Abstract
Ion channels are the gatekeepers to neuronal excitability. Retinal neurons of vertebrates and invertebrates, neurons of the suprachiasmatic nucleus (SCN) of vertebrates, and pinealocytes of non-mammalian vertebrates display daily rhythms in their activities. The interlocking transcription–translation feedback loops with specific post-translational modulations within individual cells form the molecular clock, the basic mechanism that maintains the autonomic ~24-h rhythm. The molecular clock regulates downstream output signaling pathways that further modulate activities of various ion channels. Ultimately, it is the circadian regulation of ion channel properties that govern excitability and behavior output of these neurons. In this review, we focus on the recent development of research in circadian neurobiology mainly from 1980 forward. We will emphasize the circadian regulation of various ion channels, including cGMP-gated cation channels, various voltage-gated calcium and potassium channels, Na+/K+-ATPase, and a long-opening cation channel. The cellular mechanisms underlying the circadian regulation of these ion channels and their functions in various tissues and organisms will also be discussed. Despite the magnitude of chronobiological studies in recent years, the circadian regulation of ion channels still remains largely unexplored. Through more investigation and understanding of the circadian regulation of ion channels, the future development of therapeutic strategies for the treatment of sleep disorders, cardiovascular diseases, and other illnesses linked to circadian misalignment will benefit.
Keywords: circadian, ion channels, pineal gland, retina, signaling, suprachiasmatic nucleus
General introduction of the circadian oscillators and their functions
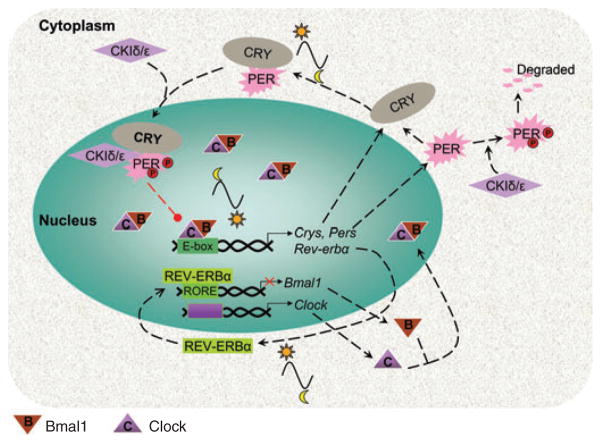
Circadian oscillators are biological clocks that exist in almost all living organisms from bacteria to humans, with persistent rhythmic periods close to 24 h (circa dian) even in the absence of external timing cues. The circadian oscillators coordinate rhythmic changes in biochemistry, physiology, and behavior of living organisms so that they can be synchronized with the 24-h oscillations of the external environment. The molecular nature of circadian oscillators, the ‘molecular clock,’ varies from species to species. A generalized model for the generation of mammalian circadian rhythms involves two interlocking transcription–translation feedback loops (Fig. 1). CLOCK (circadian locomotor output cycles kaput) and BMAL (brain and muscle, ARNT-like) 1 (Mop3) are two basic helix-loop-helix-PAS (Period-Arnt-Single-minded) transcription factors. CLOCK and BMAL1 form heterodimers and activate the transcription of downstream genes containing E-box cis regulatory enhancer sequences in their promoter regions, including the Period (Per1/Per2/Per3) and Cryptochrome (Cry1/Cry2) genes. While Bmal1 is maximally expressed during the middle of the night, the transcript levels of Pers and Crys reach their peaks during mid to late day, which are anti-phase to Bmal1 expression. The PERs, CRYs, and other proteins form heteromultimeric complexes that translocate into the nucleus and directly abrogate the transcriptional activity of the CLOCK–BMAL1 complex, thereby lowering Per and Cry mRNA levels. Thus, PERs, CRYs, and associated proteins regulate their own transcription through the inhibition of transcriptional activity of the CLOCK–BMAL1 heterodimers. Two members (Iδ and Iε) of the casein kinase family are involved in post-translational phosphorylation of PERs and ultimately contribute to their degradation. Another feedback loop includes the retinoic acid-related orphan nuclear receptor, REV–ERBα (reverse orientation c-erbA-1 gene alpha; an orphan nuclear receptor), which also is one of the downstream genes activated by CLOCK–BMAL1 heterodimers. The REV–ERBα protein represses Bmal1 gene expression through binding the retinoic acid-related orphan receptor response elements in Bmal1’s promoter. Therefore, BMAL1 attenuates its own transcription by transcriptional activation of REV–ERBα. Together, these positive and negative feedback loops are the major components of the mammalian molecular clock (Lowrey and Takahashi 2004). They play critical roles in establishing the circadian rhythm (for more detail on the molecular nature of circadian oscillators across species, please see the review by Bell-Pedersen et al. 2005). More recently, the role of micro-RNAs serving as post-transcriptional regulators, as well as other post-translational modulations, including phosphorylation/dephosphorylation, acetylation, and ubiquitination have been observed to play important parts in the molecular mechanisms underlying the circadian clock (Kim et al. 2003; Cheng et al. 2007; Hirayama et al. 2007; Jakubcakova et al. 2007). Collectively, these components comprise the core oscillator or the circadian oscillator (Dunlap 1999; Shearman et al. 2000; Glossop and Hardin 2002; Cyran et al. 2003).
Fig. 1.
This diagram illustrates a general model of the mammalian circadian oscillator in the SCN. The molecular clock is composed of interlocking transcription–translation feedback loops. CLOCK and BMAL1 (Mop3) are two basic helix-loop-helix-PAS (Period-Arnt-Single-minded) transcription factors, and Bmal1 is maximally expressed during the middle of the night. CLOCK and BMAL1 form heterodimers and activate the transcription of downstream genes containing E-box cis regulatory enhancer sequences in their promoter regions, including the Period (Per1/Per2/Per3) and Cryptochrome (Cry1/Cry2) genes. The transcript levels of both Pers and Crys reach their peak during mid to late day, which are anti-phase to Bmal1 expression. Two members of the casein kinase Iδ and Iε family are involved in post-translational phosphorylation of PERs and ultimately contribute to their degradation. The PERs, CRYs, and other proteins form heteromultimeric complexes that translocate into the nucleus and directly abrogate the transcriptional activity of the CLOCK–BMAL1 complex, thereby lowering Per and Cry mRNA levels. Thus, PERs, CRYs, and associated proteins regulate their own transcription through the inhibition of transcriptional activity of the CLOCK–BMAL1 heterodimers. Another feedback loop includes the retinoic acid-related orphan nuclear receptor, REV–ERBα, which also is one of the downstream genes activated by CLOCK–BMAL1 heterodimers. The REV–ERBα protein represses Bmal1 gene expression through binding the retinoic acid-related orphan receptor response elements (ROREs) in Bmal1’s promoter. Therefore, BMAL1 attenuates its own transcription by transcriptional activation of REV–ERBα. Collectively, these positive and negative feedback loops are the major components of the mammalian molecular clock. They play critical roles in establishing the circadian rhythm (figure is modified from Lowrey and Takahashi 2004).
The circadian oscillators in the mammalian suprachiasmatic nucleus
In higher vertebrates, circadian oscillators exist in the brain as well as in other organs or tissues (Bell-Pedersen et al. 2005). The ‘master clock’ that coordinates the activities of other oscillators is located in the suprachiasmatic nuclei (SCN) of the hypothalamus, while circadian oscillators existing in other brain areas or tissues are called peripheral clocks (or slave clocks) and are under the influence of the SCN (Balsalobre et al. 2000; Cheng et al. 2002). The SCN receives light information from the retina through the retinohypothalamic tract (Nauta and Haymaker 1969; Moore and Lenn 1972), which allows for the setting of SCN circadian oscillators to external light cues (Johnson et al. 1988). Surgical ablation of the SCN in mammals causes animals to become arrhythmic in locomotor activities, endocrine output, and other biochemical and physiological processes (Turek 1985). Transplantation of SCN tissue to SCN-lesioned animals restores circadian rhythms (Ralph et al. 1990). Furthermore, individual SCN neurons can generate self-sustained molecular and physiological oscillations, including circadian rhythmicity of action potential firing rates (Green and Gillette 1982; Shibata and Moore 1988; Welsh et al. 1995; Quintero et al. 2003). In addition, the increase in spontaneous firing during the daytime in mammalian SCN neurons is associated with autonomic circadian regulation of ion channel activities (discussed in detail later; Meredith et al. 2006; Wang and Huang 2006). Hence, the SCN is the major pacemaker responsible for setting the phase and period of the biological rhythms in higher vertebrates (Bell-Pedersen et al. 2005).
The circadian oscillators in the retina
As a unique photosensitive tissue, the retina possesses its own independent circadian oscillators, which allow the retina to anticipate the daily cycling light (Green and Besharse 2004). In addition, communication between the retina and SCN through the retinohypothalamic tract affects the circadian phase of the SCN (Berson et al. 2002). Visual systems have to detect images despite large daily changes in ambient illumination between day and night, and intrinsic circadian oscillators in the retina provide such a mechanism for visual systems to initiate more sustained adaptive changes throughout the course of the day (Cahill and Besharse 1995; Green and Besharse 2004). The retina is heterogeneous with multiple cell types organized in several layers. Early studies of circadian regulation in Xenopus and chicken retinas indicated that retinal circadian clocks are mainly located in the photoreceptors (Korenbrot and Fernald 1989; Cahill and Besharse 1993; Pierce et al. 1993; Green et al. 1995a). Later research revealed that there are multiple oscillators present in other retinal cells including bipolar, ganglion, amacrine, and horizontal cells in a species-dependent manner (photo-receptors: Xenopus-Constance et al. 2005; fish-Halstenberg et al. 2005; Li et al. 2005; Menger et al. 2005; Yu et al. 2007; avian-Bernard et al. 1997; Chong et al. 1998; Ivanova and Iuvone 2003a; Ko et al. 2001; Manglapus et al. 1998; rats-Tosini et al. 2008; bipolar cells: fish-Hull et al. 2006; mice-Ruan et al. 2006; ganglion cells: mice-Garbarino-Pico et al. 2004a,b; Ruan et al. 2006; amacrine cells: mice-Dorenbos et al. 2007; horizontal cells: mice-Ruan et al. 2006; fish-Wang and Mangel 1996). The overall circadian regulation of the retina relies on the synaptic circuitry and feedback modulation among different retinal oscillators, so that the retina is able to anticipate and adapt to sustained daily illumination changes as well as acute light/dark adaptation (for more detail on the circadian regulation of the retina, please see the review by Wiechmann and Summers 2008).
The endogenous circadian oscillators in photoreceptors are able to function independently in the absence of other retinal inputs (Cahill and Besharse 1993; Thomas et al. 1993; Ko et al. 2001). These photoreceptor oscillators lead to morphological, physiological, biochemical, and molecular changes that ultimately regulate photoreceptor function and physiology in a circadian fashion. For example, in vertebrate rod and cone photoreceptors, outer segment phagocytosis, shedding, and renewal are continuous processes, but their rates are under circadian control. The circadian phase of the rhythmic shedding and renewal in both cones and rods is highly species dependent (Anderson et al. 1978, 1980; LaVail 1980; Besharse and Dunis 1983; Fisher et al. 1983; Reme et al. 1986; Bobu et al. 2006), while the photosensitive membrane undergoes daily turnover in the lateral eye of Limulus (Runyon et al. 2004). In some teleosts and anaurans, the inner segments of rod and cone photoreceptors undergo contraction and elongation (i.e. retinomotor movement) in circadian cycles as well as in response to changes in ambient illumination (Nagle and Burnside 1984; Pierce and Besharse 1985, 1988; Wagner et al. 1993; Burnside 2001; Menger et al. 2005). While cones remain in a contracted state during the day, rods contract at night. Photoreceptors form specialized ribbon synapses with secondary neurons like horizontal or bipolar cells. The number and ultrastructure of synaptic ribbons in both photoreceptors and bipolar cells undertake changes in relation to the time of day and light intensity (Vollrath et al. 1989; Vollrath and Spiwoks-Becker 1996; Adly et al. 1999; Allwardt et al. 2001; Spiwoks-Becker et al. 2004; Hull et al. 2006). In avian, amphibian, reptile, and other lower vertebrate species, the transcription of arylalkylamine N-acetyltransferase (AANAT), the melatonin synthesis enzyme, is under circadian control (Bernard et al. 1997; Iuvone et al. 1997, 1999; Ivanova and Iuvone 2003b), which leads to higher synthesis and secretion of melatonin from photoreceptors at night, while its synthesis is inhibited by light (Cahill and Besharse 1993; Bernard et al. 1997; Ivanova and Iuvone 2003b). Also, while the synthesis and release of dopamine from retinal amacrine cells show light-driven and circadian fluctuations (Iuvone et al. 1978; Wirz-Justice et al. 1984; Pierce and Besharse 1985; Manglapus et al. 1999; Ribelayga et al. 2004; Witkovsky et al. 2004), the circadian nature of dopamine is dependent on the melatonin rhythm (Doyle et al. 2002; Miranda-Anaya et al. 2002; Ribelayga et al. 2004). In addition to the circadian oscillator genes, several genes majorly expressed in photoreceptors (e.g. opsin) are also under circadian regulation (Korenbrot and Fernald 1989; Pierce et al. 1993; Bailey et al. 2004; Liang et al. 2004). The circadian oscillators also regulate ion channel and kinase activities in the photo-receptors that ultimately contribute to the circadian regulation of photoreceptor physiology and function (Green et al. 1995a,b; Hasegawa and Cahill 1999a, 2004; Ko et al. 2001, 2003, 2004, 2007; Chae et al. 2007; Chen et al. 2007).
The circadian rhythms in the pineal gland
The major function of the pineal gland is the synthesis and release of melatonin, a neurohormone, which is elevated at night and circulated throughout the body serving as a signal of time to integrate physiological functions with environmental lighting on a daily and seasonal basis (Bailey et al. 2009; for more recent reviews, please see Klein 2006; Macchi and Bruce 2004). In lower vertebrates, pinealocytes possess a photosensitive and autonomic circadian rhythm in melatonin secretion that persists in dissociated cell cultures (Robertson and Takahashi 1988a,b; Zatz and Mullen 1988a; Zatz et al. 1988). In fact, avian pinealocytes resemble retinal cone photoreceptors as they both have oil droplets, utilize opsin, arrestin, transducin, and cGMP-gated cation channels (CNGCs) for phototransduction, and display hyperpolarizing responses to brief pulses of light (Pu and Dowling 1981; Tamotsu and Morita 1986; Dryer and Henderson 1991). Interestingly, mammalian pinealocytes are not sensitive to light, and the circadian oscillation in pineal melatonin production in this case is under SCN control through a polysynaptic pathway that involves both central and peripheral neuronal structures (review in Maronde and Stehle 2007). Even though the mammalian pineal gland serves as a peripheral oscillator driven by the SCN, the machinery for the production of melatonin, including the expression of key enzymes AANAT and hydroxyindole-O-methyltransferase that synthesize melatonin, is under circadian control (Klein 2006; Maronde and Stehle 2007). Over 600 genes that are important for immunity, cell cycle and death, intracellular signaling molecules, transcription factors, and circadian rhythmicity are also under circadian regulation in the mammalian pineal gland (Bailey et al. 2009). However, the molecular mechanism underlying melatonin secretion from pinealocytes is still not well understood, and the circadian regulation of cell excitability or ion channel activity have only been documented in non-mammalian pinealocytes.
The circadian oscillators in other neuronal tissues
Circadian oscillators in other brain areas also impact the regulation of a variety of functions. For example, clock genes and other components of the molecular clock display circadian rhythmicity in the hippocampus (Stephan and Kovacevic 1978; Eckel-Mahan et al. 2008). Lesions of the SCN, clock gene knockouts, or light-induced arrhythmic animals fail to perform hippocampal-dependent learning tasks (Ruby et al. 2008). Interestingly, in Aplysia, circadian oscillators regulate complex learning and memory formation and long-term sensitization (Lyons et al. 2005). This circadian-regulated memory formation in Aplysia is possibly mediated through a glutamate transporter (Collado et al. 2007). Furthermore, circadian oscillators in Drosophila melanogaster modulate the formation of short-term associative memory (Lyons and Roman 2009). However, there are peripheral oscillators that can function independently without input from the ‘master clock,’ and the olfactory system is one of them. The first evidence of an independent olfactory oscillator was found from the Drosophila antennae (Krishnan et al. 1999). The master clocks that control locomotor activity rhythms and sleep–wake cycles in Drosophila are located in the lateral neurons (Bell-Pedersen et al. 2005). In the Drosophila antennae, odorant-evoked neuronal responses are significantly higher at night (Krishnan et al. 1999, 2001, 2008), and this observation is correlated with an increase in nocturnal sex drive (Fujii et al. 2007). Targeted ablation of lateral neurons by using apoptosis-promoting factors demonstrates that the antennae neurons are necessary and sufficient for the circadian rhythm in the Drosophila olfactory system (Tanoue et al. 2004). In mammals, odor-induced c-Fos expression in the olfactory bulbs and piriform cortex is also under circadian control (Granados-Fuentes et al. 2006). Electrolytic lesions of the SCN abolish circadian locomotor rhythms but not odor-induced c-Fos rhythms in both olfactory bulbs and piriform cortex. However, surgical removal of both olfactory bulbs shortens the free-running period of locomotor rhythms and alters the circadian phases, which indicates that olfactory oscillators in mammals may interact with the SCN to coordinate other daily behaviors (Granados-Fuentes et al. 2006).
Circadian regulation of ion channels
Ion channels are macromolecular pores that allow charged ions to move across the cell membrane and contribute to the excitability of neurons and muscles (Hille 2001). Despite what research has accomplished on the properties, modulation, and physiological functions of ion channels in various neurons and glial cells, the circadian regulation of ion channels and the resulting physiological effects have yet to be fully explored. Thus far, circadian changes in gating properties and the corresponding impacts on physiological functions have been studied in CNGCs, voltage-gated calcium channels (VGCCs), and various potassium channels in the retina or SCN. These and other ion channels have been reported to be under circadian regulation and will be discussed in detail below. As ion channels are the gatekeepers of neuronal activity, their regulation by circadian oscillators could serve as the final posts of oscillator neurons to regulate downstream targets, synchronize local oscillators, and further regulate physiological states and behaviors. On the other hand, ion influx/efflux can activate or inactivate various cellular signaling pathways that might cause changes in circadian gene expression, which in turn can lead to shifts in circadian phases. Therefore, ion channels can serve in circadian input and/or output pathways. Furthermore, the circadian regulation of ion channels can enhance the stability of circadian oscillators, which demonstrates a model that Roenneberg and Merrow (1999) presented where the elements that lead to entrainment of the core oscillators (e.g. the ion channels) can themselves be regulated by the oscillators. One feature of this model is that it contains additional feedback loops that can markedly enhance the stability of the overall oscillator system at the cellular level and possibly at the network level (e.g. the retinal oscillators). Table 1 is a summary of ion channels that are under circadian control and their functions related with circadian rhythm in various tissues and organisms.
Table 1.
List of ion channels that are under circadian control in various neuronal tissues studied in different species, as well as their functions related with circadian rhythms
| Ion channel | Cell type | Function | Organisms studied |
|---|---|---|---|
| cGMP-gated ion channels | Retinal cone photoreceptors | Circadian sensitivity to light | Chicken |
| L-type voltage-gated calcium channels | Retinal cone photoreceptors | Circadian regulation of melatonin and retinoschisin | Chicken |
| Retinal bipolar cells | Circadian regulation of synaptic ribbons | Fish | |
| Suprachiasmatic nucleus | Circadian regulation of intracellular calcium levels | Rat, Mouse | |
| Circadian regulation of spontaneous firing rates of SCN neurons | |||
| T-type voltage-gated calcium channels | Thalamus, suprachiasmatic nucleus, cerebellum | Regulation of sleep–wake cycle, circadian phase-shifting | Rat, Mouse |
| Voltage-gated potassium channels | Retinal pacemaker neurons | Circadian regulation of the compound action potential firing frequency | Aplysia |
| Basal retinal neurons | Circadian regulation of membrane conductance and membrane potential; circadian regulation of optic nerve impulses | Mollusk Bulla gouldiana | |
| Voltage-gated potassium channels: Shaker, Shaw | Neurons and neurosecretory cells | Sleep periods, rhythmic locomotor behavior, resting membrane potential | Drosophila |
| Voltage-gated potassium channels: Kv3.1, Kv3.2 | Suprachiasmatic nucleus | Circadian regulation of spontaneous firing rate of SCN neurons | Mouse |
| Calcium-activated potassium channels | Photoreceptors, neurons in the head, and neurosecretory cells | Circadian rhythm of locomotor activity | Drosophila |
| Suprachiasmatic nucleus | Circadian regulation of spontaneous firing rates of SCN neurons; Circadian locomotor activity and physiological rhythms | Rat, Mouse | |
| Na+/K+-ATPase | Suprachiasmatic nucleus | Circadian regulation of spontaneous firing rates of SCN neurons | Rat |
| Long-opening time cation channel (ILOT) | Pineal gland | Potential synchronization of pineal oscillators in non-mammalian species | Chicken |
Circadian regulation of cGMP-gated cation channels
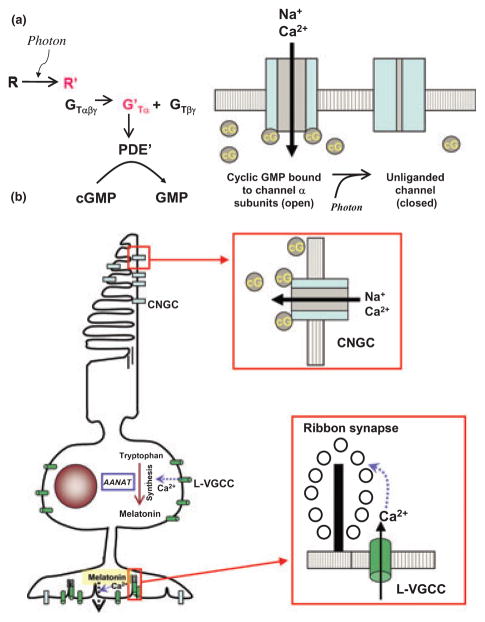
Cyclic GMP-gated cation channels are non-selective cation channels that belong to a family of cyclic nucleotide-gated channels (Kaupp and Seifert 2002). In general, cyclic nucleotide-gated channels are heterotetrameric complexes consisting of two or three different types of subunits (α, β, and γ), with important channel properties determined by the subunit composition. Activation of these channels is through direct binding of cyclic nucleotides onto the channel proteins (Kaupp and Seifert 2002). The CNGCs of rods and cones are structurally similar but have distinct α- and β-subunits (Zheng et al. 2002; Zhong et al. 2002; Bradley et al. 2005). Rods contain CNGCα1 subunits that are more sensitive to calcium-induced inhibitions, while cones contain CNGCα3 subunits that are not as sensitive to calcium (Miller and Korenbrot 1994; Korenbrot 1995; Kaupp and Seifert 2002; Krajewski et al. 2003). In the vertebrate retina, photo-transduction is mediated by a G protein-coupled cascade that results in changes in the gating of CNGCs in the outer segments of rods and cones (Zagotta and Siegelbaum 1996; Matulef and Zagotta 2003). Light initiates a fall in intracellular cGMP as the end-point of a G protein-mediated phototransduction cascade, resulting in closure of CNGCs, reduced cation influx, and membrane hyperpolarization (Fig. 2a). In the dark, the intracellular cGMP concentration is relatively high, which causes tonic activation of these channels and a steady transmembrane influx of sodium (Na+) and calcium (Ca2+) ions (Yau and Baylor 1989; Pugh and Lamb 2000). Therefore, CNGCs carry the photoreceptor ‘dark current’ and serve essential roles in the light-dependent changes in photoreceptor membrane potential and subsequent neural processing (Fig. 2b).
Fig. 2.
(a) Figure illustrate a simplified model of phototransduction in photoreceptors. In the light, after opsins/rhodopsins (R) receive photons, activated opsins/rhodopsins (R′) bind to the G protein transducin (GTαβγ). The α subunit of transducin (G′Tα) separates from the βγ subunit complex and activates phosphodiesterase. The activated phosphodiesterase (PDE′) degrades the phosphodiester bond of cGMP (cG) changing it to GMP. With less cGMP available for cGMP-gated cation channels (CNGCs), these channels close and hyperpolarize the photoreceptors. (b) In the dark (or at night), there is more intracellular cGMP available for CNGCs. After cGMP binds to the CNGCs, the channels open and allow an influx of sodium (Na+) and calcium (Ca2+) causing depolarization of the photoreceptors. Depolarization of the photoreceptor plasma membrane in turn allows calcium to enter the cells through L-type voltage-gated calcium channels (L-VGCCs). The Ca2+ influx further activates the synthesis and secretion of melatonin, and it also correlates with the arrangement of synaptic vesicles at ribbon synapses, which allows for more efficient neurotransmitter release.
Circadian regulation of cGMP-gated ion channels in retinal photoreceptors
In chicken cone photoreceptors, the apparent affinity of CNGCs for their activating ligand is under circadian regulation (Ko et al. 2001, 2003, 2004; Chae et al. 2007). There is roughly a twofold change in the apparent affinity of CNGCs for cGMP throughout the course of a day, with the affinity substantially higher at night than during the day even in constant darkness conditions after light/dark entrainment. Such changes in channel affinities can be expected to occur at the lower range of cGMP concentrations (≤ 7 μM in the dark) that are within the photoreceptor physiological range (Cobbs and Pugh 1985; Cobbs et al. 1985; Ko et al. 2001). Other biophysical features of CNGC gating, such as unitary conductance, Hill coefficient, density of channels in the plasma membrane, and maximum current amplitudes do not vary as a function of the time of day (Cobbs and Pugh 1985; Cobbs et al. 1985; Ko et al. 2001).
The gating properties of photoreceptor CNGCs can be modulated by multiple processes, including direct phosphorylation or dephosphorylation of the channel subunits and the binding of Ca2+/calmodulin or related molecules (Hsu and Molday 1993; Gordon et al. 1995; Bauer 1996; Kosolapov and Bobkov 1996). Dephosphorylation of CNGC serine/threonine (Gordon et al. 1992) or tyrosine residues (Molokanova et al. 1997, 1999a,b) causes an increase in the apparent affinity of CNGCs for their activating ligand. Binding of Ca2+/calmodulin or phosphorylation of CNGCs causes these channels to shift to a lower affinity state for cGMP (Hsu and Molday 1993; Gordon et al. 1995; Bauer 1996; Kosolapov and Bobkov 1996; Krajewski et al. 2003). The clock regulation of cone CNGCs entails a post-translational modification of the channel molecules. More specifically, it is the circadian rhythmicity of tyrosine phosphorylation that underlies the circadian modulation of cone CNGC affinity to its ligand (Ko et al. 2001, 2004; Chae et al. 2007). Inhibition of tyrosine kinases during the day increases the apparent affinity of CNGCs for cGMP, whereas inhibition of tyrosine phosphatases at night produces the opposite effect (Chae et al. 2007). While the protein expression and phosphorylation of the channel pore-forming CNGCα3 subunits remain constant throughout the day, tyrosine phosphorylation of an auxiliary subunit (probably the β subunit, ~85 kDa) displays a circadian rhythm (Chae et al. 2007). During the daytime, tyrosine phosphorylation on this 85-kDa protein is twice as high as at night. Therefore, the circadian rhythmicity of tyrosine phosphorylation on the 85-kDa auxiliary subunit of cone CNGCs provides one of the final steps in the circadian regulation of CNGCs.
The CNGCs in photoreceptors are essential components of visual phototransduction cascades. As such, it is possible that they also play a role in the light entrainment of the circadian oscillators in photoreceptors. Because the gating of CNGCs is under circadian control in cone photoreceptors, these channels represent a potential example of an entity that is both an input to and an output from the circadian oscillator. Roenneberg and Merrow (1999) have presented models of circadian oscillator systems in which pathways that lead to entrainment of the core oscillators (i.e. the circadian inputs) can themselves be regulated by the oscillators (i.e. they are also components of circadian outputs). One feature of these models is that they contain additional feedback loops that can markedly enhance the stability of the overall oscillator system. Therefore, the circadian regulation of CNGCs in retinal photoreceptors represents an adaptation to enhance the stability of the circadian oscillators in photoreceptors (Ko et al. 2001).
Signaling pathways leading to the circadian regulation of cGMP-gated ion channels
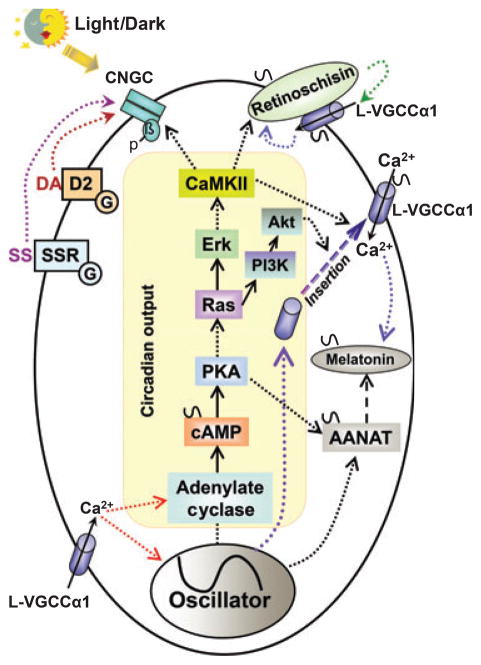
Even though the connection from the molecular oscillator to the regulation of the CNGC affinity rhythm is still not completely understood, the small GTPase Ras, the mitogen-activated protein kinase (MAPK) signaling pathway, and calcium–calmodulin kinase II (CaMKII) are involved as circadian outputs to regulate CNGC rhythms (Ko et al. 2001, 2004). The activities of Ras and MAPK are themselves under circadian control and oscillate concurrently with the CNGC affinity rhythm (Ko et al. 2001, 2004). The activity of CaMKII also displays a circadian rhythm, but it runs anti-phase to the MAPK rhythm, and CaMKII is a downstream target of MAPK (Ko et al. 2001). Perturbation of the activities of Ras, MAPK, or CaMKII causes phase-dependent changes in the gating properties of CNGCs. Furthermore, this Ras–MAPK–CaMKII pathway serves as part of a common circadian output pathway that regulates other photoreceptor molecules (Ko et al. 2007, 2008). However, neither MAPK nor CaMKII directly phosphorylate CNGCs in a circadian fashion. It is their downstream targets leading to tyrosine phosphorylation on the auxiliary subunit of CNGCs that ultimately govern the circadian regulation of CNGC gating properties (Fig. 3).
Fig. 3.
This model illustrates the circadian rhythm in chicken cone photoreceptors. Light and dark (day and night) signals from the environment enter the photoreceptor through CNGCs, which are essential in phototransduction. The light/dark signals through the ‘circadian input’ pathway entrain the photoreceptor core oscillator that cause the photoreceptor to be synchronized with the environment. From the core oscillator through the ‘circadian output,’ the activities of different molecules, including CNGCs, L-VGCCs, and retinoschisin are under circadian regulation, and the ‘circadian output’ is composed of a series of signaling pathways. Calcium influx through L-VGCCs causes changes in the signaling involved in the ‘circadian input’ pathway that regulates the core oscillator genes and cAMP signaling, and further regulates the gene and protein expression of AANAT and melatonin production (as indicated by the red dotted arrows). The circadian regulation of CNGCs and L-VGCCs in retinal photoreceptors represents an adaptation to enhance the stability of retinal circadian oscillators. It demonstrates a model that Roenneberg and Merrow (1999) presented, in which the elements that lead to entrainment or modulation of the core oscillators (e.g. CNGCs and L-VGCCs) can themselves be regulated by the oscillators. One feature of this model is that it contains additional feedback loops that can markedly enhance the stability of the overall oscillator system at the cellular level (photoreceptors only) and maybe at the retinal network level (the retinal oscillators). Dotted arrows indicate multiple steps currently not known, while the solid arrows represent known signaling. The Ras, Erk, and CaMKII signaling pathway serves as a common circadian output to regulate the circadian rhythms of CNGCs, L-VGCCs, and retinoschisin in photoreceptors. The circadian regulation of CNGCs involves tyrosine phosphorylation on an auxiliary subunit, in which the phosphorylation is high during the day corresponding to when CNGCs have a lower affinity to cGMP. The circadian phase-dependent regulation of CNGCs by dopamine and somatostatin is represented by brown and purple dotted arrows, respectively. The circadian regulation of L-VGCCs involves the regulation of L-VGCCα1 mRNA and protein expression, as well as the plasma membrane insertion and retention of L-VGCCα1. Circadian regulation of AANAT and melatonin production is partially through the circadian rhythm of cAMP production and direct regulation of AANAT mRNA expression by the core oscillator genes. The purple dotted arrow represents the circadian regulation of transcription and translation of L-VGCCα1 subunits. Functional expression of both L-VGCCα1 and retinoschisin are high at night, and there is a bidirectional feedback regulation between them. Blue dotted arrows indicate that the secretion of melatonin and retinoschisin is under the control of L-VGCCs. The green dotted arrow represents the positive feedback regulation of retinoschisin on L-VGCCα1 subunit retention in the plasma membrane. The red dotted arrow represents the circadian input from the calcium influx through the L-VGCCs. AANAT, aryl-alkylamine N-acetyltransferase; Ca2+, calcium ion; CaMKII, calcium–calmodulin kinase II; CNGC, cGMP-gated cation channel (β, the β subunit of CNGC; p, tyrosine phosphorylation on the CNGCβ subunit); DA, dopamine; D2: dopamine D2 receptor; G, G protein; L-VGCCα1, L-type voltage-gated calcium channel α1 subunit; SS, somatostatin; SSR, somatostatin receptor.
The expression and activation of adenylate cyclase in the retina is also under circadian control (Chaurasia et al. 2006), and the cAMP content in retinal photoreceptors is maximal at night (Ivanova and Iuvone 2003a,b; Chaurasia et al. 2006). In avian retinas, this cAMP rhythm not only drives the rhythm of melatonin synthesis and secretion (Ivanova and Iuvone 2003a,b; Chaurasia et al. 2006), it also serves as part of the circadian output and leads to MAPK activation and modulation of the CNGC affinity rhythm. Aside from serving as a circadian output to regulate ion channels, cAMP can stimulate retinomotor movements (Besharse et al. 1982; Burnside et al. 1982; Burnside and Ackland 1984; Burnside 2001). In Xenopus retina, cAMP signaling resets circadian oscillators within photoreceptors (Hasegawa and Cahill 1998, 1999b). Hence, the second messenger, cAMP, may well serve as part of both input and output pathways in photoreceptors.
Circadian phase-dependent modulation of cone cGMP-gated ion channels by dopamine
While circadian rhythms can occur in retinal photoreceptors cultured in the absence of other functional cell types (Cahill and Besharse 1993; Ko et al. 2001), multiple cell types contribute to the overall circadian control of the intact retina. In avian retina, melatonin inhibits the release of dopamine from a subpopulation of amacrine cells (Nowak et al. 1992). Consequently, retinal melatonin and dopamine have anti-phasic circadian cycles (Adachi et al. 1998b). Dopamine can entrain photoreceptor circadian oscillators and shift the circadian phase of photoreceptors that are similar but not identical to those of light itself (Cahill and Besharse 1991; Hasegawa and Cahill 1999b; Steenhard and Besharse 2000). Dopamine facilitates the transition between rod-dominate signaling pathways characteristic of scotopic conditions and cone-dominate pathways that operate under photopic conditions (Djamgoz and Wagner 1992; Krizaj 2000) and therefore, contributes to circadian rhythms of rod–cone dominance (Witkovsky et al. 1988; Wang and Mangel 1996; Manglapus et al. 1998, 1999). As a result, dopamine functions as a feedback signal from the inner retina that refines and modulates circadian control mechanisms within the photoreceptors (Ko et al. 2003). In addition, dopamine plays an important role in light adaptation of the retina that is independent from its effects on circadian oscillators (Besharse and Iuvone 1992; Cahill and Besharse 1995;Besharse et al. 2004).
As noted above, the synthesis and release of dopamine is under circadian control with more dopamine released during the day, but dopamine release is also stimulated after acute light exposure at night (Adachi et al. 1998a). Dopamine evokes a phase-dependent modulation of the CNGC affinity rhythm in chicken cone photoreceptors that is through the activation of D2 dopamine receptors (Ko et al. 2003). Exposure to dopamine or D2 agonists causes a significant decrease in the apparent affinity of CNGCs at night but has no effect on these channels during the day. It is well established that D2 receptors are pertussis toxin sensitive G protein- coupled receptors that mediate the inhibition of adenylate cyclase and cause a decrease in cAMP formation in the retina (Iuvone 1986, 1990; Stenkamp et al. 1994). However, the phase-dependent modulation of CNGCs in cone photoreceptors by dopamine does not involve pertussis toxin sensitive G proteins or cAMP signaling, as exposure to cAMP or pertussis toxin does not reverse the phase-dependent modulation of dopamine on CNGCs. This circadian modulation of cone CNGCs by dopamine is partially through the MAPK–CaMKII signaling pathway (Ko et al. 2003).
Modulation of cone cGMP-gated ion channels by somatostatin
Somatostatin (SS) is released from a class of amacrine cells that are immunoreactive for enkephalin, neurotensin, and SS, and thus, they are called ENSLI (enkephalin-, neurotensin-, and SS-like immunoreactive) amacrine cells (Morgan and Boelen 1996). There are two forms of SS: SS-14 with 14 amino acids and SS-28 with 28 amino acids, and both forms are released from ENSLI cells (Ishimoto et al. 1986; Dowton et al. 1994; Watt and Florack 1994; Yang et al. 1997). The release of SS from the ENSLI cells is under circadian control, which is high at night and low during the day in mammalian retinas (Webb et al. 1988; Peinado et al. 1990), and this rhythm is concurrent with the activities of these cells with a high sustained rate of activity in the dark and a low sustained rate of activity in the light (Morgan et al. 1994). The five SS receptors 1–5 are guanine nucleotide binding protein (G protein) coupled receptors. While all five subtypes of SS receptors are expressed in mammalian retina (Cristiani et al. 2002; Thermos 2003), the chicken retina does not express SS receptor 1 (Chen et al. 2007). Both SS-14 and SS-28 modulate cone CNGC sensitivity to cGMP that depend on circadian phase and immediate history of illumination. Both SS-14 and SS-28 decrease CNGC sensitivity to cGMP at night, which is similar to the action of dopamine via the D2 receptor and resistant to pertussis toxin. In addition, SS-28, but not SS-14, evokes a transient increase in CNGC affinity to cGMP only during the early part of the day in cone photoreceptors that have been exposed to light for 1–2 h. This transient effect by SS-28 is mediated by pertussis toxin sensitive G protein-coupled receptors and activation of phospholipase C and protein kinase C signaling cascades (Chen et al. 2007). Therefore, SS modulation of retinal cones may serve to reinforce circadian processes intrinsic to the photoreceptors, and may also contribute to more rapid adaptive responses to changes in ambient illumination.
Circadian regulation of L-type voltage-gated calcium channels
The L-type calcium channels mediate a voltage-dependent and depolarization-induced calcium influx and regulate diverse biological processes such as contraction, secretion, neurotransmission, differentiation, and gene expression in many different cell types (Abernethy and Soldatov 2002; Catterall et al. 2005). The L-type VGCCs (L-VGCCs) are composed of a pore-forming α1 subunit and auxiliary β, α2δ, and γ subunits, and they can be blocked by divalent cations (e.g. cobalt) and organic L-VGCC antagonists such as dihydropyridines, phenylalkylamines, and benzothiazepines (Catterall et al. 2005; Dolphin 2006). The α1 subunit serves as a voltage sensor to detect voltage changes across the plasma membrane, and it also controls the pore size to allow selective divalent cations to pass through. Mammalian α1 subunits are encoded by at least 10 distinct genes (Catterall et al. 2005; Dolphin 2006), and the α1C, α1D, and α1F subtypes (also known as Cav1.2, Cav1.3, and Cav1.4, respectively) are expressed in retina photoreceptors (Barnes and Kelly 2002; Morgans et al. 2005; Ko et al. 2007).
Circadian regulation of voltage-gated calcium channels in the retina
Photoreceptors are non-spiking neurons, and they release glutamate continuously in the dark as a result of depolarization-evoked activation of L-VGCCs (Barnes and Kelly 2002). Circadian regulation of L-VGCCs has been observed in gold fish retinal bipolar cells (Hull et al. 2006), chicken cone photoreceptors (Ko et al. 2007), and other non-retinal neurons (Pennartz et al. 2002). In both retinal cases, the average maximum current amplitudes of L-VGCCs are significantly larger at midnight than at midday. The activation voltages that elicit L-VGCC currents (i.e. current–voltage relationship) and the channel gating kinetics do not change throughout the day. In chicken retinas, the main factor that contributes to the circadian regulation of L-VGCC current amplitudes is the expression of functional L-VGCCα1 subunits, and both mRNA and protein expression of VGCCα1D are rhythmic (Ko et al. 2007). The Ras–MAPK–CaMKII signaling pathway serves as part of the circadian output to regulate L-VGCCs as well as CNGCs as described in the section, ‘Signaling pathways leading to the circadian regulation of cGMP-gated ion channels’, (Fig. 2). However, the varying maximum amplitudes of the L-VGCC currents are in stark contrast to the CNGC maximum currents, which remain constant throughout the day, and which instead exhibit changes in gating properties (Ko et al. 2001, 2007).
In addition to the Ras–MAPK–CaMKII pathway, the phosphatidylinositol 3 kinase (PI3K)–protein kinase B (Akt) signaling pathway also regulates the circadian functionality of L-VGCCs. The PI3K–Akt pathway regulates a vast array of cellular processes and biological functions (Shaw and Cantley 2006; Yoon et al. 2008) from post-translational modulation of proteins, protein and vesicle trafficking, cytoskeletal remodeling, to controlling cell cycles, proliferation, and survival (Blair and Marshall 1997; Lhuillier and Dryer 2002, 2003; Roth et al. 2004; Viard et al. 2004). Various ion channel activities are augmented following the activation of PI3K–Akt signaling that instigate channel protein trafficking and insertion into the plasma membrane, and these channels include kidney aquaporin 2 channels (Tajika et al. 2004), non-selective cation channels (Kanzaki et al. 1999), calcium-dependent potassium channels (Lhuillier and Dryer 2002), and L-VGCCs (Le Blanc et al. 2004). In chicken retina, the PI3K–Akt signaling pathway participates in the circadian phase-dependent modulation of L-VGCCs (Ko et al. 2009). Inhibition of PI3K–Akt in cultured photoreceptors decreases the maximum current amplitude of L-VGCCs at night but did not alter these currents during the day. Inhibitors of the PI3K–Akt pathway have no effect on the mRNA levels of L-VGCCα1D subunits, nor do they block the L-VGCCs directly in vitro. Cell surface biotinylation assays reveal that PI3K–Akt mainly affects the membrane insertion of VGCCα1D subunits (Ko et al. 2009). Activation of Akt requires a multistep process that includes phosphorylation of Thr308 in the kinase domain and Ser473 within the regulatory domain (Fayard et al. 2005). As a downstream target of PI3K, the overall activity of Akt is the result of an equilibrium between these two phosphorylation sites (Beaulieu et al. 2007). Circadian regulation of Akt activity occurs through phosphorylation of Thr308, but not on Ser473, in intact chicken retinas. The PI3K–Akt pathway, like Erk, is downstream of Ras but appears to be separate, yet, equally important in regulating L-VGCCs in retina photoreceptors (Ko et al. 2009). Hence, both Ras–PI3K–Akt and Ras–Erk signaling pathways serve as parallel output pathways to regulate the circadian rhythms of L-VGCCs (Fig. 3).
Functional significance of circadian regulation of voltage-gated calcium channels in the retina
A major functional significance of the L-VGCC rhythm is the circadian control of melatonin release. In many vertebrate species, melatonin is synthesized and secreted in the retina and is under circadian control with a higher level at night than during the day (Cahill and Besharse 1993; Bernard et al. 1997; Ivanova and Iuvone 2003b). One of the key enzymes leading to the synthesis of melatonin is AANAT, and both mRNA and protein levels of AANAT are elevated at night (for more detail on the circadian rhythm of melatonin, please see review by Iuvone et al. 2005). The dark-dependent increase in AANAT activity and melatonin synthesis requires calcium influx through L-VGCCs as illustrated when these channels are inhibited with dihydropyridines, which suppresses AANAT activity and therefore blocks the synthesis and release of melatonin (Iuvone and Besharse 1986; Cahill and Besharse 1993; Bernard et al. 1997; Ivanova and Iuvone 2003b). In avian retinas, the mechanism by which calcium influx controls melatonin synthesis and subsequent release is through cAMP levels, which peak at night (Ivanova and Iuvone 2003a). Calcium stimulates cAMP production through activation of calcium/calmodulin-dependent adenylate cyclases (Xia et al. 1993; Fukuhara et al. 2004; Iuvone et al. 2005), and L-VGCC blockers reverse this calcium-induced cAMP production (Iuvone et al. 1991, 2005). Cyclic AMP, in turn, can modulate the phosphorylation of transcription factors that augment the E-box-driven increase in AANAT mRNA (Iuvone et al. 2005). Thus, the circadian regulation of calcium influx through L-VGCCs may further contribute to the daily rhythm of AANAT and production and secretion of melatonin in the retina (Fig. 3). Hence, the L-VGCCs are not only subjected to circadian control through output pathways and regulating downstream targets, the calcium influx through L-VGCCs serves as part of the input pathways to modulate the core oscillators that further regulate rhythmic gene expression.
The photoreceptor components of electroretinograms (ERGs), the electrophysiological recordings of retinal physiology, recorded from humans as well as animals, show daily rhythms (Dearry and Barlow 1987; Hawlina et al. 1992; Lu et al. 1995; Hankins et al. 1998, 2001; Manglapus et al. 1998, 1999; McGoogan et al. 2000; Tuunainen et al. 2001; Miranda-Anaya et al. 2002; Rufiange et al. 2002; Ren and Li 2004). The ERG is one type of extracellular recording that record global electrical changes across the retina by placing recording electrodes on the surface of the eye. It is regularly used in clinical practice as well as research to examine the physiological status of the retina in response to various light stimulation (Lam 2005). As L-VGCCs are essential for neurotransmitter release in the retina, the circadian regulation of L-VGCCs in photoreceptors could contribute to the daily rhythm of the photoreceptor components of ERGs. One aspect of the role of L-VGCCs in the circadian regulation of synaptic transmission is the correlation of circadian rhythms in ribbon synapse morphology and L-VGCC currents recorded at the synaptic terminal. Ribbon synapses are specialized synaptic structures that allow for the tonic release of neurotransmitters under graded membrane potentials from retinal photoreceptors and bipolar cells, as well as hair cells in the auditory and vestibular system (Lenzi et al. 1999; Parsons and Sterling 2003) and are often located near clusters of L-VGCCs (Issa and Hudspeth 1994). In fish bipolar terminals, there is a diurnal difference in the organization of the vesicular halo around synaptic ribbons. At midnight, the vesicular halos surrounding the synaptic ribbons are more organized than during the daytime (Hull et al. 2006). Interestingly, this diurnal regulation of ribbon ultrastructure coincides with the diurnal rhythm of L-VGCCs in bipolar cells. The average peak amplitude of L-VGCCs recorded from bipolar cell terminals are significantly larger at midnight than at midday (Hull et al. 2006). Hence, the diurnal regulation of L-VGCCs in fish bipolar cells is associated with the daily changes in ribbon synapse ultrastructure (Fig. 2b).
Another physiological aspect of the L-VGCC rhythm is the circadian control of retinoschisin secretion. Retinoschisin is a 224-amino acid protein secreted mainly by retinal photoreceptors and bipolar cells and is important in the development and maintenance of retinal cytoarchitecture (Reid et al. 1999, 2003; Grayson et al. 2000; Molday et al. 2001; Reid and Farber 2005). Mutations in the retinoschisin gene (RS1) cause X-linked retinoschisis, a retinal dystrophy that features disorganization of retinal cell layers, disruption of the synaptic structures and neurotransmission between photoreceptors and bipolar cells, and progressive degeneration of rod and cone photoreceptors (Gehrig et al. 1999; Reid et al. 1999; Wang et al. 2002; Tantri et al. 2004; Zeng et al. 2004; Molday et al. 2007). In chicken retinas, mRNA and protein expression of retinoschisin are under circadian control, and inhibition of L-VGCCs with dihydropyridines dampens the circadian rhythm of retinoschisin secretion where only nighttime secretion is affected (Ko et al. 2008). Furthermore, there is a physical interaction between retinoschisin and the L-VGCCα1D subunit (Shi et al. 2009). Not only do retinoschisin and the N-terminal of the L-VGCCα1 subunit physically interact with one another, over-expression of a missense retinoschisin (RS1) mutant gene, R141G, in chicken cone photoreceptors causes a decrease in L-VGCC currents at night and dampens the channel rhythm. Therefore, there is a novel bidirectional relationship between L-VGCCs and retinoschisin: L-VGCCs regulate the circadian rhythm of retinoschisin secretion, while secreted retinoschisin feeds back to regulate L-VGCCs. Physical interactions between L-VGCCα1 subunits and retinoschisin play an important role in the membrane retention of L-VGCCα1 subunits and photoreceptor-bipolar synaptic transmission (Fig. 3; Shi et al. 2009).
Circadian regulation of L-voltage-gated calcium channels in the suprachiasmatic nucleus
As mentioned in the section, ‘The circadian oscillators in the mammalian suprachiasmatic nucleus’, the action potential firing rate of SCN neurons are under circadian control with increases in spontaneous firing during the day (Green and Gillette 1982; Shibata and Moore 1988; Welsh et al. 1995; Quintero et al. 2003; Wang and Huang 2004; Meredith et al. 2006). There are several ion channels that potentially contribute to the circadian rhythm of firing rates in SCN neurons, including the L-type calcium channels. There is a circadian regulation of intracellular calcium levels in SCN cells that results from a robust diurnal modulation of L-VGCC currents (Pennartz et al. 2002) and can be abolished with VGCC blockers (Colwell 2000). Pennartz et al. (2002) demonstrated that the current amplitude of L-VGCCs is significantly higher during the day and tightly coupled to spike generation in SCN neurons. However, the precise physiological role of this L-VGCC rhythm in SCN neuron spike generation is controversial, as others have reported that L-VGCCs may only contribute to the spike interval or the monophasic afterhyperpolarization but not spike generation (Pennartz et al. 2002; Cloues and Sather 2003; Jackson et al. 2004). Another possible role for SCN L-VGCCs is as a circadian input to synchronize the rhythmicity of the expression of clock genes such as Per2 and Bmal1, as inhibition of these channels abolishes the oscillatory patterns of Per2 and Bmal1 expression (Nahm et al. 2005). Hence, L-VGCCs could contribute to coordinating rhythmic clock gene expression through the input pathway, even though the channels themselves are regulated by the circadian clocks and serve as outputs (Ko et al. 2007). This dual role is similar to the circadian regulation of CNGCs serving in both the input and output pathways. Therefore, L-VGCCs may have a significant impact on maintaining the stability of the circadian oscillators at the cellular level, and such a model has been proposed for invertebrate circadian pacemaker cells (McMahon and Block 1987).
Circadian regulation of T-type voltage-gated calcium channels
The transient (T) type VGCCs (T-VGCC) are activated at low voltages for calcium influx into neurons, and they are responsible for the generation of normal brain rhythms as well as abnormal brain rhythms associated with numerous neurological disorders (Huguenard and Prince 1992; Tarasenko et al. 1997; Talley et al. 2000). The modulation of T-VGCCs has been implicated in sleep/wake cycles in animals. During slow wave sleep, relay neurons in the thalamus are relatively hyperpolarized in a range that activates T-VGCCs (Bal et al. 1995). Upon arousal, thalamic neurons become more depolarized because of influences from the brainstem resulting in less T-VGCC activity (Nordskog et al. 2006). There are diurnal regulations of gene expression of all three T-VGCC subtypes, Cav3.1, Cav3.2, and Cav3.3 in the mouse thalamus (Nordskog et al. 2006). While the gene expression of Cav3.1 peaks late at night corresponding to the early sleep period, Cav3.2 and Cav3.3 expression peak during the early night when mice are in their late inactive phase. Therefore, the fluctuations in T-VGCC activity are important for normal sleep patterns (Nordskog et al. 2006). In the rat SCN and cerebellum, the expression of T-VGCCs is also under circadian control (Nahm et al. 2005). In the SCN, gene expression of T-VGCCs is greatest during the transition period from day to night, which correlates to the late sleep period, while in the cerebellum, T-VGCC expression peaks in the middle of the night when these animals are most active (Nahm et al. 2005). Treatment with T-VGCC blockers shift the circadian phases of the SCN (Kim et al. 2005). Therefore, T-VGCCs contribute to the regulation of sleep–wake cycles and circadian phase-shifting.
Circadian regulation of potassium channels
Potassium channels are the most diverse ion channel class with over 100 genes linked to the pore-forming α-subunit identified to date (Kim and Hoffman 2008). They can dampen membrane excitability and set the resting membrane potential in neurons. There are four major families of K+ channels classified by their genetic homology and functional characteristics: voltage-gated; Ca2+-activated; inward rectifier; and leak K+ channels (Kim and Hoffman 2008). In photoreceptors, the dark inward current through CNGCs in the outer segment is counterbalanced by a K+ outward current in the inner segment (Molday and Kaupp 2000), which is mainly through voltage-gated K+ channels. The major subtypes of voltage-gated K+ channels present in photoreceptors are delayed-rectifier (Kv1.2, 1.3, and 2.1) and A-type transient K+ channels (Kv4.2; Pinto and Klumpp 1998). The Ca2+-activated K+ channels and outward-rectifying non-inactivating K+ channels (Kv10.2; eag2) are also found in photoreceptors (Jow and Jeng 2008; Pelucchi et al. 2008). Thus far, reports dealing with the circadian regulation of K+ channels in the retina have been confined mostly to invertebrate studies. In Aplysia retinal pacemaker neurons (not photoreceptors), there is a robust circadian rhythm of potassium currents carried by voltage-gated K+ channels (IKV), while the transient A-type and Ca2+-activated K+ currents remain constant throughout the day (Barnes and Jacklet 1997). When IKV peaks at late night (pre-dawn), the compound action potential firing frequency reaches its nadir. The circadian rhythm of IKV in turn contributes to the circadian control of the frequency of compound action potentials in pacemaker neurons. The basal retinal neurons in the eye of the mollusk Bulla gouldiana express a circadian rhythm in optic nerve impulses (Michel et al. 1993). It is the circadian regulation of IKV, but not other outward K+ channels, that drive the daily fluctuations in membrane conductance and membrane potential of these neurons (Michel et al. 1999).
In Drosophila, both Ca2+-activated (KCa) and voltage-gated K+ channels contribute to the circadian regulation of locomotor activity and sleep–wake cycles. The Slowpoke potassium channel is one type of big conductance KCa (BK) channel (Michel et al. 1999). The mRNA and protein expression of the Slowpoke binding protein show a circadian rhythm in photoreceptor cells as well as in Drosophila heads and in neurosecretory cells of the pars intercerebralis neurons (Jaramillo et al. 2004). Over-expression of Slowpoke binding protein alters the circadian-regulated locomotor activity rhythm. In addition, a point mutation in the Shaker gene, which encodes a voltage-gated K+ channel, causes shortened sleep periods and a reduced lifespan (Cirelli et al. 2005). Another voltage-gated K+ channel encoded by the Shaw gene is widely expressed in Drosophila central neurons and is known to regulate resting membrane potential (Hodge and Stanewsky 2008), and its over-expression in Drosophila clock neurons lead to arrhythmic locomotor behavior.
In the mammalian SCN, the BKs are also circadian regulated, with peak activity at night (Cloues and Sather 2003; Kuhlman and McMahon 2004; Meredith et al. 2006; Pitts et al. 2006). As the membrane potential, input conductance, and spontaneous firing frequency are rhythmic in the SCN (Schaap et al. 1999), the circadian regulation of BKs contributes to multiple circadian aspects of SCN neuronal activity, including suppressing spontaneous firing at night (Kent and Meredith 2008). Deletion of the Kcnma1 gene, which encodes the BK channel, dampens locomotor activity and physiological rhythms in mice (Kent and Meredith 2008). A fast delayed-rectifier potassium channel (FDR), encoded by the Kv3.1b and Kv3.2 genes, is also under circadian regulation in SCN neurons (Itri et al. 2005). The FDR potassium current peaks during the day that coincides with higher expression of Kv3.1b and Kv3.2 in SCN neurons at this time. Blocking FDR abolishes the daily rhythm in the firing rate of SCN neurons, and therefore, these channels are important for SCN neurons to sustain high firing frequencies through enabling rapid repolarization during the day (Itri et al. 2005). Taken together, one major function of the circadian regulation of the various K+ channels is to regulate neuron excitability, which could ultimately lead to the circadian output control of rhythmic behaviors.
Circadian regulation of Na+/K+-ATPase in the suprachiasmatic nucleus
The sodium-potassium pump (Na+/K+-ATPase) is an energy-transducing ionic pump located in the plasma membrane that transports 3 Na+ ions out of and 2 K+ ions into the cell at the expense of energy derived from ATP hydrolysis (Therien and Blostein 2000). The function of Na+/K+-ATPase is to maintain Na+ and K+ gradients across the plasma membrane, thereby contributing to the resting membrane potential in excitable cells (Therien and Blostein 2000). The Na+/K+-ATPase also plays an important role in the regulation of intracellular calcium homeostasis through an indirect interaction with the Na+-Ca2+ exchanger or inositol 1,4,5-triphosphate receptor mediated intracellular calcium stores (Tian and Xie 2008). In SCN neurons, there is a diurnal rhythm in Na+/K+-ATPase activity, which is higher during the day (Wang and Huang 2004, 2006). As the action potential firing rate is higher during the day, the concurrent higher activity of Na+/K+-ATPase is crucial in restoring the Na+ and K+ gradients and reestablishing the resting membrane potential.
Circadian regulation of a long-opening time cation channel (ILOT) in the avian pineal gland
As described in the section, ‘The circadian rhythms in the pineal gland’, the secretory cells (pinealocytes) of the pineal gland exhibit circadian rhythms in melatonin release that is high at night and inhibited by light (Deguchi 1979a,b,c; Zatz and Mullen 1988b; Zatz 1989). In mammals, the circadian rhythm of pineal physiology is directly controlled by the SCN. However, in other vertebrate species including avians, reptiles, and amphibians, pinealocytes are themselves photosensitive, and the light-sensitive circadian rhythms in melatonin release from pinealocytes persist in vitro (Robertson and Takahashi 1988a,b; Bell-Pedersen et al. 2005). Even though L-VGCCs are present in chicken pinealocytes (Henderson and Dryer 1992), inhibition of these channels only decreases evening melatonin by 40% with no apparent effect on daytime melatonin levels (Nikaido and Takahashi 1996), which is in stark contrast to the role of L-VGCCs on avian retinal melatonin as described previously (Iuvone and Besharse 1986; Cahill and Besharse 1993; Bernard et al. 1997; Ivanova and Iuvone 2003b). Interestingly, there is a non-selective cation channel unique to pinealocytes (D’Souza and Dryer 1996). The conductance of this channel is about 40 pS, and once open it remains so for a relatively long period of time (up to 20 s). Thus, this channel was aptly named ILOT (long-opening time). The ILOT channel is not voltage-, stretch-, or nucleotide-activated, and its gating properties persist in excised inside-out patches in the absence of Ca2+. The ILOT channel is permeable to calcium and active exclusively at night. ILOT activity is not suppressed by brief light pulses (D’Souza and Dryer 1996) but requires protein synthesis (D’Souza and Dryer 1997). Even though the biophysical properties of ILOT channels have been studied, unfortunately the molecular structure, amino acids, and gene sequence are still unknown. Moreover, ILOT channels are not activated by melatonin, activation of adenylate cyclase, or depleted internal calcium stores (D’Souza and Dryer 1996). One technical difficulty in investigating the molecular properties or the function of ILOT channels is that these channels are only observed in less than 1/4 of the pinealocyte population at night (D’Souza and Dryer 1996), and therefore, the ILOT channel is unlikely to be a regulatory factor in melatonin release from avian pineal glands. However, it is possible that ILOT channels could serve as a source of cation signals to synchronize and provide communication between pineal oscillators. More rigorous investigations will be required to further understand the nature and function of the ILOT channel.
Conclusion
In addition to the SCN as the master circadian oscillator in mammals, the retina and pineal gland are the two major areas that contain independent circadian oscillators especially in non-mammalian vertebrates. As ion channels are the gatekeepers of neuronal excitability, circadian regulation of ion channels governs the daily oscillations of plasma membrane excitability and action potential firing rates of SCN neurons; photosensitivity, melatonin release and retinoschisin secretion from retina photoreceptors; as well as melatonin release from pinealocytes. In invertebrates, circadian control of ion channels ultimately regulates the daily rhythms in locomotor behavior, sensitivity of olfaction, as well as learning and memory.
Thus far, how circadian oscillators regulate cellular function and physiology has been most intensively studied in non-mammalian retinal photoreceptors. Photoreceptors have endogenous circadian oscillators that can function independently of other retinal inputs (Cahill and Besharse 1993; Thomas et al. 1993; Ko et al. 2001). These circadian oscillators regulate retinomotor movement (Nagle and Burnside 1984; Pierce and Besharse 1985, 1988; Wagner et al. 1993; Burnside 2001), outer segment disc shedding (Besharse and Dunis 1983) and membrane renewal (Reme et al. 1986; Cahill and Besharse 1995), morphological changes at synaptic ribbons (Adly et al. 1999), gene expression (Korenbrot and Fernald 1989; Pierce et al. 1993; Yoshida et al. 1993; Green and Besharse 1996; Haque et al. 2002), a delayed-rectifier potassium channel (Michel et al. 1993), the affinity of CNGCs (Ko et al. 2001), the L-VGCCs (Ko et al. 2007), and the activities of MAPK and CaMKII (Ko et al. 2001) among other photoreceptor activities. The ERG is a graphical representation of electrical potential changes across the eye that is elicited by a light stimulus. The ERG has been used as an assessment of physical and clinical conditions of the retina, and hence, it is a useful technique for tracing retinal rhythmicity in vivo (Cameron et al. 2008). While L-VGCCs contribute to various components of ERGs, several retinal degenerative diseases are associated with dampened or abnormal circadian rhythms in ERGs. In the Royal College of Surgeons rat, an animal model with inherited retinal degeneration, the diurnal changes in ERG c-waves are dampened prior to the onset of retinal degeneration from postnatal days 17 to 24 (Hawlina et al. 1992; Strauss et al. 1998). Mutations in human cone–rod homeobox are associated with retinal diseases, including cone–rod dystrophy-2, retinitis pigmentosa, and Leber congenital amaurosis, which all lead to blindness (Furukawa et al. 1999; Morrow et al. 2005). In a mouse model of cone–rod homeobox mutation, the circadian rhythmicity of the ERG is abolished, and the mutant mice are not able to be entrained by light–dark cycles (Furukawa et al. 1999). Hence, circadian regulation of ion channels modulates the physiological states and function of the visual system.
Compared with other research areas in chronobiology, the circadian regulation of ion channels remains largely unexplored. For example, many parts of the molecular mechanisms linking the core oscillators (the ‘molecular clock’) to the rhythmic control of ion channels are still unknown. Disruption of components of the molecular clock causing abnormal or dampened circadian rhythmicities in animals has been demonstrated. However, the functional significance of circadian fluctuations in ion channel activities that ultimately lead to the daily rhythmicities in neuronal activities of controlling locomotor activity, synaptic plasticity, learning and memory, and even sleep–wake cycles, has yet to be explored. In addition, the participation of ion channels in circadian entrainment is not clear. Addressing the questions above, as well as identification and characterization of other ion channels that are potentially under circadian control, will be important for future directions of research, as ion channels have been utilized as therapeutic targets for various diseases. Understanding the circadian regulation of ion channels will ultimately benefit the future development of therapeutic strategies in the treatment of sleep disorders, cardiovascular diseases, and other illness linked to circadian misalignment (Scheer et al. 2009).
Abbreviations used
- AANAT
arylalkylamine N-acetyltransferase
- Akt
protein kinase B
- BK
big conductance KCa
- BMAL
brain and muscle, ARNT-like
- CaMKII
calcium–calmodulin kinase II
- CLOCK
circadian locomotor output cycles kaput
- CNGC
cGMP-gated cation channel
- CRY
cryptochrome
- ENSLI cells
enkephalin-, neurotensin-, and somatostatin-like immunoreactive cells
- ERG
electroretinogram
- FDR
fast delayed-rectifier
- ILOT
long-opening time cation channel
- L-VGCC
L-type VGCC
- MAPK
mitogen-activated protein kinase
- PER
period
- PI3K
phosphatidylinositol 3 kinase
- REV-ERBα
reverse orientation c-erbA-1 gene alpha; an orphan nuclear receptor
- SCN
suprachiasmatic nucleus
- SS
somatostatin
- T-VGCC
transient (T)-type VGCC
- VGCC
voltage-gated calcium channels
References
- Abernethy DR, Soldatov NM. Structure-functional diversity of human L-type Ca2+ channel: perspectives for new pharmacological targets. J Pharmacol Exp Ther. 2002;300:724–728. doi: 10.1124/jpet.300.3.724. [DOI] [PubMed] [Google Scholar]
- Adachi A, Nogi T, Ebihara S. Phase-relationship and mutual effects between circadian rhythms of ocular melatonin and dopamine in the pigeon. Brain Res. 1998a;792:361–369. doi: 10.1016/s0006-8993(98)00206-6. [DOI] [PubMed] [Google Scholar]
- Adachi K, Fujita Y, Morizane C, Akaike A, Ueda M, Satoh M, Masai H, Kashii S, Honda Y. Inhibition of NMDA receptors and nitric oxide synthase reduces ischemic injury of the retina. Eur J Pharmacol. 1998b;350:53–57. doi: 10.1016/s0014-2999(98)00317-3. [DOI] [PubMed] [Google Scholar]
- Adly MA, Spiwoks-Becker I, Vollrath L. Ultrastructural changes of photoreceptor synaptic ribbons in relation to time of day and illumination. Invest Ophthalmol Vis Sci. 1999;40:2165–2172. [PubMed] [Google Scholar]
- Allwardt BA, Lall AB, Brockerhoff SE, Dowling JE. Synapse formation is arrested in retinal photoreceptors of the zebrafish nrc mutant. J Neurosci. 2001;21:2330–2342. doi: 10.1523/JNEUROSCI.21-07-02330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DH, Fisher SK, Steinberg RH. Mammalian cones: disc shedding, phagocytosis, and renewal. Invest Ophthalmol Vis Sci. 1978;17:117–133. [PubMed] [Google Scholar]
- Anderson DH, Fisher SK, Erickson PA, Tabor GA. Rod and cone disc shedding in the rhesus monkey retina: a quantitative study. Exp Eye Res. 1980;30:559–574. doi: 10.1016/0014-4835(80)90040-8. [DOI] [PubMed] [Google Scholar]
- Bailey MJ, Beremand PD, Hammer R, Reidel E, Thomas TL, Cassone VM. Transcriptional profiling of circadian patterns of mRNA expression in the chick retina. J Biol Chem. 2004;279:52247–52254. doi: 10.1074/jbc.M405679200. [DOI] [PubMed] [Google Scholar]
- Bailey MJ, Coon SL, Carter DA, et al. Night/day changes in pineal expression of > 600 genes: central role of adrenergic/cAMP signaling. J Biol Chem. 2009;284:7606–7622. doi: 10.1074/jbc.M808394200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal T, von Krosigk M, McCormick DA. Synaptic and membrane mechanisms underlying synchronized oscillations in the ferret lateral geniculate nucleus in vitro. J Physiol. 1995;483:641–663. doi: 10.1113/jphysiol.1995.sp020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- Barnes S, Jacklet JW. Ionic currents of isolated retinal pacemaker neurons: projected daily phase differences and selective enhancement by a phase-shifting neurotransmitter. J Neurophysiol. 1997;77:3075–3084. doi: 10.1152/jn.1997.77.6.3075. [DOI] [PubMed] [Google Scholar]
- Barnes S, Kelly ME. Calcium channels at the photoreceptor synapse. Adv Exp Med Biol. 2002;514:465–476. doi: 10.1007/978-1-4615-0121-3_28. [DOI] [PubMed] [Google Scholar]
- Bauer PJ. Cyclic GMP-gated channels of bovine rod photoreceptors: affinity, density and stoichiometry of Ca(2+)-calmodulin binding sites. J Physiol. 1996;494:675–685. doi: 10.1113/jphysiol.1996.sp021523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR, Borrelli E, Caron MG. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007;27:881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard M, Iuvone PM, Cassone VM, Roseboom PH, Coon SL, Klein DC. Avian melatonin synthesis: photic and circadian regulation of serotonin N-acetyltransferase mRNA in the chicken pineal gland and retina. J Neurochem. 1997;68:213–224. doi: 10.1046/j.1471-4159.1997.68010213.x. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Dunis DA. Methoxyindoles and photoreceptor metabolism: activation of rod shedding. Science. 1983;219:1341–1343. doi: 10.1126/science.6828862. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Iuvone PM. Is dopamine a light-adaptive or a dark-adaptive modulator in retina? Neurochem Int. 1992;20:193–199. doi: 10.1016/0197-0186(92)90167-p. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Dunis DA, Burnside B. Effects of cyclic adenosine 3′, 5′-monophosphate on photoreceptor disc shedding and retinomotor movement. Inhibition of rod shedding and stimulation of cone elongation. J Gen Physiol. 1982;79:775–790. doi: 10.1085/jgp.79.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besharse JC, Zhuang M, Freeman K, Fogerty J. Regulation of photoreceptor Per1 and Per2 by light, dopamine and a circadian clock. Eur J Neurosci. 2004;20:167–174. doi: 10.1111/j.1460-9568.2004.03479.x. [DOI] [PubMed] [Google Scholar]
- Blair LA, Marshall J. IGF-1 modulates N and L calcium channels in a PI 3-kinase-dependent manner. Neuron. 1997;19:421–429. doi: 10.1016/s0896-6273(00)80950-2. [DOI] [PubMed] [Google Scholar]
- Bobu C, Craft CM, Masson-Pevet M, Hicks D. Photoreceptor organization and rhythmic phagocytosis in the nile rat Arvicanthis ansorgei: a novel diurnal rodent model for the study of cone pathophysiology. Invest Ophthalmol Vis Sci. 2006;47:3109–3118. doi: 10.1167/iovs.05-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley J, Reisert J, Frings S. Regulation of cyclic nucleotide-gated channels. Curr Opin Neurobiol. 2005;15:343–349. doi: 10.1016/j.conb.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Burnside B. Light and circadian regulation of retinomotor movement. Prog Brain Res. 2001;131:477–485. doi: 10.1016/s0079-6123(01)31038-5. [DOI] [PubMed] [Google Scholar]
- Burnside B, Ackland N. Effects of circadian rhythm and cAMP on retinomotor movements in the green sunfish, Lepomis cyanellus. Invest Ophthalmol Vis Sci. 1984;25:539–545. [PubMed] [Google Scholar]
- Burnside B, Evans M, Fletcher RT, Chader GJ. Induction of dark-adaptive retinomotor movement (cell elongation) in teleost retinal cones by cyclic adenosine 3′, 5′-monophosphate. J Gen Physiol. 1982;79:759–774. doi: 10.1085/jgp.79.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Resetting the circadian clock in cultured Xenopus eyecups: regulation of retinal melatonin rhythms by light and D2 dopamine receptors. J Neurosci. 1991;11:2959–2971. doi: 10.1523/JNEUROSCI.11-10-02959.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Circadian clock functions localized in Xenopus retinal photoreceptors. Neuron. 1993;10:573–577. doi: 10.1016/0896-6273(93)90160-s. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Circadian rhythmicity in vertebrate retinas: regulation by a photoreceptor oscillator. Prog Retinal Eye Res. 1995;14:267–291. [Google Scholar]
- Cameron MA, Barnard AR, Lucas RJ. The electro-retinogram as a method for studying circadian rhythms in the mammalian retina. J Genet. 2008;87:459–466. doi: 10.1007/s12041-008-0068-5. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII Nomenclature and structure–function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- Chae KS, Ko GY, Dryer SE. Tyrosine phosphorylation of cGMP-gated ion channels is under circadian control in chick retina photoreceptors. Invest Ophthalmol Vis Sci. 2007;48:901–906. doi: 10.1167/iovs.06-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia SS, Haque R, Pozdeyev N, Jackson CR, Iuvone PM. Temporal coupling of cyclic AMP and Ca/calmodulin-stimulated adenylyl cyclase to the circadian clock in chick retinal photoreceptor cells. J Neurochem. 2006;99:1142–1150. doi: 10.1111/j.1471-4159.2006.04154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SK, Ko GY, Dryer SE. Somatostatin peptides produce multiple effects on gating properties of native cone photoreceptor cGMP-gated channels that depend on circadian phase and previous illumination. J Neurosci. 2007;27:12168–12175. doi: 10.1523/JNEUROSCI.3541-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- Cheng HY, Papp JW, Varlamova O, et al. microRNA modulation of circadian-clock period and entrainment. Neuron. 2007;54:813–829. doi: 10.1016/j.neuron.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong NW, Cassone VM, Bernard M, Klein DC, Iuvone PM. Circadian expression of tryptophan hydroxylase mRNA in the chicken retina. Brain Res Mol Brain Res. 1998;61:243–250. doi: 10.1016/s0169-328x(98)00219-8. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Bushey D, Hill S, Huber R, Kreber R, Ganetzky B, Tononi G. Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434:1087–1092. doi: 10.1038/nature03486. [DOI] [PubMed] [Google Scholar]
- Cloues RK, Sather WA. Afterhyperpolarization regulates firing rate in neurons of the suprachiasmatic nucleus. J Neurosci. 2003;23:1593–1604. doi: 10.1523/JNEUROSCI.23-05-01593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbs WH, Pugh EN., Jr Cyclic GMP can increase rod outer-segment light-sensitive current 10-fold without delay of excitation. Nature. 1985;313:585–587. doi: 10.1038/313585a0. [DOI] [PubMed] [Google Scholar]
- Cobbs WH, Barkdoll AE, III, Pugh EN., Jr Cyclic GMP increases photocurrent and light sensitivity of retinal cones. Nature. 1985;317:64–66. doi: 10.1038/317064a0. [DOI] [PubMed] [Google Scholar]
- Collado MS, Lyons LC, Levenson JM, Khabour O, Pita-Almenar JD, Schrader L, Eskin A. In vivo regulation of an Aplysia glutamate transporter, ApGT1, during long-term memory formation. J Neurochem. 2007;100:1315–1328. doi: 10.1111/j.1471-4159.2006.04298.x. [DOI] [PubMed] [Google Scholar]
- Colwell CS. Circadian modulation of calcium levels in cells in the suprachiasmatic nucleus. Eur J Neurosci. 2000;12:571–576. doi: 10.1046/j.1460-9568.2000.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constance CM, Fan JY, Preuss F, Green CB, Price JL. The circadian clock-containing photoreceptor cells in Xenopus laevis express several isoforms of casein kinase I. Brain Res. Mol Brain Res. 2005;136:199–211. doi: 10.1016/j.molbrainres.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Cristiani R, Petrucci C, Dal Monte M, Bagnoli P. Somatostatin (SRIF) and SRIF receptors in the mouse retina. Brain Res. 2002;936:1–14. doi: 10.1016/s0006-8993(02)02450-2. [DOI] [PubMed] [Google Scholar]
- Cyran SA, Buchsbaum AM, Reddy KL, Lin MC, Glossop NR, Hardin PE, Young MW, Storti RV, Blau J. Vrille, Pdp1, and dClock form a second feedback loop in the Drosophila circadian clock. Cell. 2003;112:329–341. doi: 10.1016/s0092-8674(03)00074-6. [DOI] [PubMed] [Google Scholar]
- D’Souza T, Dryer SE. A cationic channel regulated by a vertebrate intrinsic circadian oscillator. Nature. 1996;382:165–167. doi: 10.1038/382165a0. [DOI] [PubMed] [Google Scholar]
- D’Souza T, Dryer SE. Elevated nighttime activity of chick pineal ILOT channels requires protein synthesis. Biol Signals. 1997;6:212–216. doi: 10.1159/000109149. [DOI] [PubMed] [Google Scholar]
- Dearry A, Barlow RB., Jr Circadian rhythms in the green sunfish retina. J Gen Physiol. 1987;89:745–770. doi: 10.1085/jgp.89.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi T. A circadian oscillator in cultured cells of chicken pineal gland. Nature. 1979a;282:94–96. doi: 10.1038/282094a0. [DOI] [PubMed] [Google Scholar]
- Deguchi T. Circadian rhythm of serotonin N-acetyltransferase activity in organ culture of chicken pineal gland. Science. 1979b;203:1245–1247. doi: 10.1126/science.424750. [DOI] [PubMed] [Google Scholar]
- Deguchi T. Circadian rhythms of indoleamines and serotonin N-acetyltransferase activity in the pineal gland. Mol Cell Biochem. 1979c;27:57–66. doi: 10.1007/BF00849279. [DOI] [PubMed] [Google Scholar]
- Djamgoz MB, Wagner HJ. Localization and function of dopamine in the adult vertebrate retina. Neurochem Int. 1992;20:139–191. doi: 10.1016/0197-0186(92)90166-o. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. A short history of voltage-gated calcium channels. Br J Pharmacol. 2006;147(Suppl 1):S56–S62. doi: 10.1038/sj.bjp.0706442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorenbos R, Contini M, Hirasawa H, Gustincich S, Raviola E. Expression of circadian clock genes in retinal dopaminergic cells. Vis Neurosci. 2007;24:573–580. doi: 10.1017/S0952523807070538. [DOI] [PubMed] [Google Scholar]
- Dowton M, Boelen MK, Morgan IG. Somatostatin-14 and somatostatin-28 levels are light-driven and vary during development in the chicken retina. Brain Res Dev Brain Res. 1994;78:65–69. doi: 10.1016/0165-3806(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Doyle SE, Grace MS, McIvor W, Menaker M. Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis Neurosci. 2002;19:593–601. doi: 10.1017/s0952523802195058. [DOI] [PubMed] [Google Scholar]
- Dryer SE, Henderson D. A cyclic GMP-activated channel in dissociated cells of the chick pineal gland. Nature. 1991;353:756–758. doi: 10.1038/353756a0. [DOI] [PubMed] [Google Scholar]
- Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- Eckel-Mahan KL, Phan T, Han S, Wang H, Chan GC, Scheiner ZS, Storm DR. Circadian oscillation of hippocampal MAPK activity and cAMP: implications for memory persistence. Nat Neurosci. 2008;11:1074–1082. doi: 10.1038/nn.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayard E, Tintignac LA, Baudry A, Hemmings BA. Protein kinase B/Akt at a glance. J Cell Sci. 2005;118:5675–5678. doi: 10.1242/jcs.02724. [DOI] [PubMed] [Google Scholar]
- Fisher SK, Pfeffer BA, Anderson DH. Both rod and cone disc shedding are related to light onset in the cat. Invest Ophthalmol Vis Sci. 1983;24:844–856. [PubMed] [Google Scholar]
- Fujii S, Krishnan P, Hardin P, Amrein H. Nocturnal male sex drive in Drosophila. Curr Biol. 2007;17:244–251. doi: 10.1016/j.cub.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara C, Liu C, Ivanova TN, Chan GC, Storm DR, Iuvone PM, Tosini G. Gating of the cAMP signaling cascade and melatonin synthesis by the circadian clock in mammalian retina. J Neurosci. 2004;24:1803–1811. doi: 10.1523/JNEUROSCI.4988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Li T, Davis FC, Cepko CL. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat Genet. 1999;23:466–470. doi: 10.1038/70591. [DOI] [PubMed] [Google Scholar]
- Garbarino-Pico E, Carpentieri AR, Castagnet PI, Pasquare SJ, Giusto NM, Caputto BL, Guido ME. Synthesis of retinal ganglion cell phospholipids is under control of an endogenous circadian clock: daily variations in phospholipid-synthesizing enzyme activities. J Neurosci Res. 2004a;76:642–652. doi: 10.1002/jnr.20126. [DOI] [PubMed] [Google Scholar]
- Garbarino-Pico E, Carpentieri AR, Contin MA, Sarmiento MI, Brocco MA, Panzetta P, Rosenstein RE, Caputto BL, Guido ME. Retinal ganglion cells are autonomous circadian oscillators synthesizing N-acetylserotonin during the day. J Biol Chem. 2004b;279:51172–51181. doi: 10.1074/jbc.M309248200. [DOI] [PubMed] [Google Scholar]
- Gehrig A, Weber BH, Lorenz B, Andrassi M. First molecular evidence for a de novo mutation in RS1 (XLRS1) associated with X linked juvenile retinoschisis. J Med Genet. 1999;36:932–934. [PMC free article] [PubMed] [Google Scholar]
- Glossop NR, Hardin PE. Central and peripheral circadian oscillator mechanisms in flies and mammals. J Cell Sci. 2002;115:3369–3377. doi: 10.1242/jcs.115.17.3369. [DOI] [PubMed] [Google Scholar]
- Gordon SE, Brautigan DL, Zimmerman AL. Protein phosphatases modulate the apparent agonist affinity of the light-regulated ion channel in retinal rods. Neuron. 1992;9:739–748. doi: 10.1016/0896-6273(92)90036-d. [DOI] [PubMed] [Google Scholar]
- Gordon SE, Downing-Park J, Zimmerman AL. Modulation of the cGMP-gated ion channel in frog rods by calmodulin and an endogenous inhibitory factor. J Physiol. 1995;486:533–546. doi: 10.1113/jphysiol.1995.sp020832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados-Fuentes D, Tseng A, Herzog ED. A circadian clock in the olfactory bulb controls olfactory responsivity. J Neurosci. 2006;26:12219–12225. doi: 10.1523/JNEUROSCI.3445-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson C, Reid SN, Ellis JA, Rutherford A, Sowden JC, Yates JR, Farber DB, Trump D. Retinoschisin, the X-linked retinoschisis protein, is a secreted photoreceptor protein, and is expressed and released by Weri-Rb1 cells. Hum Mol Genet. 2000;9:1873–1879. doi: 10.1093/hmg/9.12.1873. [DOI] [PubMed] [Google Scholar]
- Green CB, Besharse JC. Use of a high stringency differential display screen for identification of retinal mRNAs that are regulated by a circadian clock. Brain Res Mol Brain Res. 1996;37:157–165. doi: 10.1016/0169-328x(95)00307-e. [DOI] [PubMed] [Google Scholar]
- Green CB, Besharse JC. Retinal circadian clocks and control of retinal physiology. J Biol Rhythms. 2004;19:91–102. doi: 10.1177/0748730404263002. [DOI] [PubMed] [Google Scholar]
- Green DJ, Gillette R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res. 1982;245:198–200. doi: 10.1016/0006-8993(82)90361-4. [DOI] [PubMed] [Google Scholar]
- Green CB, Cahill GM, Besharse JC. Regulation of tryptophan hydroxylase expression by a retinal circadian oscillator in vitro. Brain Res. 1995a;677:283–290. doi: 10.1016/0006-8993(95)00166-n. [DOI] [PubMed] [Google Scholar]
- Green CB, Cahill GM, Besharse JC. Tryptophan hydroxylase is expressed by photoreceptors in Xenopus laevis retina. Vis Neurosci. 1995b;12:663–670. doi: 10.1017/s0952523800008956. [DOI] [PubMed] [Google Scholar]
- Halstenberg S, Lindgren KM, Samagh SP, Nadal-Vicens M, Balt S, Fernald RD. Diurnal rhythm of cone opsin expression in the teleost fish Haplochromis burtoni. Vis Neurosci. 2005;22:135–141. doi: 10.1017/S0952523805222022. [DOI] [PubMed] [Google Scholar]
- Hankins MW, Jones RJ, Ruddock KH. Diurnal variation in the b-wave implicit time of the human electroretinogram. Vis Neurosci. 1998;15:55–67. doi: 10.1017/s0952523898151118. [DOI] [PubMed] [Google Scholar]
- Hankins MW, Jones SR, Jenkins A, Morland AB. Diurnal daylight phase affects the temporal properties of both the b-wave and d-wave of the human electroretinogram. Brain Res. 2001;889:339–343. doi: 10.1016/s0006-8993(00)03182-6. [DOI] [PubMed] [Google Scholar]
- Haque R, Chaurasia SS, Wessel JH, III, Iuvone PM. Dual regulation of cryptochrome 1 mRNA expression in chicken retina by light and circadian oscillators. Neuroreport. 2002;13:2247–2251. doi: 10.1097/00001756-200212030-00016. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Cahill GM. Cyclic AMP resets the circadian clock in cultured Xenopus retinal photoreceptor layers. J Neurochem. 1998;70:1523–1531. doi: 10.1046/j.1471-4159.1998.70041523.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Cahill GM. Modulation of rhythmic melatonin synthesis in Xenopus retinal photoreceptors by cyclic AMP. Brain Res. 1999a;824:161–167. doi: 10.1016/s0006-8993(99)01162-2. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Cahill GM. A role for cyclic AMP in entrainment of the circadian oscillator in Xenopus retinal photoreceptors by dopamine but not by light. J Neurochem. 1999b;72:1812–1820. doi: 10.1046/j.1471-4159.1999.0721812.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Cahill GM. Regulation of the circadian oscillator in Xenopus retinal photoreceptors by protein kinases sensitive to the stress-activated protein kinase inhibitor, SB 203580. J Biol Chem. 2004;279:22738–22746. doi: 10.1074/jbc.M401389200. [DOI] [PubMed] [Google Scholar]
- Hawlina M, Jenkins HG, Ikeda H. Diurnal variations in the electroretinographic c-wave and retinal melatonin content in rats with inherited retinal dystrophy. Doc Ophthalmol. 1992;79:141–150. doi: 10.1007/BF00156573. [DOI] [PubMed] [Google Scholar]
- Henderson D, Dryer SE. Voltage- and Ca(2+)-activated ionic currents in acutely dissociated cells of the chick pineal gland. Brain Res. 1992;572:182–189. doi: 10.1016/0006-8993(92)90468-o. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sinauer; Sunderland, MA: 2001. [Google Scholar]
- Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y, Sassone-Corsi P. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature. 2007;450:1086–1090. doi: 10.1038/nature06394. [DOI] [PubMed] [Google Scholar]
- Hodge JJ, Stanewsky R. Function of the Shaw potassium channel within the Drosophila circadian clock. PLoS ONE. 2008;3:e2274. doi: 10.1371/journal.pone.0002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Molday RS. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 1993;361:76–79. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. A novel T-type current underlies prolonged Ca(2+)-dependent burst firing in GABAergic neurons of rat thalamic reticular nucleus. J Neurosci. 1992;12:3804–3817. doi: 10.1523/JNEUROSCI.12-10-03804.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, Studholme K, Yazulla S, von Gersdorff H. Diurnal changes in exocytosis and the number of synaptic ribbons at active zones of an ON-type bipolar cell terminal. J Neurophysiol. 2006;96:2025–2033. doi: 10.1152/jn.00364.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto I, Millar T, Chubb IW, Morgan IG. Somatostatin-immunoreactive amacrine cells of chicken retina: retinal mosaic, ultrastructural features, and light-driven variations in peptide metabolism. Neuroscience. 1986;17:1217–1233. doi: 10.1016/0306-4522(86)90089-8. [DOI] [PubMed] [Google Scholar]
- Issa NP, Hudspeth AJ. Clustering of Ca2+ channels and Ca(2+)-activated K+ channels at fluorescently labeled presynaptic active zones of hair cells. Proc Natl Acad Sci USA. 1994;91:7578–7582. doi: 10.1073/pnas.91.16.7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itri JN, Michel S, Vansteensel MJ, Meijer JH, Colwell CS. Fast delayed rectifier potassium current is required for circadian neural activity. Nat Neurosci. 2005;8:650–656. doi: 10.1038/nn1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuvone PM. Evidence for a D2 dopamine receptor in frog retina that decreases cyclic AMP accumulation and serotonin N-acetyl-transferase activity. Life Sci. 1986;38:331–342. doi: 10.1016/0024-3205(86)90080-9. [DOI] [PubMed] [Google Scholar]
- Iuvone PM. Development of melatonin synthesis in chicken retina: regulation of serotonin N-acetyltransferase activity by light, circadian oscillators, and cyclic AMP. J Neurochem. 1990;54:1562–1568. doi: 10.1111/j.1471-4159.1990.tb01205.x. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Besharse JC. Involvement of calcium in the regulation of serotonin N-acetyltransferase in retina. J Neurochem. 1986;46:82–88. doi: 10.1111/j.1471-4159.1986.tb12928.x. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Galli CL, Garrison-Gund CK, Neff NH. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science. 1978;202:901–902. doi: 10.1126/science.30997. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Gan J, Avendano G. K(+)-evoked depolarization stimulates cyclic AMP accumulation in photoreceptor-enriched retinal cell cultures: role of calcium influx through dihydropyridine-sensitive calcium channels. J Neurochem. 1991;57:615–621. doi: 10.1111/j.1471-4159.1991.tb03792.x. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Bernard M, Alonso-Gomez A, Greve P, Cassone VM, Klein DC. Cellular and molecular regulation of serotonin N-acetyltransferase activity in chicken retinal photoreceptors. Biol Signals. 1997;6:217–224. doi: 10.1159/000109131. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Chong NW, Bernard M, Brown AD, Thomas KB, Klein DC. Melatonin biosynthesis in chicken retina. Regulation of tryptophan hydroxylase and arylalkylamine N-acetyltransferase. Adv Exp Med Biol. 1999;460:31–41. [PubMed] [Google Scholar]
- Iuvone PM, Tosini G, Pozdeyev N, Haque R, Klein DC, Chaurasia SS. Circadian clocks, clock networks, aryl-alkylamine N-acetyltransferase, and melatonin in the retina. Prog Retinal Eye Res. 2005;24:433–456. doi: 10.1016/j.preteyeres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Ivanova TN, Iuvone PM. Circadian rhythm and photic control of cAMP level in chick retinal cell cultures: a mechanism for coupling the circadian oscillator to the melatonin-synthesizing enzyme, arylalkylamine N-acetyltransferase, in photoreceptor cells. Brain Res. 2003a;991:96–103. doi: 10.1016/j.brainres.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Ivanova TN, Iuvone PM. Melatonin synthesis in retina: circadian regulation of arylalkylamine N-acetyltransferase activity in cultured photoreceptor cells of embryonic chicken retina. Brain Res. 2003b;973:56–63. doi: 10.1016/s0006-8993(03)02540-x. [DOI] [PubMed] [Google Scholar]
- Jackson AC, Yao GL, Bean BP. Mechanism of spontaneous firing in dorsomedial suprachiasmatic nucleus neurons. J Neurosci. 2004;24:7985–7998. doi: 10.1523/JNEUROSCI.2146-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubcakova V, Oster H, Tamanini F, Cadenas C, Leitges M, van der Horst GT, Eichele G. Light entrainment of the mammalian circadian clock by a PRKCA-dependent posttranslational mechanism. Neuron. 2007;54:831–843. doi: 10.1016/j.neuron.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Jaramillo AM, Zheng X, Zhou Y, Amado DA, Sheldon A, Sehgal A, Levitan IB. Pattern of distribution and cycling of SLOB, Slowpoke channel binding protein, in Drosophila. BMC Neurosci. 2004;5:3. doi: 10.1186/1471-2202-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RF, Moore RY, Morin LP. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypo-thalamic tract. Brain Res. 1988;460:297–313. doi: 10.1016/0006-8993(88)90374-5. [DOI] [PubMed] [Google Scholar]
- Jow GM, Jeng CJ. Differential localization of rat Eag1 and Eag2 potassium channels in the retina. Neurosci Lett. 2008;431:12–16. doi: 10.1016/j.neulet.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol. 1999;1:165–170. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- Kent J, Meredith AL. BK channels regulate spontaneous action potential rhythmicity in the suprachiasmatic nucleus. PLoS ONE. 2008;3:e3884. doi: 10.1371/journal.pone.0003884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Hoffman DA. Potassium channels: newly found players in synaptic plasticity. Neuroscientist. 2008;14:276–286. doi: 10.1177/1073858408315041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Geng R, Somers DE. Circadian phase-specific degradation of the F-box protein ZTL is mediated by the proteasome. Proc Natl Acad Sci USA. 2003;100:4933–4938. doi: 10.1073/pnas.0736949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Choi HJ, Kim JS, et al. Voltage-gated calcium channels play crucial roles in the glutamate-induced phase shifts of the rat suprachiasmatic circadian clock. Eur J Neurosci. 2005;21:1215–1222. doi: 10.1111/j.1460-9568.2005.03950.x. [DOI] [PubMed] [Google Scholar]
- Klein DC. Evolution of the vertebrate pineal gland: the AANAT hypothesis. Chronobiol Int. 2006;23:5–20. doi: 10.1080/07420520500545839. [DOI] [PubMed] [Google Scholar]
- Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated cationic channels of chick retinal cones. Erk MAP Kinase and Ca2+/calmodulin-dependent protein kinase II. Neuron. 2001;29:255–266. doi: 10.1016/s0896-6273(01)00195-7. [DOI] [PubMed] [Google Scholar]
- Ko GY, Ko ML, Dryer SE. Circadian phase-dependent modulation of cGMP-gated channels of cone photoreceptors by dopamine and D2 agonist. J Neurosci. 2003;23:3145–3153. doi: 10.1523/JNEUROSCI.23-08-03145.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated channels of vertebrate cone photoreceptors: role of cAMP and Ras. J Neurosci. 2004;24:1296–1304. doi: 10.1523/JNEUROSCI.3560-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko ML, Liu Y, Dryer SE, Ko GY. The expression of L-type voltage-gated calcium channels in retinal photoreceptors is under circadian control. J Neurochem. 2007;103:784–792. doi: 10.1111/j.1471-4159.2007.04816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko ML, Liu Y, Shi L, Trump D, Ko GY. Circadian regulation of retinoschisin in the chick retina. Invest Ophthalmol Vis Sci. 2008;49:1615–1621. doi: 10.1167/iovs.07-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko ML, Jian K, Shi L, Ko GY. Phosphatidylinositol 3 kinase-Akt signaling serves as a circadian output in the retina. J Neurochem. 2009;108:1607–1620. doi: 10.1111/j.1471-4159.2009.05931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot JI. Ca2+ flux in retinal rod and cone outer segments: differences in Ca2+ selectivity of the cGMP-gated ion channels and Ca2+ clearance rates. Cell Calcium. 1995;18:285–300. doi: 10.1016/0143-4160(95)90025-x. [DOI] [PubMed] [Google Scholar]
- Korenbrot JI, Fernald RD. Circadian rhythm and light regulate opsin mRNA in rod photoreceptors. Nature. 1989;337:454–457. doi: 10.1038/337454a0. [DOI] [PubMed] [Google Scholar]
- Kosolapov AV, Bobkov YV. Modulation of the cGMP-activated conductance of the plasma membrane of photoreceptor cells by calmodulin. Biochem Mol Biol Int. 1996;38:871–877. [PubMed] [Google Scholar]
- Krajewski JL, Luetje CW, Kramer RH. Tyrosine phosphorylation of rod cyclic nucleotide-gated channels switches off Ca2+/calmodulin inhibition. J Neurosci. 2003;23:10100–10106. doi: 10.1523/JNEUROSCI.23-31-10100.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan B, Dryer SE, Hardin PE. Circadian rhythms in olfactory responses of Drosophila melanogaster. Nature. 1999;400:375–378. doi: 10.1038/22566. [DOI] [PubMed] [Google Scholar]
- Krishnan B, Levine JD, Lynch MK, Dowse HB, Funes P, Hall JC, Hardin PE, Dryer SE. A new role for cryptochrome in a Drosophila circadian oscillator. Nature. 2001;411:313–317. doi: 10.1038/35077094. [DOI] [PubMed] [Google Scholar]
- Krishnan P, Chatterjee A, Tanoue S, Hardin PE. Spike amplitude of single-unit responses in antennal sensillae is controlled by the Drosophila circadian clock. Curr Biol. 2008;18:803–807. doi: 10.1016/j.cub.2008.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D. Mesopic state: cellular mechanisms involved in pre-and post-synaptic mixing of rod and cone signals. Microsc Res Tech. 2000;50:347–359. doi: 10.1002/1097-0029(20000901)50:5<347::AID-JEMT4>3.0.CO;2-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, McMahon DG. Rhythmic regulation of membrane potential and potassium current persists in SCN neurons in the absence of environmental input. Eur J Neurosci. 2004;20:1113–1117. doi: 10.1111/j.1460-9568.2004.03555.x. [DOI] [PubMed] [Google Scholar]
- Lam BL. Electrophysiology of Vision: Clinical Testing and Applications. Taylor and Francis Group; Boca Raton, FL: 2005. [Google Scholar]
- LaVail MM. Circadian nature of rod outer segment disc shedding in the rat. Invest Ophthalmol Vis Sci. 1980;19:407–411. [PubMed] [Google Scholar]
- Le Blanc C, Mironneau C, Barbot C, Henaff M, Bondeva T, Wetzker R, Macrez N. Regulation of vascular L-type Ca2+ channels by phosphatidylinositol 3,4,5-trisphosphate. Circ Res. 2004;95:300–307. doi: 10.1161/01.RES.0000138017.76125.8b. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Runyeon JW, Crum J, Ellisman MH, Roberts WM. Synaptic vesicle populations in saccular hair cells reconstructed by electron tomography. J Neurosci. 1999;19:119–132. doi: 10.1523/JNEUROSCI.19-01-00119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhuillier L, Dryer SE. Developmental regulation of neuronal K(Ca) channels by TGFbeta1: an essential role for PI3 kinase signaling and membrane insertion. J Neurophysiol. 2002;88:954–964. doi: 10.1152/jn.2002.88.2.954. [DOI] [PubMed] [Google Scholar]
- Lhuillier L, Dryer SE. Ras is a mediator of TGFbeta1 signaling in developing chick ciliary ganglion neurons. Brain Res. 2003;982:119–124. doi: 10.1016/s0006-8993(03)03020-8. [DOI] [PubMed] [Google Scholar]
- Li P, Temple S, Gao Y, Haimberger TJ, Hawryshyn CW, Li L. Circadian rhythms of behavioral cone sensitivity and long wavelength opsin mRNA expression: a correlation study in zebrafish. J Exp Biol. 2005;208:497–504. doi: 10.1242/jeb.01424. [DOI] [PubMed] [Google Scholar]
- Liang J, Wessel JH, III, Iuvone PM, Tosini G, Fukuhara C. Diurnal rhythms of tryptophan hydroxylase 1 and 2 mRNA expression in the rat retina. Neuroreport. 2004;15:1497–1500. doi: 10.1097/01.wnr.0000131007.59315.66. [DOI] [PubMed] [Google Scholar]
- Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zoran MJ, Cassone VM. Daily and circadian variation in the electroretinogram of the domestic fowl: effects of melatonin. J Comp Physiol [A] 1995;177:299–306. doi: 10.1007/BF00192419. [DOI] [PubMed] [Google Scholar]
- Lyons LC, Roman G. Circadian modulation of short-term memory in Drosophila. Learn Mem. 2009;16:19–27. doi: 10.1101/lm.1146009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons LC, Rawashdeh O, Katzoff A, Susswein AJ, Eskin A. Circadian modulation of complex learning in diurnal and nocturnal Aplysia. Proc Natl Acad Sci USA. 2005;102:12589–12594. doi: 10.1073/pnas.0503847102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchi MM, Bruce JN. Human pineal physiology and functional significance of melatonin. Front Neuroendocrinol. 2004;25:177–195. doi: 10.1016/j.yfrne.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Manglapus MK, Uchiyama H, Buelow NF, Barlow RB. Circadian rhythms of rod-cone dominance in the Japanese quail retina. J Neurosci. 1998;18:4775–4784. doi: 10.1523/JNEUROSCI.18-12-04775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglapus MK, Iuvone PM, Underwood H, Pierce ME, Barlow RB. Dopamine mediates circadian rhythms of rod-cone dominance in the Japanese quail retina. J Neurosci. 1999;19:4132–4141. doi: 10.1523/JNEUROSCI.19-10-04132.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maronde E, Stehle JH. The mammalian pineal gland: known facts, unknown facets. Trends Endocrinol Metab. 2007;18:142–149. doi: 10.1016/j.tem.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Matulef K, Zagotta WN. Cyclic nucleotide-gated ion channels. Annu Rev Cell Dev Biol. 2003;19:23–44. doi: 10.1146/annurev.cellbio.19.110701.154854. [DOI] [PubMed] [Google Scholar]
- McGoogan JM, Wu WQ, Cassone VM. Inter-ocular interference and circadian regulation of the chick electroretinogram. Vis Res. 2000;40:2869–2879. doi: 10.1016/s0042-6989(00)00135-8. [DOI] [PubMed] [Google Scholar]
- McMahon DG, Block GD. The Bulla ocular circadian pacemaker. I Pacemaker neuron membrane potential controls phase through a calcium-dependent mechanism. J Comp Physiol [A] 1987;161:335–346. doi: 10.1007/BF00603959. [DOI] [PubMed] [Google Scholar]
- Menger GJ, Koke JR, Cahill GM. Diurnal and circadian retinomotor movements in zebrafish. Vis Neurosci. 2005;22:203–209. doi: 10.1017/S0952523805222083. [DOI] [PubMed] [Google Scholar]
- Meredith AL, Wiler SW, Miller BH, Takahashi JS, Fodor AA, Ruby NF, Aldrich RW. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci. 2006;9:1041–1049. doi: 10.1038/nn1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel S, Geusz ME, Zaritsky JJ, Block GD. Circadian rhythm in membrane conductance expressed in isolated neurons. Science. 1993;259:239–241. doi: 10.1126/science.8421785. [DOI] [PubMed] [Google Scholar]
- Michel S, Manivannan K, Zaritsky JJ, Block GD. A delayed rectifier current is modulated by the circadian pacemaker in Bulla. J Biol Rhythms. 1999;14:141–150. doi: 10.1177/074873099129000533. [DOI] [PubMed] [Google Scholar]
- Miller JL, Korenbrot JI. Differences in calcium homeostasis between retinal rod and cone photoreceptors revealed by the effects of voltage on the cGMP-gated conductance in intact cells. J Gen Physiol. 1994;104:909–940. doi: 10.1085/jgp.104.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Anaya M, Bartell PA, Menaker M. Circadian rhythm of iguana electroretinogram: the role of dopamine and melatonin. J Biol Rhythms. 2002;17:526–538. doi: 10.1177/0748730402238235. [DOI] [PubMed] [Google Scholar]
- Molday RS, Kaupp UB. Ion channels of vertebrate photoreceptors. In: Stravenga DG, Degrip WJ, Pugh EN Jr, editors. Molecular Mechanisms in Visual Transductions. Vol. 3. Elsevier Science; Amsterdam, The Netherlands: 2000. pp. 144–181. [Google Scholar]
- Molday LL, Hicks D, Sauer CG, Weber BH, Molday RS. Expression of X-linked retinoschisis protein RS1 in photoreceptor and bipolar cells. Invest Ophthalmol Vis Sci. 2001;42:816–825. [PubMed] [Google Scholar]
- Molday LL, Wu WW, Molday RS. Retinoschisin (RS1), the protein encoded by the X-linked retinoschisis gene, is anchored to the surface of retinal photoreceptor and bipolar cells through its interactions with a Na/K ATPase-SARM1 complex. J Biol Chem. 2007;282:32792–32801. doi: 10.1074/jbc.M706321200. [DOI] [PubMed] [Google Scholar]
- Molokanova E, Trivedi B, Savchenko A, Kramer RH. Modulation of rod photoreceptor cyclic nucleotide-gated channels by tyrosine phosphorylation. J Neurosci. 1997;17:9068–9076. doi: 10.1523/JNEUROSCI.17-23-09068.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molokanova E, Maddox F, Luetje CW, Kramer RH. Activity-dependent modulation of rod photoreceptor cyclic nucleotide-gated channels mediated by phosphorylation of a specific tyrosine residue. J Neurosci. 1999a;19:4786–4795. doi: 10.1523/JNEUROSCI.19-12-04786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molokanova E, Savchenko A, Kramer RH. Noncatalytic inhibition of cyclic nucleotide-gated channels by tyrosine kinase induced by genistein. J Gen Physiol. 1999b;113:45–56. doi: 10.1085/jgp.113.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. J Comp Neurol. 1972;146:1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- Morgan IG, Boelen MK. A retinal dark-light switch: a review of the evidence. Vis Neurosci. 1996;13:399–409. doi: 10.1017/s0952523800008087. [DOI] [PubMed] [Google Scholar]
- Morgan IG, Wellard JW, Boelen MK. A role for the enkephalin-immunoreactive amacrine cells of the chicken retina in adaptation to light and dark. Neurosci Lett. 1994;174:64–66. doi: 10.1016/0304-3940(94)90120-1. [DOI] [PubMed] [Google Scholar]
- Morgans C, Bayler P, Oesch N, Ren G, Akileswaran L, Taylor W. Photoreceptor calcium channels: insight from night blindness. Vis Neurosci. 2005;22:561–568. doi: 10.1017/S0952523805225038. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Furukawa T, Raviola E, Cepko CL. Synaptogenesis and outer segment formation are perturbed in the neural retina of Crx mutant mice. BMC Neurosci. 2005;6:5. doi: 10.1186/1471-2202-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle BW, Burnside B. Calmodulin-binding proteins in teleost retina, rod inner and outer segments, and rod cytoskeletons. Eur J Cell Biol. 1984;33:248–257. [PubMed] [Google Scholar]
- Nahm SS, Farnell YZ, Griffith W, Earnest DJ. Circadian regulation and function of voltage-dependent calcium channels in the suprachiasmatic nucleus. J Neurosci. 2005;25:9304–9308. doi: 10.1523/JNEUROSCI.2733-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta WJH, Haymaker W. Retino-hypothalamic connections. In: Haymaker W, Anderson E, Nauta WJH, editors. The Hypothalamus. Charles C. Thomas; Sprint-field, IL: 1969. pp. 187–189. [Google Scholar]
- Nikaido SS, Takahashi JS. Calcium modulates circadian variation in cAMP-stimulated melatonin in chick pineal cells. Brain Res. 1996;716:1–10. doi: 10.1016/0006-8993(95)01521-3. [DOI] [PubMed] [Google Scholar]
- Nordskog BK, Hammarback JA, Godwin DW. Diurnal gene expression patterns of T-type calcium channels and their modulation by ethanol. Neuroscience. 2006;141:1365–1373. doi: 10.1016/j.neuroscience.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Nowak JZ, Kazula A, Golembiowska K. Melatonin increases serotonin N-acetyltransferase activity and decreases dopamine synthesis in light-exposed chick retina: in vivo evidence supporting melatonin-dopamine interaction in retina. J Neurochem. 1992;59:1499–1505. doi: 10.1111/j.1471-4159.1992.tb08466.x. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Sterling P. Synaptic ribbon. Conveyor belt or safety belt? Neuron. 2003;37:379–382. doi: 10.1016/s0896-6273(03)00062-x. [DOI] [PubMed] [Google Scholar]
- Peinado MA, Fajardo N, Hernandez G, Puig-Domingo M, Viader M, Reiter RJ, Webb SM. Immunoreactive somatostatin diurnal rhythms in rat pineal, retina and harderian gland: effects of sex, season, continuous darkness and estrous cycle. J Neural Transm Gen Sect. 1990;81:63–72. doi: 10.1007/BF01245446. [DOI] [PubMed] [Google Scholar]
- Pelucchi B, Grimaldi A, Moriondo A. Vertebrate rod photoreceptors express both BK and IK calcium-activated potassium channels, but only BK channels are involved in receptor potential regulation. J Neurosci Res. 2008;86:194–201. doi: 10.1002/jnr.21467. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, de Jeu MT, Bos NP, Schaap J, Geurtsen AM. Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature. 2002;416:286–290. doi: 10.1038/nature728. [DOI] [PubMed] [Google Scholar]
- Pierce ME, Besharse JC. Circadian regulation of retino-motor movements. I Interaction of melatonin and dopamine in the control of cone length. J Gen Physiol. 1985;86:671–689. doi: 10.1085/jgp.86.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce ME, Besharse JC. Circadian regulation of retino-motor movements: II. The role of GABA in the regulation of cone position. J Comp Neurol. 1988;270:279–287. doi: 10.1002/cne.902700208. [DOI] [PubMed] [Google Scholar]
- Pierce ME, Sheshberadaran H, Zhang Z, Fox LE, Applebury ML, Takahashi JS. Circadian regulation of iodopsin gene expression in embryonic photoreceptors in retinal cell culture. Neuron. 1993;10:579–584. doi: 10.1016/0896-6273(93)90161-j. [DOI] [PubMed] [Google Scholar]
- Pinto LH, Klumpp DJ. Localization of potassium channels in the retina. Prog Retinal Eye Res. 1998;17:207–230. doi: 10.1016/s1350-9462(97)00011-6. [DOI] [PubMed] [Google Scholar]
- Pitts GR, Ohta H, McMahon DG. Daily rhythmicity of large-conductance Ca2+-activated K+ currents in suprachiasmatic nucleus neurons. Brain Res. 2006;1071:54–62. doi: 10.1016/j.brainres.2005.11.078. [DOI] [PubMed] [Google Scholar]
- Pu GA, Dowling JE. Anatomical and physiological characteristics of pineal photoreceptor cell in the larval lamprey, Petromyzon marinus. J Neurophysiol. 1981;46:1018–1038. doi: 10.1152/jn.1981.46.5.1018. [DOI] [PubMed] [Google Scholar]
- Pugh EN, Jr, Lamb TD. Phototransduction in vertebrate rods and cones: molecular mechanisms of amplification, recovery and light adaptation. In: Stravenga DG, Degrip WJ, Pugh EN Jr, editors. Molecular Mechanisms in Visual Transduction. Vol. 3. Elsevier Science; Amsterdam, The Netherlands: 2000. pp. 183–255. [Google Scholar]
- Quintero JE, Kuhlman SJ, McMahon DG. The biological clock nucleus: a multiphasic oscillator network regulated by light. J Neurosci. 2003;23:8070–8076. doi: 10.1523/JNEUROSCI.23-22-08070.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Reid SN, Farber DB. Glial transcytosis of a photoreceptor-secreted signaling protein, retinoschisin. Glia. 2005;49:397–406. doi: 10.1002/glia.20131. [DOI] [PubMed] [Google Scholar]
- Reid SN, Akhmedov NB, Piriev NI, Kozak CA, Danciger M, Farber DB. The mouse X-linked juvenile retinoschisis cDNA: expression in photoreceptors. Gene. 1999;227:257–266. doi: 10.1016/s0378-1119(98)00578-2. [DOI] [PubMed] [Google Scholar]
- Reid SN, Yamashita C, Farber DB. Retinoschisin, a photoreceptor-secreted protein, and its interaction with bipolar and muller cells. J Neurosci. 2003;23:6030–6040. doi: 10.1523/JNEUROSCI.23-14-06030.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reme C, Wirz-Justice A, Rhyner A, Hofmann S. Circadian rhythm in the light response of rat retinal disk-shedding and autophagy. Brain Res. 1986;369:356–360. doi: 10.1016/0006-8993(86)90550-0. [DOI] [PubMed] [Google Scholar]
- Ren JQ, Li L. A circadian clock regulates the process of ERG b- and d-wave dominance transition in dark-adapted zebra-fish. Vis Res. 2004;44:2147–2152. doi: 10.1016/j.visres.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Wang Y, Mangel SC. A circadian clock in the fish retina regulates dopamine release via activation of melatonin receptors. J Physiol. 2004;554:467–482. doi: 10.1113/jphysiol.2003.053710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LM, Takahashi JS. Circadian clock in cell culture: I. Oscillation of melatonin release from dissociated chick pineal cells in flow-through microcarrier culture. J Neurosci. 1988a;8:12–21. doi: 10.1523/JNEUROSCI.08-01-00012.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LM, Takahashi JS. Circadian clock in cell culture: II. In vitro photic entrainment of melatonin oscillation from dissociated chick pineal cells. J Neurosci. 1988b;8:22–30. doi: 10.1523/JNEUROSCI.08-01-00022.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Merrow M. Circadian systems and metabolism. J Biol Rhythms. 1999;14:449–459. doi: 10.1177/074873099129001019. [DOI] [PubMed] [Google Scholar]
- Roth U, Curth K, Unterman TG, Kietzmann T. The transcription factors HIF-1 and HNF-4 and the coactivator p300 are involved in insulin-regulated glucokinase gene expression via the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2004;279:2623–2631. doi: 10.1074/jbc.M308391200. [DOI] [PubMed] [Google Scholar]
- Ruan GX, Zhang DQ, Zhou T, Yamazaki S, McMahon DG. Circadian organization of the mammalian retina. Proc Natl Acad Sci USA. 2006;103:9703–9708. doi: 10.1073/pnas.0601940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby NF, Hwang CE, Wessells C, Fernandez F, Zhang P, Sapolsky R, Heller HC. Hippocampal-dependent learning requires a functional circadian system. Proc Natl Acad Sci USA. 2008;105:15593–15598. doi: 10.1073/pnas.0808259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufiange M, Dumont M, Lachapelle P. Correlating retinal function with melatonin secretion in subjects with an early or late circadian phase. Invest Ophthalmol Vis Sci. 2002;43:2491–2499. [PubMed] [Google Scholar]
- Runyon SL, Washicosky KJ, Brenneman RJ, Kelly JR, Khadilkar RV, Heacock KF, McCormick SM, Williams KE, Jinks RN. Central regulation of photosensitive membrane turnover in the lateral eye of Limulus, II: octopamine acts via adenylate cyclase/cAMP-dependent protein kinase to prime the retina for transient rhabdom shedding. Vis Neurosci. 2004;21:749–763. doi: 10.1017/s0952523804215097. [DOI] [PubMed] [Google Scholar]
- Schaap J, Bos NP, de Jeu MT, Geurtsen AM, Meijer JH, Pennartz CM. Neurons of the rat suprachiasmatic nucleus show a circadian rhythm in membrane properties that is lost during prolonged whole-cell recording. Brain Res. 1999;815:154–166. doi: 10.1016/s0006-8993(98)01025-7. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- Shearman LP, Sriram S, Weaver DR, et al. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- Shi L, Jian K, Ko ML, Trump D, Ko GY. Retinoschisin, a new binding partner for L-type voltage-gated calcium channels in the retina. J Biol Chem. 2009;284:3966–3975. doi: 10.1074/jbc.M806333200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Moore RY. Electrical and metabolic activity of suprachiasmatic nucleus neurons in hamster hypothalamic slices. Brain Res. 1988;438:374–378. doi: 10.1016/0006-8993(88)91367-4. [DOI] [PubMed] [Google Scholar]
- Spiwoks-Becker I, Glas M, Lasarzik I, Vollrath L. Mouse photoreceptor synaptic ribbons lose and regain material in response to illumination changes. Eur J Neurosci. 2004;19:1559–1571. doi: 10.1111/j.1460-9568.2004.03198.x. [DOI] [PubMed] [Google Scholar]
- Steenhard BM, Besharse JC. Phase shifting the retinal circadian clock: xPer2 mRNA induction by light and dopamine. J Neurosci. 2000;20:8572–8577. doi: 10.1523/JNEUROSCI.20-23-08572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenkamp DL, Iuvone PM, Adler R. Photomechanical movements of cultured embryonic photoreceptors: regulation by exogenous neuromodulators and by a regulable source of endogenous dopamine. J Neurosci. 1994;14:3083–3096. doi: 10.1523/JNEUROSCI.14-05-03083.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan FK, Kovacevic NS. Multiple retention deficit in passive avoidance in rats is eliminated by suprachiasmatic lesions. Behav Biol. 1978;22:456–462. doi: 10.1016/s0091-6773(78)92565-8. [DOI] [PubMed] [Google Scholar]
- Strauss O, Stumpff F, Mergler S, Wienrich M, Wiederholt M. The Royal College of Surgeons rat: an animal model for inherited retinal degeneration with a still unknown genetic defect. Acta Anat (Basel) 1998;162:101–111. doi: 10.1159/000046474. [DOI] [PubMed] [Google Scholar]
- Tajika Y, Matsuzaki T, Suzuki T, Aoki T, Hagiwara H, Kuwahara M, Sasaki S, Takata K. Aquaporin-2 is retrieved to the apical storage compartment via early endosomes and phosphati-dylinositol 3-kinase-dependent pathway. Endocrinology. 2004;145:4375–4383. doi: 10.1210/en.2004-0073. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Depaulis A, Perez-Reyes E, Bayliss DA. Low-voltage-activated calcium channel subunit expression in a genetic model of absence epilepsy in the rat. Brain Res Mol Brain Res. 2000;75:159–165. doi: 10.1016/s0169-328x(99)00307-1. [DOI] [PubMed] [Google Scholar]
- Tamotsu S, Morita Y. Photoreception in pineal organs of larval and adult lampreys, Lampetra japonica. J Comp Physiol [A] 1986;159:1–5. doi: 10.1007/BF00612489. [DOI] [PubMed] [Google Scholar]
- Tanoue S, Krishnan P, Krishnan B, Dryer SE, Hardin PE. Circadian clocks in antennal neurons are necessary and sufficient for olfaction rhythms in Drosophila. Curr Biol. 2004;14:638–649. doi: 10.1016/j.cub.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Tantri A, Vrabec TR, Cu-Unjieng A, Frost A, Annesley WH, Jr, Donoso LA. X-linked retinoschisis: a clinical and molecular genetic review. Surv Ophthalmol. 2004;49:214–230. doi: 10.1016/j.survophthal.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Tarasenko AN, Kostyuk PG, Eremin AV, Isaev DS. Two types of low-voltage-activated Ca2+ channels in neurones of rat laterodorsal thalamic nucleus. J Physiol. 1997;499:77–86. doi: 10.1113/jphysiol.1997.sp021912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therien AG, Blostein R. Mechanisms of sodium pump regulation. Am J Physiol Cell Physiol. 2000;279:C541–C566. doi: 10.1152/ajpcell.2000.279.3.C541. [DOI] [PubMed] [Google Scholar]
- Thermos K. Functional mapping of somatostatin receptors in the retina: a review. Vis Res. 2003;43:1805–1815. doi: 10.1016/s0042-6989(03)00169-x. [DOI] [PubMed] [Google Scholar]
- Thomas KB, Tigges M, Iuvone PM. Melatonin synthesis and circadian tryptophan hydroxylase activity in chicken retina following destruction of serotonin immunoreactive ama-crine and bipolar cells by kainic acid. Brain Res. 1993;601:303–307. doi: 10.1016/0006-8993(93)91725-8. [DOI] [PubMed] [Google Scholar]
- Tian J, Xie ZJ. The Na-K-ATPase and calcium-signaling microdomains. Physiology (Bethesda) 2008;23:205–211. doi: 10.1152/physiol.00008.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G, Pozdeyev N, Sakamoto K, Iuvone PM. The circadian clock system in the mammalian retina. BioEssays. 2008;30:624–633. doi: 10.1002/bies.20777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek FW. Circadian neural rhythms in mammals. Annu Rev Physiol. 1985;47:49–64. doi: 10.1146/annurev.ph.47.030185.000405. [DOI] [PubMed] [Google Scholar]
- Tuunainen A, Kripke DF, Cress AC, Youngstedt SD. Retinal circadian rhythms in humans. Chronobiol Int. 2001;18:957–971. doi: 10.1081/cbi-100107971. [DOI] [PubMed] [Google Scholar]
- Viard P, Butcher AJ, Halet G, Davies A, Nurnberg B, Heblich F, Dolphin AC. PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nat Neurosci. 2004;7:939–946. doi: 10.1038/nn1300. [DOI] [PubMed] [Google Scholar]
- Vollrath L, Spiwoks-Becker I. Plasticity of retinal ribbon synapses. Microsc Res Tech. 1996;35:472–487. doi: 10.1002/(SICI)1097-0029(19961215)35:6<472::AID-JEMT6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Vollrath L, Meyer A, Buschmann F. Ribbon synapses of the mammalian retina contain two types of synaptic bodies–ribbons and spheres. J Neurocytol. 1989;18:115–120. doi: 10.1007/BF01188430. [DOI] [PubMed] [Google Scholar]
- Wagner HJ, Kath D, Douglas RH, Kirsch M. Dark-adaptive cone elongation in the blue acara retina is triggered by green-sensitive cones. Vis Neurosci. 1993;10:523–527. doi: 10.1017/s0952523800004739. [DOI] [PubMed] [Google Scholar]
- Wang HY, Huang RC. Diurnal modulation of the Na+/K+-ATPase and spontaneous firing in the rat retinorecipient clock neurons. J Neurophysiol. 2004;92:2295–2301. doi: 10.1152/jn.00061.2004. [DOI] [PubMed] [Google Scholar]
- Wang YC, Huang RC. Effects of sodium pump activity on spontaneous firing in neurons of the rat suprachiasmatic nucleus. J Neurophysiol. 2006;96:109–118. doi: 10.1152/jn.01369.2005. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mangel SC. A circadian clock regulates rod and cone input to fish retinal cone horizontal cells. Proc Natl Acad Sci USA. 1996;93:4655–4660. doi: 10.1073/pnas.93.10.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Waters CT, Rothman AM, Jakins TJ, Romisch K, Trump D. Intracellular retention of mutant retinoschisin is the pathological mechanism underlying X-linked retinoschisis. Hum Mol Genet. 2002;11:3097–3105. doi: 10.1093/hmg/11.24.3097. [DOI] [PubMed] [Google Scholar]
- Watt CB, Florack VJ. A triple-label analysis demonstrating that enkephalin-, somatostatin- and neurotensin-like immuno-reactivities are expressed by a single population of amacrine cells in the chicken retina. Brain Res. 1994;634:310–316. doi: 10.1016/0006-8993(94)91935-6. [DOI] [PubMed] [Google Scholar]
- Webb SM, Peinado MA, Puig-Domingo M, Viader M, Reiter RJ. Rhythms in pineal immunoreactive somatostatin in the Syrian hamster, mouse, and gerbil. J Pineal Res. 1988;5:273–278. doi: 10.1111/j.1600-079x.1988.tb00653.x. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Summers JA. Circadian rhythms in the eye: the physiological significance of melatonin receptors in ocular tissues. Prog Retinal Eye Res. 2008;27:137–160. doi: 10.1016/j.preteyeres.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Da Prada M, Reme C. Circadian rhythm in rat retinal dopamine. Neurosci Lett. 1984;45:21–25. doi: 10.1016/0304-3940(84)90323-9. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Stone S, Besharse JC. Dopamine modifies the balance of rod and cone inputs to horizontal cells of the Xenopus retina. Brain Res. 1988;449:332–336. doi: 10.1016/0006-8993(88)91048-7. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Veisenberger E, Haycock JW, Akopian A, Garcia-Espana A, Meller E. Activity-dependent phosphorylation of tyrosine hydroxylase in dopaminergic neurons of the rat retina. J Neurosci. 2004;24:4242–4249. doi: 10.1523/JNEUROSCI.5436-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Choi EJ, Wang F, Blazynski C, Storm DR. Type I calmodulin-sensitive adenylyl cyclase is neural specific. J Neuro-chem. 1993;60:305–311. doi: 10.1111/j.1471-4159.1993.tb05852.x. [DOI] [PubMed] [Google Scholar]
- Yang DS, Boelen MK, Morgan IG. Development of the enkephalin-, neurotensin- and somatostatin-like (ENSLI) amacrine cells in the chicken retina. Brain Res Dev Brain Res. 1997;101:57–65. doi: 10.1016/s0165-3806(97)00034-5. [DOI] [PubMed] [Google Scholar]
- Yau KW, Baylor DA. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Yoon SO, Shin S, Liu Y, Ballif BA, Woo MS, Gygi SP, Blenis J. Ran-binding protein 3 phosphorylation links the Ras and PI3-kinase pathways to nucleocytoplasmic transport. Mol Cell. 2008;29:362–375. doi: 10.1016/j.molcel.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Kawamura K, Imaki J. Differential expression of c-fos mRNA in rat retinal cells: regulation by light/dark cycle. Neuron. 1993;10:1049–1054. doi: 10.1016/0896-6273(93)90053-t. [DOI] [PubMed] [Google Scholar]
- Yu CJ, Gao Y, Li P, Li L. Synchronizing multiphasic circadian rhythms of rhodopsin promoter expression in rod photoreceptor cells. J Exp Biol. 2007;210:676–684. doi: 10.1242/jeb.02694. [DOI] [PubMed] [Google Scholar]
- Zagotta WN, Siegelbaum SA. Structure and function of cyclic nucleotide-gated channels. Annu Rev Neurosci. 1996;19:235–263. doi: 10.1146/annurev.ne.19.030196.001315. [DOI] [PubMed] [Google Scholar]
- Zatz M. Relationship between light, calcium influx and cAMP in the acute regulation of melatonin production by cultured chick pineal cells. Brain Res. 1989;477:14–18. doi: 10.1016/0006-8993(89)91389-9. [DOI] [PubMed] [Google Scholar]
- Zatz M, Mullen DA. Does calcium influx regulate melatonin production through the circadian pacemaker in chick pineal cells? Effects of nitrendipine, Bay K 8644, Co2+, Mn2+, and low external Ca2+ Brain Res. 1988a;463:305–316. doi: 10.1016/0006-8993(88)90404-0. [DOI] [PubMed] [Google Scholar]
- Zatz M, Mullen DA. Photoendocrine transduction in cultured chick pineal cells. II Effects of forskolin, 8-bromocyclic AMP, and 8-bromocyclic GMP on the melatonin rhythm. Brain Res. 1988b;453:51–62. doi: 10.1016/0006-8993(88)90142-4. [DOI] [PubMed] [Google Scholar]
- Zatz M, Mullen DA, Moskal JR. Photoendocrine transduction in cultured chick pineal cells: effects of light, dark, and potassium on the melatonin rhythm. Brain Res. 1988;438:199–215. doi: 10.1016/0006-8993(88)91339-x. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Takada Y, Kjellstrom S, et al. RS-1 Gene Delivery to an Adult Rs1h Knockout Mouse Model Restores ERG b-Wave with Reversal of the Electronegative Waveform of X-Linked Retinoschisis. Invest Ophthalmol Vis Sci. 2004;45:3279–3285. doi: 10.1167/iovs.04-0576. [DOI] [PubMed] [Google Scholar]
- Zheng J, Trudeau MC, Zagotta WN. Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron. 2002;36:891–896. doi: 10.1016/s0896-6273(02)01099-1. [DOI] [PubMed] [Google Scholar]
- Zhong H, Molday LL, Molday RS, Yau KW. The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature. 2002;420:193–198. doi: 10.1038/nature01201. [DOI] [PMC free article] [PubMed] [Google Scholar]