Abstract
We have recently reported that the endogenous mGlu2/3 agonist N-acetylaspartylglutamate (NAAG) and the N-acetylated-α-linked-acidic dipepetidase (NAALADase, a NAAG degradation enzyme) inhibitor 2-PMPA significantly inhibit cocaine self-administration and cocaine-induced reinstatement of drug-seeking behavior by attenuating cocaine-enhanced extracellular dopamine and glutamate in the nucleus accumbens. However, the poor oral bioavailability of NAAG and 2-PMPA limits their practical use in humans. In the present study, we investigated the effects of the orally active NAALADase inhibitor GPI-5693 and its enantiomers on cocaine-taking and cocaine-seeking behaviors. We found that oral administration of GPI-5693 (15, 30, 60 mg/kg, p.o.) did not significantly alter intravenous cocaine self-administration under fixed-ratio (FR2) reinforcement, but significantly inhibited cocaine-induced reinstatement of the extinguished drug-seeking behavior. This inhibition was blocked by pretreatment with LY341495, a selective mGlu2/3 receptor antagonist. Pretreatment with the same doses (15, 30, 60 mg/kg, p.o.) of GPI-16476 or GPI-16477, two enantiomers of GPI-5693, also inhibited cocaine-induced reinstatement similar to GPI-5693. In contrast, GPI-5693 altered neither oral sucrose self-administration nor sucrose-triggered reinstatement of sucrose-seeking behavior. These data suggest that orally effective NAAG peptidase inhibitors deserve further study as potential agents for the treatment of cocaine addiction.
Keywords: NAAG, NAALADase, GPI-5693, cocaine, self-administration, reinstatement, relapse
1. Introduction
Cocaine addiction is characterized by high rates of relapse to drug use after abstinence. Despite extensive research, there is no effective medication available for the treatment of cocaine addiction (Shalev et al., 2002; Stewart, 2008). It is well known that cocaine priming significantly increases extracellular dopamine and glutamate in the nucleus accumbens in rats during reinstatement of drug-seeking behavior (Anderson and Pierce, 2005; Kalivas, 2004). Also, local perfusion of the group II metabotropic glutamate receptor (mGlu2/3) agonists, (2R,4S)-APDC or DCG-IV, into the nucleus accumbens inhibits dopamine and glutamate release (Hu et al., 1999; Xi et al., 2002a, 2002b). Based on this, we proposed that mGlu2/3 receptor agonists may be effective in attenuating cocaine-induced relapse by inhibiting cocaine-enhanced dopamine and glutamate in the nucleus accumbens (Xi et al., 2002a, 2002b). LY379268 is a well-characterized systemically active mGlu2/3 agonist (Imre, 2007; Gasparini and Spooren, 2007). When administered systemically or locally into the nucleus accumbens or central amygdala, LY379268 significantly inhibits intravenous cocaine self-administration, cocaine-induced reinstatement, and incubation of cocaine craving in rats and non-human primates (Adewale et al., 2006; Baptista et al., 2004; Lu et al., 2007; Peters and Kalivas, 2006). In accordance with these findings, we have recently demonstrated that intranasal administration of the endogenous mGlu2/3 agonist, N-acetylaspartylglutamate (NAAG), or intraperitoneal administration of the selective NAALADase (a NAAG degradation enzyme) inhibitor, 2-PMPA, significantly attenuates intravenous progressive-ratio cocaine self-administration (Xi et al., 2009a), cocaine-enhanced electrical brain-stimulation reward (Xi et al., 2009a) and cocaine-induced reinstatement of drug-seeking behavior (Xi et al., 2009b). However, the poor oral bioavailability of both NAAG and 2-PMPA limits their practical use in humans (Majer et al., 2003, 2006; Tsukamoto et al., 2005).
In the present study, we investigated the effects of the orally active NAALADase inhibitor GPI-5693 and its enantiomers GPI-16476 and GPI-16477 (Majer et al., 2003, 2006; Tsukamoto et al., 2005) on intravenous cocaine self-administration and cocaine-induced reinstatement of drug-seeking behavior. To determine whether a NAAG-mGlu2/3 receptor mechanism underlies such behavioral effects, we further observed the effects of LY341495, a selective mGlu2/3 receptor antagonist, administered 30 min prior to GPI-5693, on cocaine-induced reinstatement of drug-seeking behavior. Finally, to determine whether GPI-5693 also alters natural (non-drug) reward or reward-seeking, we examined the effects of GPI-5693 on oral sucrose self-administration and sucrose-triggered reinstatement of reward-seeking behavior.
2. Methods
2.1. Animals
Experimentally naïve male Long -Evans rats (Charles River Laboratories, Raleigh, NC, USA) weighing 250 to 300 g were used. Rats were housed individually in a climate-controlled room on a reversed light-dark cycle (lights on at 7:00 PM, lights off at 7:00 AM) with free access to food and water. The animal facility was fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the U.S. National Academy of Sciences, and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse of the U.S. National Institutes of Health.
2.2. Intravenous Cocaine Self-Administration
2.2.1. Surgery
All animals were prepared for experimentation by surgical catheterization of the right external jugular vein. The venous catheters were constructed of microrenathane (Braintree Scientific Inc., Braintree, MA, USA), and catheterization was performed under sodium pentobarbital anaesthesia (60 mg/kg i.p.) with aseptic surgical technique. After exiting the jugular, the catheter passed subcutaneously to the top of the skull, where it exited into a connector (a modified 24 gauge cannula; Plastics One, Roanoke, VA, USA) mounted to the skull with jeweler’s screws and dental acrylic. During experimental sessions, the catheter was connected to the injection pump via tubing encased in a protective metal spring from the head-mounted connector to the top of the experimental chamber. To help prevent clogging, the catheters were flushed daily with a gentamicin-heparin-saline solution (30 IU/ml heparin; ICN Biochemicals, Cleveland, OH, USA).
2.2.2. Apparatus
The i.v. self-administration experiments were conducted in operant response test chambers (32 × 25 × 33 cm) from MED Associates Inc. (Georgia, VT, USA). Each test chamber had 2 levers located 6.5 cm above the floor, 1 active and 1 inactive. Depression of the active lever activated the infusion pump; depression of the inactive lever was counted but had no consequence. A cue light and a speaker were located 12 cm above the active lever. The house light was turned on at the start of each 3 hour test session. When the animal performed a lever-press that resulted in a drug infusion, it was exposed to 2 drug-paired environmental cues: a cue-light and a cue-sound (tone) that lasted for the duration of the infusion. Scheduling of experimental events and data collection were accomplished using MED Associates software.
2.2.3. General procedure
After recovery from surgery, each rat was placed into a test chamber and allowed to lever-press for i.v. cocaine (1 mg/kg/injection) delivered in 0.08 ml over 4.6 seconds, on an FR1 reinforcement schedule. During the 4.6 sec injection time, additional responses on the active lever were recorded but did not lead to additional infusions. Each session lasted 3 hour. The FR1 reinforcement schedule was used for 3–5 days until stable cocaine self-administration was established. The initial cocaine dose of 1 mg/kg/infusion was chosen based on our previous experience that this dose produces the most rapid and facile acquisition of cocaine self-administration behavior. Then, animals were transitioned from FR1 to FR2 reinforcement for continuous cocaine (0.5 mg/kg/infusion) self-administration until the following criteria for stable cocaine-maintained responding were met: less than 10% variability in mean inter-response interval and less than 10% variability in mean number of presses on the active lever for at least 3 consecutive days. To avoid cocaine overdose during the self-administration period, each animal was limited to a maximum of 50 cocaine injections per session. After stable cocaine-maintained responding was achieved, each rat randomly received 1 of 3 doses of GPI-5693 (15, 30, 60 mg/kg) or vehicle (0.5% Tween-80), by gavage, 30 min prior to the test session. Animals then received an additional 5–7 days of cocaine self-administration alone until baseline response rates were re-established prior to testing the next dose of GPI-5693. The order of testing for the various doses of drug or vehicle was counterbalanced according to a Latin square design.
2.3. Cocaine-induced reinstatement of drug-seeking behavior
Additional groups of rats were used to study the effects of GPI-5693 and its enantiomers on cocaine-induced reinstatement. The general procedures for cocaine self-administration prior to behavioral extinction were as described above. After stable cocaine self-administration was established, animals were exposed to extinction conditions, during which cocaine was replaced by saline, and the cocaine-associated cue-light and tone were turned off. Active lever pressing led only to saline infusion. Daily 3 hour extinction sessions for each rat continued until that animal lever-pressed less than 10 times per 3 hour session for at least 3 consecutive days. After the animals met this established extinction criterion, they were divided into 10 dose groups (6–10 rats per group) for reinstatement testing. We chose between-subjects design, because lever responses appeared to decrease over repeated reinstatement tests, making it difficult to re-stabilize basal reinstatement responding before testing the next drug dose.
On the reinstatement test day, each group of animals received either vehicle (0.5 M HEPES, 1 ml.) or one dose of GPI-5693 (15, 30, 60 mg/kg), GPI-16476 (15, 30, 60 mg/kg) or GPI-16477 (15, 30, 60 mg/kg), by oral gavage. Three additional groups of rats were used to further determine whether mGlu2/3 receptors are involved in the pharmacological action of GPI-5693, by pretreating animals with LY341495, a selective mGlu2/3 antagonist, 30 min prior to GPI-5693 (30 mg/kg) or vehicle administration. Thirty minutes after GPI compound administration, all rats were given a priming injection of cocaine (10 mg/kg, i.p.) immediately before initiation of reinstatement testing. During the reinstatement test, the conditions were identical to those in extinction sessions. Active-lever presses (reinstatement) were recorded, although these did not lead to either cocaine infusions or presentation of the conditioned cue-light and tone. Reinstatement test sessions lasted 3 h.
2.4. Sucrose self-administration
To determine whether GPI-5693 selectively inhibits non-drug reward or reward-seeking behavior, we further observed the effects of GPI-5693 on oral sucrose self-administration and sucrose-triggered reinstatement of sucrose-seeking behavior in rats. The procedures for sucrose self-administration were identical to the procedures for cocaine self-administration except for the following: 1) no surgery was performed on the animals in the sucrose experiment; 2) active lever presses led to delivery of 0.1 ml of 5% sucrose solution into a liquid food tray on the operant chamber wall. After stable sucrose self-administration was achieved, animals were divided into three dose groups (vehicle, 30, 60 mg/kg GPI-5693, i.p., 30 min prior to sucrose self-administration), and the effects of GPI-5693 on sucrose self-administration under FR2 reinforcement conditions were assessed.
2.5. Sucrose-triggered reinstatement of sucrose-seeking behavior
The procedures for oral sucrose self-administration were the same as described above. The procedures for extinction and reinstatement testing were identical to the procedures used in the cocaine-triggered reinstatement test above except that reinstatement was triggered initially by 5 “free” sucrose deliveries, and subsequent lever presses were recorded, but these did not lead to either sucrose delivery or presentation of the conditioned cue-light and tone. After sucrose-seeking behavior was extinguished, animals were divided into three dose groups (vehicle, 30, 60 mg/kg GPI-5693, i.p., 30 min prior to reinstatement test), and the effects of GPI-5693 on sucrose-triggered reinstatement of reward-seeking behavior were assessed.
2.6. Drugs
Cocaine HCl (Sigma Chemical Co., Saint Louis, MO, USA) was dissolved in 0.9% NaCl (w/v). GPI-5693 (2-(3-mercaptopropyl)pentanedioic acid (also called 2-MPPA), GPI-16476 (R-2-(3-mercaptopropyl)pentanedioic acid) and GPI-16477 (S-2-(3-mercaptopropyl)pentanedioic acid ) were provided by Guilford Pharmaceuticals Inc. (Baltimore, MD, USA). The GPI compounds were dissolved in 0.5 M HEPES buffer (MP Biomedicals, Inc, Solon, Ohio, USA).
2.7. Data analyses
All data are presented as means (± S.E.M.). One-way or two-way analysis of variance (ANOVA) was used to analyze the effects of GPI-5693 or its enantiomers on cocaine self-administration or cocaine-induced reinstatement of drug-seeking behavior. Individual group comparisons were carried out using the Fisher LSD procedures.
3. Results
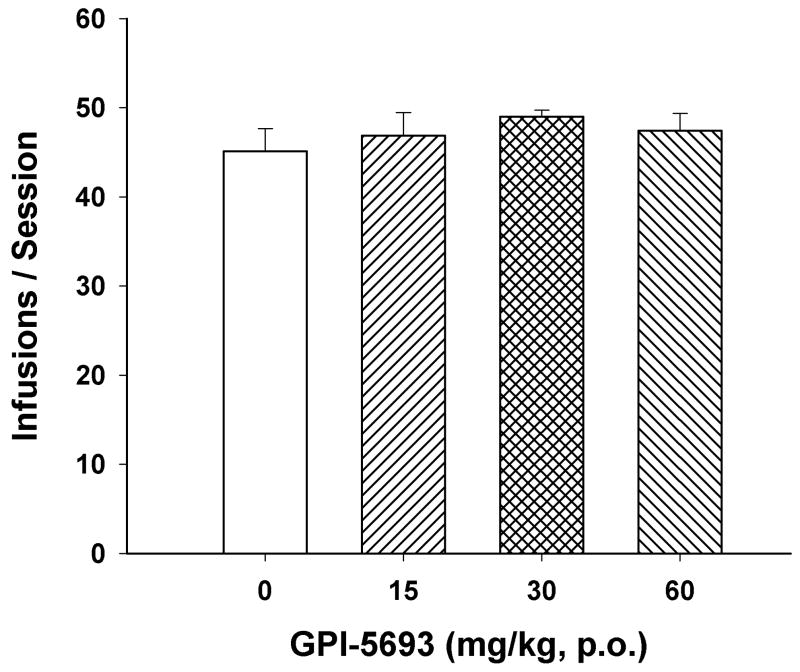
Figure 1 shows the chemical structure of GPI-5693 and 2-PMPA. Figure 2 shows that oral administration of GPI-5693 (15, 30, 60 mg/kg, p.o.) did not significantly alter i.v. cocaine self-administration (F3,29=0.75, P=NS).
Figure 1.
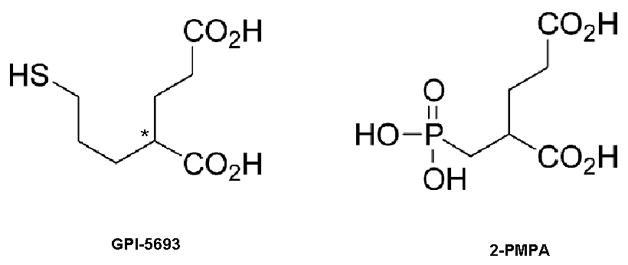
Chemical structures of GPI-5693 and 2-PMPA. GPI-5693 is the first orally active, thiol-based NAALADase inhibitor tested in humans. 2-PMPA is the first prototype phosphonate-based NAALADase inhibitor and has been extensively used in animal models studying the physiological role of NAALADase inhibition and possible therapeutic benefits. * Racemization site.
Figure 2.
Effects of GPI-5693 (15, 30, 60 mg/kg, p.o.) on intravenous cocaine self-administration under FR2 reinforcement.
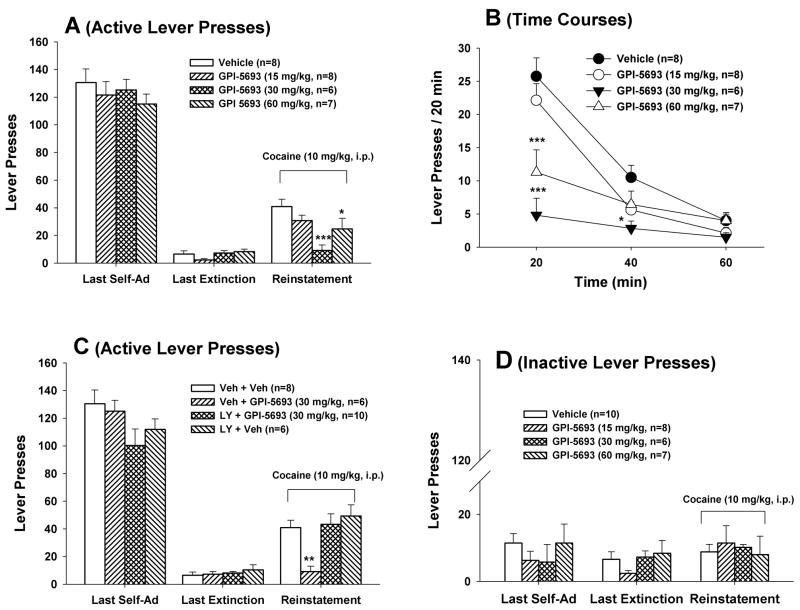
Figure 3A shows that oral administration of the same doses of GPI-5693 significantly inhibited cocaine-induced reinstatement of drug-seeking behavior (F3,25=6.46, P<0.01). Individual group comparisons revealed a statistically significant reduction in active lever presses after 30 mg/kg (t=15.18, P<0.001) and 60 mg/kg (t=14.55, P<0.05) of GPI-5693. Figure 3B shows the time courses of cocaine-induced reinstatement responding in the presence or absence of GPI-5693. Two-way ANOVA with repeated measures over time revealed a statistically significant treatment (GPI-5693 dose) main effect (F3,15=5.88, P<0.01), time main effect (F2,30=61.66, P<0.001) and treatment time interaction ( 6,30=10.98, P<0.001). Individual group comparisons revealed a significant reduction in cocaine-induced reinstatement responding at 20 min (P<0.001) and 40 min (P<0.05) after 30 mg/kg GPI-5693 or at 20 min (P<0.001) after 60 mg/kg GPI-5693, when compared to each time point in the vehicle control group. Fig. 3C shows that pretreatment with LY341495 (1 mg/kg, i.p.) completely blocked GPI-5693-induced inhibition of reinstatement responding to cocaine (F3,26=5.61, P<0.01). LY341495 alone had no effect on cocaine-induced reinstatement. Figure 3D shows the inactive lever response during the reinstatement testing, indicating that cocaine and/or GPI-5693 had no effect on inactive lever pressing.
Figure 3.
Effects of GPI-5693 on cocaine-induced reinstatement of drug-seeking behavior in rats. A: Dose-effects of GPI-5693 on cocaine-triggered reinstatement; B: Time courses of cocaine-induced reinstatement in the presence or absence of GPI-5693; C: Effects of LY341494 pretreatment on cocaine-induced reinstatement; D: Effects of inactive lever responding to cocaine priming in the presence or absence of GPI-5693. *P<0.05, **P<0.01, ***P<0.001, compared to the vehicle treatment group.
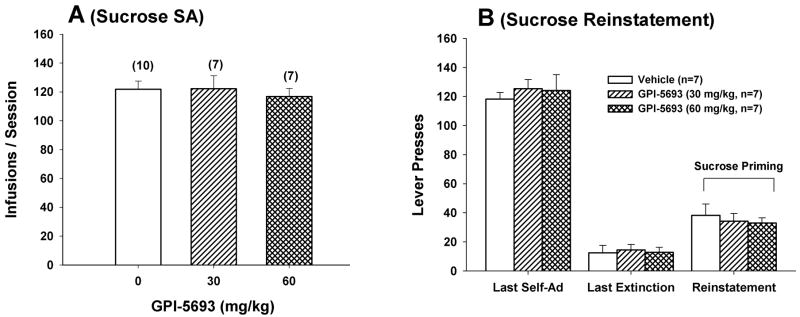
Figure 4 shows that GPI-5693 altered neither oral sucrose self-administration under FR2 reinforcement (F2,21=0.23, P=NS, Fig. 4A) nor sucrose-triggered reinstatement of sucrose- seeking behavior (F2,18=0.26, P=NS, Fig. 4B).
Figure 4.
Effects of GPI-5693 on oral sucrose self-administration and sucrose-triggered reinstatement of sucrose-seeking behavior.
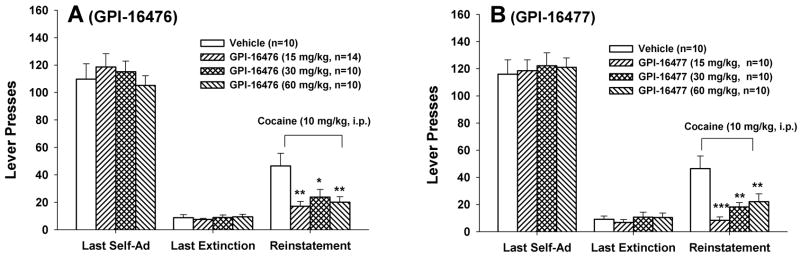
Figure 5 shows that oral administration of the enantiomer GPI-16476 (F3,39=5.37, P<0.01; Fig. 5A) or GPI-16477 (F3,36=8.17, P<0.001; Fig. 5B) also significantly inhibited cocaine- induced reinstatement. Individual group comparisons revealed a statistically significant reduction in active lever presses after 15 mg/kg (P<0.01), 30 mg/kg (P<0.05) or 60 mg/kg (P<0.01) of GPI-16476, and after 15 mg/kg (P<0.001), 30 mg/kg (P<0.01) or 60 mg/kg (P<0.01) of GPI-16477.
Figure 5.
Effects of GPI-16476 (A) or GPI-16477 (B) on cocaine-induced reinstatement of drug-seeking behavior. *P<0.05, **P<0.01, ***P<0.001, compared to the vehicle treatment group.
4. Discussion
NAAG is the most abundant peptide neurotransmitter in the brain (Neale et al., 2000). It is expressed in discrete subsets of neurons and co-released with glutamate or other neurotransmitters upon stimulation. Following its release, NAAG activates group II mGlu receptors located on presynaptic terminals and glial cells and modulates neurotransmitter or glial trophic factor release (Neale et al., 2000, 2005). NAAG is inactivated by NAALADase, which is located on brain glial cells (Baslow, 2000, 2006). In the present study, we found that oral administration of the NAALADase inhibitor, GPI-5693, and its enantiomers significantly inhibited cocaine-induced reinstatement, but not i.v. cocaine self-administration. Pretreatment with LY341495, a selective mGlu2/3 antagonist, blocked GPI-5693-induced inhibition of cocaine seeking, suggesting that GPI-5693 s effect is mediated by activation of mGlu2/3 receptors. In contrast, GPI-5693 altered neither sucrose self-administration, nor sucrose-triggered reinstatement, suggesting that GPI-5693 selectively inhibits cocaine, but not non-drug-induced reinstatement of reward-seeking behavior. These findings are consistent with previous studies indicating that GPI-5693 significantly attenuates cocaine-induced conditioned place preference (CPP) (Slusher et al., 2001), and that 2-PMPA inhibits cocaine-induced behavioral sensitization (Shippenberg et al., 2000), cocaine-induced CPP (Slusher et al., 2001), PR cocaine self-administration (Xi et al., 2009a), cocaine-enhanced brain-stimulation reward (Xi et al., 2009a) and cocaine-induced reinstatement of drug-seeking behavior (Xi et al., 2009b).
In contrast to 2-PMPA, which has poor bioavailability and a highly polar phosphonate group in its structure (Fig. 1), GPI-5693 is orally active (~70% bioavailability) with the IC50 of 90 ± 26 nM and has a less polar thiol group in its structure (Majer et al., 2003, 2006; Tsukamoto et al., 2005, 2007), suggesting that lower molecular polarility may be related to the higher oral bioavailability of GPI-5693. This high oral bioavailability may have clinical utility at the human levels.
There are several possible explanations for the ineffectiveness of GPI-5693 on FR2 cocaine self-administration. First, the cumulative doses (0.5 mg/kg/infusion × 50 maximal infusions = 25 mg/kg) of cocaine under the FR2 reinforcement conditions were higher than the single dose of cocaine (10 mg/kg i.p.) used in the reinstatement study. Thus, the higher cumulative dose of cocaine dose may overcome GPI-5693 s pharmacological effect. Second, animals may compensate for GPI-5693 s action by increasing cocaine intake or self-administration rate under FR2 reinforcement. This possibility is unlikely as we did not observe a compensatory increase in cocaine self-administration (unpublished observation). Third, the different pharmacological action of NAALADase inhibition on cocaine-enhanced dopamine (partial blockade) and glutamate (complete blockade) may contribute to its different action on cocaine self-administration versus reinstatement (Xi et al., 2009b).
GPI-5693 is a racemic mixture of GPI-14676 and GPI-14677 (Majer et al., 2003, 2006). It was expected that only one of the enantiomers would be effective (Tsukamoto et al., 2005). However, we found that both GPI-14676 and GPI-14677 significantly inhibited cocaine-induced reinstatement, with similar efficacy. This is consistent with a previous finding that both GPI-14676 and GPI-14677 exhibited similar efficacy in inhibiting NAALADase activity and neuropathic pain with the IC50 values of 85 ± 33 nM and 67 ± 29 nM, respectively (Majer et al., 2003; Tsukamoto et al., 2005).
Systemic administration of GPI-5693 did not produce a typical pharmacological dose-response relationship. That is, the highest tested dose of GPI-5693 appeared to be less effective than lower doses in attenuating the actions of cocaine on reinstatement. The mechanisms for the loss of efficacy at high doses are unclear. At least two possibilities arise. First, it is possible that high doses of GPI-5693 may interact non-selectively with other functional proteins and/or receptors. Second, a high level of brain NAAG may act on other receptor signaling systems and this action may attenuate the pharmacological action of NAAG on mGlu2/3 receptors.
Another important finding of the present study is that blockade of mGlu2/3 receptors by LY341495 blocked the antagonism by GPI-5693 of cocaine-induced reinstatement, suggesting that a NAAG-mGlu2/3 mechanism underlies the pharmacological action of GPI-5693. This is congruent with previous findings that NAALADase inhibition (with 2-PMPA or ZJ-43) selectively elevates brain NAAG levels (Nagel et al., 2006; Slusher et al., 1999; Zhong et al., 2006), and that blockade of mGlu2/3 by LY341495 blocks 2-PMPA- or NAAG-induced inhibition of cocaine-induced reinstatement or GPI-5693-induced inhibition of glutamate release in the hippocampus (Sanabria et al., 2004; Xi et al., 2009a, 2009b). We note recent reports that the electrophysiological actions of NAAG in vitro cell lines may be mediated by glutamate in unpurified NAAG preparations, suggesting that NAAG may be not a selective mGlu3 receptor agonist (Chopra et al., 2009; Fricker et al., 2009). However, this view is not supported by our findings in vivo that intranasal microinjections, by which the drug is directly delivered from the nose into the brain, of the same doses of NAAG or 2-PMPA, but not glutamate, inhibited cocaine-induced reinstatement of drug-seeking behavior and cocaine-enhanced electrical brain-stimulation reward in rats (Xi et al., 2009a, 2009b).
Given the important role of both dopamine and glutamate in cocaine-triggered reinstatement of drug-seeking behavior (Kalivas, 2004; Knackstedt and Kalivas, 2009), and our recent finding that NAALADase inhibition dose-dependently attenuates both cocaine-induced reinstatement of drug-seeking and cocaine-induced increases in nucleus accumbens dopamine and glutamate (Xi et al., 2009a, 2009b), we hypothesize that a similar NAAG-mGlu2/3-glutamate/dopamine mechanism may underlie the presently-observed antagonism by GPI-5693 of cocaine-induced reinstatement.
In conclusion, the present study demonstrates that oral administration of GPI-5693 significantly inhibits cocaine-induced reinstatement of drug-seeking behavior in male Long Evans rats. Since GPI-5693 has been shown to be safe and well-tolerated in human clinical trials (Van der Post et al., 2005), the present finding supports the potential use of this compound or other orally active NAALADase inhibitors in preventing relapse to drug-seeking behavior.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services. We thank Ajit G. Thomas and Barbara S. Slusher of Guilford Pharmaceuticals Inc., Baltimore, MD for providing the drug GPI-5693 and its enantiomers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adewale AS, Platt DM, Spealman RD. Pharmacological stimulation of group ii metabotropic glutamate receptors reduces cocaine self-administration and cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2006;318:922–931. doi: 10.1124/jpet.106.105387. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Pierce RC. Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacol Ther. 2005;106:389–403. doi: 10.1016/j.pharmthera.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baslow MH. Functions of N-acetyl-L-aspartate and N-acetyl-L-aspartylglutamate in the vertebrate brain: role in glial cell-specific signaling. J Neurochem. 2000;75:453–459. doi: 10.1046/j.1471-4159.2000.0750453.x. [DOI] [PubMed] [Google Scholar]
- Baslow MH. NAAG peptidase as a therapeutic target: Potential for regulating the link between glucose metabolism and cognition. Drug News Perspect. 2006;19:145–150. doi: 10.1358/dnp.2006.19.3.985930. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Adam G, Chaboz S, Henningsen R, Kemp JA, Klingelschmidt A, Metzler V, Monsma F, Schaffhauser H, Wichmann J, Mutel V. Characterization of [3H]-(2S,2′R,3′R)-2-(2′,3′-dicarboxy-cyclopropyl)glycine ([3H]-DCG IV) binding to metabotropic mGlu2 receptor-transfected cell membranes. Br J Pharmacol. 1998;123:497–504. doi: 10.1038/sj.bjp.0701647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra M, Yao Y, Blake TJ, Hampson DR, Johnson EC. The neuroactive peptide N-acetylaspartylglutamate is not an agonist at the metabotropic glutamate receptor subtype 3 of metabotropic glutamate receptor. J Pharmacol Exp Ther. 2009;330:212–219. doi: 10.1124/jpet.109.152553. [DOI] [PubMed] [Google Scholar]
- Fricker AC, Mok MH, de la Flor R, Shah AJ, Woolley M, Dawson LA, Kew JN. Effects of N-acetylaspartylglutamate (NAAG) at group II mGluRs and NMDAR. Neuropharmacology. 2009;56:1060–1067. doi: 10.1016/j.neuropharm.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Gasparini F, Spooren W. Allosteric Modulators for mGlu Receptors. Curr Neuropharmacol. 2007;5:187–194. doi: 10.2174/157015907781695900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Duffy P, Swanson C, Ghasemzadeh MB, Kalivas PW. The regulation of dopamine transmission by metabotropic glutamate receptors. J Pharmacol Exp Ther. 1999;289:412–416. [PubMed] [Google Scholar]
- Imre G. The preclinical properties of a novel group II metabotropic glutamate receptor agonist LY379268. CNS Drug Rev. 2007;13:444–464. doi: 10.1111/j.1527-3458.2007.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Curr Opin Pharmacol. 2009;9:59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Majer P, Hin B, Stoermer D, Adams J, Xu W, Duvall BR, Delahanty G, Liu Q, Stathis MJ, Wozniak KM, Slusher BS, Tsukamoto T. Structural optimization of thiol-based inhibitors of glutamate carboxypeptidase II by modification of the P1′ side chain. J Med Chem. 2006;49:2876–2885. doi: 10.1021/jm051019l. [DOI] [PubMed] [Google Scholar]
- Majer P, Jackson PF, Delahanty G, Grella BS, Ko YS, Li W, Liu Q, Maclin KM, Polakova J, Shaffer KA, Stoermer D, Vitharana D, Wang EY, Zakrzewski A, Rojas C, Slusher BS, Wozniak KM, Burak E, Limsakun T, Tsukamoto T. Synthesis and biological evaluation of thiol-based inhibitors of glutamate carboxypeptidase II: discovery of an orally active GCP II inhibitor. J Med Chem. 2003;46:1989–1996. doi: 10.1021/jm020515w. [DOI] [PubMed] [Google Scholar]
- Nagel J, Belozertseva I, Greco S, Kashkin V, Malyshkin A, Jirgensons A, Shekunova E, Eilbacher B, Bespalov A, Danysz W. Effects of NAAG peptidase inhibitor 2-PMPA in model chronic pain - relation to brain concentration. Neuropharmacology. 2006;51:1163–1171. doi: 10.1016/j.neuropharm.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Neale JH, Bzdega T, Wroblewska B. N-Acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J Neurochem. 2000;75:443–452. doi: 10.1046/j.1471-4159.2000.0750443.x. [DOI] [PubMed] [Google Scholar]
- Neale JH, Olszewski RT, Gehl LM, Wroblewska B, Bzdega T. The neurotransmitter N-acetylaspartylglutamate in models of pain, ALS, diabetic neuropathy, CNS injury and schizophrenia. Trends Pharmacol Sci. 2005;26:477–484. doi: 10.1016/j.tips.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl) 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- Sanabria ER, Wozniak KM, Slusher BS, Keller A. GCP II (NAALADase) inhibition suppresses mossy fiber-CA3 synaptic neurotransmission by a presynaptic mechanism. J Neurophysiol. 2004;91:182–193. doi: 10.1152/jn.00465.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Rea W, Slusher BS. Modulation of behavioral sensitization to cocaine by NAALADase inhibition. Synapse. 2000;38:161–166. doi: 10.1002/1098-2396(200011)38:2<161::AID-SYN7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Slusher BS, Thomas A, Paul M, Schad CA, Ashby CR., Jr Expression and acquisition of the conditioned place preference response to cocaine in rats is blocked by selective inhibitors of the enzyme N-acetylated-alpha-linked-acidic dipeptidase (NAALADase) Synapse. 2001;41:22–28. doi: 10.1002/syn.1056. [DOI] [PubMed] [Google Scholar]
- Slusher BS, Vornov JJ, Thomas AG, Hurn PD, Harukuni I, Bhardwaj A, Traystman RJ, Robinson MB, Britton P, Lu XC, Tortella FC, Wozniak KM, Yudkoff M, Potter BM, Jackson PF. Selective inhibition of NAALADase, which converts NAAG to glutamate, reduces ischemic brain injury. Nat Med. 1999;5:1396–1402. doi: 10.1038/70971. [DOI] [PubMed] [Google Scholar]
- Stewart J. Review. Psychological and neural mechanisms of relapse. Philos Trans R Soc Lond B Biol Sci. 2008;363:3147–3158. doi: 10.1098/rstb.2008.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto T, Majer P, Vitharana D, Ni C, Hin B, Lu XC, Thomas AG, Wozniak KM, Calvin DC, Wu Y, Slusher BS, Scarpetti D, Bonneville GW. Enantiospecificity of glutamate carboxypeptidase II inhibition. J Med Chem. 2005;48:2319–2324. doi: 10.1021/jm049258g. [DOI] [PubMed] [Google Scholar]
- Tsukamoto T, Wozniak KM, Slusher BS. Progress in the discovery and development of glutamate carboxypeptidase II inhibitors. Drug Discov Today. 2007;12:767–776. doi: 10.1016/j.drudis.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Van der Post JP, de Visser SJ, de Kam ML, Woelfler M, Hilt DC, Vornov J, Burak ES, Bortey E, Slusher BS, Limsakun T, Cohen AF, van Gerven JM. The central nervous system effects, pharmacokinetics and safety of the NAALADase-inhibitor GPI 5693. Br J Clin Pharmacol. 2005;60:128–136. doi: 10.1111/j.1365-2125.2005.02396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroblewska B, Wroblewski JT, Pshenichkin S, Surin A, Sullivan SE, Neale JH. N-acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. J Neurochem. 1997;69:174–181. doi: 10.1046/j.1471-4159.1997.69010174.x. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther. 2002a;300:162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006a;26:8531–8536. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Kiyatkin M, Li X, Peng XQ, Wiggins A, Spiller K, Li J, Gardner EL. N-acetylaspartylglutamate (NAAG) inhibits intravenous cocaine self-administration and cocaine-enhanced brain-stimulation reward in rats. Neuropharmacology. 2009a doi: 10.1016/j.neuropharm.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Jr, Gitajn L, Gardner EL. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology. 2006b;31:1393–1405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Li X, Peng XQ, Li J, Gardner EL, Thomas AG, Slusher BS, Ashby CR., Jr Inhibition of N-acetylated-alpha-Linked-Acidic Dipeptidase (NAALADase) Attenuates Cocaine-Induced Relapse in Rats: A NAAG-mGluR2/3-Mediated Mechanism. J Neurochem. 2009b doi: 10.1111/j.1471-4159.2009.06478.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther. 2002b;303:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- Zhong C, Zhao X, Van KC, Bzdega T, Smyth A, Zhou J, Kozikowski AP, Jiang J, O’Connor WT, Berman RF, Neale JH, Lyeth BG. NAAG peptidase inhibitor increases dialysate NAAG and reduces glutamate, aspartate and GABA levels in the dorsal hippocampus following fluid percussion injury in the rat. J Neurochem. 2006;97:1015–1025. doi: 10.1111/j.1471-4159.2006.03786.x. [DOI] [PubMed] [Google Scholar]