Abstract
Platelet hyper-reactivity and a systemic prothrombotic state are associated with atherosclerosis and other inflammatory conditions. CD36, a member of the Type 2 scavenger receptor family, is a multiligand pattern recognition receptor that recognizes specific oxidized phospholipids, molecules expressed on microbial pathogens, apoptotic cells, and cell-derived microparticles. Recent studies have demonstrated that CD36 binding to oxidized LDL or microparticles activates a specific signaling pathway that induces platelet activation. This pathway is activated in vivo in the setting of hyperlipidemia and oxidant stress. Genetic deletion of CD36 protects mice from pathological thrombosis associated with hyperlipidemia without any apparent effect on normal hemostasis. Targeting CD36 or its signaling pathway could potentially lead to the development of novel antithrombotic therapies for patients with atheroinflammatory disorders.
Keywords: CD36, microparticles, oxidized LDL, platelets, thrombosis
Platelets & hyperlipidemia
For many years platelet activation has been postulated to contribute to the pathogenesis of atherosclerosis and platelet hyper-reactivity has been thought to play a role in acute atherothrombotic disorders such as myocardial infarction and stroke. Clinical studies support an association between platelet reactivity and prognosis in patients with coronary disease [1] and numerous studies have linked hyperlipidemia to atherothrombotic risk [2]. In 1974, a potential connection between these two risk factors was identified by Carvalho et al., who demonstrated that platelets from patients with familial hypercholesterolemia were more sensitive to activation by epinephrine or ADP, although the mechanism of this phenomenon was not explored [3]. Shortly thereafter, Shattil and colleagues demonstrated in a series of in vitro experiments that artificial loading of platelet membranes with cholesterol increased sensitivity to agonists, while cholesterol depletion reduced sensitivity [4]. They postulated that changes in membrane fluidity or dynamics might account for these differences and that similar changes could occur in the setting of hyperlipidemia [5]. In retrospect, it is more likely that these manipulations modulated the structure and function of cholesterol-rich membrane microdomains thereby nonspecifically altering cell signaling mediated by G-protein-coupled receptors (GPCR) and src-family kinases that reside in these domains.
In the 35-year period since these seminal observations, advances in cell biology and genome science coupled with the development of numerous mouse genetic knockout strains that have been tested in robust models of in vivo thrombosis have led to understanding specific mechanisms by which lipids interact with platelets. A critical event in the evolution of our knowledge arrived with the development of mouse models of hyperlipidemia, such as the apoe-null strain developed by the Breslow laboratory [6]. When fed a diet replicating a typical human high-fat western diet these mice develop severe hypercholesterolemia and atherosclerotic lesions that closely resemble those observed in humans. Importantly, Eitzman and colleagues demonstrated that apoe-null mice fed a western diet and then subjected to arterial injury displayed shortened time to form occlusive thrombi compared with wild-type mice [7]. These studies are consistent with the human clinical data and demonstrated that the apoe-null mouse model could be used to probe pathophysiologic mechanisms of hyperlipidemia-associated hypercoagulability.
Although increased plasma levels of plasminogen activator inhibitor-1 have been implicated in some aspects of the prothrombotic phenotype of the apoe-null mice [8], it is also clear that platelet hyperactivity plays a significant role. In that context much attention has been paid to identifying and characterizing receptors on the platelet surface that recognize specific classes of lipids and lipoproteins. In some cases the signaling pathways linked to these receptors have been partially characterized and shown to either activate or inhibit platelet function. The receptors fall into two main groups; pattern recognition receptors common to the innate immune system and GPCRs. Among the GPCRs are at least two members of the EDG/lysophosphatidic acid (LPA) family of receptors for LPA, the platelet-activating factor receptor, and the thromboxane receptor [9–11]. Among the pattern-recognition receptors are members of the Toll-like receptor (TLR) family, including TLR4 [12], which is the receptor for bacterial lipopolysacharide, members of the scavenger receptor (SR) B-1 family [13] (as well as CD36 [14]), and up to three splice variants of the LDL receptor-related protein-8 (LRP-8), also known as apoER2 [15]. This paper focuses mainly on CD36, a receptor for oxidized phospholipids that has been implicated by studies from our laboratory and others as a major modulator of platelet reactivity with particular relevance to hyperlipidemia and oxidant stress [16].
CD36, hyperlipidemia & oxidant stress
Phospholipid components of circulating lipoproteins [17–19], including HDL and LDL, are highly susceptible to oxidation in vivo and an abundance of experimental data link oxidized LDL (oxLDL) to important components of the pathogenesis of atherosclerosis and thrombosis. OxLDL can be detected in atherosclerotic plaque and in the circulating plasma of experimental animals and patients with atherosclerosis and hyperlipidemia [16,19]. Macrophages recognize oxLDL primarily via CD36 and SRA1 [20], leading to activation of signaling cascades that inhibit migration [21] and that promote internalization of the lipoprotein particles [22], formation of foam cells and a proinflammatory response [23,24]. Studies of mouse gene knockouts have shown that loss of CD36 confers substantial protection against atherosclerosis in the apoe-null model [25–28] and suggest that CD36 is the most relevant scavenger receptor in atherogenesis. Although much is known about the mechanism of oxLDL interactions with macrophages, only recently have studies demonstrated that CD36 also mediates platelet responses to oxLDL and contributes to the pro-coagulant state associated with hyperlipidemia and oxidative stress [16].
CD36 structure
CD36 was initially identified in platelets and was termed glycoprotein IV as it was the fourth major band observed on SDS-PAGE gels of platelet membranes [14]. It is sometimes referred to as glycoprotein IIIB in older literature, but most reports now use the CD designation, which was assigned when it was shown to be identical to the antigen recognized by the antimonocyte/macrophage monoclonal antibody OKM5 [29]. It is the defining member of a small gene family [30,31] that in vertebrates contains two other members, lysosomal integral membrane protein (LIMP)-2 and CLA-1 (CD36 and LIMP-2 analogs), which is also known as SRB-1. The primary structure of CD36 family members is conserved in mammals, and multiple ortho-logs have been identified in insects, nematodes, sponges and slime mold [32–36], suggesting that the common ancestral gene is very ancient.
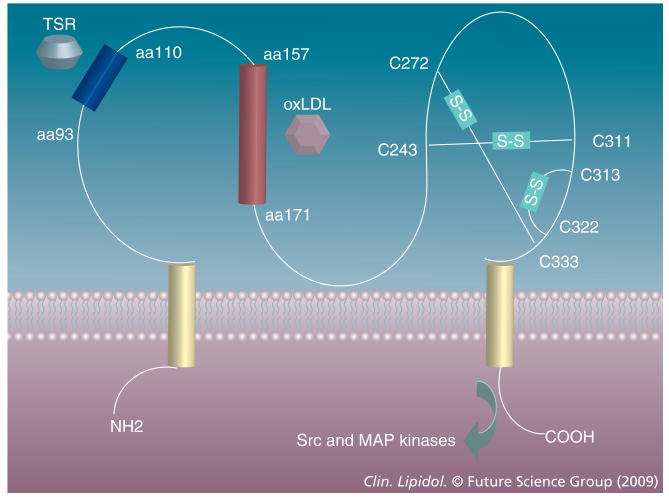
CD36 is 88,000 Da in molecular weight and has two transmembrane domains, short intracytoplasmic domains of five to seven and 11–13 amino acids, and a large extracellular domain with six conserved cysteines linked in three disulfide bridges (Figure 1) [37,38]. The extracellular domain is glycosylated on at least nine of the ten asparagine residues, accounting for the observed molecular weight 30–40,000 Da greater than predicted by the cDNA. Recent studies suggest that glycosylation is necessary for correct trafficking to the plasma membrane [39]. The extracellular domain also contains a hydrophobic stretch that may be membrane-associated and a consensus protein kinase C phosphorylation site with a threonine residue at position 92 that may be phosphorylated on certain cells, including platelets [40,41]. Both of the intracellular domains contain paired cysteine residues that are lipid acylated and thus probably tightly associated with the inner leaflet of the plasma membrane [42]. CD36 can be ubiquitinated on two lysines (amino acids 469 and 472) in its C-terminal domain [43] and this provides a level of control of expression by targeting it to lysosomes; this pathway is sensitive to insulin and fatty acids.
Figure 1. CD36 has two transmembrane domains (yellow), two short cytoplasmic domains and a large extracellular domain.
The latter contains three disulfide bonds clustered near the N-terminal transmembrane domain and independent binding sites for TSR-containing proteins (blue) and oxLDL (red) on the N-terminal half. The C-terminal cytoplasmic domains directs intracellular signaling via activation of specific Src-family and MAP kinases. TSR: Thrombospondin type I repeat.
It is now clear that CD36 is a true ‘multifunctional’ protein recognizing at least three classes of ligand; modified phospholipids [17,19,44]; a subset of proteins containing a structural domain termed the thrombospondin type I repeat (TSR) [30,45,46]; and free fatty acids [47,48]. CD36 affects very different cellular processes depending on the nature of the ligand and the type and location of the cell that expresses it. Cross competition experiments suggest that the binding sites for the three types of ligand are independent. Studies with recombinant and synthetic peptides identified the linear sequence between amino acids 93 and 110 as the likely site for TSR protein binding [49,50]. This peptide contains a negatively charged sequence that is conserved among the three members of the human CD36 family, rodent CD36 and invertebrate orthologs. We termed this domain CD36, LIMP2, drosophila epithelial membrane protein and SRB-1 homology (CLESH) domain and demonstrated that it is present in several unrelated proteins where it also mediates TSR protein binding [51,52]. Interestingly, ectodomain phosphorylation of threonine 92 has been shown to regulate binding of thrombospondin-1 to platelet CD36 [40,41]. The oxidized phospholipid binding site on CD36 remains incompletely characterized, but recent studies from Podrez's group and others suggest that the region between amino acids 157–171 contains a major binding site and that lysine residues at positions 164 and 166 are critical [53,54].
CD36 expression
CD36 is present on many mammalian cell types, including platelets, erythroid precursors and microvascular endothelium; most ‘professional’ phagocytes (macrophages, dendritic cells, microglia and retinal pigment epithelium); hepatocyes; adipocytes; cardiac and skeletal myocytes; and epithelia of the gut, breasts and kidneys [55]. Expression in monocytes has been studied by our group and others and found to be highly regulated at the transcriptional level [56]. Expression increases by as much as five- to ten-fold as peripheral blood monocytes differentiate in culture to macrophages or dendritic cells [57,58], and also in response to exposure to Il-4, macrophage colony-stimulating factor, granulocyte macrophage colony-stimulating factor, oxLDL and PPAR-γ agonists [56,59,60]. Expression decreases rapidly and dramatically after exposure to lipopolysaccharide, dexamethasone or TGF-β [56,61]. In vivo, monocyte CD36 expression levels increase in response to lipid infusion [62]. CD36 expression in macrophages is increased by exposure to hyperglycemia [63,64] and certain HIV protease inhibitor drugs [65]. The glucose effect is not transcriptional, but rather due to increased efficiency of CD36 mRNA translation [63]. In the liver, the lipogenic transcription factors PPAR-γ, liver X receptor (LXR) and pregnane X receptor (PXR) [59,61] cooperate to regulate CD36 expression [66].
CD36 function may also be regulated posttranslationally by movement to and from the plasma membrane surface from an intracellular pool. In muscle cells, insulin, glucose, fatty acids, ischemia and contraction have been shown to regulate CD36 in this manner [67,68]. Regulation of CD36 expression in other cell types, including endothelial cells and megakaryocytes/platelets is not well studied, although one report suggests that platelet CD36 levels in humans may be influenced by lipid-lowering statin drugs [69].
Null mutations in the human CD36 gene are surprisingly common. It has been estimated that 5–10% of Asians and Africans carry such mutations and therefore as many as 1% of these populations are CD36 null [38]. CD36 function as a receptor for Plasmodium falciparum malaria-infected erythrocytes and have been postulated to be the selective pressure for these mutations. In addition to the CD36-null genotypes, approximately 7–10% of Japanese and other Asian populations carry the so-called Naka-blood group polymorphism associated with lack of platelet CD36 expression. Interestingly, CD36 expression is preserved on other cell types in these individuals. At present, there is no definitive phenotype associated with the absence of CD36, although studies of small numbers of subjects suggest defects in cardiac muscle uptake of fatty acids and perhaps an association with insulin resistance [70–73]. Bleeding or thrombotic diatheses have not been reported.
CD36 regulates angiogenesis
Several TSR-containing proteins, including thrombospondin-1 and -2, and vasculostatin are potent inhibitors of angiogenesis in vivo and of microvascular endothelial cell responses to angiogenic factors in vitro [74,75]. These proteins play important roles in regulating angiogenesis in pathological settings including cancer. Their antiangiogenic activity is contained in the TSR domains, and our group in collaboration with Bouck and colleagues identified CD36 as the endothelial cell receptor that mediates this activity [76]. CD36-mediated antiangiogenesis is caused by its ability to activate a specific signaling cascade that results in diversion of a proangiogenic response to an apoptotic response [77]. This cascade involves activation of specific members of the mitogen-activated protein kinase (MAPK) and Src kinase families (p38, c-jun N-terminal kinase [JNK] and fyn) resulting in direct activation of caspases as well as induction of expression of other proapoptotic proteins, such as Fas ligand and TNF receptor [78,79]. Recently, we identified a circulating protein, histidine-rich glycoprotein that contains a TSR-binding CLESH domain and that acts as a soluble decoy to block the antiangiogenic activities of TSPs, thereby promoting angiogenesis [74].
CD36 function in lipid metabolism
CD36 functions on adipocytes, muscle cells, enterocytes and hepatocytes as a facilitator of long-chain fatty acid transport [47,66,80–82]. In the gut, CD36 promotes absorption of long-chain fatty acids [81,82] and participates in carotenoid uptake for vitamin A metabolism [83]. In adipocytes, CD36 participates in lipid storage while in muscle it participates in lipid oxidation. The mechanism by which CD36 performs these transport functions remain poorly understood [47,48] but cd36-null mice show abnormal plasma lipid and lipoprotein profiles and resting hypoglycemia [84], attributable partly to a marked impairment of fatty acid utilization in cardiac and striated muscle and fatty acid uptake by adipose tissues [80]. Studies in rodents and humans suggest that CD36 fatty acid interactions may contribute to the pathogenesis of metabolic disorders, such as insulin resistance, obesity and nonalcoholic hepatic steatosis [63,64,85–88]. Studies of rodent models also demonstrated that CD36 in taste bud cells mediates behavioral preferences for fatty food [89], implicating CD36 in a complex neurosignaling network. This is consistent with the findings that CD36 orthologs in sensory cells in insect antennae participate in behavioral responses to lipid pheromones [33–35].
CD36 scavenger functions
On professional phagocytes, CD36 functions as a pattern recognition/scavenger receptor; which is one of a group of structurally unrelated proteins that evolved with the innate immune system as primitive receptors involved in helping the organism eliminate ‘foreign’ agents (e.g., bacteria, parasites and viruses) during an infection [90,91]. The hallmark of these receptors is their ability to recognize specific classes of molecular patterns presented by pathogens or by pathogen-infected cells. CD36 recognizes specific lipid and lipoprotein components of bacterial cell walls [92], particularly those of staphylococcal and mycobacterial organisms [93], β-glucans on fungal species [94], and erythrocytes infected with P. falciparum malaria [30,95] and thereby triggers a reaction that results in opsonin-independent pathogen internalization. Scavenging is an evolutionarily ancient function of CD36; orthologs in Drosophila are involved in recognition and clearance of apoptotic cells and mycobacteria, and an ortholog on cells of the worm Caenorhabditis elegans participates in the innate immune response to fungi [94].
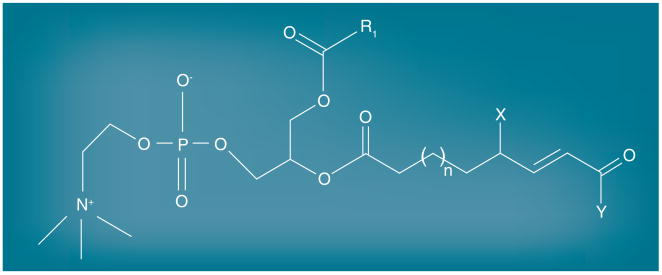
As with many other scavenger receptors, CD36 also recognizes endogenously derived ligands, including apoptotic cells [58,96,97], cell-derived microparticles (MP) [98], shed photoreceptor outer segments [44,99], oxidized lipoproteins [17,18], glycated proteins and amyloid-forming peptides [100,101]. Research carried out in Hazen's laboratory utilizing a combination of HPLC, mass spectrometry and synthetic chemistry identified the structural characteristics of the oxidized phospholipids in oxLDL that are recognized by CD36. Truncated oxidized unsaturated fatty acids in the sn-2 position of glycerol-phospholipids that contain a terminal aldehyde or carboxylic acid, a double bond in the β position, and a ketone or alcohol in the γ position are high affinity ligands for CD36 (Figure 2) [19]. These structures, termed oxPCCD36, are present in atheroma, in oxLDL circulating in patients and mice with hyperlipidemia, and on the surface of apoptotic cells [16,19,44]. Structural modeling based on hydrogen-deuterium mass spectrometry suggests that the CD36 ligand structures protrude from the lipoprotein particle or apoptotic cell surface into the plasma milieu where they can be recognized by CD36-bearing cells [102].
Figure 2. oxPCCD36 structure.
Truncated oxidized unsaturated fatty acids in the sn-2 position of glycerol-phospholipids containing a terminal aldehyde or carboxylic acid, a double bond in the β position, and a ketone or alcohol in the γ position are ligands for CD36. X is OH or =O; Y is H or OH.
Studies with the cd36-null mouse strain generated in our laboratory have led to considerable insight into the function of CD36 scavenging function in vivo. Our group demonstrated that CD36 plays a role in retinal pigment epithelial cell phagocytosis of shed photoreceptor outer segments [99], antigen presentation [58] by dendritic cells, and ischemia–reperfusion injury in the brain [103]. Loss of CD36 confers protection from diet-induced atherosclerosis [25] and limits inflammation and tissue infarction associated with acute cerebrovascular occlusion [103], but may increase susceptibility to certain infections [92]. The atheroprotective role of CD36 deficiency has been controversial [104–106], but the preponderance of evidence supports an important role for CD36 in mediating proatherogenic macrophage responses, including foam cell formation [22] and inhibition of migration [21], and studies in several in vivo model systems often show dramatically smaller plaque lesions [25–28] and/or less complex lesions with less aortic cholesterol in the absence of CD36 [106].
CD36 mediates platelet hyperreactivity induced by hyperlipidemia & oxidant stress
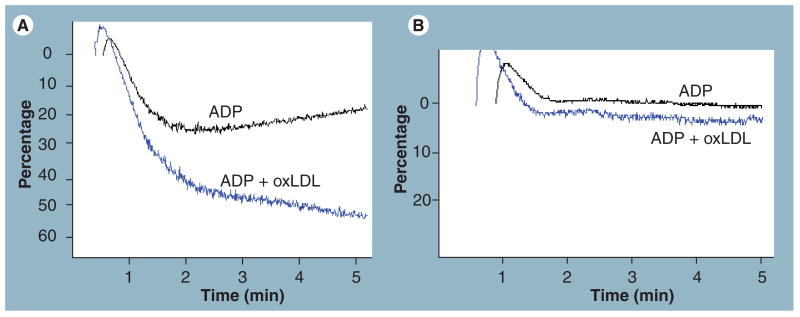
Although CD36 has been recognized as a major platelet surface glycoprotein for more than 30 years [14], only recently have functional roles been identified. As early as 1999, Volf et al. noted that oxLDL could bind to platelet surfaces and induce activation [107], but mechanisms were not identified. Later studies using competitive ligands for different scavenger receptors suggested that SRA1 was not a likely candidate to serve as the platelet oxLDL receptor [108]. The development of highly specific ligands for CD36 along with mouse models of cd36 deficiency has now helped clarify this field by demonstrating a central role for platelet CD36. In collaboration with Podrez, Byzova and Hazen, our laboratory definitively identified CD36 as the platelet receptor for oxLDL and demonstrated that oxLDL induced platelet activation in a CD36-dependent manner [16]. OxLDL bound to the surface of platelets in a saturable, concentration and time-dependent manner that was inhibited by monoclonal antibodies to CD36 but not to SRA1. Importantly, specific binding was not observed with platelets from CD36-negative humans or mice. Unilammelar phospholipid liposomes containing the synthetic oxPCCD36 species previously described also bound platelets in a CD36-dependent manner. At saturating concentrations, oxLDL or oxPCCD36 induced activation of platelets detected by flow cytometry assays for expression of the specific activation markers P-selectin and activated integrin αIIbβ3. These agents did not induce activation of platelets from cd36-null mice or from human CD36-null donors. These observations are consistent with pharmacologic studies from Göpfert et al. demonstrating that platelet activation by specific oxidized phospholipids was not mediated by platelet-activating factor receptor, thromboxane receptor or LPA receptors [109]. At lower concentrations (e.g., observed in individuals with atherosclerosis), we demonstrated that oxLDL augmented platelet aggregation responses to low doses of ‘classic’ platelet agonists, such as ADP and collagen (Figure 3) [16,110]. Based on these observations we hypothesized that CD36, owing to its unique specificity for ligands generated during host responses to atheroinflammatory diseases (that is oxidized phospholipids and apoptotic cells), may function as a ‘disease sensor’ capable of triggering a signaling cascade that could influence platelet responses and contribute to disease pathology. To test this hypothesis and show in vivo relevance of the studies described previously, we studied western diet-fed apoe-null mice as a model of the hyperlipidemic, atherogenic, prothrombotic state [16]. Platelet-rich plasma from these mice showed enhanced aggregation responses to low doses of ADP, and when washed platelets from wild-type mice were resuspended in plasma obtained from the western diet-fed apoe-null mice, they showed the same increase in sensitivity to low doses of ADP as did platelets incubated with oxLDL. Platelets from cd36-null mice did not show a proaggregation response to oxLDL or hyperlipidemic plasma. As noted previously, apoe-null mice fed the high-fat ‘western’ diet have shortened tail vein bleeding times (an assay for ‘normal’ hemostasis) and shortened times to form occlusive thrombi in carotid arteries or mesenteric arterioles and venules in response to chemical injury (an assay for pathological thrombosis) [7]. When cd36-null mice were bred into the apoe-null background to create a double null strain, this prothrombotic phenotype disappeared (i.e., the bleeding times and occlusion times returned to that observed in wild-type mice) [16]. Associated with this phenotypic ‘rescue’, we also demonstrated that the platelet hyper-reactivity in vitro disappeared. These studies thus defined CD36 as a receptor that provides a mechanistic link between oxidant stress, hyperlipidemia and thrombosis.
Figure 3. Oxidized LDL sensitizes platelets in plasma to activation by low doses of physiologic agonists.
Washed platelets were incubated with 50 μM copper oxidized LDL (blue tracings) or native LDL (black tracings) for 30 min and then ADP added at 1 μM at 37°C under stirring conditions. Aggregation was detected as increase in light transmission over time. (A) OxLDL enhanced the aggregation response in CD36-positive donors, (B) but not CD36-negative donors.
These studies do not imply that CD36 is the only functional receptor on platelets for lipoproteins or modified lipoproteins. Studies using so-called ‘minimally’ oxidized LDL implicated LPA as a platelet reactive substance in oxLDL [9–11,111,112]. While these nonoxidized, modified phospholipids can be detected in atherosclerotic plaque and some oxLDL preparations [113], and platelets are known to express at least three different G-protein-coupled LPA receptors [9,11], the in vivo relevance of this system has not been demonstrated. However, ex vivo studies demonstrated that minimally oxidized LDL at high concentrations could induce platelet shape change and sensitize platelets to aggregate in response to other agonists [112].
Studies of HDL effects on platelet function have been inconsistent, with some reports showing inhibition [114,115] and others showing activation [116]. LRP-8 (particularly a splice variant known as apoER2′) binds lipidated apoE resulting in enhanced nitric oxide production and inhibition of platelet activation by ADP and other agonists [117–119]. Platelets from lrp8-null mice or normal platelets treated with a general LRP antagonist showed partially abrogation of this effect [15]. On the other hand, platelets from mice haplo-insufficient for lrp8 showed lower reactivity to ADP and the mice displayed prolongation of thrombosis times after carotid injury [15]. These inconsistencies may be related to the relative low affinity of platelets for HDL, the ability of HDL particles to ‘carry’ a wide array of bioactive molecules, and also to the capacity of LRP-8 to bind apoB-containing lipoproteins [120,121]. The latter has been shown to result in thromboxane generation and sensitization of platelets to other agonists. Another potential confounder is that HDL is at least as susceptible to oxidation in vitro and in vivo as LDL. The Podrez laboratory demonstrated that oxHDL, but not native HDL inhibits platelet reactivity to multiple agonists, including ADP, collagen and thrombin [122]. Using antibodies and null mice, they demonstrated that this inhibitory effect was mediated by SRB1 (not CD36) and was not dependent on nitric oxide generation. They hypothesized that the balance between oxLDL and oxHDL may determine platelet reactivity in patients with hyperlipidemia and oxidant stress. However, in contrast to these studies, Assinger et al. demonstrated that oxHDL-activated platelets in a manner that was inhibited by antibodies to CD36 [116].
CD36 modulates thrombosis in the absence of hyperlipidemia: role of microparticles
Although neither CD36-deficient mice nor humans have a noticeable bleeding diathesis, we observed a subtle hemostatic phenotype in cd36-null mice subjected to models of in vivo vascular injury and thrombosis, even in the absence of hyperlipidemia. When carotid arteries in cd36-null mice were subjected to oxidant-induced injury with a low concentration of ferric chloride (7.5% compared with the ‘standard’ 10–12.5% concentrations used in most of the published literature) the time to form an occlusive thrombus was nearly doubled compared with wild-type mice [98]. Thrombosis times were also significantly prolonged in mesenteric arterioles and venules injured with the lower dose. Direct visualization of thrombus formation in vivo using video microscopy and measurement of blood flow with a Doppler probe suggested that thrombi formed more slowly in cd36-null mice, but were not less stable when they finally formed. To determine the mechanism of these observations, we demonstrated that platelets from CD36-defcient human subjects had a subtle defect in activation, detected as a rightward shift of dose–response curves to agonists such as ADP.
Based on these discoveries, we suggested that CD36 must recognize endogenous ligands generated during vascular injury and hypothesized that cell-derived MP could be one such ligand. Previous studies from our laboratory and others demonstrated that CD36-dependent phagocyte recognition and uptake of apoptotic cells and/or shed photoreceptor outer segments was mediated by binding of CD36 to phosphatidylserine (PS) and/or oxPS on their surfaces [44,99]. A characteristic feature of MP generation is the loss of membrane asymmetry resulting in surface expression of PS. Thus, we hypothesized that PS or oxPS on MP might also act as a ligand for platelet CD36 and thereby promote platelet activation.
Microparticles are vesicular fragments that bud off cells during either activation or apoptosis [123]. They are 200–1000 nm in size and possess different antigenic properties depending on the type of cell from which they are derived. MP can be generated from platelets, monocytes, erythrocytes, leukocytes and endothelial cells during vascular injury and have been shown to become incorporated in developing thrombi in vivo [124]. MP, mostly of platelet origin, can be detected in the circulation of normal human subjects, but markedly increased numbers of circulating MP have been reported in patients with a variety of inflammatory and prothrombotic disorders including cancer, acute coronary syndrome [125], sickle cell disease [126], diabetes mellitus [127,128], thrombotic thrombocytopenic purpura [129], vasculitis, antiphospholipid antibody syndrome [130], hypertension [131] and hematopoietic stem cell transplantation [132]. MPs have been postulated to participate in thrombus formation as PS on their surfaces can be a site for catalytic assembly of the prothrombinase complex [133] and because MP derived from some cells (e.g., monocytes and tumor cells) are a source of circulating tissue factor [134]. In our studies, we used MP derived in vitro from cultured endothelial cells, purified human monocytes or platelets, or isolated from plasma of normal human subjects as model systems and showed using fluorescence-based assays that they bound specifically to platelets in a concentration-dependent manner. Binding was inhibited by anti-CD36 antibody or competitive CD36 ligands (e.g., oxLDL), not observed with platelets from CD36-null donors, and inhibited by blockade of exposed PS on the MP surface by annexin V or a monoclonal anti-PS IgM antibody [98].
The interaction of MP with platelets has important functional implications. Similar to the effect of oxLDL, preincubation of platelets with MP led to CD36-dependent augmentation of platelet activation in response to low doses of ADP, assessed by measuring integrin α2bβ3 activation, P-selectin expression and aggregation. Immunofluorescence confocal microscpopic analysis of murine cartotid thrombi from CD36-null mice showed a significant decrement in endothelial antigen accumulation, suggesting that CD36 plays a role in MP recruitment into thrombi [98]. Importantly, the platelet-activating effect of MP was independent of tissue factor, and thus these data define a novel role for CD36 in thrombosis that may be particularly relevant to the increased risk of pathological thrombosis observed in settings where circulating MP levels are known to be increased.
Mechanisms of CD36 signal transduction in platelets
The mechanisms by which CD36, a receptor with minimal intracellular presence, no intrinsic kinase or phosphatase activity, no known intracellular scaffolding domain(s), and no direct link to GTPases, activates multiple signaling pathways remain incompletely understood, but are under intense study. CD36 signaling in response to its ligands leads to multiple outcomes, including triggering phagocytes to internalize (scavenge) bound ligands [58,95–97], inducing leukocyte proinflammatory responses [21,23,24,101,103], inducing microvascular endothelial antiangiogenic responses [77–79], and promoting platelet granule secretion and integrin activation [16,18,110]. In all cases studied to date, the intracellular signals involve recruitment and activation of Src family nonreceptor protein tyrosine kinases and serine/threonine kinases of the MAPK family, but the specific signaling partners differ depending on the cellular context; for example, the Src family member Lyn and the MAPK JNK2 are critical effectors of macrophage responses [22] while the Src family member Fyn and p38 MAPK are the primary mediators of endothelial cell responses [77].
Korporaal and colleagues recently demonstrated that oxLDL-induced phosphorylation of p38 MAPK in platelets in a pathway that involved both SRA1 and CD36 [135]. We demonstrated by immunoprecipitation that the active phosphorylated forms of Fyn and Lyn were recruited to CD36 in platelets exposed to oxLDL [110]. We also demonstrated that JNK2 and its upstream activator MAPK kinase-4 were phosphorylated after exposure to oxLDL; this was not observed in platelets from CD36-null donors, showing that the effect was CD36 dependent. Pharmacological inhibition of Src kinases reduced JNK phosphorylation by oxLDL and inhibition of either JNK or Src abolished platelet activation by oxLDL in vitro. We also found that JNK was constitutively phosphorylated in ‘resting’ platelets from western diet-fed apoe-null mice, but not from cd36/apoe-double-null mice. When carotid artery thrombi induced by ferric chloride injury were analyzed by immunohistochemistry, we found significantly less phosphorylated JNK in the thrombi formed in cd36-null mice than the wild-type. Importantly, pharmacological inhibition of JNK prolonged thrombosis times in wild-type but not cd36-null mice in vivo [110]. These findings demonstrate that a specific CD36-dependent signaling pathway is activated in platelets by oxLDL.
It is highly likely that the C-terminal cytoplasmic domain of CD36 directs its associations with Src and MAPKs. Point mutations of specific tyrosine or cysteine residues (Y463 or C464) in this domain [136] result in the loss of response to ligands in cell lines and a recombinant protein containing this cytoplasmic domain was shown to precipitate a multiprotein complex from monocyte lysates that contained Lyn, a MAPK kinase, and several as yet unidentified proteins [22]. As CD36 resides in cholesterol-rich, detergent-insoluble lipid raft domains and co-purifies with caveolae from some tissues [137–140], it is possible that CD36 signaling relates to its localization in these membrane regions in which signaling molecules, such as Src, accumulate. It is also likely that CD36 may affect signal transduction, in part, by interacting with other membrane receptors, such as integrins, tetraspanins [141] and TLR [142]. The latter was elegantly demonstrated in studies showing cooperation between CD36 and TLR2 or TLR6 in macrophage recognition and response to some bacteria and bacterial cell wall components [142,143]. However, uptake and proinflammatory responses in response to others did not require TLR-mediated activation of NF-κB and was entirely dependent on CD36-JNK signaling [144,145]. Similarly, TLRs are not required for CD36-dependent uptake of oxLDL [22] or apoptotic cells. Several CD36 functions require coexpression of integrins and both β2[100,146] and β3[141] integrins coimmunoprecipitate with anti-CD36 antibodies. Internalization of apoptotic cells and photoreceptor outer segments require integrins – αvβ3 in macrophages [96] and αvβ5 in dendritic cells [58] and retinal pigment epithelia [147]. Microglial responses to αβ amyloid require β2 integrins and the spreading of brain tumor cells on thrombospondin-1 seems controlled by a functional interaction between β1 integrins and CD36 [148]. The potential role of integrins, TLRs, tetraspanins and membrane microdomains in platelet CD36 signaling has not been studied.
The signaling partners downstream of Src and MAP kinases in platelets are not well described. In other cells, studies have implicated focal adhesion components, including the tyrosine kinases Pyk2 and focal adhesion kinase and the adaptor proteins p130cas and paxillin, in CD36 signaling [21,149]. For example, after macrophage exposure to oxLDL, focal adhesion kinase undergoes prolonged phosphorylation and activation [21] owing to direct activation by Src family kinases coupled with inactivation of a specific phosphatase src homology (SH) domain 2 containing tyrosine phosphatase-2 (SHP-2). The latter was the result of intracellular generation of reactive oxygen species and subsequent oxidative inactivation of a critical cysteine residue in the enzymatic active site. The net result of these intracellular events was enhanced actin polymerization, increased cellular spreading and inhibition of migration. The Vav family of proteins may also link CD36 to downstream events [146]. These proteins are known substrates for Fyn and Lyn and when phosphorylated function as guanine nucleotide exchange factors for Rho and Rac GTPases. Vavs are large multidomain proteins that contain SH3 domains flanking a single SH2 domain and, thus, they function as scaffolds as well as guanine nucleotide exchange factors. Vavs are phosphorylated in macrophages, microglial cells and platelets in a CD36-dependent manner and may be an important link between CD36 and responses requiring small molecular weight G-proteins.
Conclusion & future perspective
The studies reviewed in this paper provide convincing evidence of a significant role for platelet CD36 in modulating platelet reactivity and for promoting a prothrombotic state in settings where CD36 ligands are present. These ligands, such as oxLDL, MP and apoptotic cells are generated in vivo by oxidant stress, hyperlipidemia, inflammation and cancer; and thus, targeting CD36 and/or its signaling pathway may provide a novel approach for development of antithrombotic therapies relevant to specific high-risk states. Importantly, CD36 deficiency in humans is not rare and is not associated with any significant pathologic phenotype, including excess bleeding. Similarly, cd36-null mice are phenotypically normal under most conditions. These observations strongly suggest that targeting CD36 pharmacologically is feasible. As CD36 is expressed on many different cell types, further study of CD36 signaling in platelets is warranted to identify unique features that could be targeted to ‘spare’ the effects of blocking CD36 globally.
Executive summary.
Platelet CD36 is a receptor for oxLDL & cell-derived microparticles
Platelet CD36 activates a signaling cascade involving fyn and lyn kinase, JNK2 and Vav, resulting in platelet activation.
Platelet CD36 signaling cascade is activated in vivo by hyperlipidemia and oxidant stress.
CD36 is thromboprotective in mice
Genetic deletion of CD36 abrogates the hypercoagulable state induced by hyperlipidemia and oxidant stress and protects against formation of occlusive thrombi induced by chemical vascular injury.
CD36 deficiency is not associated with a bleeding diathesis.
Acknowledgments
Roy L Silverstein receives financial support from the National Institute of Health (grants NIH P50 HL81011 and NIH P01 HL087018).
Footnotes
Financial & competing interests disclosure: The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Kabbani SS, Watkins MW, Ashikaga T, et al. Platelet reactivity characterized prospectively: a determinant of outcome 90 days after percutaneous coronary intervention. Circulation. 2001;104:181–186. doi: 10.1161/01.cir.104.2.181. [DOI] [PubMed] [Google Scholar]
- 2.Everett CJ, Mainous AG, 3rd, Koopman RJ, Diaz VA. Predicting coronary heart disease risk using multiple lipid measures. Am J Cardiol. 2005;95:986–988. doi: 10.1016/j.amjcard.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho AC, Colman RW, Lees RS. Platelet function in hyperlipoproteinemia. N Engl J Med. 1974;290(8):434–438. doi: 10.1056/NEJM197402212900805. [DOI] [PubMed] [Google Scholar]
- 4.Shattil SJ, Anaya-Galindo R, Bennett J, Colman RW, Cooper RA. Platelet hypersensitivity induced by cholesterol incorporation. J Clin Invest. 1975;55(3):636–643. doi: 10.1172/JCI107971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shattil SJ, Cooper RA. Membrane microviscosity and human platelet function. Biochemistry. 1976;15:4832–4837. doi: 10.1021/bi00667a012. [DOI] [PubMed] [Google Scholar]
- 6.Plump AS, Smith JD, Hayek T, et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 7.Eitzman DT, Westrick RJ, Xu Z, Tyson J, Ginsburg D. Hyperlipidemia promotes thrombosis after injury to atherosclerotic vessels in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:1831–1834. doi: 10.1161/01.atv.20.7.1831. [DOI] [PubMed] [Google Scholar]; ▪ Demonstrates the value of mouse hyperlipidemia and in vivo thrombosis models to study platelet hyper-reactivity.
- 8.Schafer K, Müller K, Hecke A, et al. Enhanced thrombosis in atherosclerosis-prone mice is associated with increased arterial expression of plasminogen activator inhibitor-1. Arterioscler Thromb Vasc Biol. 2003;23:2097–2103. doi: 10.1161/01.ATV.0000097766.36623.DF. [DOI] [PubMed] [Google Scholar]
- 9.Rother E, Brandl R, Baker DL, et al. Subtype-selective antagonists of lysophosphatidic acid receptors inhibit platelet activation triggered by the lipid core of atherosclerotic plaques. Circulation. 2003;108:741–747. doi: 10.1161/01.CIR.0000083715.37658.C4. [DOI] [PubMed] [Google Scholar]
- 10.Siess W, Tigyi G. Thrombogenic and atherogenic activities of lysophosphatidic acid. J Cell Biochem. 2004;92:1086–1094. doi: 10.1002/jcb.20108. [DOI] [PubMed] [Google Scholar]
- 11.Williams JR, Khandoga AL, Goyal P, et al. Unique ligand selectivity of the GPR92/LPA5 lysophosphatidate receptor indicates role in human platelet activation. J Biol Chem. 2009;284:17304–17319. doi: 10.1074/jbc.M109.003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang G, Han J, Welch EJ, et al. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J Immunol. 2009;182:7997–8004. doi: 10.4049/jimmunol.0802884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imachi H, Murao K, Cao W, et al. Expression of human scavenger receptor B1 on and in human platelets. Arterioscler Thromb Vasc Biol. 2003;23:898–904. doi: 10.1161/01.ATV.0000067429.46333.7B. [DOI] [PubMed] [Google Scholar]
- 14.Clemetson KJ, Pfueller ST, Luscher EF, Jenkins CSP. Isolation of the membrane glycoproteins of human blood platelets by lection affinity chromatography. Biochim Biophys Acta. 1977;464:493–508. doi: 10.1016/0005-2736(77)90025-6. [DOI] [PubMed] [Google Scholar]
- 15.Robertson JO, Li W, Silverstein RL, Topol EJ, Smith JD. Deficiency of LRP8 in mice is associated with altered platelet function and prolonged time for in vivo thrombosis. Thromb Res. 2009;123:644–652. doi: 10.1016/j.thromres.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Podrez EA, Byzova TV, Febbraio M, et al. Platelet CD36 links hyperlipidemia, oxidant stress and a pro-thrombotic phenotype. Nat Med. 2007;13:1086–1095. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ ▪ Defines a role for CD36 in platelet function and demonstrates its important role in mediating a hyper-reactive state in vivo and in vivo in response to oxLDL.
- 17.Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidised low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- 18.Podrez EA, Febbraio M, Sheibani N, et al. The macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J Clin Invest. 2000;105:1095–1108. doi: 10.1172/JCI8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Podrez EA, Batyreva E, Shen Z, et al. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J Biol Chem. 2002;277:38517–38523. doi: 10.1074/jbc.M205924200. [DOI] [PubMed] [Google Scholar]
- 20.Kunjathoor VV, Febbraio M, Podrez EA, et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 21.Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and contributes to macrophage trapping in the arterial intima. J Clin Invest. 2009;119:136–145. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. CD36-dependent activation of c-Jun N-terminal kinase by oxidized LDL is required for macrophage foam cell formation. Cell Metabol. 2006;4:211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore KJ, El Khoury J, Medeiros LA, et al. A CD36-initiated signaling cascade mediates inflammatory effects of β-amyloid. J Biol Chem. 2002;277:47373–47379. doi: 10.1074/jbc.M208788200. [DOI] [PubMed] [Google Scholar]
- 24.Janabi M, Yamashita S, Hirano K, et al. Oxidized LDL-induced NF-κB activation and subsequent expression of proinflammatory genes are defective in monocyte-derived macrophages from CD36-deficient patients. Arterio Thromb Vasc Biol. 2000;20:1953–1960. doi: 10.1161/01.atv.20.8.1953. [DOI] [PubMed] [Google Scholar]
- 25.Febbraio M, Podrez EA, Smith JD, et al. Targeted disruption of the class B scavenger receptor, CD36, protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Defines a critical role for CD36 in atherogenesis.
- 26.Febbraio M, Guy E, Silverstein RL. Stem cell transplantation reveals that absence of macrophage CD36 is protective against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:2333–2338. doi: 10.1161/01.ATV.0000148007.06370.68. [DOI] [PubMed] [Google Scholar]
- 27.Guy E, Kuchibhotla S, Silverstein RL, Febbraio M. Continued inhibition of atherosclerotic lesion development in long term Western diet fed CD36°/apoE° mice. Atherosclerosis. 2007;192:123–130. doi: 10.1016/j.atherosclerosis.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 28.Kuchibhotla S, Vanegas D, Kennedy EJ, et al. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc Res. 2008;78:185–196. doi: 10.1093/cvr/cvm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knowles DM, Tolidijian B, Marboe C, Agati VD, Grimes M, Chess L. Monoclonal anti-human monocyte antibodies OKM1 and OKM5 possess distinctive tissue distributions including differential reactivity with vascular endothelium. J Immunol. 1984;132:2170–2173. [PubMed] [Google Scholar]
- 30.Oquendo P, Hundt E, Lawler J, Seed B. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell. 1994;58:95–101. doi: 10.1016/0092-8674(89)90406-6. [DOI] [PubMed] [Google Scholar]
- 31.Calvo D, Dopazo J, Vega MA. The CD36, CLA-1 (CD36L1), and LIMPII (CD36L2) gene family: cellular distribution, chromosomal location, and genetic evolution. Genomics. 1995;25:100–106. doi: 10.1016/0888-7543(95)80114-2. [DOI] [PubMed] [Google Scholar]
- 32.Hart K, Wilcox MA. Drosophila gene encoding an epithelial membrane protein with homology to CD36/LIMP II. J Mol Biol. 1993;234:249–253. doi: 10.1006/jmbi.1993.1580. [DOI] [PubMed] [Google Scholar]
- 33.Nichols Z, Vogt RG. The SNMP/CD36 gene family in Diptera, Hymenoptera and Coleoptera: Drosophila melanogaster, D. pseudoobscura, Anopheles gambiae, Aedes aegypti, Apis mellifera, and Tribolium castaneum. Insect Biochem Mol Biol. 2008;38:398–415. doi: 10.1016/j.ibmb.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Jin X, Ha TS, Smith DP. SNMP is a signaling component required for pheromone sensitivity in Drosophila. Proc Natl Acad Sci USA. 2008;105:10996–11001. doi: 10.1073/pnas.0803309105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benton R, Vannice KS, Vosshall LB. An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature. 2007;450:289–293. doi: 10.1038/nature06328. [DOI] [PubMed] [Google Scholar]
- 36.Müller WEG, Thakur NL, Ushijima H, et al. Matrix-mediated canal formation in primmorphs from the sponge Suberites domuncula involves the expression of a CD36 receptor-ligand system. J Cell Sci. 2004;117:2579–2590. doi: 10.1242/jcs.01083. [DOI] [PubMed] [Google Scholar]
- 37.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2(72):re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rac ME, Safranow K, Poncyljusz W. Molecular basis of human CD36 gene mutations. Mol Med. 2007;13:288–296. doi: 10.2119/2006-00088.Rac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoosdally SJ, Andress EJ, Wooding C, Martin CA, Linton KJ. The human scavenger receptor CD36: glycosylation status and its role in trafficking and function. J Biol Chem. 2009;284(24):16277–16288. doi: 10.1074/jbc.M109.007849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asch AS, Liu I, Briccetti FM, et al. Analysis of CD36 binding domains: ligand specificity controlled by dephosphorylation of an ectodomain. Science. 1993;262:1436–1440. doi: 10.1126/science.7504322. [DOI] [PubMed] [Google Scholar]; ▪ Demonstrates that platelet CD36 function may be regulated by post-translational modification.
- 41.Ho M, Hoang HL, Lee KM, et al. Ectophosphorylation of CD36 regulates cytoadherence of Plasmodium falciparum to microvascular endothelium under flow conditions. Infect Immun. 2005;73:8179–8187. doi: 10.1128/IAI.73.12.8179-8187.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tao N, Wagner SJ, Lublin DM. CD36 is palmitoylated on both N- and C-terminal cytoplasmic tails. J Biol Chem. 1996;271:22315–22320. doi: 10.1074/jbc.271.37.22315. [DOI] [PubMed] [Google Scholar]
- 43.Smith J, Su X, El-Maghrabi R, Stahl PD, Abumrad NA. Opposite regulation of CD36 ubiquitination by fatty acids and insulin: effects on fatty acid uptake. J Biol Chem. 2008;283:13578–13585. doi: 10.1074/jbc.M800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun M, Finnemann SC, Febbraio M, et al. Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for CD36-mediated phagocytosis by retinal pigment epithelium: a potential mechanism for modulating outer segment phagocytosis under oxidant stress condition. J Biol Chem. 2006;281:4222–4230. doi: 10.1074/jbc.M509769200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asch AS, Barnwell J, Silverstein RL, Nachman RL. Isolation of the thrombospondin membrane receptor. J Clin Invest. 1987;79:1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silverstein RL, Baird M, Yesner L. Sense and anti-sense cDNA transfection of glycoprotein IV (CD36) in melanoma cells: role of CD36 as a thrombospondin receptor. J Biol Chem. 1992;267:16607–16612. [PubMed] [Google Scholar]
- 47.Abumrad NA, el-Maghrabi MA, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993;268:17665–17668. [PubMed] [Google Scholar]
- 48.Baillie AG, Coburn CT, Abumrad NA. Reversible binding of long-chain fatty acids to purified FAT, the adipose CD36 homolog. J Membr Biol. 1996;153:75–81. doi: 10.1007/s002329900111. [DOI] [PubMed] [Google Scholar]
- 49.Pearce SFA, Wu J, Silverstein RL. Recombinant fusion proteins define a thrombospondin binding domain: evidence for a single calcium-dependent binding site on CD36. J Biol Chem. 1995;270:2981–2986. doi: 10.1074/jbc.270.7.2981. [DOI] [PubMed] [Google Scholar]
- 50.Leung LL, Li WX, McGregor JL, Albrecht G, Howard RJ. CD36 peptides enhance or inhibit CD36-thrombospondin binding. A two-step process of ligand-receptor interaction. J Biol Chem. 1992;267:18244–18250. [PubMed] [Google Scholar]
- 51.Crombie AR, Silverstein RL. Lysosomal integral membrane protein II binds thrombospondin1: evidence of functional homology with the cell adhesion molecule CD36. J Biol Chem. 1998;273:4855–4864. doi: 10.1074/jbc.273.9.4855. [DOI] [PubMed] [Google Scholar]
- 52.Crombie AR, Silverstein RL, MacLow C, Pearce SFA, Nachman RL, Laurence J. Identification of a CD36-related thrombospondin-1 binding domain in HIV-1 envelope glycoprotein gp120: relationship to HIV-specific inhibitory factors in human saliva. J Exp Med. 1998;187:25–35. doi: 10.1084/jem.187.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kar NS, Ashraf MS, Valiyaveettil M, Podrez EA. Mapping and characterization of the binding site for specific oxidized phospholipids and oxidized low density lipoprotein of scavenger receptor CD36. J Biol Chem. 2008;283:8765–8771. doi: 10.1074/jbc.M709195200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pearce SFA, Roy P, Febbraio M, Nicholson AC, Hajjar DP, Silverstein RL. Recombinant GST/CD36 fusion proteins define an oxidized LDL binding domain. J Biol Chem. 1998;273:34875–34881. doi: 10.1074/jbc.273.52.34875. [DOI] [PubMed] [Google Scholar]
- 55.Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation and lipid metabolism. J Clin Inv. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yesner LM, Huh HY, Pearce SFA, Silverstein RL. Regulation of thrombospondin and CD36 expression in human monocytes by soluble mediators. Arterioscler Thromb Vasc Biol. 1996;16:1019–1025. doi: 10.1161/01.atv.16.8.1019. [DOI] [PubMed] [Google Scholar]
- 57.Huh HY, Pearce SF, Yesner LM, Schindler JL, Silverstein RL. Regulated expression of CD36 during monocyte to macrophage differentiation: Potential role of CD36 in foam cell formation. Blood. 1996;87:2020–2028. [PubMed] [Google Scholar]
- 58.Albert ML, Pearce SFA, Francisco L, Sauter B, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to CTLs. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 60.Han J, Hajjar DP, Febbraio M, Nicholson AC. Native and modified low density lipoproteins increase the functional expression of the macrophage class B scavenger receptor, CD36. J Biol Chem. 1997;272:21654–21659. doi: 10.1074/jbc.272.34.21654. [DOI] [PubMed] [Google Scholar]
- 61.Han J, Hajjar DP, Tauras JM, Feng J, Gotto AM, Jr, Nicholson AC. Transforming growth factor-β1 (TGF-β1) and TGF-β2 decrease expression of CD36, the type B scavenger receptor, through mitogen-activated protein kinase phosphorylation of peroxisome proliferator-activated receptor-γ. J Biol Chem. 2000;275:1241–1246. doi: 10.1074/jbc.275.2.1241. [DOI] [PubMed] [Google Scholar]
- 62.Kashyap SR, Ioachimescu A, Gornik HL, et al. Lipid induced insulin resistance is associated with increased monocyte expression of scavenger receptor CD36 and internalization of oxidized LDL. Obesity. 2009 doi: 10.1038/oby.2009.179. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Griffin E, Re A, Hamel N, et al. A link between diabetes and atherosclerosis: glucose regulates expression of CD36 at the level of translation. Nat Med. 2001;7:840–846. doi: 10.1038/89969. [DOI] [PubMed] [Google Scholar]
- 64.Liang CP, Han S, Okamoto H, et al. Increased CD36 protein as a response to defective insulin signaling in macrophages. J Clin Invest. 2004;113:764–773. doi: 10.1172/JCI19528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dressman J, Kincer J, Matveev SV, et al. HIV protease inhibitors promote atherosclerotic lesion formation independent of dyslipidemia by increasing CD36-dependent cholesteryl ester accumulation in macrophages. J Clin Invest. 2003;111:389–397. doi: 10.1172/JCI16261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou J, Febbraio M, Zhai Y, et al. LXR, PXR, and PPARγ cooperate in regulating fatty acid transporter CD36 and promoting hepatic lipogenesis. Gastroenter. 2008;134:556–567. doi: 10.1053/j.gastro.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 67.Bonen A, Dyck DJ, Ibrahimi A, Abumrad NA. Muscle contractile activity increases fatty acid metabolism and transport and FAT/CD36. Am J Physiol. 1999;276:E642–E649. doi: 10.1152/ajpendo.1999.276.4.E642. [DOI] [PubMed] [Google Scholar]
- 68.Nickerson JG, Momken I, Benton CR, et al. Protein-mediated fatty acid uptake: regulation by contraction, AMP-activated protein kinase, and endocrine signals. Appl Physiol Nutr Metab. 2007;32:865–873. doi: 10.1139/H07-084. [DOI] [PubMed] [Google Scholar]
- 69.Bruni F, Pasqui AL, Pastorelli M, et al. Different effect of statins on platelet oxidized-LDL receptor (CD36 and LOX-1) expression in hypercholesterolemic subjects. Clin Appl Thromb Hemost. 2005;11:417–428. doi: 10.1177/107602960501100408. [DOI] [PubMed] [Google Scholar]
- 70.Kajihara S, Hisatomi A, Ogawa Y, et al. Association of the Pro90Ser CD36 mutation with elevated free fatty acid concentrations but not with insulin resistance syndrome in Japanese. Clin Chim Acta. 2001;314:125–130. doi: 10.1016/s0009-8981(01)00658-1. [DOI] [PubMed] [Google Scholar]
- 71.Furuhashi M, Ura N, Nakata T, Shimamoto K. Insulin sensitivity and lipid metabolism in human CD36 deficiency. Diabetes Care. 2003;26:471–474. doi: 10.2337/diacare.26.2.471. [DOI] [PubMed] [Google Scholar]
- 72.Yanai H, Chiba H, Morimoto M, Jamieson GA, Matsuno K. Type I CD36 deficiency in humans is not associated with insulin resistance syndrome. Thromb Haemost. 2000;83:786. [PubMed] [Google Scholar]
- 73.Love-Gregory L, Sherva R, Sun L, et al. Variants in the CD36 gene associate with the metabolic syndrome and high-density lipoprotein cholesterol. Hum Mol Genet. 2008;17:1695–1704. doi: 10.1093/hmg/ddn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silverstein RL, Febbraio M. CD36-TSP-HRGP interactions in the regulation of angiogenesis. Curr Pharm Des. 2007;13:3559–3567. doi: 10.2174/138161207782794185. [DOI] [PubMed] [Google Scholar]
- 75.Kaur B, Sandberg EM, Devi NS, et al. Vasculostatin inhibits intracranial glioma growth and negatively regulates in vivo angiogenesis through a CD36-dependent mechanism. Canc Res. 2009;69(3):1212–1220. doi: 10.1158/0008-5472.CAN-08-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dawson DW, Pearce SFA, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck NP. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 78.Volpert OV, Zaichuk T, Zhou W, et al. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nat Med. 2002;8:349–357. doi: 10.1038/nm0402-349. [DOI] [PubMed] [Google Scholar]
- 79.Rege TA, Stewart J, Jr, Dranka B, Benveniste EN, Silverstein RL, Gladson CL. Thrombospondin-1-induced apoptosis of brain microvascular endothelial cells can be mediated by TNF-R1. J Cell Phys. 2009;218:94–103. doi: 10.1002/jcp.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coburn CT, Knapp FF, Jr, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long-chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem. 2000;275:32523–32529. doi: 10.1074/jbc.M003826200. [DOI] [PubMed] [Google Scholar]
- 81.Drover VA, Nguyen DV, Bastie CC, et al. CD36 mediates both cellular uptake of very long chain fatty acids and their intestinal absorption in mice. J Biol Chem. 2008;283:13108–13115. doi: 10.1074/jbc.M708086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nassir F, Wilson B, Han X, Gross RW, Abumrad NA. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem. 2007;282:19493–19501. doi: 10.1074/jbc.M703330200. [DOI] [PubMed] [Google Scholar]
- 83.van Bennekum A, Werder M, Thuahnai ST, et al. Class B scavenger receptor-mediated intestinal absorption of dietary β-carotene and cholesterol. Biochem. 2005;44:4517–4525. doi: 10.1021/bi0484320. [DOI] [PubMed] [Google Scholar]
- 84.Febbraio M, Abumrad NA, Hajjar DP, et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274:19055–19062. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- 85.Glazier AM, Scott J, Aitman TJ. Molecular basis of the CD36 chromosomal deletion underlying SHR defects in insulin action and fatty acid metabolism. Mamm Genome. 2002;13:108–113. doi: 10.1007/s00335-001-2132-9. [DOI] [PubMed] [Google Scholar]
- 86.Miyaoka K, Kuwasako T, Hirano K, Nozaki S, Yamashita S, Matsuzawa Y. CD36 deficiency associated with insulin resistance. Lancet. 2001;357:686–687. doi: 10.1016/s0140-6736(00)04138-6. [DOI] [PubMed] [Google Scholar]
- 87.Corpeleijn E, van der Kallen CJ, Kruijshoop M, et al. Direct association of a promoter polymorphism in the CD36/FAT fatty acid transporter gene with Type 2 diabetes mellitus and insulin resistance. Diabet Med. 2006;23:907–911. doi: 10.1111/j.1464-5491.2006.01888.x. [DOI] [PubMed] [Google Scholar]
- 88.Greco D, Kotronen A, Westerbacka J, et al. Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1281–G12817. doi: 10.1152/ajpgi.00074.2008. [DOI] [PubMed] [Google Scholar]
- 89.Laugerette F, Passilly-Degrace P, Patris B, et al. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115:3177–3184. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van Berkel TJ, Out R, Hoekstra M, Kuiper J, Biessen E, van Eck M. Scavenger receptors: friend or foe in atherosclerosis? Curr Opin Lipidol. 2005;16:525–535. doi: 10.1097/01.mol.0000183943.20277.26. [DOI] [PubMed] [Google Scholar]
- 91.Akira A, Takeda S. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 92.Hoebe K, Georgel P, Rutschmann S, et al. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 93.Philips JA, Rubin EJ, Perrimon N. Drosophila RNAi screen reveals CD36 family member required for mycobacterial infection. Science. 2005;309:1251–1253. doi: 10.1126/science.1116006. [DOI] [PubMed] [Google Scholar]
- 94.Means TK, Mylonakis E, Tampakakis E, et al. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J Exp Med. 2009;206:637–653. doi: 10.1084/jem.20082109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smith TG, Serghides L, Patel S, Febbraio M, Silverstein RL, Kain KC. CD36-mediated non-opsonic phagocytosis of erythrocytes infected with stage I and IIA gametocyes of Plasmodium falciparum. Infect Immun. 2003;71:393–400. doi: 10.1128/IAI.71.1.393-400.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Savill J, Hogg N. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992;90:1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ren Y, Silverstein RL, Allen J, Savill J. CD36 gene transfer confers capacity for phagocytosis of cells undergoing apoptosis. J Exp Med. 1995;181:1857–1862. doi: 10.1084/jem.181.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ghosh A, Li W, Febbraio M, Espinola RG, McCrae K, Silverstein RL. Platelet CD36 mediates interactions with endothelial cell-derived microparticles and contributes to thrombosis in vivo. J Clin Invest. 2008;11:8, 1934–1943. doi: 10.1172/JCI34904. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ This paper identifies endogenous microparticles as ligands for platelet CD36 and characterizes an in vivo role during thrombus formation.
- 99.Ryeom S, Sparrow J, Silverstein RL. CD36 Participates in the phagocytosis of rod outer segments on retinal pigment epithelium. J Cell Sci. 1996;109:387–395. doi: 10.1242/jcs.109.2.387. [DOI] [PubMed] [Google Scholar]
- 100.Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar β-amyloid mediates microglial activation. J Neurosci. 2003;23:2665–7264. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.El Khoury JB, Moore KJ, Means TK, et al. CD36 mediates the innate host response to β-amyloid. J Exp Med. 2003;197:1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Greenberg ME, Li XM, Gugiu BG, et al. The lipid whisker model of the structure of oxidized cell membranes. J Biol Chem. 2008;283:2385–2396. doi: 10.1074/jbc.M707348200. [DOI] [PubMed] [Google Scholar]; ▪ This elegant paper uses sophisticated structural biology tools to characterize the oxidized phospholipid ligands for CD36.
- 103.Cho S, Park EM, Febbraio M, et al. The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. J Neurosci. 2005;25:2504–2512. doi: 10.1523/JNEUROSCI.0035-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moore KJ, Kunjathoor VV, Koehn SL, et al. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol. 2006;26:1702–1711. doi: 10.1161/01.ATV.0000229218.97976.43. [DOI] [PubMed] [Google Scholar]
- 106.Manning-Tobin JJ, Moore KJ, Seimon TA, et al. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 2009;29:19–26. doi: 10.1161/ATVBAHA.108.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Volf I, Moeslinger T, Cooper J, Schmid W, Koller E. Human platelets exclusively bind oxidized low density lipoprotein showing no specificity for acetylated low density lipoprotein. FEBS Lett. 1999;449:141–145. doi: 10.1016/s0014-5793(99)00437-8. [DOI] [PubMed] [Google Scholar]
- 108.Volf I, Roth A, Cooper J, Moeslinger T, Koller E. Hypochlorite modified LDL are a stronger agonist for platelets than copper oxidized LDL. FEBS Lett. 2000;483:155–159. doi: 10.1016/s0014-5793(00)02104-9. [DOI] [PubMed] [Google Scholar]
- 109.Göpfert MS, Siedler F, Siess W, Sellmayer A. Structural identification of oxidized acyl-phosphatidylcholines that induce platelet activation. J Vasc Res. 2005;42:120–132. doi: 10.1159/000083461. [DOI] [PubMed] [Google Scholar]
- 110.Chen K, Febbraio M, Li W, Silverstein RL. A specific CD36-dependent signaling pathway is required for platelet activation by oxidized LDL. Circ Res. 2008;102:1512–1519. doi: 10.1161/CIRCRESAHA.108.172064. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ This paper characterizes a specific intracellular signaling pathway activated in platelets by CD36.
- 111.Siess W, Zangl KJ, Essler M, et al. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci USA. 1999;96:6931–6936. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haserück N, Erl W, Pandey D, et al. The plaque lipid lysophosphatidic acid stimulates platelet activation and platelet-monocyte aggregate formation in whole blood: involvement of P2Y1 and P2Y12 receptors. Blood. 2004;103:2585–2592. doi: 10.1182/blood-2003-04-1127. [DOI] [PubMed] [Google Scholar]
- 113.Retzer M, Essler M. Lysophosphatidic acid-induced platelet shape change proceeds via Rho/Rho kinase-mediated myosin light-chain and moesin phosphorylation. Cell Signal. 2000;12:645–648. doi: 10.1016/s0898-6568(00)00108-x. [DOI] [PubMed] [Google Scholar]
- 114.Desai K, Bruckdorfer KR, Hutton RA, Owen JS. Binding of apoE-rich high density lipoprotein particles by saturable sites on human blood platelets inhibits agonist-induced platelet aggregation. J Lipid Res. 1989;30:831–840. [PubMed] [Google Scholar]
- 115.Pedreño J, de Castellarnau C, Masana L. Platelet HDL(3) binding sites are not related to integrin α(IIb)β(3) (GPIIb-IIIa) Atherosclerosis. 2001;154:23–29. doi: 10.1016/s0021-9150(00)00442-1. [DOI] [PubMed] [Google Scholar]
- 116.Assinger A, Schmid W, Eder S, Schmid D, Koller E, Volf I. Oxidation by hypochlorite converts protective HDL into a potent platelet agonist. FEBS Lett. 2008;582:778–784. doi: 10.1016/j.febslet.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 117.Chen LY, Mehta JL. Inhibitory effect of high-density lipoprotein on platelet function is mediated by increase in nitric oxide synthase activity in platelets. Life Sci. 1994;55:1815–1821. doi: 10.1016/0024-3205(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 118.Mehta JL, Chen LY. Reversal by high-density lipoprotein of the effect of oxidized low-density lipoprotein on nitric oxide synthase protein expression in human platelets. J Lab Clin Med. 1996;127:287–295. doi: 10.1016/s0022-2143(96)90097-9. [DOI] [PubMed] [Google Scholar]
- 119.Nofer JR, Walter M, Kehrel B, et al. HDL3-mediated inhibition of thrombin-induced platelet aggregation and fibrinogen binding occurs via decreased production of phosphoinositide-derived second messengers 1,2-diacylglycerol and inositol 1,4,5-tris-phosphate. Arterioscler Thromb Vasc Biol. 1998;18:861–869. doi: 10.1161/01.atv.18.6.861. [DOI] [PubMed] [Google Scholar]
- 120.Korporaal SJ, Relou IA, van Eck M, et al. Binding of low density lipoprotein to platelet apolipoprotein E receptor 2′ results in phosphorylation of p38MAPK. J Biol Chem. 2004;279:52526–52534. doi: 10.1074/jbc.M407407200. [DOI] [PubMed] [Google Scholar]
- 121.Korporaal SJ, Akkerman JW. Platelet signaling induced by lipoproteins. Cardiovasc Hematol Agents Med Chem. 2006;4:93–109. doi: 10.2174/187152506776369944. [DOI] [PubMed] [Google Scholar]
- 122.Valiyaveettil M, Kar N, Ashraf MZ, Byzova TV, Febbraio M, Podrez EA. Oxidized high-density lipoprotein inhibits platelet activation and aggregation via scavenger receptor BI. Blood. 2008;111:1962–1971. doi: 10.1182/blood-2007-08-107813. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Identifies roles for oxidized HDL and scavenger receptor BI in platelet function.
- 123.Combes V, Simon AC, Grau GE, et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest. 1999;104:93–102. doi: 10.1172/JCI4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Falati S, Liu Q, Gross P, et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197:1585–1598. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bernal-Mizrachi L, Jy W, Jimenez JJ, et al. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am Heart J. 2003;145:962–970. doi: 10.1016/S0002-8703(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 126.Shet AS, Aras O, Gupta K, et al. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–2683. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 127.Davi G, Ferroni P. Microparticles in Type 2 diabetes mellitus. J Thromb Haemost. 2005;3:1166–1167. doi: 10.1111/j.1538-7836.2005.01196.x. [DOI] [PubMed] [Google Scholar]
- 128.Koga H, Sugiyama S, Kugiyama K, et al. Elevated levels of VE-cadherin-positive endothelial microparticles in patients with Type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. 2005;45:1622–1630. doi: 10.1016/j.jacc.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 129.Jimenez JJ, Jy W, Mauro LM, Horstman LL, Ahn YS. Elevated endothelial microparticles in thrombotic thrombocytopenic purpura: findings from brain and renal microvascular cell culture and patients with active disease. Br J Haematol. 2001;112:81–90. doi: 10.1046/j.1365-2141.2001.02516.x. [DOI] [PubMed] [Google Scholar]
- 130.Dignat-George F, Camoin-Jau L, Sabatier F, et al. Endothelial microparticles: a potential contribution to the thrombotic complications of the antiphospholipid syndrome. Thromb Haemost. 2004;91:667–673. doi: 10.1160/TH03-07-0487. [DOI] [PubMed] [Google Scholar]
- 131.Gonzalez-Quintero VH, Smarkusky LP, Jimenez JJ, et al. Elevated plasma endothelial microparticles: preeclampsia versus gestational hypertension. Am J Obstet Gynecol. 2004;191:1418–1424. doi: 10.1016/j.ajog.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 132.Pihusch V, Rank A, Steber R, et al. Endothelial cell-derived microparticles in allogeneic hematopoietic stem cell recipients. Transplant. 2006;81:1405–1409. doi: 10.1097/01.tp.0000209218.24916.ba. [DOI] [PubMed] [Google Scholar]
- 133.Sims PJ, Wiedmer T, Esmon CT, Weiss HJ, Shattil SJ. Assembly of the platelet prothrombinase complex is linked to vesiculation of the platelet plasma membrane. Studies in Scott syndrome: an isolated defect in platelet procoagulant activity. J Biol Chem. 1989;264:17049–17057. [PubMed] [Google Scholar]
- 134.Sabatier F, Roux V, Anfosso F, Camoin L, Sampol J, Dignat-George F. Interaction of endothelial microparticles with monocytic cells in vitro induces tissue factor-dependent procoagulant activity. Blood. 2002;99:3962–3970. doi: 10.1182/blood.v99.11.3962. [DOI] [PubMed] [Google Scholar]
- 135.Korporaal SJ, van Eck M, Adelmeijer J, et al. Platelet activation by oxidized low density lipoprotein is mediated by CD36 and scavenger receptor-A. Arterioscler Thromb Vasc Biol. 2007;27:2476–2483. doi: 10.1161/ATVBAHA.107.150698. [DOI] [PubMed] [Google Scholar]; ▪ This paper suggests that CD36 and scavenger receptor-A may cooperate in platelet signaling in response to oxidized LDL.
- 136.Stuart LM, Deng J, Silver JM, et al. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J Cell Biol. 2005;170:477–485. doi: 10.1083/jcb.200501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lisanti MP, Scherer PE, Vidugiriene J. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol. 1994;126:111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pohl J, Ring A, Korkmaz U, Ehehalt R, Stremmel W. FAT/CD36-mediated long-chain fatty acid uptake in adipocytes requires plasma membrane rafts. Mol Biol Cell. 2005;16:24–31. doi: 10.1091/mbc.E04-07-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zeng Y, Tao N, Chung KN, Heuser JE, Lublin DM. Endocytosis of oxidized low density lipoprotein through scavenger receptor CD36 utilizes a lipid raft pathway that does not require caveolin-1. J Biol Chem. 2003;278:45931–45936. doi: 10.1074/jbc.M307722200. [DOI] [PubMed] [Google Scholar]
- 140.Kincer JF, Uittenbogaard A, Dressman J, et al. Hypercholesterolemia promotes a CD36-dependent and endothelial nitric-oxide synthase-mediated vascular dysfunction. J Biol Chem. 2002;277:23525–23533. doi: 10.1074/jbc.M202465200. [DOI] [PubMed] [Google Scholar]
- 141.Miao WM, Vasile E, Lane WS, Lawler J. CD36 associates with CD9 and integrins on human blood platelets. Blood. 2001;97:1689–1696. doi: 10.1182/blood.v97.6.1689. [DOI] [PubMed] [Google Scholar]
- 142.Triantafilou M, Gamper FG, Haston RM, et al. Membrane sorting of Toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J Biol Chem. 2006;281:31002–31011. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- 143.Nilsen NJ, Deininger S, Nonstad U, et al. Cellular trafficking of lipoteichoic acid and Toll-like receptor 2 in relation to signaling: role of CD14 and CD36. J Leukoc Biol. 2008;84:280–291. doi: 10.1189/jlb.0907656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hashimoto M, Tawaratsumida K, Kariya H, et al. Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus. J Immunol. 2006;177:3162–3169. doi: 10.4049/jimmunol.177.5.3162. [DOI] [PubMed] [Google Scholar]
- 145.Baranova IN, Kurlander R, Bocharov AV, et al. Role of human CD36 in bacterial recognition, phagocytosis, and pathogen-induced JNK-mediated signaling. J Immunol. 2008;181:7147–7156. doi: 10.4049/jimmunol.181.10.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wilkinson B, Koenigsknecht-Talboo J, Grommes C, Lee CY, Landreth G. Fibrillar β-amyloid-stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem. 2006;281:20842–20850. doi: 10.1074/jbc.M600627200. [DOI] [PubMed] [Google Scholar]
- 147.Finnemann SC, Silverstein RL. Differential roles of CD36 and αvβ5 integrin in photoreceptor phagocytosis by the retinal pigment epithelium. J Exp Med. 2001;194:1289–1298. doi: 10.1084/jem.194.9.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pijuan-Thompson V, Grammar JR, Stewart J, et al. Retenoic acid alters the mechanism of attachment of malignant astrocytoma and neuroblatoma cells to TSP-1. Exp Cell Res. 1999;249:86–101. doi: 10.1006/excr.1999.4458. [DOI] [PubMed] [Google Scholar]
- 149.Stuart LM, Bell SA, Stewart CR, et al. CD36 signals to the actin cytoskeleton and regulates microglial migration via a p130Cas complex. J Biol Chem. 2007;282:27392–27301. doi: 10.1074/jbc.M702887200. [DOI] [PubMed] [Google Scholar]