorly to LNs although they emigrate normally from peripheral tissues (Qu et al., 2004).
Fig. 4.
DC networks and migratory pathways of DCs within the LN. This schematic depicts the DC network and anatomic features within the LN, including (clockwise from top left), the afferent lymphatics/LN entry point (inset 1), subcapsular sinus and the peri-follicular region (inset 2), the B cell follicle and T&B cell border (inset 3), the efferent lymphatics and LN exit point (inset 4), and the T cell zone/HEV (inset 5). Major structural features of the LN are depicted, in addition to T cells (grey circles), B cells (brown circles), and free flowing or processed Ag (black diamonds). The major trafficking pathways and chemotactic molecules guiding migratory and resident DC subsets within each zone are highlighted.
CCR7 is also believed to be involved in guiding migrating DCs deeper into the cortex where CCL19 and CCL21 are most strongly expressed in and around HEVs. This may setup an interstitial gradient along which CCR7+ DCs travel. Thus, within one day after departing from peripheral tissues, lymph-derived mature DCs become concentrated around HEVs (Bajenoff et al., 2003). This conspicuous localization may allow newly arrived DCs to function like a “welcoming committee” by presenting recently acquired peripheral Ags to both B and T cells that constantly diapedese across HEVs from the blood (Mempel et al., 2004; Qi et al., 2006). In addition to following chemokine gradients, DCs within the T cell area can themselves produce CCL19, thus providing a gradient not only for T cells but also for other mature DCs to reach the T cell zone and perhaps engage in DC-DC encounters that might lead to the transfer of antigenic material (Cyster, 1999).
Within the migrating pool, different DCs colonize distinct areas of the LN. For example, a subset of skin-derived DCs upregulates CXCR5 and becomes responsive to CXCL13, which attracts them into the follicular region (Saeki et al., 2000) independently of CCR7 (Forster et al., 1999). Dermal DCs migrate to the outer paracortex below B cell follicles, whereas slower migrating LCs colonize preferentially the inner paracortex (Kissenpfennig et al., 2005). The specific cues that confine dermal DC and LC positioning to these specific areas and the immunological consequences of this differential distribution remain unknown. The first migratory DCs require 12–18h to reach the draining LN after Ag-challenge in the skin (Kissenpfennig et al., 2005). By contrast, soluble free Ag can enter the lymph much more quickly and is processed by LN-resident DCs that initiate early T cell priming events within the first four hours after Ag challenge. However, the slower moving wave of Ag-bearing skin-derived DCs is required to induce full-fledged effector responses (Itano et al., 2003).
Recent MP-IVM studies have provided a glimpse into the motility and distribution of DCs in LNs. Some have described the behavior of DCs that had recently arrived through afferent lymphatics (Bousso and Robey, 2003; Mempel et al., 2004; Miller et al., 2004b), while others have imaged LN-resident DCs (Hugues et al., 2004; Lindquist et al., 2004). Peripheral migratory DCs follow random trajectories within the T cell area, and exhibit high motility reaching a peak ~24h after their entry into the LN that subsides during the following day (Mempel et al., 2004). By contrast, the LN-resident DCs are organized in a sprawling network that permeates the perifollicular region and extends into T cell zone. Most of these resident DCs appear to be anchored in place although many display highly motile dendrites that are thought to enhance the contact frequency with surrounding T cells (Lindquist et al., 2004).
The extensive dendritic probings and motility exhibited by LN-resident DCs is dependent on the dynamics of the actin cytoskeleton regulated by Rho family GTPases. Inhibition of the Rac-cdc42-Ral pathway markedly reduces dendritic probings as well as short and long-term contacts with T cells in vitro (Swetman et al., 2002). Moreover, DCs from Rac1−/− or Rac2−/− mice show severe alterations in dendrite formation and defective migration in vivo (Benvenuti et al., 2004). Similarly, interference in a key downstream effector of the Cdc42 pathway, the Wiskott-Aldrich syndrome protein (WASp), leads to defects in DC trafficking from skin to draining LNs and in DC localization within LNs (de Noronha et al., 2005). Additional regulation in cytoskeletal dynamics is exerted by phosphoinositide 3- kinases (PI3Ks), which serve as a 'compass' by controlling F-actin localization and leukocyte polarity towards chemoattractants (Rickert et al., 2000). Accordingly, PI3K−/− DCs migrate poorly towards chemotactic factors in vitro and in vivo, leading to defective cutaneous hypersensitivity responses in PI3K−/− mice (Del Prete et al., 2004).
The available MP-IVM observations suggest that newly arriving migratory DCs initially display much higher motility as compared to their LN-resident sessile counterparts, but they slow down over time and eventually join the established DC network. The “motor” that drives DC motility in LNs has not been identified. However, intranodal T cell motility depends, in part, on CCR7, which interacts with ligands that are probably immobilized on the branched FRC network, a collagen-rich organized ECM meshwork that is ensheathed by fibroblastic reticular cells (Okada and Cyster, 2007; Worbs et al., 2007). This network directs and confines the seemingly random migration of T cells in the paracortex (Bajenoff et al., 2006) and probably has a role in both the guidance of migratory DCs and as a rigid scaffold for sessile DCs. One could speculate in this context that the gradual loss of DC motility after arrival in the LN might be caused by progressive attenuation of CCR7 signaling either due to desensitization or downregulation of the receptor.
DCs have also been detected in small numbers in TD lymph (Bell, 1979; Cavanagh et al., 2005), suggesting that not all DCs that enter LNs stay there. There is evidence that some DCs in efferent lymph originate from peripheral tissues, but it is not clear whether and to what extent the LN-resident pool contributes to this migratory population (Bonasio et al., 2006; Cavanagh et al., 2005; Dandie et al., 1994). It is also not clear whether tissue-derived migratory DCs trek through the LN parenchyma, or whether they merely flow through the SCS and/or trabecular sinuses, to enter efferent lymph vessels. Likewise, whether and how DCs within the node enter into lymphatic sinusoids to exit the node has not been determined.
DC Network in Spleen
Multiple signals influence DC positioning and migration within the spleen. In mice, resting CD8α− DCs localize mainly to the MZ, while CD8α+ DCs are enriched in the T cell area (Leenen et al., 1998). Additionally, some scattered CD11c+ cells can be found in the red pulp, although the mechanisms that guide them there are not clear (Metlay et al., 1990). Although DCs have not been found in the B follicles of resting spleens in mice, some DCs are detectable in human B cell follicles (Pack et al., 2008). As human spleen samples are not ‘resting’ when compared to experimental mice that are typically kept in a specific pathogen-free environment, it is possible that DCs in human B follicles represent a subset of activated DCs. This idea is supported by findings in mice where a small fraction of DCs that bind the cysteine-rich domain of the mannose receptor express CXCR5 upon activation and are attracted to B follicles by CXCL13 (Yu et al., 2002). However, the vast majority of splenic DCs enter the T cell zone upon activation because they upregulate CCR7, which allows them to respond to CCL19 and CCL21 gradients emanating from the PALS (Reis e Sousa et al., 1997). There is also evidence that S1P contributes to the positioning of some splenic DC subsets, although the role of S1P depends on the immunological context as pharmacologic interruption of the S1P receptor, S1P1, affects the positioning of immature but not mature DCs even though mature DCs express higher levels of S1P1 (Czeloth et al., 2007). Finally, the intrasplenic distribution of DCs is also influenced by B cell-derived lymphotoxin (LT)-α1β2; DCs must express LTβR to accumulate in the MZ (Wu et al., 1999). However, it is controversial whether LTβR is needed for DC positioning within the spleen or for DC homeostasis (Kabashima et al., 2005).
Concluding remarks
Here, we have summarized our current understanding of the mechanisms and consequences of DC migration. The emerging picture is that of a sophisticated roadmap where distinct DC subsets and their precursors follow site-specific and context-dependent traffic signals that have one single-minded purpose: the efficient dissemination and targeted delivery of biological information that determines the very nature of both cellular and humoral immune responses. Circulating and recirculating DC precursors provide a flexible supply of fresh cells to target tissues throughout the body where the differentiating DCs acquire an organ-specific phenotype. Tissue-resident DCs then collect antigenic material while simultaneously gathering intelligence about the presence and character of innocuous and noxious events in their surroundings. DCs possess the unique capacity to integrate and translate the collected information into molecular recognition patterns for lymphocytes. They then seek out their cellular audience in lymphoid tissues to provide multi-facetted instructions that may prevent, promote or modify immune responses, as the case may be.
Our roadmap still lacks many of the all-important details. At every turn along the way we find more questions than answers. How do DCs and their precursors decide to leave tissues in the steady state? How do they enter the blood stream? The thoracic duct is one established route, but how and where might extravascular cells migrate directly into blood vessels, especially in the BM, which is thought to be devoid of draining lymph vessels? What are the specific molecular events that constitute the multi-step adhesion cascades for DC recruitment from the blood? Are there tissue-tropic DC subsets in the circulation, analogous to the organ-specific effector and memory lymphocytes? What are the environmental signals that prompt newly homed DC progenitors to assume a subset-specific phenotype? Why do some DCs, especially the CD8α+ subset, fail to gain access to lymph vessels in peripheral tissues? To what extent do lymph vessels exert a gate-keeper function for DC migration and how do DCs interact with lymphatic endothelial cells? What signals do DCs perceive while in the lymph? Is their movement through lymphatics merely a passive ride to the downstream LNs or can DCs interact with and perhaps even emigrate from lymphatics while en route? What penotypic and migratory changes do DCs undergo after they have accessed SLOs and how do these changes affect their immunogenicity? Why are LNs so remarkably efficient in retaining migrating DCs, but not lymphocytes even though both express CCR7 and S1P1? How do some rare lymph-borne DCs manage to bypass or depart from LNs and enter the efferent lymph? Once these escapees have returned to the blood what is their overall contribution to systemic immunity and tolerance? One answer, in particular, is especially coveted: that we as scientists and clinicians can one day exploit these migratory pathways and drive DCs into the realm of modern medicine where many are waiting to go along for the ride.
Acknowledgements
This work was supported, in part, by NIH grants RO1 AI069259, RO1 AI072252, PO1 AI078897 and PO1 HL56949 (to U.H.v.A.), a Fellowship from the Canadian Institutes of Health Research (to D.A.) and a Fellowship from the Austrian Academy of Sciences (to E.H.V.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antonopoulos C, Cumberbatch M, Dearman RJ, Daniel RJ, Kimber I, Groves RW. Functional caspase-1 is required for Langerhans cell migration and optimal contact sensitization in mice. J. Immunol. 2001;166:3672–3677. doi: 10.4049/jimmunol.166.6.3672. [DOI] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- Austyn JM, Kupiec-Weglinski JW, Hankins DF, Morris PJ. Migration patterns of dendritic cells in the mouse. Homing to T cell-dependent areas of spleen, and binding within marginal zone. J. Exp. Med. 1988;167:646–651. doi: 10.1084/jem.167.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajenoff M, Granjeaud S, Guerder S. The Strategy of T Cell Antigen-presenting Cell Encounter in Antigen-draining Lymph Nodes Revealed by Imaging of Initial T Cell Activation. J. Exp. Med. 2003;198:715–724. doi: 10.1084/jem.20030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Baumjohann D, Lutz MB. Non-invasive imaging of dendritic cell migration in vivo. Immunobiology. 2006;211:587–597. doi: 10.1016/j.imbio.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Bell EB. Antigen-laden cells in thoracic duct lymph. Implications for adoptive transfer experiments. Immunology. 1979;38:797–808. [PMC free article] [PubMed] [Google Scholar]
- Benvenuti F, Hugues S, Walmsley M, Ruf S, Fetler L, Popoff M, Tybulewicz VL, Amigorena S. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 2004;305:1150–1153. doi: 10.1126/science.1099159. [DOI] [PubMed] [Google Scholar]
- Blazar BR, Lindberg FP, Ingulli E, Panoskaltsis-Mortari A, Oldenborg PA, Iizuka K, Yokoyama WM, Taylor PA. CD47 (integrin-associated protein) engagement of dendritic cell and macrophage counterreceptors is required to prevent the clearance of donor lymphohematopoietic cells. J. Exp. Med. 2001;194:541–549. doi: 10.1084/jem.194.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat. Immunol. 2006;7:1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- Bonasio R, von Andrian UH. Generation, migration and function of circulating dendritic cells. Curr. Opin. Immunol. 2006;18:503–511. doi: 10.1016/j.coi.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Borkowski TA, Letterio JJ, Farr AG, Udey MC. A role for endogenous transforming growth factor beta 1 in Langerhans cell biology: the skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans cells. J. Exp. Med. 1996;184:2417–2422. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchon A, Hernandez-Munain C, Cella M, Colonna M. A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J. Exp. Med. 2001;194:1111–1122. doi: 10.1084/jem.194.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousso P, Robey E. Dynamics of CD8(+) T cell priming by dendritic cells in intact lymph nodes. Nat. Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- Brand CU, Hunziker T, Braathen LR. Studies on human skin lymph containing Langerhans cells from sodium lauryl sulphate contact dermatitis. J. Invest. Dermatol. 1992;99:109S–110S. doi: 10.1111/1523-1747.ep12669997. [DOI] [PubMed] [Google Scholar]
- Bujdoso R, Hopkins J, Dutia BM, Young P, McConnell I. Characterization of sheep afferent lymph dendritic cells and their role in antigen carriage. J. Exp. Med. 1989;170:1285–1302. doi: 10.1084/jem.170.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, Hogquist KA. Identification of a novel population of Langerin+ dendritic cells. J. Exp. Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan MD, Parker I, Wei SH, Miller MJ. Two-photon tissue imaging: seeing the immune system in a fresh light. Nat. Rev. Immunol. 2002;2:872–880. doi: 10.1038/nri935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh LL, Bonasio R, Mazo IB, Halin C, Cheng G, van der Velden AW, Cariappa A, Chase C, Russell P, Starnbach MN, et al. Activation of bone marrow-resident memory T cells by circulating, antigen-bearing dendritic cells. Nat. Immunol. 2005;6:1029–1037. doi: 10.1038/ni1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- Cera MR, Del Prete A, Vecchi A, Corada M, Martin-Padura I, Motoike T, Tonetti P, Bazzoni G, Vermi W, Gentili F, et al. Increased DC trafficking to lymph nodes and contact hypersensitivity in junctional adhesion molecule-A-deficient mice. J. Clin. Invest. 2004;114:729–738. doi: 10.1172/JCI21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin BL, Morelli AE, Logar AJ, Lau AH, Thomson AW. Comparative evaluation of CC chemokine-induced migration of murine CD8alpha+ and CD8alpha- dendritic cells and their in vivo trafficking. J. Leukoc. Biol. 2004;75:275–285. doi: 10.1189/jlb.1202613. [DOI] [PubMed] [Google Scholar]
- Cumberbatch M, Dearman RJ, Kimber I. Interleukin 1 beta and the stimulation of Langerhans cell migration: comparisons with tumour necrosis factor alpha. Arch. Dermatol. Res. 1997;289:277–284. doi: 10.1007/s004030050193. [DOI] [PubMed] [Google Scholar]
- Cumberbatch M, Dearman RJ, Kimber I. Langerhans cell migration in mice requires intact type I interleukin 1 receptor (IL-1RI) function. Arch. Dermatol. Res. 1999;291:357–361. doi: 10.1007/s004030050422. [DOI] [PubMed] [Google Scholar]
- Cumberbatch M, Kimber I. Tumour necrosis factor-alpha is required for accumulation of dendritic cells in draining lymph nodes and for optimal contact sensitization. Immunology. 1995;84:31–35. [PMC free article] [PubMed] [Google Scholar]
- Cyster JG. Chemokines and the homing of dendritic cells to the T cell areas of lymphoid organs. J. Exp. Med. 1999;189:447–450. doi: 10.1084/jem.189.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- Czeloth N, Bernhardt G, Hofmann F, Genth H, Forster R. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J. Immunol. 2005;175:2960–2967. doi: 10.4049/jimmunol.175.5.2960. [DOI] [PubMed] [Google Scholar]
- Czeloth N, Schippers A, Wagner N, Muller W, Kuster B, Bernhardt G, Forster R. Sphingosine-1 phosphate signaling regulates positioning of dendritic cells within the spleen. J. Immunol. 2007;179:5855–5863. doi: 10.4049/jimmunol.179.9.5855. [DOI] [PubMed] [Google Scholar]
- Dandie GW, Watkins FY, Ragg SJ, Holloway PE, Muller HK. The migration of Langerhans' cells into and out of lymph nodes draining normal, carcinogen and antigen-treated sheep skin. Immunol. Cell Biol. 1994;72:79–86. doi: 10.1038/icb.1994.12. [DOI] [PubMed] [Google Scholar]
- Darmanin S, Chen J, Zhao S, Cui H, Shirkoohi R, Kubo N, Kuge Y, Tamaki N, Nakagawa K, Hamada J, et al. All-trans retinoic acid enhances murine dendritic cell migration to draining lymph nodes via the balance of matrix metalloproteinases and their inhibitors. J. Immunol. 2007;179:4616–4625. doi: 10.4049/jimmunol.179.7.4616. [DOI] [PubMed] [Google Scholar]
- de Noronha S, Hardy S, Sinclair J, Blundell MP, Strid J, Schulz O, Zwirner J, Jones GE, Katz DR, Kinnon C, Thrasher AJ. Impaired dendritic-cell homing in vivo in the absence of Wiskott-Aldrich syndrome protein. Blood. 2005;105:1590–1597. doi: 10.1182/blood-2004-06-2332. [DOI] [PubMed] [Google Scholar]
- de Vries IJ, Lesterhuis WJ, Barentsz JO, Verdijk P, van Krieken JH, Boerman OC, Oyen WJ, Bonenkamp JJ, Boezeman JB, Adema GJ, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat. Biotechnol. 2005;23:1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- Del Prete A, Shao WH, Mitola S, Santoro G, Sozzani S, Haribabu B. Regulation of dendritic cell migration and adaptive immune response by leukotriene B4 receptors: a role for LTB4 in up-regulation of CCR7 expression and function. Blood. 2007;109:626–631. doi: 10.1182/blood-2006-02-003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete A, Vermi W, Dander E, Otero K, Barberis L, Luini W, Bernasconi S, Sironi M, Santoro A, Garlanda C, et al. Defective dendritic cell migration and activation of adaptive immunity in PI3Kgamma-deficient mice. EMBO J. 2004;23:3505–3515. doi: 10.1038/sj.emboj.7600361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rosa F, Pabst R. The bone marrow: a nest for migratory memory T cells. Trends Immunol. 2005;26:360–366. doi: 10.1016/j.it.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Diacovo TG, Blasius AL, Mak TW, Cella M, Colonna M. Adhesive mechanisms governing interferon-producing cell recruitment into lymph nodes. J. Exp. Med. 2005;202:687–696. doi: 10.1084/jem.20051035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Ait-Yahia S, Briere F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieu-Nosjean MC, Massacrier C, Homey B, Vanbervliet B, Pin JJ, Vicari A, Lebecque S, Dezutter-Dambuyant C, Schmitt D, Zlotnik A, Caux C. Macrophage inflammatory protein 3alpha is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J. Exp. Med. 2000;192:705–718. doi: 10.1084/jem.192.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donskoy E, Goldschneider I. Two developmentally distinct populations of dendritic cells inhabit the adult mouse thymus: demonstration by differential importation of hematogenous precursors under steady state conditions. J. Immunol. 2003;170:3514–3521. doi: 10.4049/jimmunol.170.7.3514. [DOI] [PubMed] [Google Scholar]
- Elbe A, Tschachler E, Steiner G, Binder A, Wolff K, Stingl G. Maturational steps of bone marrow-derived dendritic murine epidermal cells. Phenotypic and functional studies on Langerhans cells and Thy-1+ dendritic epidermal cells in the perinatal period. J. Immunol. 1989;143:2431–2438. [PubMed] [Google Scholar]
- Enk AH, Angeloni VL, Udey MC, Katz SI. An essential role for Langerhans cell-derived IL-1 beta in the initiation of primary immune responses in skin. J. Immunol. 1993;150:3698–3704. [PubMed] [Google Scholar]
- Enk AH, Katz SI. Early molecular events in the induction phase of contact sensitivity. Proc. Natl. Acad. Sci. U. S. A. 1992;89:1398–1402. doi: 10.1073/pnas.89.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann I, Scheidegger EP, Koch FK, Heinzerling L, Odermatt B, Burg G, Lowe JB, Kundig TM. Fucosyltransferase VII-deficient mice with defective E-, P-, and L-selectin ligands show impaired CD4+ and CD8+ T cell migration into the skin, but normal extravasation into visceral organs. J. Immunol. 2002;168:2139–2146. doi: 10.4049/jimmunol.168.5.2139. [DOI] [PubMed] [Google Scholar]
- Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL. Plasmacytoid dendritic cells (natural interferon- alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am. J. Pathol. 2001;159:237–243. doi: 10.1016/s0002-9440(10)61689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Krooshoop DJ, Bleijs DA, van Vliet SJ, van Duijnhoven GC, Grabovsky V, Alon R, Figdor CG, van Kooyk Y. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat. Immunol. 2000;1:353–357. doi: 10.1038/79815. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Revy P, Regnault A, Lepelletier Y, Dy M, Brousse N, Amigorena S, Hermine O, Durandy A. TGF-beta 1 prevents the noncognate maturation of human dendritic Langerhans cells. J. Immunol. 1999;162:4567–4575. [PubMed] [Google Scholar]
- Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, Helft J, Ochando J, Kissenpfennig A, Malissen B, Grisotto M, et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J. Exp. Med. 2007;204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, Stanley ER, Randolph GJ, Merad M. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollmann G, Neuwirt H, Tripp CH, Mueller H, Konwalinka G, Heufler C, Romani N, Tiefenthaler M. Sphingosine-1-phosphate receptor type-1 agonism impairs blood dendritic cell chemotaxis and skin dendritic cell migration to lymph nodes under inflammatory conditions. Int. Immunol. 2008 doi: 10.1093/intimm/dxn050. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Granucci F, Ferrero E, Foti M, Aggujaro D, Vettoretto K, Ricciardi-Castagnoli P. Early events in dendritic cell maturation induced by LPS. Microbes Infect. 1999;1:1079–1084. doi: 10.1016/s1286-4579(99)00209-9. [DOI] [PubMed] [Google Scholar]
- Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT, Nakano H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 1999;189:451–460. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halin C, Rodrigo Mora J, Sumen C, von Andrian UH. In vivo imaging of lymphocyte trafficking. Annu. Rev. Cell Dev. Biol. 2005;21:581–603. doi: 10.1146/annurev.cellbio.21.122303.133159. [DOI] [PubMed] [Google Scholar]
- Hammad H, Lambrecht BN. Lung dendritic cell migration. Adv. Immunol. 2007;93:265–278. doi: 10.1016/S0065-2776(06)93007-7. [DOI] [PubMed] [Google Scholar]
- Heinzel K, Benz C, Bleul CC. A silent chemokine receptor regulates steady-state leukocyte homing in vivo. Proc. Natl. Acad. Sci. U. S. A. 2007;104:8421–8426. doi: 10.1073/pnas.0608274104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt PG, Haining S, Nelson DJ, Sedgwick JD. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J. Immunol. 1994;153:256–261. [PubMed] [Google Scholar]
- Hugues S, Fetler L, Bonifaz L, Helft J, Amblard F, Amigorena S. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat. Immunol. 2004;5:1235–1242. doi: 10.1038/ni1134. [DOI] [PubMed] [Google Scholar]
- Ichiyasu H, McCormack JM, McCarthy KM, Dombkowski D, Preffer FI, Schneeberger EE. Matrix metalloproteinase-9-deficient dendritic cells have impaired migration through tracheal epithelial tight junctions. Am. J. Respir. Cell Mol. Biol. 2004;30:761–770. doi: 10.1165/rcmb.2003-0370OC. [DOI] [PubMed] [Google Scholar]
- Idzko M, Hammad H, van Nimwegen M, Kool M, Muller T, Soullie T, Willart MA, Hijdra D, Hoogsteden HC, Lambrecht BN. Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J. Clin. Invest. 2006;116:2935–2944. doi: 10.1172/JCI28295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzko M, Panther E, Corinti S, Morelli A, Ferrari D, Herouy Y, Dichmann S, Mockenhaupt M, Gebicke-Haerter P, Di Virgilio F, et al. Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. FASEB J. 2002;16:625–627. doi: 10.1096/fj.01-0625fje. [DOI] [PubMed] [Google Scholar]
- Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat. Rev. Immunol. 2004;4:432–444. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4(+) T cells. J. Exp. Med. 1997;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- Iwasaki A. Mucosal dendritic cells. Annu. Rev. Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- Jahnsen FL, Lund-Johansen F, Dunne JF, Farkas L, Haye R, Brandtzaeg P. Experimentally induced recruitment of plasmacytoid (CD123high) dendritic cells in human nasal allergy. J. Immunol. 2000;165:4062–4068. doi: 10.4049/jimmunol.165.7.4062. [DOI] [PubMed] [Google Scholar]
- Jakob T, Udey MC. Regulation of E-cadherin-mediated adhesion in Langerhans cell-like dendritic cells by inflammatory mediators that mobilize Langerhans cells in vivo. J. Immunol. 1998;160:4067–4073. [PubMed] [Google Scholar]
- Janatpour MJ, Hudak S, Sathe M, Sedgwick JD, McEvoy LM. Tumor necrosis factor-dependent segmental control of MIG expression by high endothelial venules in inflamed lymph nodes regulates monocyte recruitment. J. Exp. Med. 2001;194:1375–1384. doi: 10.1084/jem.194.9.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang A, Bloom O, Ono S, Cui W, Unternaehrer J, Jiang S, Whitney JA, Connolly J, Banchereau J, Mellman I. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27:610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johng HM, Yoo JS, Yoon TJ, Shin HS, Lee BC, Lee C, Lee JK, Soh KS. Use of magnetic nanoparticles to visualize threadlike structures inside lymphatic vessels of rats. Evid. Based. Complement Alternat. Med. 2007;4:77–82. doi: 10.1093/ecam/nel057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J. Exp. Med. 2006;203:2763–2777. doi: 10.1084/jem.20051759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashima K, Banks TA, Ansel KM, Lu TT, Ware CF, Cyster JG. Intrinsic lymphotoxin-beta receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity. 2005;22:439–450. doi: 10.1016/j.immuni.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Kabashima K, Shiraishi N, Sugita K, Mori T, Onoue A, Kobayashi M, Sakabe J, Yoshiki R, Tamamura H, Fujii N, et al. CXCL12-CXCR4 engagement is required for migration of cutaneous dendritic cells. Am. J. Pathol. 2007;171:1249–1257. doi: 10.2353/ajpath.2007.070225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath AT, Pooley J, O'Keeffe MA, Vremec D, Zhan Y, Lew AM, D'Amico A, Wu L, Tough DF, Shortman K. The development, maturation, and turnover rate of mouse spleen dendritic cell populations. J. Immunol. 2000;165:6762–6770. doi: 10.4049/jimmunol.165.12.6762. [DOI] [PubMed] [Google Scholar]
- Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Klesney-Tait J, Colonna M. Uncovering the TREM-1-TLR connection. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;293:L1374–L1376. doi: 10.1152/ajplung.00415.2007. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Matsumoto M, Kotani M, Makino T. Possible involvement of matrix metalloproteinase-9 in Langerhans cell migration and maturation. J. Immunol. 1999;163:5989–5993. [PubMed] [Google Scholar]
- Kowala MC, Schoefl GI. The popliteal lymph node of the mouse: internal architecture, vascular distribution and lymphatic supply. J. Anat. 1986;148:25–46. [PMC free article] [PubMed] [Google Scholar]
- Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132:612–630. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- Lämmermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Söldner R, Hirsch K, Keller M, Förster R, Critchley DR, Fässler R, Sixt M. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J. Immunol. 2007;178:2000–2007. doi: 10.4049/jimmunol.178.4.2000. [DOI] [PubMed] [Google Scholar]
- Lebre MC, Kalinski P, Das PK, Everts V. Inhibition of contact sensitizer-induced migration of human Langerhans cells by matrix metalloproteinase inhibitors. Arch. Dermatol. Res. 1999;291:447–452. doi: 10.1007/s004030050436. [DOI] [PubMed] [Google Scholar]
- Leenen PJ, Radosevic K, Voerman JS, Salomon B, van Rooijen N, Klatzmann D, van Ewijk W. Heterogeneity of mouse spleen dendritic cells: in vivo phagocytic activity, expression of macrophage markers, and subpopulation turnover. J. Immunol. 1998;160:2166–2173. [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Lim YC, Snapp K, Kansas GS, Camphausen R, Ding H, Luscinskas FW. Important contributions of P-selectin glycoprotein ligand-1-mediated secondary capture to human monocyte adhesion to P-selectin, E-selectin, and TNF-alpha-activated endothelium under flow in vitro. J. Immunol. 1998;161:2501–2508. [PubMed] [Google Scholar]
- Lin CL, Suri RM, Rahdon RA, Austyn JM, Roake JA. Dendritic cell chemotaxis and transendothelial migration are induced by distinct chemokines and are regulated on maturation. Eur. J. Immunol. 1998;28:4114–4122. doi: 10.1002/(SICI)1521-4141(199812)28:12<4114::AID-IMMU4114>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, Nussenzweig MC. Visualizing dendritic cell networks in vivo. Nat. Immunol. 2004;5:1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat. Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12694–12699. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Matsuyuki H, Shimano K, Kataoka H, Sugahara K, Chiba K. Migration of CD4 T cells and dendritic cells toward sphingosine 1-phosphate (S1P) is mediated by different receptor subtypes: S1P regulates the functions of murine mature dendritic cells via S1P receptor type 3. J. Immunol. 2007;178:3437–3446. doi: 10.4049/jimmunol.178.6.3437. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat. Rev. Immunol. 2006;6:907–918. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]
- Martin-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez de la Torre Y, Locati M, Buracchi C, Dupor J, Cook DN, Bonecchi R, Nebuloni M, Rukavina D, Vago L, Vecchi A, et al. Increased inflammation in mice deficient for the chemokine decoy receptor D6. Eur. J. Immunol. 2005;35:1342–1346. doi: 10.1002/eji.200526114. [DOI] [PubMed] [Google Scholar]
- Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, von Andrian UH. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- Merad M, Hoffmann P, Ranheim E, Slaymaker S, Manz MG, Lira SA, Charo I, Cook DN, Weissman IL, Strober S, Engleman EG. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat. Med. 2004;10:510–517. doi: 10.1038/nm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, Engleman EG. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlay JP, Witmer-Pack MD, Agger R, Crowley MT, Lawless D, Steinman RM. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J. Exp. Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJ, Hejazi AS, Wei SH, Cahalan MD, Parker I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc. Natl. Acad. Sci. U. S. A. 2004a;101:998–1003. doi: 10.1073/pnas.0306407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J. Exp. Med. 2004b;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Nakano H, Aritomi K, Wang CR, Gunn MD, Kakiuchi T. Mice lacking expression of the chemokines CCL21-ser and CCL19 (plt mice) demonstrate delayed but enhanced T cell immune responses. J. Exp. Med. 2001;193:207–218. doi: 10.1084/jem.193.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse MA, Coleman RE, Akabani G, Niehaus N, Coleman D, Lyerly HK. Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res. 1999;59:56–58. [PubMed] [Google Scholar]
- Mullins DW, Sheasley SL, Ream RM, Bullock TN, Fu YX, Engelhard VH. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J. Exp. Med. 2003;198:1023–1034. doi: 10.1084/jem.20021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, Carotta S, O'Keeffe M, Bahlo M, Papenfuss A, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat. Immunol. 2007;8:1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- Nakano H, Gunn MD. Gene duplications at the chemokine locus on mouse chromosome 4: multiple strain-specific haplotypes and the deletion of secondary lymphoid-organ chemokine and EBI-1 ligand chemokine genes in the plt mutation. J. Immunol. 2001;166:361–369. doi: 10.4049/jimmunol.166.1.361. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, Burg G, Liu YJ, Gilliet M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, Forster R. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Okada T, Cyster JG. CC Chemokine Receptor 7 Contributes to Gi-Dependent T Cell Motility in the Lymph Node. J. Immunol. 2007;178:2973–2978. doi: 10.4049/jimmunol.178.5.2973. [DOI] [PubMed] [Google Scholar]
- Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat. Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- Pack M, Trumpfheller C, Thomas D, Park CG, Granelli-Piperno A, Munz C, Steinman RM. DEC-205/CD205+ dendritic cells are abundant in the white pulp of the human spleen, including the border region between the red and white pulp. Immunology. 2008;123:438–446. doi: 10.1111/j.1365-2567.2007.02710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palframan RT, Jung S, Cheng G, Weninger W, Luo Y, Dorf M, Littman DR, Rollins BJ, Zweerink H, Rot A, von Andrian UH. Inflammatory chemokine transport and presentation in HEV: A remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 2001;194:1361–1374. doi: 10.1084/jem.194.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendl GG, Robert C, Steinert M, Thanos R, Eytner R, Borges E, Wild MK, Lowe JB, Fuhlbrigge RC, Kupper TS, et al. Immature mouse dendritic cells enter inflamed tissue, a process that requires E- and P-selectin, but not P-selectin glycoprotein ligand 1. Blood. 2002;99:946–956. doi: 10.1182/blood.v99.3.946. [DOI] [PubMed] [Google Scholar]
- Penna G, Sozzani S, Adorini L. Cutting edge: selective usage of chemokine receptors by plasmacytoid dendritic cells. J. Immunol. 2001;167:1862–1866. doi: 10.4049/jimmunol.167.4.1862. [DOI] [PubMed] [Google Scholar]
- Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J. Exp. Med. 2007;204:3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- Qu C, Edwards EW, Tacke F, Angeli V, Llodra J, Sanchez-Schmitz G, Garin A, Haque NS, Peters W, van Rooijen N, et al. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J. Exp. Med. 2004;200:1231–1241. doi: 10.1084/jem.20032152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzinger G, Stoitzner P, Ebner S, Lutz MB, Layton GT, Rainer C, Senior RM, Shipley JM, Fritsch P, Schuler G, Romani N. Matrix metalloproteinases 9 and 2 are necessary for the migration of Langerhans cells and dermal dendritic cells from human and murine skin. J. Immunol. 2002;168:4361–4371. doi: 10.4049/jimmunol.168.9.4361. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickert P, Weiner OD, Wang F, Bourne HR, Servant G. Leukocytes navigate by compass: roles of PI3Kgamma and its lipid products. Trends Cell Biol. 2000;10:466–473. doi: 10.1016/s0962-8924(00)01841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Fuhlbrigge RC, Kieffer JD, Ayehunie S, Hynes RO, Cheng G, Grabbe S, von Andrian UH, Kupper TS. Interaction of dendritic cells with skin endothelium: A new perspective on immunosurveillance. J. Exp. Med. 1999;189:627–636. doi: 10.1084/jem.189.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu. Rev. Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- Ruedl C, Koebel P, Bachmann M, Hess M, Karjalainen K. Anatomical origin of dendritic cells determines their life span in peripheral lymph nodes. J. Immunol. 2000;165:4910–4916. doi: 10.4049/jimmunol.165.9.4910. [DOI] [PubMed] [Google Scholar]
- Saeki H, Moore AM, Brown MJ, Hwang ST. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J. Immunol. 1999;162:2472–2475. [PubMed] [Google Scholar]
- Saeki H, Wu MT, Olasz E, Hwang ST. A migratory population of skin-derived dendritic cells expresses CXCR5, responds to B lymphocyte chemoattractant in vitro, and co-localizes to B cell zones in lymph nodes in vivo. Eur. J. Immunol. 2000;30:2808–2814. doi: 10.1002/1521-4141(200010)30:10<2808::AID-IMMU2808>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR, Qin S, Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 1998;28:2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Sato K, Kawasaki H, Nagayama H, Enomoto M, Morimoto C, Tadokoro K, Juji T, Takahashi TA. TGF-beta 1 reciprocally controls chemotaxis of human peripheral blood monocyte-derived dendritic cells via chemokine receptors. J. Immunol. 2000;164:2285–2295. doi: 10.4049/jimmunol.164.5.2285. [DOI] [PubMed] [Google Scholar]
- Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- Silberberg-Sinakin I, Thorbecke GJ, Baer RL, Rosenthal SA, Berezowsky V. Antigen-bearing langerhans cells in skin, dermal lymphatics and in lymph nodes. Cell. Immunol. 1976;25:137–151. doi: 10.1016/0008-8749(76)90105-2. [DOI] [PubMed] [Google Scholar]
- Smith AL, Fazekas de St Groth B. Antigen-pulsed CD8alpha+ dendritic cells generate an immune response after subcutaneous injection without homing to the draining lymph node. J. Exp. Med. 1999;189:593–598. doi: 10.1084/jem.189.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JB, McIntosh GH, Morris B. The migration of cells through chronically inflamed tissues. J. Pathol. 1970;100:21–29. doi: 10.1002/path.1711000104. [DOI] [PubMed] [Google Scholar]
- Sozzani S, Allavena P, D'Amico G, Luini W, Bianchi G, Kataura M, Imai T, Yoshie O, Bonecchi R, Mantovani A. Cutting edge: Differential regulation of chemokine receptors during dendritic cell maturation: A model for their trafficking properties. J. Immunol. 1998;161:1083–1086. [PubMed] [Google Scholar]
- Sozzani S, Luini W, Borsatti A, Polentarutti N, Zhou D, Piemonti L, D'Amico G, Power CA, Wells TNC, Gobbi M, et al. Receptor expression and responsiveness of human dendritic cells to a defined set of CC and CXC chemokines. J. Immunol. 1997;159:1993. [PubMed] [Google Scholar]
- Sozzani S, Sallusto F, Luini W, Zhou D, Piemonti L, Allavena P, Van Damme J, Valitutti S, Lanzavecchia A, Mantovani A. Migration of dendritic cells in response to formyl peptides, C5a, and a distinct set of chemokines. J. Immunol. 1995;155:3292–3295. [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multi-step paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Stumbles PA, Strickland DH, Pimm CL, Proksch SF, Marsh AM, McWilliam AS, Bosco A, Tobagus I, Thomas JA, Napoli S, et al. Regulation of dendritic cell recruitment into resting and inflamed airway epithelium: use of alternative chemokine receptors as a function of inducing stimulus. J. Immunol. 2001;167:228–234. doi: 10.4049/jimmunol.167.1.228. [DOI] [PubMed] [Google Scholar]
- Stutte S, Jux B, Esser C, Forster I. CD24a expression levels discriminate Langerhans cells from dermal dendritic cells in murine skin and lymph nodes. J. Invest. Dermatol. 2008;128:1470–1475. doi: 10.1038/sj.jid.5701228. [DOI] [PubMed] [Google Scholar]
- Swartz MA. The physiology of the lymphatic system. Adv. Drug Deliv. Rev. 2001;50:3–20. doi: 10.1016/s0169-409x(01)00150-8. [DOI] [PubMed] [Google Scholar]
- Swetman CA, Leverrier Y, Garg R, Gan CH, Ridley AJ, Katz DR, Chain BM. Extension, retraction and contraction in the formation of a dendritic cell dendrite: distinct roles for Rho GTPases. Eur. J. Immunol. 2002;32:2074–2083. doi: 10.1002/1521-4141(200207)32:7<2074::AID-IMMU2074>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takegahara N, Takamatsu H, Toyofuku T, Tsujimura T, Okuno T, Yukawa K, Mizui M, Yamamoto M, Prasad DV, Suzuki K, et al. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol. 2006;8:615–622. doi: 10.1038/ncb1416. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherins: A molecular family important in selective cell- cell adhesion. Annu. Rev. Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- Tang A, Amagai M, Granger LG, Stanley JR, Udey MC. Adhesion of epidermal Langerhans cells to keratinocytes mediated by E- cadherin. Nature. 1993;361:82–85. doi: 10.1038/361082a0. [DOI] [PubMed] [Google Scholar]
- Tomasello E, Desmoulins PO, Chemin K, Guia S, Cremer H, Ortaldo J, Love P, Kaiserlian D, Vivier E. Combined natural killer cell and dendritic cell functional deficiency in KARAP/DAP12 loss-of-function mutant mice. Immunity. 2000;13:355–364. doi: 10.1016/s1074-7613(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Tomura M, Yoshida N, Tanaka J, Karasawa S, Miwa Y, Miyawaki A, Kanagawa O. Monitoring cellular movement in vivo with photoconvertible fluorescence protein "Kaede" transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 2008;105:10871–10876. doi: 10.1073/pnas.0802278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J. Leukoc. Biol. 2007;82:1375–1381. doi: 10.1189/jlb.0607338. [DOI] [PubMed] [Google Scholar]
- Van VQ, Lesage S, Bouguermouh S, Gautier P, Rubio M, Levesque M, Nguyen S, Galibert L, Sarfati M. Expression of the self-marker CD47 on dendritic cells governs their trafficking to secondary lymphoid organs. EMBO J. 2006;25:5560–5568. doi: 10.1038/sj.emboj.7601415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J. Exp. Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassileva G, Soto H, Zlotnik A, Nakano H, Kakiuchi T, Hedrick JA, Lira SA. The reduced expression of 6Ckine in the plt mouse results from the deletion of one of two 6Ckine genes. J. Exp. Med. 1999;190:1183–1188. doi: 10.1084/jem.190.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermi W, Riboldi E, Wittamer V, Gentili F, Luini W, Marrelli S, Vecchi A, Franssen JD, Communi D, Massardi L, et al. Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. J. Exp. Med. 2005;201:509–515. doi: 10.1084/jem.20041310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol. Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J. Immunol. 2000;164:2978–2986. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- Walzer T, Galibert L, De Smedt T. Dendritic cell function in mice lacking Plexin C1. Int. Immunol. 2005;17:943–950. doi: 10.1093/intimm/dxh274. [DOI] [PubMed] [Google Scholar]
- Wang B, Fujisawa H, Zhuang L, Kondo S, Shivji GM, Kim CS, Mak TW, Sauder DN. Depressed Langerhans cell migration and reduced contact hypersensitivity response in mice lacking TNF receptor p75. J. Immunol. 1997;159:6148–6155. [PubMed] [Google Scholar]
- Wendland M, Czeloth N, Mach N, Malissen B, Kremmer E, Pabst O, Forster R. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc. Natl. Acad. Sci. U. S. A. 2007;104:6347–6352. doi: 10.1073/pnas.0609180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wethmar K, Helmus Y, Luhn K, Jones C, Laskowska A, Varga G, Grabbe S, Lyck R, Engelhardt B, Bixel MG, et al. Migration of immature mouse DC across resting endothelium is mediated by ICAM-2 but independent of beta2-integrins and murine DC-SIGN homologues. Eur. J. Immunol. 2006;36:2781–2794. doi: 10.1002/eji.200526311. [DOI] [PubMed] [Google Scholar]
- Wong AW, Brickey WJ, Taxman DJ, van Deventer HW, Reed W, Gao JX, Zheng P, Liu Y, Li P, Blum JS, et al. CIITA-regulated plexin-A1 affects T-cell-dendritic cell interactions. Nat. Immunol. 2003;4:891–898. doi: 10.1038/ni960. [DOI] [PubMed] [Google Scholar]
- Worbs T, Mempel TR, Bolter J, von Andrian UH, Forster R. CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo. J. Exp. Med. 2007;204:489–495. doi: 10.1084/jem.20061706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J. Exp. Med. 2002;195:1145–1154. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Shortman K. Heterogeneity of thymic dendritic cells. Semin. Immunol. 2005;17:304–312. doi: 10.1016/j.smim.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Wu Q, Wang Y, Wang J, Hedgeman EO, Browning JL, Fu YX. The requirement of membrane lymphotoxin for the presence of dendritic cells in lymphoid tissues. J. Exp. Med. 1999;190:629–638. doi: 10.1084/jem.190.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Guan H, Zu G, Bullard D, Hanson J, Slater M, Elmets CA. The role of ICAM-1 molecule in the migration of Langerhans cells in the skin and regional lymph node. Eur. J. Immunol. 2001;31:3085–3093. doi: 10.1002/1521-4141(2001010)31:10<3085::AID-IMMU3085>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagami S, Hamrah P, Miyamoto K, Miyazaki D, Dekaris I, Dawson T, Lu B, Gerard C, Dana MR. CCR5 chemokine receptor mediates recruitment of MHC class II-positive Langerhans cells in the mouse corneal epithelium. Invest. Ophthalmol. Vis. Sci. 2005;46:1201–1207. doi: 10.1167/iovs.04-0658. [DOI] [PubMed] [Google Scholar]
- Yanagihara S, Komura E, Nagafune J, Watarai H, Yamaguchi Y. EBI1/CCR7 is a new member of dendritic cell chemokine receptor that is up-regulated upon maturation. J. Immunol. 1998;161:3096–3102. [PubMed] [Google Scholar]
- Yen JH, Khayrullina T, Ganea D. PGE2-induced metalloproteinase-9 is essential for dendritic cell migration. Blood. 2008;111:260–270. doi: 10.1182/blood-2007-05-090613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama H, Matsuno K, Zhang Y, Nishiwaki T, Kitabatake M, Ueha S, Narumi S, Morikawa S, Ezaki T, Lu B, et al. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int. Immunol. 2004;16:915–928. doi: 10.1093/intimm/dxh093. [DOI] [PubMed] [Google Scholar]
- Yu P, Wang Y, Chin RK, Martinez-Pomares L, Gordon S, Kosco-Vibois MH, Cyster J, Fu YX. B cells control the migration of a subset of dendritic cells into B cell follicles via CXC chemokine ligand 13 in a lymphotoxin-dependent fashion. J. Immunol. 2002;168:5117–5123. doi: 10.4049/jimmunol.168.10.5117. [DOI] [PubMed] [Google Scholar]
- Zabel BA, Silverio AM, Butcher EC. Chemokine-like receptor 1 expression and chemerin-directed chemotaxis distinguish plasmacytoid from myeloid dendritic cells in human blood. J. Immunol. 2005;174:244–251. doi: 10.4049/jimmunol.174.1.244. [DOI] [PubMed] [Google Scholar]