Abstract
The intestinal Fc receptor, FcRn, functions in the maternofetal transfer of gamma globulin (IgG) in the neonatal rodent. In humans, most of this transfer is presumed to occur in utero via the placenta. Although the fetus swallows amniotic fluid that contains immunoglobulin, it is unknown whether this transfer also occurs via the fetal intestine. A human FcRn has been identified in the syncytiotrophoblast that mediates the maternofetal transfer of antibody. It has also been identified in human fetal intestine and is postulated to function in IgG transport. We hypothesize that the human fetal intestinal FcRn may play a role in IgG transport from the amniotic fluid into the fetal circulation. The aim of this study was to characterize the distribution of the FcRn along the human fetal intestine. Lysates prepared from human fetal intestine and from a nonmalignant human fetal intestinal epithelial cell line (H4) were subjected to Western blot analysis and probed using anti-FcRn antibodies. A 42-kD band, consistent with the known molecular weight of the FcRn, was detected along the human fetal intestine and in H4 cells. Expression of the human FcRn was confirmed with immunohistochemistry. Our study demonstrates the expression of FcRn along the human fetal intestine and in a human nonmalignant fetal intestinal epithelial cell line (H4), which by location indicates that FcRn could play a role in the uptake and transport of IgG in the human fetus.
Humoral immunity acquired via maternofetal transfer of antibody is critical in the prevention of perinatal infections. In rodents, this transfer occurs through the yolk sac and the intestine via a Fc receptor for IgG (FcRn). This receptor has been considered important in the maternofetal transmission of IgG in the yolk sac of mice and rats and also in the human placental syncytiotrophoblast (1). The FcRn is also expressed in the small intestine of suckling mice and rats, where the receptor functions in the uptake of IgG from ingested maternal milk. Although a FcRn has been demonstrated on the human fetal small intestine, a similar function for this receptor in the human fetal intestine has not been demonstrated to date.
The acquisition of humoral immunity early in life may have a significant lifelong impact on the prevention of infectious and inflammatory diseases. Because IgG synthesis in the human fetus is reduced, most IgG in the blood of newborns is maternal in origin. Low levels of IgG have been detected in the plasma of 12-wk-old fetuses, with levels reaching maternal concentrations by 26 wk of gestation. This rise in serum IgG in the fetus occurs in parallel with the rise in IgG in amniotic fluid, with highest concentrations reached between 15 and 33 wk, around the time when the fetus begins to swallow (2, 3). Because the major placental transfer of IgG via the FcRn occurs after 22 wk, it is possible that an earlier transfer from swallowed amniotic fluid may occur via the fetal intestine (2, 3).
Although an “Fc binding site” has been identified in the human small intestine (3) with a structure that seems to be identical to the cloned human syncytiotrophoblast Fc receptor (1), the distribution of this receptor throughout the fetal intestine has not been clearly defined. Accordingly, the aim of this study was to further characterize the expression of the FcRn in human fetal intestine as a prelude to determining its role in the protective function of maternal IgG in the newborn. Using human fetal intestinal models established in this laboratory, we sought to characterize the distribution of the FcRn along the human fetal intestine.
MATERIALS AND METHODS
Human cell lines
Caco2 and T84 cell lines were used to represent human intestinal epithelial cells. The H4 cell line is a primary nonmalignant fetal small intestinal epithelial cell line that has been characterized in our laboratory (4). It was used as a model for fetal enterocytes. Cell passages of H4 cells from 20 to 34 were studied. H4 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; GIBCO, Grand Island, NY, U.S.A.) supplemented with 5% fetal bovine serum (FBS) (BioWhittaker, Walkersville, MD, U.S.A.), 2 mM glutamine (GIBCO), 100,000 U/L penicillin, and 100 mg/L streptomycin (GIBCO). The Caco2 cell line was purchased from the American Type Culture Collection (Manassas, VA, U.S.A.). Caco2 cells were grown in 10% FBS, 2 mM glutamine, 0.1 mM nonessential amino acids, 100,000 U/L penicillin, 100 mg/L streptomycin, and 10 mM HEPES buffer solution under a humidified atmosphere at 37°C and 5% CO2. T84 cells were generously provided by Dr. Beth McCormick of the Mucosal Immunology Laboratory at Massachusetts General Hospital East, Charlestown, MA. T84 cells were grown in media containing DMEM with l-glutamine, Ham’s F12 Nutrient mixture with l-glutamine, 0.014 M sodium bicarbonate, 0.015 M N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES) buffer solution, 0.04 g/L penicillin, 0.09g/L streptomycin, and 10% FCS.
Human intestinal tissue
All human tissue was used after obtaining informed consent according to the regulations of the Human Studies Committee at the Brigham and Women’s Hospital and Massachusetts General Hospital, Boston. Human fetal intestine was derived from aborted fetuses, aged 18–22 wk. Fetal gastrointestinal tissue was snap frozen in liquid nitrogen for cryosectioning. Intestinal biopsies from infants and children were obtained after informed consent from parents, during diagnostic endoscopic procedures done in the Combined Program of Pediatric Gastroenterology and Nutrition at Massachusetts General Hospital, Boston. All tissue, fetal, and intestinal biopsies were approved by the institutional review board.
Antibodies
Anti-FcRn antibodies purified from rabbit antipeptide antisera, raised against the α2 (88–177 amino acids), α3 (178–274 amino acids) (5), or against amino acids 176–190 domains of the human FcRn (6) were used. Polyclonal antisera was also raised in mice against the α2 domains of the human FcRn expressed as a GST fusion protein in Escherichia coli. These antisera did not cross-react with either major histocompatibility complex (MHC) class I or CD1d, as determined by Western blotting of 721.220, 721.220-CD1d transfectant, Jurkat, THP1, and HeLa cells as previously described (5). An endosomal antibody raised in mice to an early endosomal antigen EEA1 (BD Biosciences, San Jose, CA, U.S.A.) was used for endosomal localization.
Western blot analysis
H4, T84, and Caco2 cells, as well as fetal and normal human small intestinal cells, were extracted in 5% SDS in water. Protein extracts were resolved on gradient polyacrylamide denaturing 10–20% Tris-HCl gels (Bio-Rad, Hercules, CA, U.S.A.) and transferred onto PVDF (polyvinylidene difluoride) membrane (Amersham Pharmacia Biotech, Piscataway, NJ, U.S.A.). Blots were probed with affinity-purified anti-FcRn peptide antibody, and bound antibody was detected with horseradish peroxidase–conjugated goat anti-rabbit antibody (Sigma Chemical, St. Louis, MO, U.S.A.) and enhanced chemiluminescence by Super Signal, West Femto, Sensitivity Substrate (Pierce Chemical, Rockford, IL, U.S.A.). All reactions were normalized to a monoclonal mouse anti-rabbit GAPDH (Research Diagnostics, Flanders, NJ, U.S.A.) antibody.
Immunohistochemistry and confocal microscopy
Normal human fetal intestine (18–22 wk gestation) was embedded in Tissue-Tek OCT Compound (Sakura-Finetek, Torrance, CA, U.S.A.) for frozen sectioning on a Leica cryomicrotome (Leica, Nussloch, Germany). Frozen sections (5 µm) were air-dried at room temperature, fixed in 4% paraformaldehyde in PBS, washed in PBS, and blocked in 10% normal goat serum (Zymed Laboratories, South San Francisco, CA, U.S.A.). Sections were stained with an affinity-purified rabbit anti-human FcRn peptide antibody (amino acids 176–190) or a mouse polyclonal anti-FcRn antiserum diluted in blocking solution and detected with appropriate fluorophore-conjugated secondary antibodies for confocal microscopy. T84 cells and H4 cells were grown on glass coverslips and similarly stained. All staining reactions were accompanied by a negative control that consisted of an affinity-purified, isotype-matched nonspecific antiserum. All sections prepared for fluorescent microscopy were mounted in ProLong antifade reagent (Molecular Probes, Eugene, OR, U.S.A.) and viewed using a Leica TCS NT confocal microscope (Leica Microsystems, Inc., Deerfield, IL, U.S.A.). Electronic images were captured and edited in Adobe Photoshop (Adobe Systems Inc., Mountain View, CA, U.S.A.).
RESULTS
Western blot analysis
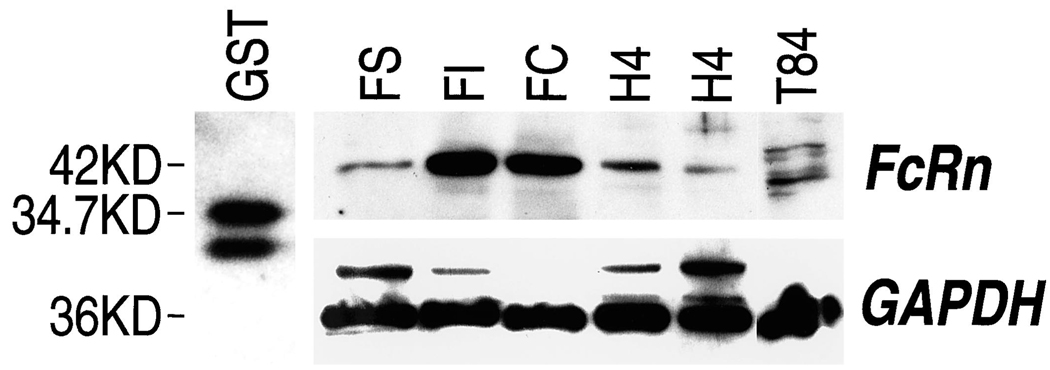
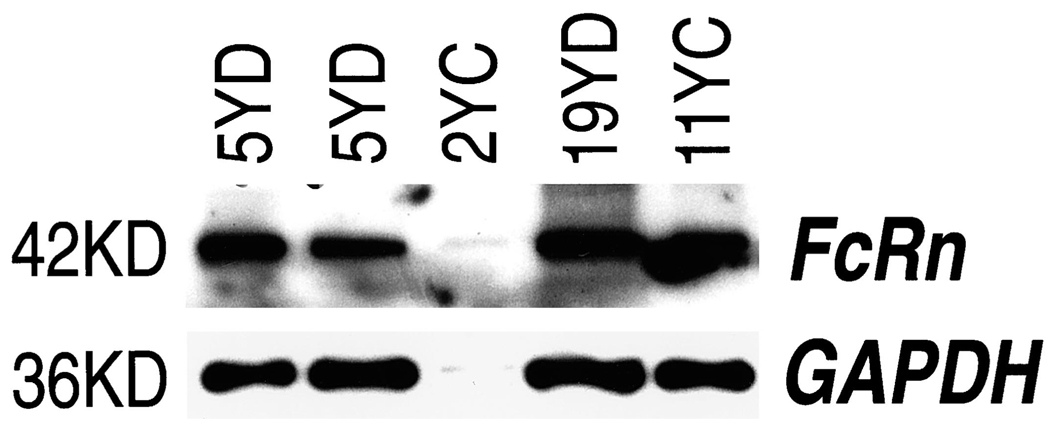
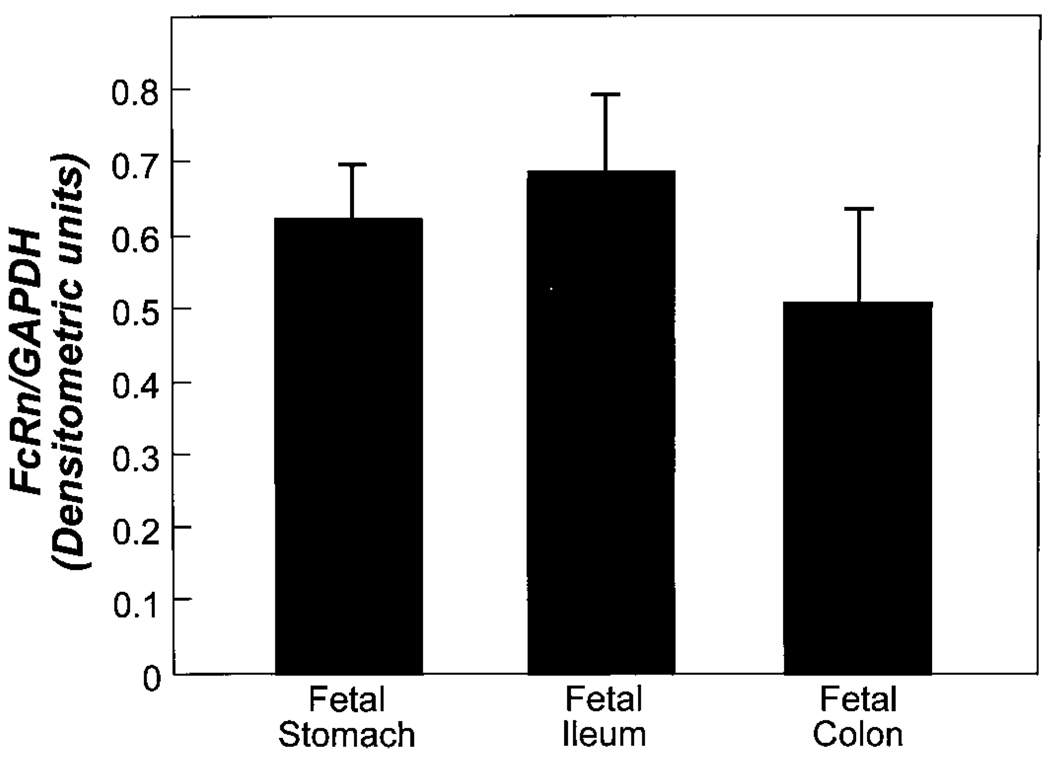
Lysates were prepared from T84, Caco2, and H4 cells grown in culture and from human fetal stomach, duodenum, ileum, and colon derived from fetuses of 18 and 22 wk gestational age. Lysates were also prepared from duodenal biopsies from a 5-y-old child and a 19-y-old adolescent and colonic biopsies from a 2-y-old and an 11-y-old child. These lysates were measured by Western blot analysis (5). Blots were probed with affinity purified anti-FcRn antibodies from rabbit antipeptide sera (6) and polyclonal antisera raised in mice against the α2 domain of FcRn expressed as a GST fusion protein (5). A 42-kD band consistent with the known molecular weight of the FcRn heavy chain was detected along different segments of the gastrointestinal tract from fetuses of varying ages. The FcRn was demonstrated along the intestine of a 22-wk-old fetus, as shown in Figure 1. The FcRn was also detected from different segments of the gastrointestinal tract of older children (7) (Fig. 2). Lysates derived from the human fetal intestinal cell line H4 and T84 cells also expressed the FcRn (Fig. 1) as did Caco2 cells (data not shown). The GST-FcRn-tail fusion protein that produces a band approximately 35 kD in size was used as a control (Fig. 1) (5). These results were normalized against GAPDH and analyzed by densitometry (Fig. 3). Although preliminary results suggested an enhanced expression of the FcRn in the fetal ileum and a reduced expression in the fetal stomach, densitometry, however, using pooled sample results from several experiments, failed to show a significant difference in expression along the gastrointestinal tract of the fetus. The SE bars seem excessive, and we presume that this is likely the result of a difference in autolysis among fetal segments at the time of lysate preparation. An enhanced expression of the FcRn was demonstrated in intestinal biopsies derived from older children and adolescents. However, because these were single biopsies, a statistical analysis of densitometric measurements could not be obtained. The biopsies obtained by endoscopy from the older children and an adolescent were histologically normal and were obtained as part of a diagnostic workup for abdominal pain.
Figure 1.
Western blot analysis of the FcRn in lysates prepared from fetal stomach (FS), intestine (FI), and colon (FC) derived from a 22-wk-old fetus, and H4 and T84 cells. The primary antibody used to detect the FcRn was a rabbit polyclonal antibody raised to amino acids 176–190 of the human FcRn. A GST-fusion protein was used as a positive control. The lysates were normalized against mouse anti-rabbit GAPDH in the lower panel. A lower band of approximately 40 kD was also identified in some lysates and likely represents a cross-reactive protein.
Figure 2.
Western blot analysis of FcRn in lysates prepared from duodenal biopsies from a 5-y-old (5YD) and a 19-y-old (19YD) and colonic biopsies from a 2-y-old (2YC) and 11-y-old (11YC) child.
Figure 3.
Densitometric analysis of Western blots are expressed as the ratio of FcRn to GAPDH and are shown as the mean and SEM.
Immunohistochemical analysis
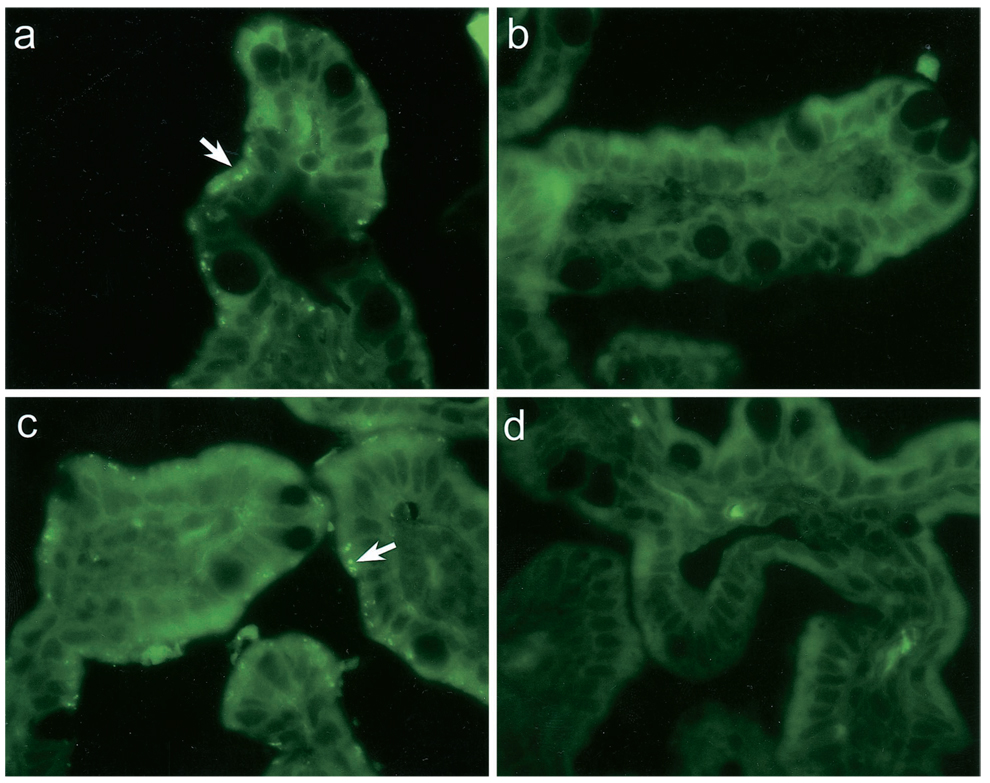
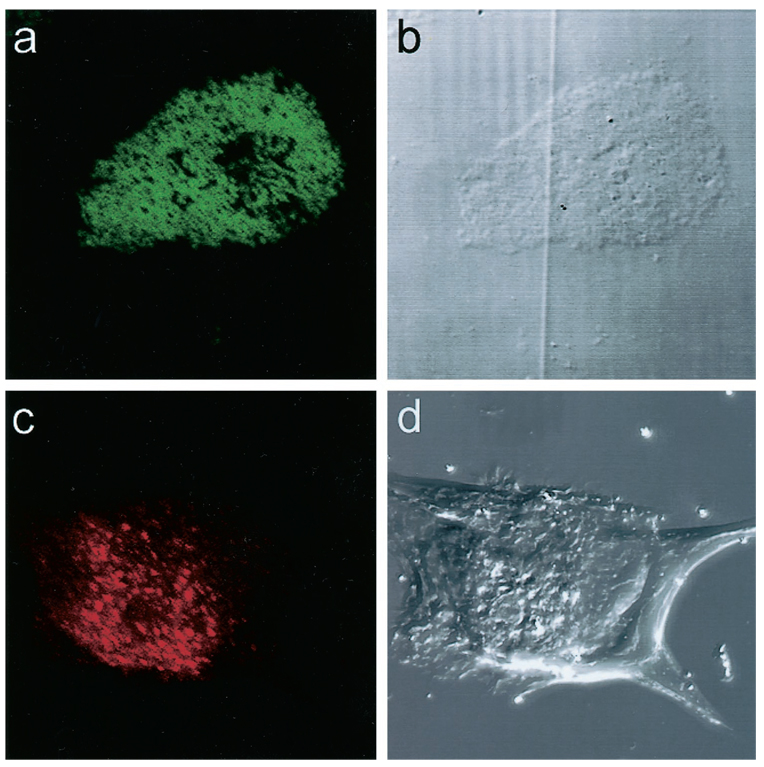
The expression of the human FcRn in H4 and T84 cells and human fetal intestine was confirmed by immunohistochemistry with antibodies raised against the FcRn. Frozen sections of fetal intestinal mucosa showed distinct villous and crypt staining patterns similar to that previously described (7). Villous enterocytes showed a delicate punctate staining located at the apical membrane. (Fig. 4, a and c). A similar pattern of staining for the FcRn has been recently demonstrated in adult intestine as well (5). Staining with neonatal mouse intestine was used as a positive control for the tissue and corroborated previous data (8). In contrast to the human fetal intestine, where staining for the FcRn was punctate, there was a more linear, apical expression of the FcRn on enterocytes in neonatal mouse intestine.
Figure 4.
Immunofluorescence using a FITC-conjugated anti-FcRn antibody and confocal microscopy for the FcRn in human fetal intestine (a, c) with negative control (b, d). A punctate apical pattern of staining is seen on the villi (arrows).
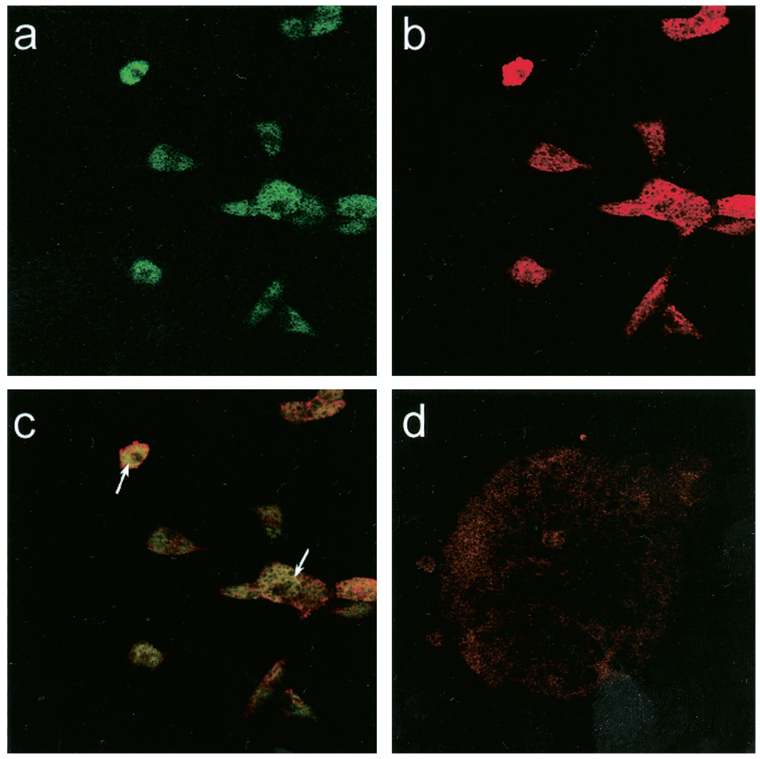
T84 cells, stained with anti-FcRn antibodies, showed a punctate staining pattern as previously described (5). H4 cells showed a similar punctate staining with a cytoplasmic distribution (Figs. 5a) suggestive of endosomal localization. Positive staining for the early endosomal antibody, EEA1, was also demonstrated in H4 cells (Fig. 5c) and T84 cells (data not shown). Double staining for EEA1 showed a co-localization with the FcRn in H4 (Fig. 6c) and T84 cells (data not shown). A positive immunofluorescence for the FcRn was not present in identical T84 and H4 cells or in sections treated with nonimmune serum and an irrelevant affinity-purified antiserum (Fig. 4, b and d, and Fig. 6d).
Figure 5.
Immunofluorescence using a FITC-conjugated anti-FcRn antibody of an H4 cell as seen with a 40× lens, zoom 4 magnification using a confocal microscope, showing the cytoplasmic localization of the FcRn (a) with a Nomarski image in (b). Double-staining of the H4 cell with a rhodamine red-conjugated EEA1 (c) with the Nomarski image (d).
Figure 6.
H4 cells immunostained with a FITC-conjugated anti-FcRn antibody (a) and a rhodamine red-conjugated EEA1 (b), showing co-localization (c) and absence of staining with an isotype-matched irrelevant antiserum (d).
DISCUSSION
This study further characterizes the distribution of expression of the FcRn in different segments of the human fetal gastrointestinal tract and demonstrates that the FcRn is expressed in the stomach, small intestine, and colon of the human fetus as well as in intestinal biopsies derived from the small and large intestine of older children (Fig. 1 and Fig. 2). Although preliminary data suggested that there might be an enhancement of FcRn expression cephalocaudally, densitometry analysis of pooled sample data from lysates derived from various fetuses failed to demonstrate a significant difference in expression (Fig. 3). The intestinal tissue used for these experiments was derived from fetuses of varying gestational ages of between 18 and 22 wk. A difference in age did not seem to alter the pattern of expression in the human fetus. However, an increase in the FcRn was suggested from intestinal biopsy tissue derived from the small intestine as well as the colon of an older child and adolescent (Fig. 2). The subapical, punctate pattern of staining for the FcRn by immunohistochemistry in the human fetal intestine of 18–22 wk gestational age is similar to that demonstrated previously in adult intestine (Fig. 4) (3, 5), with the FcRn expressed on both villous and crypt enterocytes. The FcRn was also detected in fetal stomach, with a distribution along the glands and in the fetal colon, where staining was found to be diffuse and along the crypts.
The H4 cell line is a primary nonmalignant human fetal intestinal epithelial cell line that has been developed at our laboratory. The cells retain features of intestinal enterocytes and express cytokeratins and villin (4). Demonstration of the FcRn on H4 cells is very encouraging, suggesting that this cell line may be used as a model to further study the cellular events associated with FcRn function. The FcRn may bind its ligand in the acidic media of the endosomal compartment (5). The cytoplasmic punctate staining for FcRn in H4 cells (Fig. 5) and T84 cells had led us to investigate the possibility that FcRn may be localized endosomally within the cell. Co-localization of an antibody against endosomal EEA1 with the FcRn in H4 cells supports this conclusion (Fig. 6).
The finding of a Fc receptor in a subapical location in the human fetal intestine establishes a potential entry site for IgG. Most of the IgG in the blood of newborns is maternal in origin, with a rise in serum IgG in the fetus occurring in parallel with a rise of IgG in amniotic fluid (2, 3). Because the majority of placental transfer of IgG via the FcRn in the syncytiotrophoblast occurs after 22 wk, it is possible that an earlier transfer also takes place from swallowed amniotic fluid via an FcRn in the fetal intestine (2, 3).
Because the development of the neonatal rat intestine, which is known to have an FcRn, is morphologically and enzymatically (7–9) quite similar to the human fetal intestine, we hypothesize that a similar IgG-FcRn shuttle may exist in the human fetus. Our laboratory has previously reported that the human fetal intestine binds IgG with affinity characteristics similar to that seen for neonatal rodent FcRn (3). The FcRn receptor has been cloned in the rat, mouse, and human (10) and is expressed in various adult tissues, including adult intestine (3, 5, 11). With its expression as a heterodimer of a glycosylated heavy chain associated with β2 microglobulin, our laboratory has previously provided evidence for a possible protective role for the FcRn by establishing an increased catabolism of IgG seen in β2 microglobulin-deficient mice (12). A differential regulation of the cellular distribution of FcRn expression has also been demonstrated on endothelial cells (13) and in human monocytes, macrophages, and dendritic cells (14).
In vitro studies have shown bidirectional transport of IgG in human malignant intestinal epithelial cell lines that is specific to the FcRn (5) and dependent on endosome acidification (5). In suckling rats, the FcRn enters intestinal cells by endocytosis and recent data suggests that tryptophan and dileucine motifs in the cytoplasmic domain of the rat FcRn may function as endocytotic signals (15). We postulate that the FcRn may serve to bind IgG at the cell surface or transport immune complexes in the opposite direction to lamina propria lymphocytes. Such a transport process, existing in neonatal rodents, may also exist in human neonates and may be developmentally regulated (16). Hence, research planned in the future will include the use of two additional human fetal models, a human fetal intestinal xenograft transplant model (17) and fetal organ culture (18), to investigate the function and developmental regulation of the FcRn in the developing human intestine.
Breast milk, colostrum, and amniotic fluid contain a variety of cytokines and trophic factors such as IL-6 and TGF-β, which are hypothesized to contribute to their immunomodulatory properties and to development in the newborn (19). With further advances made in the analysis and cloning of the gene encoding the human neonatal Fc receptor (20, 21), the identification of a binding motif for cytokine-inducible transcription factors, NF-IL6 (nuclear factor IL-6), NF1(nuclear factor 1), and TGF-β upstream of the gene encoding the FcRn α-chain, suggests that raised levels of these cytokines might increase FcRn transcription and hence increase serum IgG concentrations. This process might be of importance during an acute inflammatory response in which IL-6 is known to be operational (22). For example, IL-6 is known to be elevated in amniotic fluid from mothers with intrauterine infections and in those with preterm labor (23). It is therefore conceivable that these inflammatory cytokines may up-regulate the FcRn to maintain IgG at the luminal surface or to transport it into the fetal circulation.
It is well recognized that infants are born relatively hypogammaglobulinemic (24), and the more premature the neonate, the lower are the Ig levels. With improving neonatal care, a greater percentage of premature infants are now able to survive. The isolation of a ubiquitously expressed homolog of the rodent FcRn from the human placenta (10) and intestine (12) together with its conservation across species of IgG residues that interact with the FcRn (25) indicates that the FcRn may play a role in IgG homeostasis and transport in the fetus. This would have far-reaching therapeutic implications. An example of such an application would be the oral use of Ig and specific antibodies in the vulnerable premature neonate to prevent necrotizing enterocolitis (26, 27). A detailed understanding of the function of the FcRn in the neonatal human intestine and the various factors and cytokines that regulate its expression may have significant clinical application in the management of perinatal disease.
Acknowledgments
The authors thank Nanda Nanthakumar and Gerburg Spiekermann for technical support and advice.
Supported by National Institutes of Health grants R37-HD12437, RO1-HD 31852, PO1-DK 33506, and P30-DK40561. U.S. supported by a National Institutes of Health training grant (T32-DK07477).
Abbreviations
- FcRn
neonatal Fc receptor for IgG
- GST
glutathione-S-transferase
- GAPDH
glyceraldehyde phosphate dehydrogenase
- EEA1
early endosomal antigen 1
- TGF-β
transforming growth factor beta
REFERENCES
- 1.Simister NE, Story CM, Chen HL, Hunt JS. An IgG transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur J Immunol. 1996;26:1527–1531. doi: 10.1002/eji.1830260718. [DOI] [PubMed] [Google Scholar]
- 2.Quan CP, Forestier F, Bouvet JP. Immunoglobulins of the human amniotic fluid. Am J Reprod Immunol. 1999;42:219–225. doi: 10.1111/j.1600-0897.1999.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 3.Israel EJ, Simister NE, Freiberg E, Caplan A, Walker WA. IgG binding sites on human fetal intestine: a possible mechanism for the passive transfer of immunity from mother to infant. Immunology. 1993;79:77–81. [PMC free article] [PubMed] [Google Scholar]
- 4.Sanderson IR, Ezzel RM, Kedinger M, Erlanger M, Xu Z, Pringault E, Leon-Robin S, Louvard D, Walker WA. Human fetal enterocytes in vitro: modulation of the phenotype by extracellular matrix. Proc Natl Acad Sci U S A. 1996;93:7717–7712. doi: 10.1073/pnas.93.15.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickinson BL, Badizadegan K, Wu Z, Ahouse JC, Zhu X, Simister NE, Blumberg RS, Lencer WI. Bi-directional FcRn dependent IgG transport in a polarized human intestinal epithelial cell line. J Clin Invest. 1999;104:903–911. doi: 10.1172/JCI6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy KM, Yoong Y, Simister NE. Bi-directional transcytosis of IgG by the rat neonatal Fc receptor expressed in a rat kidney cell line: a system to study protein transport across epithelia. J Cell Sci Apr. 2000;113:1277–1285. doi: 10.1242/jcs.113.7.1277. [DOI] [PubMed] [Google Scholar]
- 7.Israel EJ, Taylor S, Wu Z, Mizoguchi E, Blumberg RS, Bhan A, Simister NE. Expression of the neonatal Fc receptor FcRn on human intestinal epithelial cells. Immunology. 1997;92:69–74. doi: 10.1046/j.1365-2567.1997.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodewald R, Kraehenbuhl JP. Receptor mediated transport of IgG. J Cell Biol. 1984;99:159–6519. doi: 10.1083/jcb.99.1.159s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryant MG, Buchan AM, Gregor M, Ghatei MA, Polak JM, Bloom SR. Development of intestinal regulatory peptides in the human fetus. Gastroenterology. 1982;83:47–54. [PubMed] [Google Scholar]
- 10.Story CM, Mikulska JE, Simister NE. A MHC class 1 like Fc receptor cloned from human placenta: possible role in transfer of IgG from mother to fetus. J Exp Med. 1994;180:2377–2381. doi: 10.1084/jem.180.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haymann JP, Levraud JP, Bouet S, Kappes V, Hagege J, Nguyen G, Xu Y, Rondeau E, Sraer JD. Characterization and localization of the neonatal Fc receptor in adult human kidney. Am J Soc Nephrol. 2000;11:632–639. doi: 10.1681/ASN.V114632. [DOI] [PubMed] [Google Scholar]
- 12.Israel EJ, Wilsker DF, Hayes KC, Schonfeld D, Simister NE. Increased clearance of IgG in mice that lack β2 microglobulin: possible protective role of FcRn. Immunology. 1996;89:573–578. doi: 10.1046/j.1365-2567.1996.d01-775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borvak J, Richardson J, Madesan C, Antohe F, Radu C, Simonescu M, Ghetie V, Ward VS. Functional expression of the Class 1 MHC receptor FcRn in endothelial cells of mice. Int Immunol. 1998;10:1289. doi: 10.1093/intimm/10.9.1289. [DOI] [PubMed] [Google Scholar]
- 14.Zhu X, Meng G, Dickinson BL, Li X, Mizoguchi E, Miao L, Wang Y, Robert C, Wu B, Smith PD, Lencer WI, Blumberg RS. MHC Class 1 related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages and dendritic cells. J Immunol. 2001;166:3266–3276. doi: 10.4049/jimmunol.166.5.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z, Simister NE. Tryptophan and dileucine based endocytosis signals in the neonatal Fc receptor. J Biol Chem. 2001;276:5240–5247. doi: 10.1074/jbc.M006684200. [DOI] [PubMed] [Google Scholar]
- 16.Martin GM, Wu V, Walsh JH. Ontogenetic development and distribution of antibody transport and Fc receptor mRNA expression in rat intestine. Dig Dis Sci. 1997;42:1062–1069. doi: 10.1023/a:1018853506830. [DOI] [PubMed] [Google Scholar]
- 17.Savidge TC, Morey AL, Ferguson DJP, Fleming KA, Shamkov AN, Phillips AD. Human intestinal development in a severe combined immunodeficient xenograft model. Differentiation. 1995;58:361–371. doi: 10.1046/j.1432-0436.1995.5850361.x. [DOI] [PubMed] [Google Scholar]
- 18.Macdonald TT, Spencer J. Evidence that gut mucosal T cells play a role in the pattern of enteropathy in human small intestine. J Exp Med. 1988;167:1341–1349. doi: 10.1084/jem.167.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawkes JS, Bryan DL, James MJ, Gibson RA. Cytokines IL-1β, IL6, TNFα, TGFβ and prostaglandin E2 in human milk during the first three months postpartum. Pediatr Res. 1999;46:194–199. doi: 10.1203/00006450-199908000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Mikulska JE, Simister NE. Analysis of the promoter region of the human FcRn gene. Biochim Biophys Acta. 2000;1492:180–184. doi: 10.1016/s0167-4781(00)00068-3. [DOI] [PubMed] [Google Scholar]
- 21.Mikulska JE, Pablo L, Canal J, Simister NE. Cloning and analysis of the gene encoding the human neonatal Fc receptor. Eur J Immunogenet. 2000;27:231–240. doi: 10.1046/j.1365-2370.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 22.Kandil E, Noguchi M, Isibashi T, Kasahara M. Structure and phylogenetic analysis of the MHC class I like Fc gene. J Immunol. 1995;154:5907–5918. [PubMed] [Google Scholar]
- 23.Saji F, Samejima Y, Kamiura S, Sawai K, Shimoya K, Kimura T. Cytokine production in chorioamnionitis. J Reprod Immunol. 2000;47:185–196. doi: 10.1016/s0165-0378(00)00064-4. [DOI] [PubMed] [Google Scholar]
- 24.Ballow N, Cates KL, Rowe JC, Goetz C, Debonnet C. Development of the immune system of very low birth weight infants: concentrations of plasma immunoglobulins and patterns of infections. Pediatr Res. 1986;20:899–904. doi: 10.1203/00006450-198609000-00019. [DOI] [PubMed] [Google Scholar]
- 25.Kim JK, Firan M, Radu CG, Kim CH, Ghetie V, Ward ES. Mapping the site on human IgG for binding of the MHC class 1 related receptor, FcRn. Eur J Immunol. 1999;29:2819–2825. doi: 10.1002/(SICI)1521-4141(199909)29:09<2819::AID-IMMU2819>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 26.Israel EJ. Neonatal necrotizing enterocolitis, a disease of the immature intestinal mucosal barrier. Acta Paediatr Suppl. 1994;396:27–32. doi: 10.1111/j.1651-2227.1994.tb13238.x. [DOI] [PubMed] [Google Scholar]
- 27.Claud EC, Walker WA. Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. FASEB J. 2001;15:1398–1403. doi: 10.1096/fj.00-0833hyp. [DOI] [PubMed] [Google Scholar]