Abstract
Tumor metabolism consists of complex interactions between oxygenation states, metabolites, ions, the vascular network and signaling cascades. Accumulation of lactate within tumors has been correlated with poor clinical outcomes. While its production has negative implications, potentially contributing to tumor progression, the implications of the ability of tumors to utilize lactate can offer new therapeutic targets for the future. Monocarboxylate transporters (MCTs) of the SLC16A gene family influence substrate availability, the metabolic path of lactate and pH balance within the tumor. CD147, a chaperone to some MCT subtypes, contributes to tumor progression and metastasis. The implications and consequences of lactate utilization by tumors are currently unknown; therefore future research is needed on the intricacies of tumor metabolism. The possibility of metabolic modification of the tumor microenvironment via regulation or manipulation of MCT1 and CD147 may prove to be promising avenues of therapeutic options.
Keywords: Cancer, CD147, lactate, MCT1, MCT1 inhibitors, MCT4, tumor metabolism
Much of the past research on lactate in cancer has focused on its production. Under the influence of hypoxia, glucose is catabolized to lactate via glycolysis to yield ATP; mitochondria cannot produce ATP in oxidative phosphorylation without oxygen to aid in electron transport. This type of production is termed the ‘Pasteur effect.’ Cancer cells can also produce lactate under aerobic conditions. The switch from a ‘normal’ metabolic profile, consisting of glycolysis followed by oxidative phosphorylation, to cytosolic glycolysis in the presence of adequate oxygen, is termed the ‘Warburg effect.’ The discovery of aerobic glycolysis resulting in increased lactate production within tumors in the 1920–1930s by Otto Warburg led to a reevaluation of the potential role and significance of lactate in cancer studies [1–4]. Since the pioneering work of Warburg, production and accumulation of lactate has been documented to have clinical significance in a number of different cancer types. In the 1970s, lactate metabolism in malignant metastatic carcinomas and colorectal cancers was beginning to be explored with blood samples from patients that were injected or infused with radio-labeled glucose and pyruvate [5,6]. These studies indicated that lactate levels in plasma and venous blood were elevated in patients with metastatic cancer [6]. Nuclear magnetic resonance (NMR) studies following the fate of 13C-labeled lactate are still used today.
In the 1980s, quantitative bioluminescence imaging was used to assess metabolites such as lactate and glucose from flash-frozen biopsies to study ischemia in brain tissue [7,8]. This technique was later applied to assess these same metabolites in cancer [9–11]. More recent studies investigating lactate accumulation in human tumors have found that cervical tumors, head and neck cancers and rectal adenocarcinomas, with metastatic spread, exhibited a wider range and significantly higher levels of lactate than nonmetastatic tumors [12–14]. Subsequently, it was found that elevated lactate levels correlate with poorer patient prognosis, poor disease-free or metastasis-free survival and poor overall survival in human cervical cancers [15], head and neck cancer [16], high-grade gliomas [9,17,18] and non-small-cell lung cancer [19] (Table 1). Mean lactate content can vary widely between individual tumors, even if tumors are of the same size, grade or entity [13,15]. The reason and mechanisms behind this variation are still poorly understood; further research on monocarboxylate transporters’ (MCTs’) functions and interactions within the tumor microenvironment could help to explain this variation. Elucidating the intricacies of lactate metabolism and MCT interaction, we may have the potential to modify the metabolism of the tumor. Coaxing tumors to utilize lactate (decreasing its accumulation) or finding ways to reduce glycolysis and lactate formation may affect tumor aggressiveness and invasiveness. In summary, lactate has been demonstrated to be a prognostic indicator across a wide variety of tumors and types of cancer; this feature makes lactate metabolism of interest for further investigation, not only as a biological marker, but also as a potential therapeutic end point or target.
Table 1.
MCT1, MCT4, CD147 expression and lactate levels within tumors.
| Type of cancer | MCT1 expression | MCT4 expression | CD147 expression | Lactate levels |
|---|---|---|---|---|
| Cervical | ↑ From preinvasive to invasive [44] | ↑ From preinvasive to invasive [44] | ↑ With metastases [12], poor DFS, OS [15] | |
| Head and neck | (+) [Wergin M, Kennedy K, Dedeugd C, Dewhirst MW. Duke University Medical Center, NC, USA. Unpublished data] | (+) [Wergin M et al. Unpublished data] | ↑ With metastases [13], poor DFS, OS [16]. HMRS studies: lactate SI did not correlate with tumor pO2, treatment response or locoregional response [167] | |
| Colorectal | (+) In tumor cells in samples tested [37], ↑ in tumor cells compared to normal epithelium [36] (−) Or loss from normalcy to malignancy [123] | Weak (+) in tumor cells [37], ↑ in tumor cells compared to normal epithelium [36] | (+) Increased cell death with silencing [165] | ↑ With metastases [14] |
| Liver | Hepatocellular carcinoma: (+), correlated with ↑ invasiveness [168], multidrug resistance [143] and ↑ MMP (?) | Utilization in rat hepatomas [84,85] | ||
| Melanoma | (+) [161] | (+) [161] | ↑ Correlated with ↑ invasiveness and ↑ MMP production [140], linked to metastasis [160], increased cell death with silencing [165] and decreased VEGF with silencing [161] | |
| Breast | ↓ In MDA-MB-231 and some clinical samples by hypermethylation of CGI upstream [121] | ↑ In MDA-MB-231 and metastatic breast cancer [40] | ↑ With multidrug resistance, in vitro invasiveness [164], histopathological risk factors [144] and resistance to anoikis [145] | |
| Prostate | ↑ Correlates with ↑ invasiveness [163] +/↑ correlative to TNM stage, histological subtype and poor prognosis, independent prognostic factor [169,170] | |||
| Brain | (+) In gliomas, silencing leads to cell death [114] | ↑ In gliomas [7,9]. Utilization in rat glioma [57,86,88] | ||
| Esophageal, ESSC | ↑ With ↑ MMP2 resulted in poor prognosis in ESCC [130] | |||
| Lung | (+) In tumor cells, (−) in normal cells [125], cytoplasmic accumulation in alveolar soft-part sarcoma [171] | (+) In tumor cells, (−) in normal [125] | Cytoplasmic accumulation in alveolar soft part sarcoma [171] | [Lactate] significantly ↑ in patients with recurrence, and correlates with poorer survival in men [19] |
| Gallbladder carcinoma | ↑ Levels predict for poor prognosis and overall survival [141] | |||
| Bladder | (+)/↑ Correlates with TNM stage, histological subtype and poor prognosis, Independent prognostic factor [170] | |||
| Renal | +/↑ Correlates with TNM stage, histological subtype and poor prognosis, Independent prognostic factor [170] | |||
| OSSC | ↑ In OSCC compared to normal, linked with ↑ multidrug resistance [172] | |||
| Pancreatic | (+) [39] | (+) [39] | ↑ Compared to normal tissue, Expression in tumor and stromal cells [173] (+), linked to invasiveness and tumorigenic potential [39] | |
| Ovarian | (+)/↑ Confers invasiveness [162] | |||
| Neuroendocrine | ↑ In neuroblastoma [150] |
The (+) sign indicates that the protein has been shown experimentally to be expressed (positive for expression), but information regarding whether it is upregulated as compared to normal tissue was not found. Likewise, the (−) sign indicates that there was found to be no expression (negative for expression), as opposed to downregulation. ↑: Increase; ↓: Decrease.
CGI: CpG island; DFS: Disease-free survival; ESCC: Esophageal squamous cell carcinoma; MMP: Matrix metalloproteinase; OS: Overall survival; OSSC: Oral squamous cell carcinoma; TNM: Tumor node metastasis.
Characteristics of the tumor microenvironment
Tumor microenvironment pathology is influenced by several key physiological factors: oxygen transport, vascular structure/perfusion, nutrient and metabolite transport and pH. Oncogenic and tumor suppressor genomic regulation also greatly contribute to the phenotype [20,21], but will not be emphasized in this review. Features of tumor physiology that affect oxygen transport can be summarized in ‘seven points of regulation’, as described by Dewhirst et al. [22]. Compared to normal tissues, these points are:
The steep longitudinal oxygen gradients within the tumor;
Increased intravascular gradients;
Shunt flow in the vascular structure to evade the tumor tissue;
Lower vascular density;
Inefficient and unorganized vascular orientation and structure;
Imbalance between oxygen consumption and delivery rates;
Slower blood flow due to increased blood viscosity from intravascular hypoxia [22].
While these effects develop gradually, temporal oxygen fluctuations can occur in a matter of minutes [23,24].
A consequence of tumor hypoxia is the upregulation of the α-subunit of the heterodimer hypoxia-inducible transcription factor (HIF-1). Both the overlying physiological and the underlying temporal influences promote hypoxia; in turn, HIF-1α is stabilized [25]. HIF-1α participates in genetic regulation and signaling cascades that control angiogenesis, invasiveness, oxidative stress resistance, treatment-resistance and the glycolytic switch to anaerobic metabolism [22,26].
The physiological influences mentioned above, paired with upregulation of genetic factors that promote glycolysis, lead to altered ionic and metabolic gradients within the tumor [27]. Spatial relationships between vasculature, ATP and metabolites exist within the tumor; lactate accumulation often occurring within areas of hypoxia and/or necrosis [28]. Metabolic conditions exacerbate and induce prosurvival pathways through transcriptional or translational regulation of tumor suppressor genes and oncogenes. This can result in cancer cell proliferation due to loss of proapoptotic factors and an increase in prosurvival and growth signaling [29].
What has become more apparent in the last few decades of metabolism research is that lactate is more than merely a byproduct of glycolysis; it has the ability to influence angiogenesis, expression of surface molecules, genetic response, ATP generation and cell migration.
Lactate metabolism introduction
There are five requisite conditions for lactate oxidation:
Lactate must enter the cell or be produced internally by the cell;
The movement of lactate in (and out) of the cell requires a membrane-bound monocarboxylate transporter, such as MCT1;
Lactate dehydrogenase (LDH) is required to convert lactate to pyruvate before it can enter into the tricarboxcylic acid (TCA) cycle;
Adequate concentration of oxygen is required – lactate cannot be consumed anaerobically;
Healthy, functional mitochondria are required to carry out the TCA cycle and the electron transport chain (ETC).
Beyond this, there are other secondary influences on lactate metabolism. For example: pyruvate dehydrogenase complex (PDC) regulation by phosphorylation via pyruvate dehydrogenase kinase (PDK); PDK inhibitors/phosphatases determine if pyruvate is converted to acetyl coA for the first step of TCA cycle; and regulation of glycolysis via phosphofructokinase (PFK) and other glycolytic enzymes or intermediaries will contribute to overall cell metabolism. Other factors to mention are pH, ratios of ATP:ADP, AMP:cAMP and NAD+:NADH, and 5′ adenosine monophosphate-activated protein kinase (AMPK) and inorganic phosphate (Pi) levels [30,31]. Lactate utilization can encompass oxidation for energetic purposes, but utilization also includes amino acid production or shuttles for anabolic purposes.
Monocarboxylate transporter family
Proton-coupled monocarboxylate transporters are 12-span transmembrane proteins with N-terminus and C-terminus in the cytosolic domain. While there are 14 members/subtypes of MCTs coded by the SLC16A gene family, not all of these are currently characterized [32]. MCTs account for 70–90% of muscle lactate transport [33]. While it could be said that the distinguishing common feature among members of this family was the transport of monocarboxylates (lactate, pyruvate, butyrate, acetate and propionate), it has been found that certain subtypes have the ability to transport a wider range of substrates such as ketone bodies, some aromatic amino acids, and even some pharmacological agents, such as γ-hydroxybutyrate (GHB) and statins, among others [34,35]. Other members, MCT6, MCT8 and MCT10, will transport substrates such as diuretics, thyroid hormones T3 and T4, and aromatic amino acids, respectively [32,34].
Of the characterized family members of SLC16A, MCT1 is reported to have the most ubiquitous tissue expression [32] (Table 2). Other subtypes demonstrate tissue-specific expression. Expression and regulation of MCT1, MCT2, MCT3 and MCT4 have been reported in a wide variety of cancers [36–44]. For brevity and clarity, we will be addressing the subtypes of proton-coupled MCTs 1–4 best characterized in human tissue, largely focusing on MCT1 and MCT4 with only passing mention of MCT2 and MCT3 when relevant.
Table 2.
MCT1 and MCT4 expression tissue distribution.
| Tissue expression/distribution | High expression | |
|---|---|---|
| MCT1 | Ubiquitous | Heart, oxidative skeletal muscle |
| MCT4 | Skeletal muscle, chondrocytes, leukocytes, testis, lung, heart, placenta, skin, retina, brain | Glycolytic skeletal muscle, astrocytes, leukocytes |
Sodium-coupled MCTs (SMCTs) of the SLC5A gene family were discovered in the early to mid 2000s, namely SLC5A8 [45,46] and SLC5A12 [47]. SMCTs transport similar substrates as many of the SLC16A MCT family members; however, they show differences in patterns of tissue distribution and their potential roles in cancer or cancer progression [34,48]. SLC5A members will be mentioned when relevant, but the focus for this review will be SLC16A MCTs.
Lactate consumption in normal tissue
Studies on direct utilization of lactate in normal cells can provide a window into the potential metabolic processes in cancer cells; therefore, we will briefly explore lactate metabolism in normal tissue. Lactate movement and metabolism as participating in a cell-to-cell shuttle system was first intimated by the work of Carl and Gerty Cori with their discovery of the Cori cycle, a cycle in which lactate picked up from the bloodstream by the liver undergoes gluconeogenesis [49]. Later, in 1984, Brooks initiated the new wave of lactate shuttle research [50]. The basic observations of lactate transport and utilization were first studied in muscle. From here, as Gladden summarizes [51], the lactate shuttle theory has grown to include the cell-to-cell lactate shuttle within muscle, intracellular lactate shuttle [52–54], the astrocyte–neuron lactate shuttle [55–58], a lactate–alanine shuttle [59,60], a peroxisomal lactate shuttle [61] and the spermatogenic lactate shuttles [62].
Lactate utilization in muscle
Muscle cells have the ability to produce and export lactate, as well as import and directly utilize lactate. During exercise, myocytes, which normally participate in oxidative phosphorylation, will begin to undergo glycolysis as the energy demand increases and oxygen supply decreases. Cells will export the accumulated intracellular lactate concentrations. Lactate will filter into the bloodstream and enter the liver, primarily, to undergo the Cori cycle for gluconeogenesis [31,49]. What is of more interest for this review is the ability of myocytes to directly consume lactate.
Myocytes are efficient in their ATP generation, utilizing glucose, lactate, fatty acids and ketone bodies as energy sources. Briefly, the muscle–lactate shuttle occurs when lactate generated and excreted by exercising muscle is taken up by other muscle cells via bloodstream delivery, or directly by nearby resting muscle. Of the lactate generated during exercise, 55–75% will be oxidized by muscle [63]. Muscle cells express both MCT1 and MCT4, although the expression pattern and regulation in tissue is isoform-specific. Expression of MCT1 (Km~3.5 mM for lactate) is significantly higher in heart [64] and oxidative muscle fibers compared with glycolytic muscle fibers, and strongly correlates with lactate uptake [65], while MCT4 (Km~34 mM for lactate) expression is seen primarily in glycolytic muscle [64]. Lactate accounts for up to 40% of oxidative substrate utilized by the heart at rest, and this portion climbs to 60% with work [66]. Experiments in rabbits demonstrate that oxidative myocytes take up and oxidize lactate at 2.5 mM, while glycolytic myocytes use lactate for gluconeogenesis and will not take it up until the concentration reaches 4 mM [67]. Other studies have demonstrated that lactate transport capacity in slow twitch muscle fibers is nearly twice that of fast twitch fibers [33].
Tumor cells may demonstrate similar heterogeneity in the ability to take up and utilize lactate; some tumor types may take up lactate as a threshold mechanism at high lactate concentrations, while others may be avid consumers, utilizing as little or as much lactate as they are exposed to without the requirement for high concentrations to force uptake [68]. Similar to muscle, this may depend on which MCTs are expressed on the cell surface.
Lactate uptake rates
Many of the Km values cited for MCT sub-type 1 and 4 are relatively consistent among the literature. Determination of Km values for lactate uptake can be found in studies conducted with Xenopus laevis oocytes. In vitro, oocytes expressing MCT1 demonstrated a rate of 828 ± 79 pmol/10 min for uptake of 5 mM lactate [69]. In a separate study, MCT4-expressing oocytes took up lactate at a rate of 323 ± 25 pmol/10 min per oocyte when incubated with 1 mM lactate, which is still higher than oocytes without MCT4 expression (60 ± 3 pmol/10 min) [70]. When comparing one MCT subtype to another, many studies use their Km or Vmax values as a basis for comparison. As stated above, MCT1 as well as MCT2 both have a lower Km than MCT4 for lactate, illustrating that subtye 1 and 2 have a greater affinity for lactate than MCT4 [32]; however, the affinity for lactate in MCT4 can be increased (and the Km decreased) by exposure to lower pH values. When X. laevis oocytes were incubated in a pH of 7, 6 and 5 the MCT4 Km value for lactate dropped from 34 ± 5 mM to 10 ± 2 mM to 1.4 ± 0.5 mM, respectively [70].
Lactate utilization in brain
Although glucose was thought of as the primary energy substrate of normal, healthy brain tissue, recent studies have found that brain tissue is an avid consumer of lactate, with the capability of taking up lactate from the bloodstream after exercise, as well as direct consumption by neurons from astrocytic production of lactate [71]. Neurons account for approximately 90–95% of energy consumption as compared to glial cells, and there is evidence that lactate is a preferential substrate to glucose by neurons [72]. Lactate and glucose administered in equimolar concentrations in vitro are utilized for neuronal oxidative metabolism, 90% (direct lactate) and 10% (glucose-derived pyruvate), respectively [57]. In a metabolic model within the brain, astrocytes, which express MCT1 and MCT4, undergo aerobic glycolysis, exporting a considerable amount of lactate into the extracellular space. The lactate is then taken up to be used as an energy source by neighboring neurons via the lactate transporter, MCT2 (Km for lactate ~0.7 mM) [32] expressed on the surface [73].
Not all lactate is of equal energetic value. Studies in the early 2000s have demonstrated that neurons in culture have a kinetic preference for extracellular lactate over intracellular lactate (or pyruvate) generated via glycolysis from previously acquired glucose [74]. This supports a two-compartmental theory of lactate utilization (discussed in more detail below). To support the assertion that lactate is being used as an energy substrate and not merely shuttled back out of the cell, NMR studies in neurons have demonstrated the output of labeled TCA cycle intermediates and amino acids following administration of 13C-lactate [60]. Further evidence for lactate as a preferential substrate for brain comes from in vivo studies with NMR, demonstrating decreased utilization of glucose after raising plasma lactate levels [75,76].
Lactate in wound healing
Induction of cell proliferation and intracellular oxidant production are seen in fibroblasts exposed to exogenous lactate during wound healing [77]. This induction of oxidants may be due to increased oxidation of lactate through oxidative phosphorylation, generating the free radicals and ATP necessary to meet the energetic demands of wound healing. During wound healing, lactate functions to stimulate production of VEGF, as well as a number of cytokines and growth-promoting factors that assist in angiogenesis and collagen synthesis [78].
The growth-promoting effects of lactate that are seen in wound healing likely occur within the tumor environment as well; lactate may act as a growth or survival signal to the surrounding stromal fibroblasts in tumors with lactate accumulation.
Lactate utilization in tumors
Although a majority of studies on lactate in relation to cancer have predominantly focused on its presence and accumulation, a number of studies have found that tumor cells have the ability to take up lactate and utilize it for energetic purposes as well as amino acid formation. Lactate uptake is not to be confused with its metabolism to generate ATP, as it is possible that the cell can take up lactate that may then participate in the lactate–alanine shuttle or another mechanism that is not related to energetic metabolism. Here we will focus on its uptake and metabolism with mention of the lactate–alanine shuttle when appropriate. Lactate uptake occurs in vitro in cervical cancer SiHa cells [68] and breast cancer MDA-MB-231 cells in a pH-dependent manner [79]. If lactate uptake in tumor tissue is similar to muscle, areas with increased blood flow would show greater uptake [80]. While muscle and brain tissue may be able to make use of lactate as an efficient substrate, the microenvironment of a tumor often does not have adequate oxygenation or an efficient and functional vascular network. Therefore, tumor utilization of lactate is dependent upon oxygen availability, lactate concentrations, presence of healthy mitochondria and appropriate MCT subtype expression for lactate uptake [50,81].
In 1991, metabolic studies were conducted on EMT6/Ro multicellular spheroids. A benefit of this method is that the spheroid mimics tumor micro-areas in vivo. Since the cells are not grown in a monolayer, they are exposed to varying degrees of oxygenation and nutrient availability based on their spatial distribution. Metabolic data on glucose and lactate levels were measured with Boehringer Mannheim enzymatic tests after spheroids were subjected to progressively increasing concentrations of lactate under conditions of 20% O2 and 5% O2. Cells showed lactate consumption at the highest lactate concentration (20 mM) tested in both oxygenation states [82]. Other experiments in this same study demonstrated a depressed cell growth rate when exposed to high lactate as compared to glucose, but yet the viable rim of the spheroids showed high metabolic activity and O2 consumption in the presence of high lactate concentrations [82]. Likewise, studies conducted in the 1970s found that three strains of Ehrlich ascites tumor cells oxidized exogenous 14C-labeled lactate resulting in incorporation of 14C in amino acids, acetate and CO2 to varying proportions among the strains [83]. Approximately a third of a series of 29 rat hepatomas were demonstrated to utilize lactate based on calculations of arteriovenous substrate differences. The production or utilization of lactate was dependent on arterial lactate concentration and not arterial glucose concentration or glucose utilization [84,85]. These results demonstrated that tumor utilization of lactate occurred when the arterial lactate concentrations rose above 2–3 mM [85]. The ‘metabolic fate’ of the lactate removed from the arterial blood was not investigated in this particular study, and the mechanisms underlying this observation were not fully understood at the time.
Later studies with in vitro glioma cell lines and in vivo rat gliomas demonstrated that exogenous lactate was found to be a major source for oxidative metabolism in spite of net lactate production from aerobic glycolysis [86,87]. In vitro studies using NMR with [3-13C]L-lactate and [1-13C]D-glucose in C6 glioma cells were conducted. Cells were incubated with either 5.5 mM of labeled glucose, 11 mM of labeled lactate or a combination of 5.5 mM of labeled glucose with 11 mM of unlabeled lactate for 4 h before NMR samples were collected. Accounting for the fact that [1-13C] D-glucose metabolizes to 1 M of unlabeled lactate and 1 M of [3-13C]L-lactate, they found there was a high yield of glucose conversion to lactate [86]. From the NMR spectrum, peaks corresponding to 3C alanine and 4C glutamate were also seen; the former indicating [3-13C]L-lactate conversion to alanine, and the latter indicating [3-13C]L-lactate entering the TCA cycle to produce glutamate. Comparing the results from the dishes with lone-labeled substrates, they found a high yield of 13C incorporation into glutamate when starting from [3-13C]L-lactate [86]. Additionally, their findings suggest that lactate was a better precursor for alanine and glutamate than glucose [86]. From these results, we can conclude that certain tumors have the ability to utilize the lactate they take up for either energetic metabolism, as indicated by the labeled glutamate metabolite, or for amino acid formation, as indicated by the labeled alanine metabolite.
Based on observations that lactate production and utilization can occur simultaneously and/or shift from net lactate production to net lactate uptake based on the external lactate concentrations [82,86], a two-compartmental theory was proposed. Briefly, it described two separate metabolic pools of both lactate and pyruvate within the cell; one pool of lactate being a result of cytosolic glycolysis that is separate from oxidative metabolism, and the other pool being the imported exogenous lactate to be used for oxidative phosphorylation [86,88]. Using NMR, results from a 2001 study in perfused rat hearts support the idea of lactate compartmentalization. Glycolytically-derived lactate efflux can occur simultaneously with exogenous labeled lactate uptake, demonstrating that these metabolic pathways function separately [89].
These studies beg the question of what effects lactate utilization will have on tumor survival, aggressiveness and clinical profile. If accumulation of lactate correlates with increased metastasis and poor disease-free and overall survival [9,12–16,18,19], does the ability of the tumor to utilize lactate contribute to its survival, furthering cancer progression? Or does the ability to utilize lactate reduce the accumulation of lactate, and, in turn, lessen the negative effect of a hostile tumor environment? Does the ability to utilize lactate indicate a less aggressive tumor that shows a more ‘normal’ metabolic ability, namely, using available oxygen as opposed to undergoing the ‘Warburg effect’? Or will the increased oxygen consumption necessary for lactate oxidation ultimately lead to a more hypoxic tumor with increased reactive oxygen species? Of course, lactate may not be utilized for oxidation or energetic purposes; it may contribute to amino acid production through transamination reactions. In this case, will it serve to promote tumor survival, providing the tumor with the building blocks of protein synthesis? These are important questions in the field of cancer metabolism – ones that strongly encourage more research on the intricacies of tumor metabolism.
Lactosis versus acidosis
Lactate and acidity are concepts that are often assumed as a singular phenomenon. Although the molecule is often referred to as ‘lactic acid,’ lactate exists primarily in ionic form within the body at physiological pH, with over 99% of it being dissociated from the proton [51]. Upon transport, the proton and lactate anion are associated as they cross the cell membrane by MCTs [80]. Although MCTs have the ability to contribute to pH regulation, there are other transporters on the cell surface that specialize in pH regulation, such as the vacuolar proton pump (V-ATPase), sodium-proton exchangers (NHE1) and bicarbonate transporters (BCT). Many of these pH-regulatory transporters undergo expression or regulatory changes with malignant transformation, contributing to enhanced growth signaling, motility and multidrug resistance [90]. Low extracellular pH (pHe) within the tumor cannot be primarily attributed to lactate or the ionic transport of MCTs. Table 3 provides a summary of the relevance of pH regulators to the tumor microenvironment, including their basic functions, expression in tumors, influences on cell migration and downstream targets, and a brief reference to potential inhibitors.
Table 3.
pH regulators relevant to tumor progression.
| pH regulator* | Function/mechanism | Expression in tumors | Cell migration | Associated/regulated molecules | Inhibitors |
|---|---|---|---|---|---|
| Na+/H+ exchanger: NHEs (1) | Regulates pHe and pH Expels H+ from cytosol, promotes cell polarity [174] necessary for cell proliferation and stable cell volume [90] | Antiapoptotic activity [90] | ↑ Invasiveness of breast carcinoma cells [174,175], required for migration, accumulates on leading edge of lamellipodium [174,176] → tumor cell pseudopodia | MMP activity dependent on NHE1 activity [174] Ras-mediated ERK, G protein-coupled receptors, PKC, RhoA and integrin receptors regulate NHE1 activity [90] | Amiloride and derivatives Guamidine and derivatives |
| Bicarbonate transporters: BCTs: SLC4 = Na+-HCO3− cotransporters (NBCs 1–4), Cl−/HCO3− exchangers (NCBE) and anion exchangers (AE1–4) SLC26 | Regulates pHi and cell volume ↑ in NHE1-deficient cells [174,177] | NBC has less influence on cell migration compared with NHE1, but will contribute to migration during acute intracellular acid load [177] | Coexpression with CA9 for AE1–3 increases AE-mediated bicarbonate transport [178] | ||
| Carbonic anhydrases: CAs (9 and 12) | Extracellular production of HCO3− is transported into cytosol H+ ions remain at cell surface, ↓ pHe [174] | CA 9 ↑ in solid tumors, ↑ tumor cell growth [174] | CA9 regulated by HIF-1 [174] | ||
| Monocarboxylate transporters: SLC16A = MCTs (1 and 4) | Facilitated passive H+ transport with monocarboxylates | Refer to Table 1 | MCT4 colocalized with β1 integrin at focal adhesions, expressed on leading edge of migrating cells [153,174] | CD147 chaperone MCT4 regulated by HIF-1 | Aromatic monocarboxylates Anion transport inhibitors Bioflavenoids Others [90] |
| V-ATPases | H+ pump, cytoplasmic pH homeostasis Endocytosis, intracellular transport [90] | ↑ In multidrug resistant cells [90] | C subunit interacts with β1 integrin [90] | E subunit interacts with mSos-1 → Ras and Rac-1 signaling [90] |
Names of pH regulators are given in normal text, bold typeface denotes abbreviations, italicizing denotes a gene family, and relevant isoforms or subtypes are given in parentheses.
↑: Increased; ↓: Decreased; MCT: Monocarboxylate transporter; MMP: Matrix metalloproteinase; NBC: Sodium-bicarbonate cotransporter; NHE1: Na+/H+ exchanger; PKC: Protein kinase C.
Glycolysis-generated lactate itself is not inherently a source of acidity; acidification can occur without glycolysis. Two different studies were conducted comparing LDH-competent verses LDH-deficient cell lines [91] and glycolysis-competent verses glycolysis-impaired ras94− and 7ras3− cell lines [92] in both in vitro and in vivo systems. The non-lactate producing cells from both studies showed acidification of the culture media and tumor. The drop in pHe tended to be greater in the LDH-competent cells than the LDH-deficient cells although this finding was not statistically significant. However, the amount of 14CO2 produced from either 14C glucose or 14C alanine was significant when comparing the cell lines; the LDH-deficient cells, showed a significantly greater fraction of their 14CO2 production from 14C alanine rather than from 14C glucose [91]. In vivo, the pHe of the tumor tissue was similar for both LDH-competent (7.03 ± 0.03) and LDH-deficient (7.03 ± 0.05) tumors, despite the wide disparity in lactate content (1877 ± 135 vs 488 ± 91 μg/g wet weight, respectively) [91]. Studies with the glycolysis-impaired ras94− and 7ras3− cells demonstrated similar results to the LDH-deficient cells in regards to media acidification. The H3O+:lactate ratio was significantly higher in the glycolysis-impaired cell lines, indicating that there was a source of acidity besides lactate. In vivo studies with tumors of these cell lines demonstrated a mean interstitial pH that was not significantly different between the glycolysis-competent verses glycolysis-impaired. After measuring metabolites in the tumor interstitial fluid of parental and glycolysis-impaired tumors, it was found that lactate levels were 28% lower in the glycolysis-impaired tumors, although CO2and pH levels were comparable with the parental tumors [92].
Implicated in the increased acidification of the abovementioned studies was increased levels of CO2 [92], as hydrated CO2 generates bicarbonate (HCO3−) and H+ [93]. Briefly, a recent review highlights three sources of CO2 that can lead to acidification the extracellular milieu. CO2 can be generated from the TCA cycle since one carbon atom that passes through will generate one molecule of CO2. The pentose phosphate shunt is another pathway that generates CO2. Finally, as indicated at the beginning of this paragraph, titration of bicarbonate with protons can lead to increased CO2 [93]. This discussion is expanded and detailed in a review by Swietach et al. [93]. Carbonic anhydrase (CA) catalyzes this reaction; there are 14 mammalian isoforms. Certain CA isoforms, such as 9 and 12, are increased in many cancers and are HIF-1α-regulated [94]. Bicarbonate transport has been found to be increased through CA colocalization and interaction with some bicarbonate transporters [95,96], which is partially regulated by protein kinase C (PKC) [97]. Additionally, when a sodium-bicarbonate cotransporter (NBC) was expressed together with MCT1 in Xenopus oocytes, the transport of lactate and H+ by MCT1 increased almost twofold, suggesting that the buffering capability of NBC reduced H+ intracellular accumulation, which would normally hinder MCT1 activity [98]. It is clear that no one transporter is responsible for cellular pH regulation, and not only can many different transporters fulfill the same function, they can also assist or enhance the activity of other transporters.
Although lactate accumulates within tumors via the Pasteur effect, the cellular response to hypoxia is not the same as with lactosis or acidosis. Both hypoxia and accumulated lactate show correlation to more aggressive cancers, poor patient prognosis and disease-free and overall survival [99–101]. However, in studies that carefully controlled for conditions of hypoxia, (nonlactate) acidosis, lactosis and lactic acidosis, the range of cell responses was intriguing. A strong lactic acidosis genomic response has been found to not only negatively correlate with a hypoxic response score, but additionally the lactic acidosis response predicts a favorable prognosis in a series of clinical breast cancer trials [102]. Found within the same data set, the cellular response to high lactic acidosis was upregulated genes associated with aerobic metabolism, including factors involved in the TCA cycle and electron transport, while the response for low lactic acidosis showed an upregulation in genes associated with matrix metalloproteinases (MMP) [102], enzymes implicated in many aggressive cancers that are also induced by HIF [103].
MCT1 & MCT4 expression & regulation
The characteristics of these two transporters are being covered together since they share some common traits. Regulation is summarized in Figure 1. It is known that the function of both transporters is dependent upon interactions with other proteins, such as the chaperone CD147 [32]. Many studies have demonstrated the tight association of CD147 with MCT1 and MCT4 with coimmunoprecipitation, and immunohistochemical techniques have demonstrated co-expression on the plasma and mitochondrial membranes [104–107]. MCT1, MCT3 and MCT4 depend on association with the mature, glycosylated form of CD147 in order for these MCTs to be expressed and functional on either plasma or mitochondrial membranes [107,108]. Conversely, it appears that CD147 maturation is affected by MCT expression. In MCT1, knockdown experiments in Caco-2 cells, accumulation of the immature, core-glycosylated form and disappearance of the mature glycosylated form occurred with no difference in the mRNA [107]. In other experiments, abrogation of fully glycosylated of CD147 was observed and the core-glycosylated form was restricted to the endoplasmic reticulum when MCT4 siRNA was added to MDA-MB-231 cells [40].
Figure 1. Monocarboxylate transporter 1 and 4 (MCT1 and MCT4) regulation.
Blue boxes indicate upregulation of the specific MCT subtype while green boxes indicate a downregulation. There is no distinction between transcriptional versus translational regulation.
HIF: Hypoxia-inducible transcription factor; MCT: Monocarboxylate transporter; NO: Nitric oxide.
Studies on rat and human muscle cells have demonstrated that MCT1 mRNA is upregulated in response to 10 mM lactate in vitro [53], or in response to the increase of local lactate in exercising muscle, in vivo [109]. The increase in MCT1 mRNA also corresponds to significant increases in PGC-1α mRNA [53]; PGC-1α signaling is important to muscle metabolism, promoting oxidation [110]. Total and mitochondrial protein levels of MCT1 and CD147 have been demonstrated to increase significantly with exposure to exogenous lactate, while MCT4 expression remains unchanged [53]. In vivo exercise studies found that while MCT1 and MCT4 will increase after training, the increase of MCT1 correlates with citrate synthase activity, indicating an active TCA cycle [109]. MCT4 did not show the same correlation, indicating that MCT1 regulation can be important to the ability of the muscle cell to directly consume lactate to be used in TCA cycle/oxidative phosphorylation while MCT4 regulation is more likely to correspond to lactate efflux from the more glycolytic myocyte. This begs the question of whether cancer cells also have the ability to consume lactate or if the upregulation of MCT1 is more likely an adaptation to higher concentrations of intracellular lactate generated from increased glycolysis in cancer cells.
Lactate is not the only substrate that can upregulate MCT1 expression. Butyrate, a short chain fatty acid synthesized by bacterial fermentation in the colon, has been demonstrated to stimulate MCT1 promoter activity [111]. Hormones act to regulate the expression of MCT1; both testosterone [112] and thyroid-stimulating hormone (TSH) [113] will stimulate tissue protein expression of MCT1. The metabolite milieu is affected by the expression and function of MCTs. Inhibition of MCT1 and MCT2 via siRNA showed significant decrease in malignant glioma cell viability and lactate efflux (i.e., glycolysis) in vitro; these effects were increased when both were inhibited simultaneously [114]. MCT1 overexpression in rat β-cells has been demonstrated to increase pyruvate oxidation, and when co-overexpressed with lactate dehydrogenase A, islet cells will additionally increase lactate oxidation [115]. A recent study in cultured astrocytes found that basal lactate uptake was decreased with MCT1 silencing, but not with MCT4 silencing. The effect was more profound than CD147 silencing, which decreased basal lactate influx and efflux and glutamate-activated lactate release. The results from this study indicate the possibility of an uncharacterized MCT subtype or an unidentified transporter functioning within astrocytes [116].
MCTs & cancer
MCT expression in cancer is summarized in Table 1. Colorectal cancer provides intriguing information regarding MCT expression in cancer. MCT1 is expressed in normal colonic epithelium to facilitate the transport of butyrate, the primary energy source for these cells [117]. Butyrate has been demonstrated to be important in proliferation, apoptosis and differentiation, with declining levels indicative of colon cancer progression [118]. Since butyrate is imperative for differentiation of colon cells [119,120], as well as breast cells [121], it makes sense that low levels of this substrate would contribute to carcinogenic transition or progression. Butyrate unavailability may be due to microenvironmental levels as well as a decreased ability of the cells to take up the substrate. The loss or silencing of MCT1 has been demonstrated to correlate with the deregulation of butyrate-responsive genes involved in differentiation and apoptosis [43,122], transition from normalcy to malignancy in colonic epithelium [123], along with a simultaneous increase in GLUT-1 expression [37,123], supporting the hypothesis that the primary energy source for cancerous colon cells switches from butyrate to glucose. SLC5A8, termed SMCT1, is also responsible for mediating uptake of butyrate [48] and has been demonstrated to act as a tumor suppressor in colon epithelium, with its silencing via aberrant correlating to neoplastic transformation of colon cells [124]. An opposing finding on MCT expression in colon tissue indicates that there is an increase in MCT1, MCT2 and MCT4 in colorectal carcinoma compared with normal colonic epithelium [36]. Expression patterns of MCT1 within a separate study demonstrated that both membrane and cytoplasmic MCT1 expression was seen in both normal colonic tissue as well as in colonic tumor cells and tumor-associated fibroblasts [37]. Studies mentioned used immunohistochemical analysis to assess MCT1 abundance; no conclusion can be drawn about the discrepancy between results. Further studies are needed to elucidate MCT expression and its implications in colorectal cancer.
Differential expression of MCT1 has been seen in clinical breast samples with hypermethylation of a CpG island (CGI) in the 5′ upstream region of MCT1 accounting for silencing, similar to the expression seen in the MDA-MB-231 cell line [121]. In lung cancer, MCT1, MCT2 and MCT4 expression are found among tumor cells while very weak expression was seen in stroma, and no MCT expression was found in normal lung tissue [125]. Although MCT signaling pathways are not completely known, MCT2 expression/regulation has been linked to IGF-1 [126] and PI3k/Akt or mTor pathways [127], which may also be relevant to other subtypes. Interestingly, current research on SMCT1 demonstrates a more consistent expression pattern in different cancers compared with MCT1; SMCT1 is reputedly silenced in thyroid, stomach, brain, breast, pancreas and kidney cancers [48]. Perhaps this indicates that the SMCTs may have an unambiguous expression pattern in cancers compared with proton-coupled MCTs; further research on the expression and function of SMCT and MCT subtypes in cancer is warranted to elucidate these possibilities.
Monocarboxylate transporter subtypes may be specifically expressed among tumor cell types, with MCT1 being more abundant in cells with the potential for lactate oxidation while MCT4 is restricted to the more glycolytic cells. SiHa cells, which were found to be able to take in lactate, express abundant MCT1 and little MCT4, while the reverse was found for the more glycolytic cell line WiDr [68]. Hypoxia, specifically HIF-1α, will upregulate MCT4 mRNA and protein expression while having no effect on MCT1 expression [128]. Our laboratory has seen similar results in 0.5% oxygen conditions with MCT4 upregulation and MCT1 downregulation in A549 and HT1080 cells [Wergin M, Kennedy K, Dedeugd C, Dewhirst MW. Duke University Medical Center, NC, USA. Unpublished data]. In sections of some human tumors, an inverse spatial distribution of MCT1 and MCT4 was found when compared to the distribution of hypoxia marker, EF5: MCT4 showing a positive correlation to EF5 staining and MCT1 showing a negative correlation [Wergin M et al. Unpublished data]. To support the concept of an inverse spatial relationship between MCT1/aerobic tissue and MCT4/hypoxic tissue, non-small-cell lung cancer biopsies showed that MCT1 did not colocalize with EF5, a hypoxia marker drug [68].
CD147 & cancer
It is well established that cancer cells show enhanced expression of CD147 [129] (Table 1). Esophageal squamous cell carcinomas (ESCC) (stage 3 and 4) and endometrial (97.3% incidence) carcinoma show significant upregulation of tissue expression of CD147, while CD147 is not expressed in the respective normal tissues [130,131]. CD147 holds promise as a reliable biomarker in cancer progression, showing a significant expression increase in carcinomas compared with sarcomas and normal epithelial tissues, as well as positively correlating with tumor recurrence and progression and negatively correlating to survival in breast cancer patients in a retrospective study [132].
Much of the influence of CD147 on the invasiveness and metastatic potential of tumor cells is related to the induction of MMPs, which break down extracellular matrix proteins. This induction of MMPs by CD147 occurs largely in the adjacent fibroblasts found within the stroma or adjacent normal tissue [130,133]. MMP subtypes under CD147 induction include 1, 2, 3, 9, 11, 14, and 15 [134] in both fibroblasts and endothelial cells. Many subtypes are notorious for their association and correlation to malignant cancers [135–138]. A study in breast cancer cells has demonstrated that disruption of the homophilic CD147 interaction with antibody treatment inhibited MMP2 production from tumor cells and MMP2-dependent invasion in vitro through matrigel [139]. Invasive properties conferred to melanoma tumor cells by CD147 are a result of stimulation of MMPs [140]. CD147 has been demonstrated, in gallbladder carcinoma, to predict for poor prognosis and overall survival, especially in association with MMP-2 expression [141]. Adding to the tumorigenic effects of CD147 is its ability to stimulate VEGF via the PI3K/Akt pathway [142]. VEGF will likewise be upregulated by increased MMP production (Figure 2).
Figure 2. CD147 signaling and interactions.
Black arrows indicate stimulation/activation. Gray arrows indicate associated molecules and their additional signaling or augmentation of effect. MCT: Monocarboxylate transporter; MMP: Matrix metalloproteinase.
Invasiveness and multidrug resistance of hepatocellular carcinoma cells is mediated by CD147 and shows increased levels of MMP11, which are both decreased with CD147 RNAi [143]. In human breast cancer, positive CD147 correlates with high tumor grade and volume, increased mitotic index, decreased tumor-specific survival and negative estrogen receptor (ER) and progesterone receptor (PR) staining [144]. CD147 facilitates survival of suspended breast carcinoma cells MDA-MB-231s, while knockdown of CD147 decreases viability of suspended cells. CD147 inhibits anoikis, and when silenced, MDA MB 231 cells undergo apoptosis. Anoikis is due to CD147 activating the MAPK pathway with phosphorylation of Erk1/2 leading to a decreased level of Bim (pro-apoptotic) by proteosomal degradation, which confers survival. This partially explains why increased levels of CD147 in cancer correlate with metastasis [145].
Additionally, CD147 is associated with a number of accessory or regulatory proteins beyond MCTs, including β1-integrins, cyclophilin A, caveolin-1 [135] and transporters CD98 heavy chain-LAT1 complex, ASCT2 (amino acid transporter) and EpCAM (epithelial cell adhesion molecule, a regulator of cell proliferation) [146]. CD147 will induce production hyaluronan from adjacent cells [147]. Another large complex recently explored is the CD44 variant hyaluronan-associated CD147/MCT complex that will ultimately affect lactate transport [148].
Larger cell membrane CD147-associated complexes include the MCT/CD147-CD98hc/LAT1-ASCT2-EpCAM, which likely suppresses AMPK, as indirectly supported by CD147/CD98 RNAi experiments demonstrating that AMPK activation reduces expression of the large complex [146]. AMPK affects many metabolic operations, including stimulating fatty acid oxidation and glucose uptake, which lead to increased lactate production [30,149]. Even as an indirect target of CD147-associated complexes, the activation or suppression of AMPK will impact a myriad of metabolic effects. This indicates indirect participation of molecules contribute to complex metabolic signaling pathways, and that manipulating one target can change the metabolic phenotype in potentially unexpected ways.
MCTs & CD147 as potential targets
Rationale behind MCT inhibition is multi-factorial (Figure 3). Inhibition of MCTs will have a direct effect on monocarboxylate transport and pH. It has been demonstrated that MCT1 inhibition decreases intracellular pH, resulting in cell death [68,90,150,151]. Since MCT1 is bidirectional, inhibition of this transporter may also cause the extracellular environment to become more acidic. While this effect is usually associated with a more aggressive phenotype, the increase in acid allows for more lactate uptake [79] and may have additional aid in treatment if candidate drugs require lower extracellular pH to enhance their uptake [152].
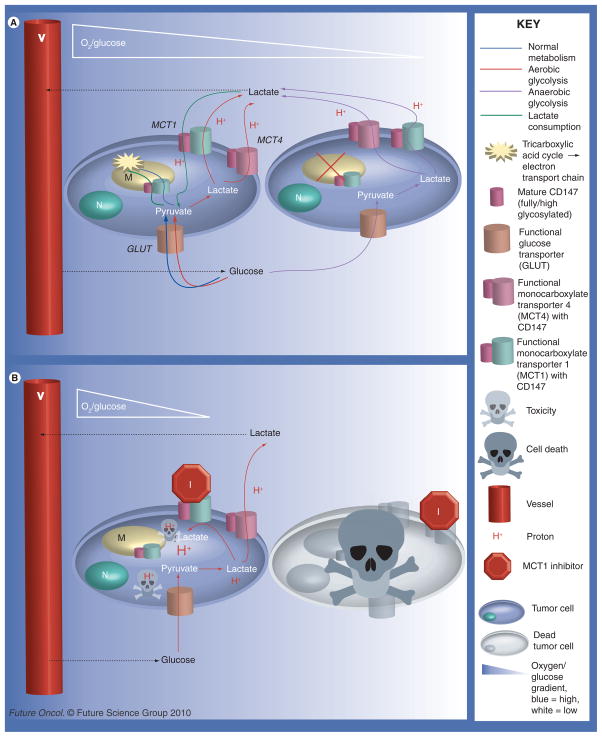
Figure 3. Potential metabolic pathways between tumor cells (see left).
Cells close to vessels will have the advantage of high/adequate concentrations of oxygen and nutrients, such as glucose. Cells farther from vessels will experience varying degrees of hypoxia and starvation. (A) The metabolic pathways possible between cancer cells without MCT1 inhibition when the aerobic cells are able to consume/utilize lactate. Shown here is the Pasteur effect (anaerobic glycolysis) in the hypoxic cells farther from vessels. The well-oxygenated cells close to vessels may undergo healthy oxidative phosphorylation, possibly the Warburg effect (aerobic glycolysis), or lactate utilization. (B) Illustrates the consequences of MCT1 inhibition on cell-to-cell metabolism and intracellular pH. MCT4, having a high Km, is unlikely to take up lactate unless there is a very high extracellular concentration of lactate. Excluded from this diagram are pH regulators other than MCTs, such as Na+/H+ exchanger (NHE1), which would serve to remove some of the H+ from the cell. MCT1 inhibition can lead to cell death by two different means: the hypoxic cells are starved since cells close to vessels are forced to only take up glucose since the ability of lactate consumption is blocked, as seen by comparing (A) to (B) or the decrease in intracellular pH leads to toxicity, indicated by the protons represented in the diagram.
Another hypothesis regarding potential efficacy of MCT1 inhibition as a cancer treatment is in relation to the proposed ‘metabolic symbiont’ model between hypoxic and aerobic cells within the tumor microenvironment. Briefly, this model states that aerobic cells, which have the ability to consume lactate, can confer a survival advantage to the hypoxic tumor cells by allowing glucose to reach the hypoxic cells farther from vessels. By inhibiting MCT1, lactate is no longer available to the aerobic cells, forcing them to take up the glucose. This starves the more treatment-resistant hypoxic cells [68]. Inhibition of MCT1 has been found to decrease tumor growth rate [68] and elicit cell death via apoptosis and necrosis when silenced in conjunction with MCT2 [114].
Finally, inhibition of MCTs may suppress cell migration. Silencing of MCT4 via siRNA has been demonstrated to reduce transwell migration of MDA-MB-231 cells by as much as 85% [40]. More recent studies by Philp’s group have illustrated the coimmunoprecipitation and colocalization of MCT4 and β-1-integrin in the leading edge lamellapodia of migrating cells [153]. Both CD147 and MCT4 interact closely with β-1-integrin; these findings, paired with the dependency of CD147 expression on MCT4 expression [40], indicate that MCT4 may be exerting more of an influence on focal adhesion- and integrin-associated cell migration than previously imagined.
There are several small molecules that inhibit MCT function. The efficiency of inhibition of MCT4 function tested under conditions of 30 mM lactate for the following inhibitors has been evaluated: α-cyano-4-hydroxycinnamate (CHC or CINN) (Ki ~27 μM), phloretin (Ki ~1.4 μM), 5-nitro-2-(3-phenylpropylamino) benzoate, 3-isobutyl-1-methylxanthine and p-chloromercuribenzene sulfonate (pCMBS) (Ki < 1.0 μM), which inhibits MCT1 via reaction with CD147 as opposed to direct MCT1 interaction [154,155]. There are some variations of efficiency of functional inhibition of the different inhibitors based on the subtype. CHC is supposedly a specific MCT1 inhibitor [154]. Of these CHC, pCMBS and phloretin are well-established MCT1 inhibitors, which reduces lactate influx by up to approximately 80% in normal hepatocytes in a dose-dependent manner, starting at concentrations of 5 mM. Similar effects have been demonstrated with 4,4′-dibenzamidostilbene-2,2′-disulfonate and 4,4′-diisothiocyanostilbene-2,2′-disulfonate [156] and with the tumor cell line Ehrlich–Lettre, although with some variation [157,158]. A recent MCT1-specific inhibitor, AR-C117977, has been found to have immunosuppressive properties that will significantly prolong skin graft and heart allograft survival in mice [159]. Some of these inhibitors can be used as broad spectrum pH regulator inhibitors, and likewise will inhibit lactate and proton efflux via MCTs. More MCT-specific inhibitors hold promise in facilitating tumor metabolism manipulation, as well as potentially acting as immunosuppressors.
Inhibition studies on CD147 with RNAi (Figure 4) have demonstrated significant decreases in invasiveness [39,160–162], MMP secretion [163], multidrug resistance [143,162,164] and increased cell death, both through apoptosis and necrosis [145,165]. Inhibition by a mouse monoclonal antibody led to specific cancer cell death while sparing normal fibroblast; this same antibody also disrupted the CD147–MCT1 association [165]. Since some MCT subtypes rely on CD147 for expression and regulate pH via the monocarboxylate symport, CD147 inhibition helps to kill cells by indirectly inducing a drop in intracellular pH, as in the case of pCMBS. CD147 regulation has been demonstrated to effect lactate influx/efflux [116].
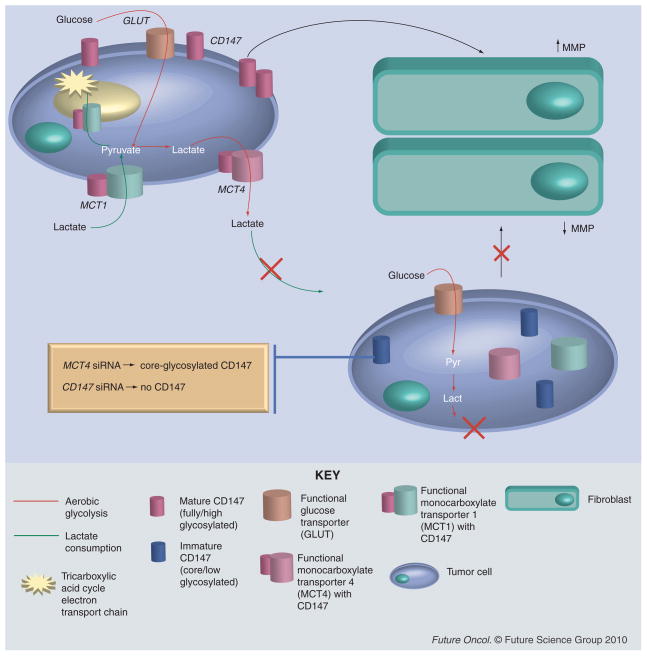
Figure 4. Influence of CD147 silencing on MCT expression, tumor cell lactate metabolism and MMP regulation in neighboring fibroblasts.
Above tumor cell (left) has upregulated CD147 expression on the surface and adequate membrane expression of MCT subtypes 1 and 4, which allows various metabolic options for the cell (see Figure 3). Overexpression of CD147 can lead to increased matrix metalloproteinase production by neighboring fibroblasts (right). Bottom tumor cell shows the consequences of CD147 silencing with either CD147 siRNA or monocarboxylate transporter 4 (MCT4) siRNA. The gold bubble indicates the difference between these two. With MCT4 siRNA, CD147 is present in the cell, but in the low glycosylated form and therefore restricted to the cytosol. With CD147 siRNA, CD147 expression is ablated. Both scenarios lead to decreased MCT1 and MCT4 membrane expression, which will prevent monocarboxylate transport in and out of the cell. Lack of mature CD147 expression on the cell surface will also reduce MMP production by fibroblasts in comparison with a tumor cell with high CD147 expression.
MCT: Monocarboxylate transporter; MMP: Matrix metalloproteinase.
When considering MCTs as a target for therapy, it is imperative to bear in mind and evaluate toxicity to normal tissue. These molecules have the potential for altering metabolism, inflammatory response, intracellular pH, and angiogenic response on a broad spectrum. Local delivery may be required to prevent deleterious whole-body effects. Systemic delivery of an MCT inhibitor, and specifically MCT1, could affect almost every organ of the body, with the most drastic effects on cardiac and skeletal muscle. Possible side effects in skeletal muscle include muscle fatigue and inability to tolerate moderate- to high-intensity exercise due to the build-up of intracellular lactate as well as hydrogen ions [166]. In the worst-case scenario, the side effects of MCT inhibition in the heart may lead to exercise intolerance or other more severe cardiac toxicities [155,166]. Since MCT1 is one of the transporters that will take up butyrate, it is possible that with MCT1 inhibition there may be potential problems with proper differentiation or cell proliferation may be reduced in the colon. Inhibition of CD147 systemically may severely interfere with a number of the body’s systems and cell processes. For perspective, CD147-null female mice will have severely impaired embryonic implantation, and CD147-null male mice are sterile [135]. It is obvious that for these molecules to have prospects for treatment, local delivery to the tumor may be preferable.
Future perspective
While some MCT inhibitors demonstrate potential for clinical usage, there are none that are currently in clinical trials. Local delivery of MCT inhibitors is an important first step towards clinical use. Once that hurdle is cleared, future prospects could include tumor-targeted combined therapies of CD147 silencing and/or MCT subtype-specific inhibition by small molecules. With further research on gene therapy, manipulation of CD147, MCT or SMCT expression may be possible for treatment of cancer for future generations. Manipulation of CD147 may drastically reduce tumor cell invasion, ECM breakdown, metastases and multi-drug resistance. Therapeutic change in MCT expression could reduce cell migration, increase cell death through intracellular acidification or through hypoxic cell starvation. Reintroducing SMCT expression in cases of loss of expression through hypermethylation may re-establish the tumor-suppressing effects of SMCT1. While ultimately it is unknown what future place MCT-, SMCT- or CD147-related therapies will have in the clinic, the manipulation of these molecules have and will continue to grant us great insight into connections between tumor metabolism, microenvironment and metastatic propensity.
Executive summary
Physiologic tumor microenvironment
Unorganized vascular structure and shunt flow.
Inefficient red blood cell flux and oxygen transport can lead to hypoxia and increased HIF-1α levels.
-
Lactate accumulation:
– Anaerobic glycolysis (Pasteur effect).
– Aerobic glycolysis (Warburg effect).
Requirements for lactate utilization & sites of regulation
Membrane transporter (MCTs) – regulated by metabolites, chaperones and transcription factors.
Lactate dehydrogenase: lactate ↔ pyruvate.
Adequate oxygen – regulated by consumption and delivery.
Healthy, functional mitochondria.
Pyruvate dehydrogenase complex: pyruvate can be converted into acetyl CoA, pyruvate dehydrogenase complex is regulated by PDK1.
Lactate in cancer
Accumulation correlates with poor prognosis, metastases-free and overall survival in many cancer types.
Lactate has been demonstrated to spatially correlate to hypoxic regions in tumor sections in some tumors.
Mean lactate content has a wide variation between tumor samples and within individual tumors.
Certain cancer cell lines have the ability to take up lactate.
Hepatomas and gliomas have been documented to utilize lactate in vivo according to nuclear magnetic resonance studies.
Strong lactic acidosis genomic response predicted for favorable prognosis of clinical breast cancer trials.
MCTs & CD147 in cancer
MCTs demonstrate variable regulation by several stimuli.
MCT regulation profiles differ among cancer types.
CD147 has been found to be upregulated in most cancers.
CD147 stimulates matrix metalloproteinases that degrade extracellular matrix components and facilitate invasiveness.
CD147 stimulates VEGF production, potentially increasing angiogenesis.
MCTs and CD147 are involved in several large membrane complexes that can be under regulation of hyaluronan or 5′ adenosine monophosphate-activated protein kinase.
Targeting MCTs & CD147
-
MCT1 inhibition:
– Decreases pHi leading to cell death.
– Blocks aerobic tumor cell lactate utilization, starving hypoxic cells of glucose.
MCT4 siRNA decreases tumor cell migration.
CD147 siRNA decreases MMP secretion, multidrug resistance and tumor cell invasiveness.
CD147 siRNA increases cell death by apoptosis and necrosis.
Acknowledgments
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Contributor Information
Kelly M Kennedy, Pathology department, Research Drive, Duke University Medical Center, NC, USA.
Mark W Dewhirst, Email: dewhi001@mc.duke.edu, Box 3455, Room 201 MSRB, Research Drive, Duke University Medical Center, Durham, NC 27710, USA, Tel.: +1 919 684 4180, Fax: +1 919 684 8718.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269–270. [PubMed] [Google Scholar]
- 3.Warburg OH, Dickens F. The metabolism of tumours: investigations from the Kaiser Wilhelm Institute for Biology, Berlin-Dahlem. Constable; London, UK: 1930. Kaiser–Wilhelm-Institut Fuir Biologie. [Google Scholar]
- 4.Warburg OH. Ãœber den stoffwechsel der tumoren: arbeiten aus dem kaiser wilheim-institut fã¼r biologie, berlin-dahlem. Julius Springer; Berlin, Germany: 1926. Kaiser-Wilhelm-Institut Fuì̂R Biologie. [Google Scholar]
- 5.Waterhouse C. Lactate metabolism in patients with cancer. Cancer. 1974;33(1):66–71. doi: 10.1002/1097-0142(197401)33:1<66::aid-cncr2820330113>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Holroyde CP, Axelrod RS, Skutches CL, Haff AC, Paul P, Reichard GA. Lactate metabolism in patients with metastatic colorectal cancer. Cancer Res. 1979;39(12):4900–4904. [PubMed] [Google Scholar]
- 7.Paschen W. Regional quantitative determination of lactate in brain sections. A bioluminescent approach. J Cereb Blood Flow Metab. 1985;5(4):609–612. doi: 10.1038/jcbfm.1985.90. [DOI] [PubMed] [Google Scholar]
- 8.Paschen W, Mies G, Kloiber O, Hossmann KA. Regional quantitative determination of brain glucose in tissue sections: a bioluminescent approach. J Cereb Blood Flow Metab. 1985;5(3):465–468. doi: 10.1038/jcbfm.1985.63. [DOI] [PubMed] [Google Scholar]
- 9.Paschen W, Djuricic B, Mies G, Schmidt-Kastner R, Linn F. Lactate and pH in the brain: association and dissociation in different pathophysiological states. J Neurochem. 1987;48(1):154–159. doi: 10.1111/j.1471-4159.1987.tb13140.x. [DOI] [PubMed] [Google Scholar]
- 10.Mueller-Klieser W, Kroeger M, Walenta S, Rofstad EK. Comparative imaging of structure and metabolites in tumours. Int J Radiat Biol. 1991;60(1–2):147–159. doi: 10.1080/09553009114551741. [DOI] [PubMed] [Google Scholar]
- 11.Tamulevicius P, Streffer C. Metabolic imaging in tumours by means of bioluminescence. Br J Cancer. 1995;72(5):1102–1112. doi: 10.1038/bjc.1995.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwickert G, Walenta S, Sundfor K, Rofstad EK, Mueller-Klieser W. Correlation of high lactate levels in human cervical cancer with incidence of metastasis. Cancer Res. 1995;55(21):4757–4759. [PubMed] [Google Scholar]
- 13.Walenta S, Salameh A, Lyng H, et al. Correlation of high lactate levels in head and neck tumors with incidence of metastasis. Am J Pathol. 1997;150(2):409–415. [PMC free article] [PubMed] [Google Scholar]
- 14.Walenta S, Chau TV, Schroeder T, et al. Metabolic classification of human rectal adenocarcinomas: a novel guideline for clinical oncologists? J Cancer Res Clin Oncol. 2003;129(6):321–326. doi: 10.1007/s00432-003-0450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walenta S, Wetterling M, Lehrke M, et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000;60(4):916–921. [PubMed] [Google Scholar]
- 16.Brizel DM, Schroeder T, Scher RL, et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51(2):349–353. doi: 10.1016/s0360-3016(01)01630-3. [DOI] [PubMed] [Google Scholar]
- 17.Fulham MJ, Bizzi A, Dietz MJ, et al. Mapping of brain tumor metabolites with proton MR spectroscopic imaging: clinical relevance. Radiology. 1992;185(3):675–686. doi: 10.1148/radiology.185.3.1438744. [DOI] [PubMed] [Google Scholar]
- 18.Hossmann KA, Mies G, Paschen W, Szabo L, Dolan E, Wechsler W. Regional metabolism of experimental brain tumors. Acta Neuropathol. 1986;69(1–2):139–147. doi: 10.1007/BF00687050. [DOI] [PubMed] [Google Scholar]
- 19.Yokota H, Guo J, Matoba M, Higashi K, Tonami H, Nagao Y. Lactate, choline, and creatine levels measured by vitro 1H-MRS as prognostic parameters in patients with non-small-cell lung cancer. J Magn Reson Imaging. 2007;25(5):992–999. doi: 10.1002/jmri.20902. [DOI] [PubMed] [Google Scholar]
- 20.Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell. 2007;12(2):108–113. doi: 10.1016/j.ccr.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 2009;23(5):537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8(6):425–437. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardenas-Navia LI, Braun R, Lewis K, Dewhirst M. Comparison of fluctuations of oxygen tension in FSA, 9L, and R3230AC tumors in rats. Adv Exp Med Biol. 2003;510:7–12. doi: 10.1007/978-1-4615-0205-0_2. [DOI] [PubMed] [Google Scholar]
- 24.Cardenas-Navia LI, Mace D, Richardson RA, Wilson DF, Shan S, Dewhirst MW. The pervasive presence of fluctuating oxygenation in tumors. Cancer Res. 2008;68(14):5812–5819. doi: 10.1158/0008-5472.CAN-07-6387. [DOI] [PubMed] [Google Scholar]
- 25.Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1) α: Its protein stability and biological functions. Exp Mol Med. 2004;36(1):1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- 26.Cardenas-Navia LI, Richardson RA, Dewhirst MW. Targeting the molecular effects of a hypoxic tumor microenvironment. Front Biosci. 2007;12:4061–4078. doi: 10.2741/2372. [DOI] [PubMed] [Google Scholar]
- 27.Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol. 2004;14(3):198–206. doi: 10.1016/j.semradonc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Schroeder T, Yuan H, Viglianti BL, et al. Spatial heterogeneity and oxygen dependence of glucose consumption in R3230AC and fibrosarcomas of the Fischer 344 rat. Cancer Res. 2005;65(12):5163–5171. doi: 10.1158/0008-5472.CAN-04-3900. [DOI] [PubMed] [Google Scholar]
- 29.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 30.Bergeron R, Russell RR, 3rd, Young LH, et al. Effect of AMPK activation on muscle glucose metabolism in conscious rats. Am J Physiol. 1999;276(5 Pt 1):E938–E944. doi: 10.1152/ajpendo.1999.276.5.E938. [DOI] [PubMed] [Google Scholar]
- 31.Garrett RH. GCM: Biochemistry. 3. Vol. 1216. Brooks Cole; CA, USA: 2004. [Google Scholar]
- 32▪.Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004;447(5):619–628. doi: 10.1007/s00424-003-1067-2. A thorough review on monocarboxylate transporters of the SLC16A family. [DOI] [PubMed] [Google Scholar]
- 33.Poortmans JR. Principles of exercise biochemistry. 3. Karger Publishers; Basel, Switzerland: 2003. [Google Scholar]
- 34.Morris ME, Felmlee MA. Overview of the proton-coupled MCT (SLC16A) family of transporters: characterization, function and role in the transport of the drug of abuse γ-hydroxybutyric acid. AAPS J. 2008;10(2):311–321. doi: 10.1208/s12248-008-9035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhattacharya I, Boje KM. Potential γ-hydroxybutyric acid (GHB) drug interactions through blood–brain barrier transport inhibition: a pharmacokinetic simulation-based evaluation. J Pharmacokinet Pharmacodyn. 2006;33(5):657–681. doi: 10.1007/s10928-006-9029-x. [DOI] [PubMed] [Google Scholar]
- 36.Pinheiro C, Longatto-Filho A, Scapulatempo C, et al. Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows Arch. 2008;452(2):139–146. doi: 10.1007/s00428-007-0558-5. [DOI] [PubMed] [Google Scholar]
- 37.Koukourakis MI, Giatromanolaki A, Harris AL, Sivridis E. Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma. Cancer Res. 2006;66(2):632–637. doi: 10.1158/0008-5472.CAN-05-3260. [DOI] [PubMed] [Google Scholar]
- 38.Lin RY, Vera JC, Chaganti RS, Golde DW. Human monocarboxylate transporter 2 (MCT2) is a high affinity pyruvate transporter. J Biol Chem. 1998;273(44):28959–28965. doi: 10.1074/jbc.273.44.28959. [DOI] [PubMed] [Google Scholar]
- 39.Schneiderhan W, Scheler M, Holzmann KH, et al. CD147 silencing inhibits lactate transport and reduces malignant potential of pancreatic cancer cells in in vivo and in vitro models. Gut. 2009;58(10):1391–1398. doi: 10.1136/gut.2009.181412. [DOI] [PubMed] [Google Scholar]
- 40.Gallagher SM, Castorino JJ, Wang D, Philp NJ. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Res. 2007;67(9):4182–4189. doi: 10.1158/0008-5472.CAN-06-3184. [DOI] [PubMed] [Google Scholar]
- 41.Chan MM, Lu X, Merchant FM, Iglehart JD, Miron PL. Gene expression profiling of NMU-induced rat mammary tumors: cross species comparison with human breast cancer. Carcinogenesis. 2005;26(8):1343–1353. doi: 10.1093/carcin/bgi100. [DOI] [PubMed] [Google Scholar]
- 42.Dai Z, Lakshmanan RR, Zhu WG, et al. Global methylation profiling of lung cancer identifies novel methylated genes. Neoplasia. 2001;3(4):314–323. doi: 10.1038/sj.neo.7900162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daly K, Cuff MA, Fung F, Shirazi-Beechey SP. The importance of colonic butyrate transport to the regulation of genes associated with colonic tissue homoeostasis. Biochem Soc Trans. 2005;33(Pt 4):733–735. doi: 10.1042/BST0330733. [DOI] [PubMed] [Google Scholar]
- 44.Pinheiro C, Longatto-Filho A, Ferreira L, et al. Increasing expression of monocarboxylate transporters 1 and 4 along progression to invasive cervical carcinoma. Int J Gynecol Pathol. 2008;27(4):568–574. doi: 10.1097/PGP.0b013e31817b5b40. [DOI] [PubMed] [Google Scholar]
- 45.Gopal E, Fei YJ, Sugawara M, et al. Expression of SLC5A8 in kidney and its role in Na(+)-coupled transport of lactate. J Biol Chem. 2004;279(43):44522–44532. doi: 10.1074/jbc.M405365200. [DOI] [PubMed] [Google Scholar]
- 46.Miyauchi S, Gopal E, Fei YJ, Ganapathy V. Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na(+)-coupled transporter for short-chain fatty acids. J Biol Chem. 2004;279(14):13293–13296. doi: 10.1074/jbc.C400059200. [DOI] [PubMed] [Google Scholar]
- 47.Srinivas SR, Gopal E, Zhuang L, et al. Cloning and functional identification of SLC5A12 as a sodium-coupled low-affinity transporter for monocarboxylates (smct2) Biochem J. 2005;392(Pt 3):655–664. doi: 10.1042/BJ20050927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48▪.Ganapathy V, Thangaraju M, Gopal E, et al. Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J. 2008;10(1):193–199. doi: 10.1208/s12248-008-9022-y. A review on sodium-coupled monocarboxylate transporters (SMCTs) and their emerging and potential role in normal tissue and cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cori C. Mammalian carbohydrate metabolism. Physiol Rev. 1931;11(2):143–275. [Google Scholar]
- 50.Brooks GA. Intra- and extra-cellular lactate shuttles. Med Sci Sports Exerc. 2000;32(4):790–799. doi: 10.1097/00005768-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 51▪.Gladden LB. Lactate metabolism: a new paradigm for the third millennium. J Physiol. 2004;558(Pt 1):5–30. doi: 10.1113/jphysiol.2003.058701. Provides extensive information on lactate metabolism in many systems. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hashimoto T, Hussien R, Cho HS, Kaufer D, Brooks GA. Evidence for the mitochondrial lactate oxidation complex in rat neurons: demonstration of an essential component of brain lactate shuttles. PLoS ONE. 2008;3(8):E2915. doi: 10.1371/journal.pone.0002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. Lactate sensitive transcription factor network in l6 cells: Activation of MCT1 and mitochondrial biogenesis. FASEB J. 2007;21(10):2602–2612. doi: 10.1096/fj.07-8174com. [DOI] [PubMed] [Google Scholar]
- 54.Gladden LB. Lactic acid: new roles in a new millennium. Proc Natl Acad Sci USA. 2001;98(2):395–397. doi: 10.1073/pnas.98.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pellerin L, Pellegri G, Bittar PG, et al. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci. 1998;20(4–5):291–299. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- 56.Pellerin L, Pellegri G, Martin JL, Magistretti PJ. Expression of monocarboxylate transporter mRNAs in mouse brain: support for a distinct role of lactate as an energy substrate for the neonatal vs adult brain. Proc Natl Acad Sci USA. 1998;95(7):3990–3995. doi: 10.1073/pnas.95.7.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bouzier-Sore AK, Voisin P, Canioni P, Magistretti PJ, Pellerin L. Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J Cereb Blood Flow Metab. 2003;23(11):1298–1306. doi: 10.1097/01.WCB.0000091761.61714.25. [DOI] [PubMed] [Google Scholar]
- 58.Pellerin L. Lactate as a pivotal element in neuron-glia metabolic cooperation. Neurochem Int. 2003;43(4–5):331–338. doi: 10.1016/s0197-0186(03)00020-2. [DOI] [PubMed] [Google Scholar]
- 59.Zwingmann C, Richter-Landsberg C, Brand A, Leibfritz D. NMR spectroscopic study on the metabolic fate of [3-(13)c] alanine in astrocytes, neurons, and cocultures: implications for glia-neuron interactions in neurotransmitter metabolism. Glia. 2000;32(3):286–303. doi: 10.1002/1098-1136(200012)32:3<286::aid-glia80>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 60.Waagepetersen HS, Sonnewald U, Larsson OM, Schousboe A. A possible role of alanine for ammonia transfer between astrocytes and glutamatergic neurons. J Neurochem. 2000;75(2):471–479. doi: 10.1046/j.1471-4159.2000.0750471.x. [DOI] [PubMed] [Google Scholar]
- 61.McClelland GB, Khanna S, Gonzalez GF, Butz CE, Brooks GA. Peroxisomal membrane monocarboxylate transporters: evidence for a redox shuttle system? Biochem Biophys Res Commun. 2003;304(1):130–135. doi: 10.1016/s0006-291x(03)00550-3. [DOI] [PubMed] [Google Scholar]
- 62.Storey BT, Kayne FJ. Energy metabolism of spermatozoa VI Direct intramitochondrial lactate oxidation by rabbit sperm mitochondria. Biol Reprod. 1977;16(4):549–556. [PubMed] [Google Scholar]
- 63.Gaesser GA, Brooks GA. Metabolic bases of excess post-exercise oxygen consumption: a review. Med Sci Sports Exerc. 1984;16(1):29–43. [PubMed] [Google Scholar]
- 64.Bonen A. The expression of lactate transporters (MCT1 and MCT4) in heart and muscle. Eur J Appl Physiol. 2001;86(1):6–11. doi: 10.1007/s004210100516. [DOI] [PubMed] [Google Scholar]
- 65.Mccullagh KJ, Poole RC, Halestrap AP, O’Brien M, Bonen A. Role of the lactate transporter (MCT1) in skeletal muscles. Am J Physiol. 1996;271(1 Pt 1):E143–E150. doi: 10.1152/ajpendo.1996.271.1.E143. [DOI] [PubMed] [Google Scholar]
- 66.Stanley WC. Myocardial lactate metabolism during exercise. Med Sci Sports Exerc. 1991;23(8):920–924. [PubMed] [Google Scholar]
- 67.Pagliassotti MJ, Donovan CM. Role of cell type in net lactate removal by skeletal muscle. Am J Physiol. 1990;258(4 Pt 1):E635–E642. doi: 10.1152/ajpendo.1990.258.4.E635. [DOI] [PubMed] [Google Scholar]
- 68.Sonveaux P, Vegran F, Schroeder T, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118(12):3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Broer S, Schneider HP, Broer A, Rahman B, Hamprecht B, Deitmer JW. Characterization of the monocarboxylate transporter 1 expressed in xenopus laevis oocytes by changes in cytosolic pH. Biochem J. 1998;333(Pt 1):167–174. doi: 10.1042/bj3330167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dimmer KS, Friedrich B, Lang F, Deitmer JW, Broer S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J. 2000;350(Pt 1):219–227. [PMC free article] [PubMed] [Google Scholar]
- 71.Ide K, Schmalbruch IK, Quistorff B, Horn A, Secher NH. Lactate, glucose and O2 uptake in human brain during recovery from maximal exercise. J Physiol. 2000;522(Pt 1):159–164. doi: 10.1111/j.1469-7793.2000.t01-2-00159.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pellerin L, Magistretti PJ. Neuroenergetics: calling upon astrocytes to satisfy hungry neurons. Neuroscientist. 2004;10(1):53–62. doi: 10.1177/1073858403260159. [DOI] [PubMed] [Google Scholar]
- 73.Pellerin L, Bergersen LH, Halestrap AP, Pierre K. Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. J Neurosci Res. 2005;79(1–2):55–64. doi: 10.1002/jnr.20307. [DOI] [PubMed] [Google Scholar]
- 74.Itoh Y, Esaki T, Shimoji K, et al. Dichloroacetate effects on glucose and lactate oxidation by neurons and astroglia in vitro and on glucose utilization by brain in vivo. Proc Natl Acad Sci USA. 2003;100(8):4879–4884. doi: 10.1073/pnas.0831078100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith D, Pernet A, Hallett WA, Bingham E, Marsden PK, Amiel SA. Lactate: A preferred fuel for human brain metabolism in vivo. J Cereb Blood Flow Metab. 2003;23(6):658–664. doi: 10.1097/01.WCB.0000063991.19746.11. [DOI] [PubMed] [Google Scholar]
- 76.Bouzier AK, Thiaudiere E, Biran M, Rouland R, Canioni P, Merle M. The metabolism of [3-(13)c]lactate in the rat brain is specific of a pyruvate carboxylase-deprived compartment. J Neurochem. 2000;75(2):480–486. doi: 10.1046/j.1471-4159.2000.0750480.x. [DOI] [PubMed] [Google Scholar]
- 77.Wagner S, Hussain MZ, Hunt TK, Bacic B, Becker HD. Stimulation of fibroblast proliferation by lactate-mediated oxidants. Wound Repair Regen. 2004;12(3):368–373. doi: 10.1111/j.1067-1927.2004.012315.x. [DOI] [PubMed] [Google Scholar]
- 78.Trabold O, Wagner S, Wicke C, et al. Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound Repair Regen. 2003;11(6):504–509. doi: 10.1046/j.1524-475x.2003.11621.x. [DOI] [PubMed] [Google Scholar]
- 79.Wang Q, Morris ME. The role of monocarboxylate transporter 2 and 4 in the transport of γ-hydroxybutyric acid in mammalian cells. Drug Metab Dispos. 2007;35(8):1393–1399. doi: 10.1124/dmd.107.014852. [DOI] [PubMed] [Google Scholar]
- 80.Gladden LB. Muscle as a consumer of lactate. Med Sci Sports Exerc. 2000;32(4):764–771. doi: 10.1097/00005768-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 81▪.Gladden LB. A lactatic perspective on metabolism. Med Sci Sports Exerc. 2008;40(3):477–485. doi: 10.1249/MSS.0b013e31815fa580. Provides extensive information on lactate metabolism in many systems. [DOI] [PubMed] [Google Scholar]
- 82.Bourrat-Floeck B, Groebe K, Mueller-Klieser W. Biological response of multicellular EMT6 spheroids to exogenous lactate. Int J Cancer. 1991;47(5):792–799. doi: 10.1002/ijc.2910470528. [DOI] [PubMed] [Google Scholar]
- 83.Katz J, Brand K, Golden S, Rubinstein D. Lactate and pyruvate metabolism and reducing equivalent transfer in Ehrlich ascites tumor. Cancer Res. 1974;34(4):872–877. [PubMed] [Google Scholar]
- 84.Sauer LA, Dauchy RT. Ketone body, glucose, lactic acid, and amino acid utilization by tumors in vivo in fasted rats. Cancer Res. 1983;43(8):3497–3503. [PubMed] [Google Scholar]
- 85.Sauer LA, Stayman JW, 3rd, Dauchy RT. Amino acid, glucose, and lactic acid utilization in vivo by rat tumors. Cancer Res. 1982;42(10):4090–4097. [PubMed] [Google Scholar]
- 86▪.Bouzier AK, Voisin P, Goodwin R, Canioni P, Merle M. Glucose and lactate metabolism in C6 glioma cells: evidence for the preferential utilization of lactate for cell oxidative metabolism. Dev Neurosci. 1998;20(4–5):331–338. doi: 10.1159/000017328. This study and [87,88] provide more extensive and detailed research on lactate utilization in tumors as well as information on nuclear magnetic resonance (NMR) [DOI] [PubMed] [Google Scholar]
- 87▪.Bouzier-Sore AK, Canioni P, Merle M. Effect of exogenous lactate on rat glioma metabolism. J Neurosci Res. 2001;65(6):543–548. doi: 10.1002/jnr.1184. This study and [86,88] provide more extensive and detailed research on lactate utilization in tumors as well as information on NMR. [DOI] [PubMed] [Google Scholar]
- 88▪.Bouzier AK, Goodwin R, De Gannes FM, et al. Compartmentation of lactate and glucose metabolism in C6 glioma cells. A 13C and 1H NMR study. J Biol Chem. 1998;273(42):27162–27169. doi: 10.1074/jbc.273.42.27162. This study and [86,87] provide more extensive and detailed research on lactate utilization in tumors, as well as information on NMR. [DOI] [PubMed] [Google Scholar]
- 89.Chatham JC, Des Rosiers C, Forder JR. Evidence of separate pathways for lactate uptake and release by the perfused rat heart. Am J Physiol. 2001;281(4):E794–802. doi: 10.1152/ajpendo.2001.281.4.E794. [DOI] [PubMed] [Google Scholar]
- 90.Izumi H, Torigoe T, Ishiguchi H, et al. Cellular pH regulators: potentially promising molecular targets for cancer chemotherapy. Cancer Treat Rev. 2003;29(6):541–549. doi: 10.1016/s0305-7372(03)00106-3. [DOI] [PubMed] [Google Scholar]
- 91.Yamagata M, Hasuda K, Stamato T, Tannock IF. The contribution of lactic acid to acidification of tumours: studies of variant cells lacking lactate dehydrogenase. Br J Cancer. 1998;77(11):1726–1731. doi: 10.1038/bjc.1998.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Helmlinger G, Sckell A, Dellian M, Forbes NS, Jain RK. Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin Cancer Res. 2002;8(4):1284–1291. [PubMed] [Google Scholar]
- 93.Swietach P, Vaughan-Jones RD, Harris AL. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 2007;26(2):299–310. doi: 10.1007/s10555-007-9064-0. [DOI] [PubMed] [Google Scholar]
- 94.Wykoff CC, Beasley NJ, Watson PH, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60(24):7075–7083. [PubMed] [Google Scholar]
- 95.Vince JW, Carlsson U, Reithmeier RA. Localization of the Cl−/HCO3− anion exchanger binding site to the amino-terminal region of carbonic anhydrase II. Biochemistry. 2000;39(44):13344–13349. doi: 10.1021/bi0015111. [DOI] [PubMed] [Google Scholar]
- 96.Vince JW, Reithmeier RA. Identification of the carbonic anhydrase II binding site in the Cl(−)/HCO(3)(−) anion exchanger AE1. Biochemistry. 2000;39(18):5527–5533. doi: 10.1021/bi992564p. [DOI] [PubMed] [Google Scholar]
- 97.Alvarez BV, Vilas GL, Casey JR. Metabolon disruption: a mechanism that regulates bicarbonate transport. EMBO J. 2005;24(14):2499–2511. doi: 10.1038/sj.emboj.7600736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Becker HM, Broer S, Deitmer JW. Facilitated lactate transport by MCT1 when coexpressed with the sodium bicarbonate cotransporter (NBC) in xenopus oocytes. Biophys J. 2004;86(1 Pt 1):235–247. doi: 10.1016/S0006-3495(04)74099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38(2):285–289. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 100▪.Moon EJ, Brizel DM, Chi JT, Dewhirst MW. The potential role of intrinsic hypoxia markers as prognostic variables in cancer. Antioxid Redox Signal. 2007;9(8):1237–1294. doi: 10.1089/ars.2007.1623. Provides excellent information on hypoxia markers, and is highly relevant to cancer research. [DOI] [PubMed] [Google Scholar]
- 101.Rofstad EK, Sundfor K, Lyng H, Trope CG. Hypoxia-induced treatment failure in advanced squamous cell carcinoma of the uterine cervix is primarily due to hypoxia-induced radiation resistance rather than hypoxia-induced metastasis. Br J Cancer. 2000;83(3):354–359. doi: 10.1054/bjoc.2000.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102▪.Chen JL, Lucas JE, Schroeder T, et al. The genomic analysis of lactic acidosis and acidosis response in human cancers. PLoS Genet. 2008;4(12):E1000293. doi: 10.1371/journal.pgen.1000293. One of the first studies we have come across comparing cellular response to hypoxia versus lactosis versus lactic acidosis versus acidosis in both normal and cancer cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Petrella BL, Lohi J, Brinckerhoff CE. Identification of membrane type-1 matrix metalloproteinase as a target of hypoxia-inducible factor-2 α in von hippel-lindau renal cell carcinoma. Oncogene. 2005;24(6):1043–1052. doi: 10.1038/sj.onc.1208305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hashimoto T, Masuda S, Taguchi S, Brooks GA. Immunohistochemical analysis of MCT1, MCT2 and MCT4 expression in rat plantaris muscle. J Physiol. 2005;567(Pt 1):121–129. doi: 10.1113/jphysiol.2005.087411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kirk P, Wilson MC, Heddle C, Brown MH, Barclay AN, Halestrap AP. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 2000;19(15):3896–3904. doi: 10.1093/emboj/19.15.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hashimoto T, Hussien R, Brooks GA. Colocalization of MCT1, CD147, and LDH in mitochondrial inner membrane of l6 muscle cells: evidence of a mitochondrial lactate oxidation complex. Am J Physiol. 2006;290(6):E1237–1244. doi: 10.1152/ajpendo.00594.2005. [DOI] [PubMed] [Google Scholar]
- 107.Deora AA, Philp N, Hu J, Bok D, Rodriguez-Boulan E. Mechanisms regulating tissue-specific polarity of monocarboxylate transporters and their chaperone CD147 in kidney and retinal epithelia. Proc Natl Acad Sci USA. 2005;102(45):16245–16250. doi: 10.1073/pnas.0504419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Philp NJ, Ochrietor JD, Rudoy C, Muramatsu T, Linser PJ. Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse. Invest Ophthalmol Vis Sci. 2003;44(3):1305–1311. doi: 10.1167/iovs.02-0552. [DOI] [PubMed] [Google Scholar]
- 109.Dubouchaud H, Butterfield GE, Wolfel EE, Bergman BC, Brooks GA. Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am J Physiol. 2000;278(4):E571–E579. doi: 10.1152/ajpendo.2000.278.4.E571. [DOI] [PubMed] [Google Scholar]
- 110.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1(6):361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 111.Borthakur A, Saksena S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Regulation of monocarboxylate transporter 1 (MCT1) promoter by butyrate in human intestinal epithelial cells: involvement of Nf-κB pathway. J Cell Biochem. 2008;103(5):1452–1463. doi: 10.1002/jcb.21532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Enoki T, Yoshida Y, Lally J, Hatta H, Bonen A. Testosterone increases lactate transport, monocarboxylate transporter (MCT) 1 and MCT4 in rat skeletal muscle. J Physiol. 2006;577(Pt 1):433–443. doi: 10.1113/jphysiol.2006.115436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fanelli A, Grollman EF, Wang D, Philp NJ. MCT1 and its accessory protein CD147 are differentially regulated by TSH in rat thyroid cells. Am J Physiol. 2003;285(6):E1223–1229. doi: 10.1152/ajpendo.00172.2003. [DOI] [PubMed] [Google Scholar]
- 114.Mathupala SP, Parajuli P, Sloan AE. Silencing of monocarboxylate transporters via small interfering ribonucleic acid inhibits glycolysis and induces cell death in malignant glioma: an in vitro study. Neurosurgery. 2004;55(6):1410–1419. doi: 10.1227/01.neu.0000143034.62913.59. discussion 1419. [DOI] [PubMed] [Google Scholar]
- 115.Ishihara H, Wang H, Drewes LR, Wollheim CB. Overexpression of monocarboxylate transporter and lactate dehydrogenase alters insulin secretory responses to pyruvate and lactate in β cells. J Clin Invest. 1999;104(11):1621–1629. doi: 10.1172/JCI7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maekawa F, Minehira K, Kadomatsu K, Pellerin L. Basal and stimulated lactate fluxes in primary cultures of astrocytes are differentially controlled by distinct proteins. J Neurochem. 2008;107(3):789–798. doi: 10.1111/j.1471-4159.2008.05650.x. [DOI] [PubMed] [Google Scholar]
- 117.Cuff MA, Lambert DW, Shirazi-Beechey SP. Substrate-induced regulation of the human colonic monocarboxylate transporter, MCT1. J Physiol. 2002;539(Pt 2):361–371. doi: 10.1113/jphysiol.2001.014241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Clausen MR, Bonnen H, Mortensen PB. Colonic fermentation of dietary fibre to short chain fatty acids in patients with adenomatous polyps and colonic cancer. Gut. 1991;32(8):923–928. doi: 10.1136/gut.32.8.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Scheppach W, Bartram HP, Richter F. Role of short-chain fatty acids in the prevention of colorectal cancer. Eur J Cancer. 1995;31A(7–8):1077–1080. doi: 10.1016/0959-8049(95)00165-f. [DOI] [PubMed] [Google Scholar]
- 120.Kruh J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biochem. 1982;42(2):65–82. doi: 10.1007/BF00222695. [DOI] [PubMed] [Google Scholar]
- 121.Asada K, Miyamoto K, Fukutomi T, et al. Reduced expression of GNA11 and silencing of MCT1 in human breast cancers. Oncology. 2003;64(4):380–388. doi: 10.1159/000070297. [DOI] [PubMed] [Google Scholar]
- 122.Cuff M, Dyer J, Jones M, Shirazi-Beechey S. The human colonic monocarboxylate transporter isoform 1: its potential importance to colonic tissue homeostasis. Gastroenterology. 2005;128(3):676–686. doi: 10.1053/j.gastro.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 123.Lambert DW, Wood IS, Ellis A, Shirazi-Beechey SP. Molecular changes in the expression of human colonic nutrient transporters during the transition from normality to malignancy. Br J Cancer. 2002;86(8):1262–1269. doi: 10.1038/sj.bjc.6600264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li H, Myeroff L, Smiraglia D, et al. SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc Natl Acad Sci USA. 2003;100(14):8412–8417. doi: 10.1073/pnas.1430846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Koukourakis MI, Giatromanolaki A, Bougioukas G, Sivridis E. Lung cancer: a comparative study of metabolism related protein expression in cancer cells and tumor associated stroma. Cancer Biol Ther. 2007;6(9):1476–1479. doi: 10.4161/cbt.6.9.4635. [DOI] [PubMed] [Google Scholar]
- 126.Chenal J, Pierre K, Pellerin L. Insulin and IGF-1 enhance the expression of the neuronal monocarboxylate transporter MCT2 by translational activation via stimulation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin pathway. Eur J Neurosci. 2008;27(1):53–65. doi: 10.1111/j.1460-9568.2007.05981.x. [DOI] [PubMed] [Google Scholar]
- 127.Chenal J, Pellerin L. Noradrenaline enhances the expression of the neuronal monocarboxylate transporter MCT2 by translational activation via stimulation of PI3K/Akt and the mTOR/S6K pathway. J Neurochem. 2007;102(2):389–397. doi: 10.1111/j.1471-4159.2007.04495.x. [DOI] [PubMed] [Google Scholar]
- 128.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1α-dependent mechanism. J Biol Chem. 2006;281(14):9030–9037. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- 129.Ellis SM, Nabeshima K, Biswas C. Monoclonal antibody preparation and purification of a tumor cell collagenase-stimulatory factor. Cancer Res. 1989;49(12):3385–3391. [PubMed] [Google Scholar]
- 130.Ishibashi Y, Matsumoto T, Niwa M, et al. CD147 and matrix metalloproteinase-2 protein expression as significant prognostic factors in esophageal squamous cell carcinoma. Cancer. 2004;101(9):1994–2000. doi: 10.1002/cncr.20593. [DOI] [PubMed] [Google Scholar]
- 131.Ueda K, Yamada K, Urashima M, et al. Association of extracellular matrix metalloproteinase inducer in endometrial carcinoma with patient outcomes and clinicopathogenesis using monoclonal antibody 12C3. Oncol Rep. 2007;17(4):731–735. [PubMed] [Google Scholar]
- 132.Li Y, Xu J, Chen L, et al. HAb18G (CD147), a cancer-associated biomarker and its role in cancer detection. Histopathology. 2009;54(6):677–687. doi: 10.1111/j.1365-2559.2009.03280.x. [DOI] [PubMed] [Google Scholar]
- 133.Tang Y, Kesavan P, Nakada MT, Yan L. Tumor-stroma interaction: positive feedback regulation of extracellular matrix metalloproteinase inducer (emmprin) expression and matrix metalloproteinase-dependent generation of soluble emmprin. Mol Cancer Res. 2004;2(2):73–80. [PubMed] [Google Scholar]
- 134.Yan L, Zucker S, Toole BP. Roles of the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour progression. Thromb Haemost. 2005;93(2):199–204. doi: 10.1160/TH04-08-0536. [DOI] [PubMed] [Google Scholar]
- 135.Iacono KT, Brown AL, Greene MI, Saouaf SJ. CD147 immunoglobulin superfamily receptor function and role in pathology. Exp Mol Pathol. 2007;83(3):283–295. doi: 10.1016/j.yexmp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pritchard SC, Nicolson MC, Lloret C, et al. Expression of matrix metalloproteinases 1, 2, 9 and their tissue inhibitors in stage II non-small cell lung cancer: implications for MMP inhibition therapy. Oncol Rep. 2001;8(2):421–424. [PubMed] [Google Scholar]
- 137.Murray GI, Duncan ME, O’Neil P, McKay JA, Melvin WT, Fothergill JE. Matrix metalloproteinase-1 is associated with poor prognosis in oesophageal cancer. J Pathol. 1998;185(3):256–261. doi: 10.1002/(SICI)1096-9896(199807)185:3<256::AID-PATH115>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 138.Murray GI, Duncan ME, O’Neil P, Melvin WT, Fothergill JE. Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat Med. 1996;2(4):461–462. doi: 10.1038/nm0496–461. [DOI] [PubMed] [Google Scholar]
- 139.Sun J, Hemler ME. Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res. 2001;61(5):2276–2281. [PubMed] [Google Scholar]
- 140.Kanekura T, Chen X, Kanzaki T. Basigin (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int J Cancer. 2002;99(4):520–528. doi: 10.1002/ijc.10390. [DOI] [PubMed] [Google Scholar]
- 141.Wu W, Wang R, Liu H, et al. Prediction of prognosis in gallbladder carcinoma by CD147 and MMP-2 immunohistochemistry. Med Oncol. 2009;26(2):117–123. doi: 10.1007/s12032-008-9087-6. [DOI] [PubMed] [Google Scholar]
- 142.Tang Y, Nakada MT, Rafferty P, et al. Regulation of vascular endothelial growth factor expression by emmprin via the PI3K-AKT signaling pathway. Mol Cancer Res. 2006;4(6):371–377. doi: 10.1158/1541-7786.MCR-06-0042. [DOI] [PubMed] [Google Scholar]
- 143.Jia L, Xu H, Zhao Y, Jiang L, Yu J, Zhang J. Expression of CD147 mediates tumor cells invasion and multidrug resistance in hepatocellular carcinoma. Cancer Invest. 2008;26(10):977–983. doi: 10.1080/07357900802072723. [DOI] [PubMed] [Google Scholar]
- 144.Reimers N, Zafrakas K, Assmann V, et al. Expression of extracellular matrix metalloproteases inducer on micrometastatic and primary mammary carcinoma cells. Clin Cancer Res. 2004;10(10):3422–3428. doi: 10.1158/1078-0432.CCR-03-0610. [DOI] [PubMed] [Google Scholar]
- 145.Yang JM, O’Neill P, Jin W, et al. Extracellular matrix metalloproteinase inducer (CD147) confers resistance of breast cancer cells to anoikis through inhibition of bim. J Biol Chem. 2006;281(14):9719–9727. doi: 10.1074/jbc.M508421200. [DOI] [PubMed] [Google Scholar]
- 146.Xu D, Hemler ME. Metabolic activation-related CD147–CD98 complex. Mol Cell Proteomics. 2005;4(8):1061–1071. doi: 10.1074/mcp.M400207-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Marieb EA, Zoltan-Jones A, Li R, et al. Emmprin promotes anchorage-independent growth in human mammary carcinoma cells by stimulating hyaluronan production. Cancer Res. 2004;64(4):1229–1232. doi: 10.1158/0008-5472.can-03-2832. [DOI] [PubMed] [Google Scholar]
- 148.Slomiany MG, Grass GD, Robertson AD, et al. Hyaluronan, CD44, and emmprin regulate lactate efflux and membrane localization of monocarboxylate transporters in human breast carcinoma cells. Cancer Res. 2009;69(4):1293–1301. doi: 10.1158/0008-5472.CAN-08-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Winder WW. Energy-sensing and signaling by AMP-activated protein kinase in skeletal muscle. J Appl Physiol. 2001;91(3):1017–1028. doi: 10.1152/jappl.2001.91.3.1017. [DOI] [PubMed] [Google Scholar]
- 150.Fang J, Quinones QJ, Holman TL, et al. The H+-linked monocarboxylate transporter (MCT1/SLC16A1): a potential therapeutic target for high-risk neuroblastoma. Mol Pharmacol. 2006;70(6):2108–2115. doi: 10.1124/mol.106.026245. [DOI] [PubMed] [Google Scholar]
- 151.Wahl ML, Owen JA, Burd R, et al. Regulation of intracellular pH in human melanoma: potential therapeutic implications. Mol Cancer Ther. 2002;1(8):617–628. [PubMed] [Google Scholar]
- 152.Mahoney BP, Raghunand N, Baggett B, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics I Acid pH affects the distribution of chemotherapeutic agentsin vitro. Biochem Pharmacol. 2003;66(7):1207–1218. doi: 10.1016/s0006-2952(03)00467-2. [DOI] [PubMed] [Google Scholar]
- 153.Gallagher SM, Castorino JJ, Philp NJ. Interaction of monocarboxylate transporter 4 with β1-integrin and its role in cell migration. Am J Physiol Cell Physiol. 2009;296(3):C414–C421. doi: 10.1152/ajpcell.00430.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Manning Fox JE, Meredith D, Halestrap AP. Characterisation of human monocarboxylate transporter 4 substantiates its role in lactic acid efflux from skeletal muscle. J Physiol. 2000;529(Pt 2):285–293. doi: 10.1111/j.1469-7793.2000.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Meredith D, Christian HC. Xenobiotica; the fate of foreign compounds in biological systems. 7–8. Vol. 38. Informa Healthcare; London, UK: 2008. The SLC16 monocaboxylate transporter family; pp. 1072–1106. [DOI] [PubMed] [Google Scholar]
- 156.Jackson VN, Halestrap AP. The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver cells determined using the fluorescent intracellular ph indicator, 2′,7′-bis(carboxyethyl)-5(6)-carboxyfluorescein. J Biol Chem. 1996;271(2):861–868. doi: 10.1074/jbc.271.2.861. [DOI] [PubMed] [Google Scholar]
- 157.Carpenter L, Halestrap AP. The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich–Lettre tumour cells studied with the intracellular pH indicator BCECF. Biochem J. 1994;304(Pt 3):751–760. doi: 10.1042/bj3040751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wilson MC, Jackson VN, Heddle C, et al. Lactic acid efflux from white skeletal muscle is catalyzed by the monocarboxylate transporter isoform MCT3. J Biol Chem. 1998;273(26):15920–15926. doi: 10.1074/jbc.273.26.15920. [DOI] [PubMed] [Google Scholar]
- 159.Bueno V, Binet I, Steger U, et al. The specific monocarboxylate transporter (MCT1) inhibitor, ar-c117977, a novel immunosuppressant, prolongs allograft survival in the mouse. Transplantation. 2007;84(9):1204–1207. doi: 10.1097/01.tp.0000287543.91765.41. [DOI] [PubMed] [Google Scholar]
- 160.Chen X, Lin J, Kanekura T, et al. A small interfering CD147-targeting RNA inhibited the proliferation, invasiveness, and metastatic activity of malignant melanoma. Cancer Res. 2006;66(23):11323–11330. doi: 10.1158/0008-5472.CAN-06-1536. [DOI] [PubMed] [Google Scholar]
- 161.Su J, Chen X, Kanekura T. A CD147-targeting siRNA inhibits the proliferation, invasiveness, and VEGF production of human malignant melanoma cells by down-regulating glycolysis. Cancer Lett. 2009;273(1):140–147. doi: 10.1016/j.canlet.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 162.Zou W, Yang H, Hou X, Zhang W, Chen B, Xin X. Inhibition of CD147 gene expression via RNA interference reduces tumor cell invasion, tumorigenicity and increases chemosensitivity to paclitaxel in HO-8910pm cells. Cancer Lett. 2007;248(2):211–218. doi: 10.1016/j.canlet.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 163.Wang L, Wu G, Yu L, et al. Inhibition of CD147 expression reduces tumor cell invasion in human prostate cancer cell line via RNA interference. Cancer Biol Ther. 2006;5(6):608–614. doi: 10.4161/cbt.5.6.2661. [DOI] [PubMed] [Google Scholar]
- 164.Li QQ, Wang WJ, Xu Jd, et al. Involvement of CD147 in regulation of multidrug resistance to P-gp substrate drugs and in vitro invasion in breast cancer cells. Cancer Sci. 2007;98(7):1064–1069. doi: 10.1111/j.1349-7006.2007.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Baba M, Inoue M, Itoh K, Nishizawa Y. Blocking CD147 induces cell death in cancer cells through impairment of glycolytic energy metabolism. Biochem Biophys Res Commun. 2008;374(1):111–116. doi: 10.1016/j.bbrc.2008.06.122. [DOI] [PubMed] [Google Scholar]
- 166.Fishbein WN. Lactate transporter defect: a new disease of muscle. Science. 1986;234(4781):1254–1256. doi: 10.1126/science.3775384. [DOI] [PubMed] [Google Scholar]
- 167.Le QT, Koong A, Lieskovsky YY, et al. In vivo 1h magnetic resonance spectroscopy of lactate in patients with stage IV head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2008;71(4):1151–1157. doi: 10.1016/j.ijrobp.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li Y, Shang P, Qian AR, Wang L, Yang Y, Chen ZN. Inhibitory effects of antisense RNA of HAB18G/CD147 on invasion of hepatocellular carcinoma cells in vitro. World J Gastroenterol. 2003;9(10):2174–2177. doi: 10.3748/wjg.v9.i10.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Han ZD, Bi XC, Qin WJ, et al. CD147 expression indicates unfavourable prognosis in prostate cancer. Pathol Oncol Res. 2008 doi: 10.1007/s12253-008-9131-z. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 170.Han ZD, He HC, Bi XC, et al. Expression and clinical significance of CD147 in genitourinary carcinomas. J Surg Res. 2008 doi: 10.1016/j.jss.2008.11.838. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 171.Ladanyi M, Antonescu CR, Drobnjak M, et al. The precrystalline cytoplasmic granules of alveolar soft part sarcoma contain monocarboxylate transporter 1 and CD147. Am J Pathol. 2002;160(4):1215–1221. doi: 10.1016/S0002-9440(10)62548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kuang YH, Chen X, Su J, et al. Proteome analysis of multidrug resistance of human oral squamous carcinoma cells using CD147 silencing. J Proteome Res. 2008;7(11):4784–4791. doi: 10.1021/pr800355b. [DOI] [PubMed] [Google Scholar]
- 173.Zhang W, Erkan M, Abiatari I, et al. Expression of extracellular matrix metalloproteinase inducer (emmprin/CD147) in pancreatic neoplasm and pancreatic stellate cells. Cancer Biol Ther. 2007;6(2):218–227. doi: 10.4161/cbt.6.2.3623. [DOI] [PubMed] [Google Scholar]
- 174▪.Stock C, Schwab A. Protons make tumor cells move like clockwork. Pflugers Arch. 2009;458(5):981–992. doi: 10.1007/s00424-009-0677-8. Stock and Schwab’s review on pH regulators is well-done and provides a wealth of information on the influence of pH regulators on cancer. [DOI] [PubMed] [Google Scholar]
- 175.Reshkin SJ, Bellizzi A, Albarani V, et al. Phosphoinositide 3-kinase is involved in the tumor-specific activation of human breast cancer cell Na(+)/H(+) exchange, motility, and invasion induced by serum deprivation. J Biol Chem. 2000;275(8):5361–5369. doi: 10.1074/jbc.275.8.5361. [DOI] [PubMed] [Google Scholar]
- 176.Stock C, Mueller M, Kraehling H, et al. pH nanoenvironment at the surface of single melanoma cells. Cell Physiol Biochem. 2007;20(5):679–686. doi: 10.1159/000107550. [DOI] [PubMed] [Google Scholar]
- 177.Schwab A, Rossmann H, Klein M, et al. Functional role of Na+-HCO3− cotransport in migration of transformed renal epithelial cells. J Physiol. 2005;568(Pt 2):445–458. doi: 10.1113/jphysiol.2005.092957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Morgan PE, Pastorekova S, Stuart-Tilley AK, Alper SL, Casey JR. Interactions of transmembrane carbonic anhydrase, caix, with bicarbonate transporters. Am J Physiol Cell Physiol. 2007;293(2):C738–748. doi: 10.1152/ajpcell.00157.2007. [DOI] [PubMed] [Google Scholar]