Abstract
The seed is an important organ in higher plants, it is an important organ for plant survival and species dispersion. The transition between seed dormancy and germination represents a critical stage in the plant life cycle and it is an important ecological and commercial trait. A dynamic balance of synthesis and catabolism of two antagonistic hormones, abscisic acid (ABA) and giberellins (GAs), controls the equilibrium between seed dormancy and germination. Embryonic ABA plays a central role in induction and maintenance of seed dormancy and also inhibits the transition from embryonic to germination growth. Therefore, the ABA metabolism must be highly regulated at both temporal and spatial levels during phase of dessication tolerance. On the other hand, the ABA levels do not depend exclusively on the seeds because sometimes it becomes a strong sink and imports it from the roots and rhizosphere through the xylem and/or phloem. These events are discussed in depth here. Likewise, the role of some recently characterized genes belonging to seeds of woody species and related to ABA signaling are also included. Finally, although four possible ABA receptors have been reported, not much is known about how they mediate ABA signaling transduction. However, new publications seem to show that almost all these receptors lack several properties to consider them as such.
Key words: ABA/GA balance, ABA in woody plants, ABA-receptors, biosynthetic ABA mutants, rhizosphere ABA, seed dormancy
Introduction
The seed is the dispersal unit emerged in the course of plant evolution. The biology of seeds can be divided in three important phases: development that includes zygotic embryogenesis, dormancy that prevents seeds from germinating under unfavorable conditions and germination (seed emergence). The transition between dormancy and germination represents a critical stage in the life cycle of higher plants and it is an important ecological and commercial trait. Seed germination is regulated by endogenous hormonal cues and external environmental signals such as water, low temperature and light, which influence whether an imbibed seed completes germination or remains dormant (reviewed in refs. 1 and 2). Seed dormancy, a temporary quiescent state that is observed in seeds from many plants species, prevents untimely germination and ensures plant survival by adjusting vegetative development to seasonal changes in the environment.1,3 A dynamic balance between synthesis and catabolism of the abscisic acid (ABA) and gibberellins (GAs) controls the equilibrium between dormancy and germination.4 At the molecular level, the ABA/GA balance is in part determined by the antagonistic control of ABA and GA on each other through their reciprocal regulation of the transcription of their metabolic genes.5–7
The ABA, derived from epoxycarotenoid cleavage, serves as a plant-specific signal during development and in response to environmental stresses such as cold, drought and high concentrations of salt in the soil (reviewed in ref. 8). The ABA also elicits, among others numerous physiological functions, the closure of stomatal pores to restrict transpiration, adjustment of metabolism to tolerate desiccation and cold temperatures, and inhibition seedlings growth. Likewise, ABA represses germination and is presumed to function to stabilize the dormant state (reviewed in refs. 1, 9–11). ABA, like other hormones, functions through a complex network of signaling pathways where the cell response is initiated by ABA perception which triggers downstream signaling cascades to induce the final physiological effects. Numerous downstream components involved in ABA signal transduction have been identified by genetic approaches (reviewed in refs. 12 and 13). Signaling pathways are usually made of regulatory networks of transcription factors (TFs) which specifically bind short DNA sequences (cis-elements) in the regulatory regions (promoters) of their target genes to regulate their expression levels in response to hormonal/environmental signals.14 Recently, it was demonstrated that the N-end rule pathway (i.e., one of several proteolytic pathways of ubiquitin system) promotes seed germination in Arabidopsis through removal of ABA sensitivity.15 In this review, we mainly discuss recent findings that are related with ABA controlled dormancy and signaling at the receptor level.
ABA Biosynthesis Pathway and Temporal and Spatial Expression of its Biosynthetic Genes in Seeds
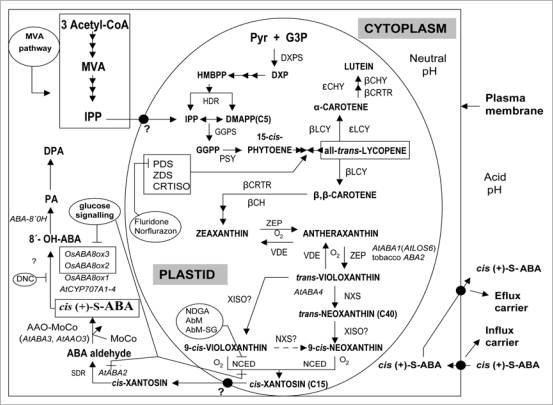
The knowledge of ABA biosynthesis pathway notably advanced in the past few years (updated in Fig. 1). Thus, most intermediates and enzymes involved in its synthesis have been identified and a large number of genes and mutants related to the ABA biosynthetic pathway were also isolated and characterized.8,16–19 But their regulation has mainly been studied in vegetative tissues, often in relation to hydric stress, and expression studies in seeds are still incomplete. ABA is a sexquiterpene derived from oxidative cleavage of phytoene, a C40 common precursor of all plant carotenoids which are synthesized in plastids by nuclear-encoded enzymes.20 The phytoene is synthesized by phytoene-synthase after condensation of two molecules of geranylgeranyl diphosphate (GGPP), a C20 formed from isopentenyl diphosphate (IPP) and its isomer dimethylallyl diphosphate (DMAPP). The IPP can be synthesized from mevalonic acid (MVA), via the cytosolic MVA pathway and subsequently sent to the plastid,21 or alternatively formed from 1-deoxy-D-xylulose 5-phosphate (DXP) which is synthesized in plastid from pyruvate and glyceraldehide 3-phosphate, via the methylerythritol phosphate pathway.22 The all-trans-lycopene synthesized from phytoene is successively converted in β-carotene and zeaxanthin, the first oxygenated carotenoid precursor of ABA, which produces successively antheraxanthin and trans-violaxanthin mediated by ZEP. The all-trans-violaxanthin is either converted to 9-cis-violoxanthin or to 9′-cis-neoxanthin, both C40 carotenoids being cleaved in the plastid to the C15 aldehyde xanthosine and a C25 compound.13 The ABA1/ZEP gene is ubiquitously expressed during A. thaliana seed maturation but their expression becomes restricted to the embryo and endosperm during desiccation.23 Enzyme(s) involved in the conversion of all-transviolaxanthin to 9-cis-violoxanthin or 9′-cis-neoxanthin are yet to be identified. However, the enzymatic step that catalyzes the all-trans-violoxanthin to the all-trans-neoxanthin is the latest to be solved by positional cloning of the AtABA4 gene.24
Figure 1.
ABA biosynthesis pathway, inhibitors and intracellular compartmentalization in higher plants. AbM, abamine; DMAPP, dimethylallyl di-P; DNC, diniconazole; DPA, dihydrophaseic acid; DXP, 1-deoxy-D-xylulose-5-P; G3P, glyceraldehyde-3-P; GGPP, geranylgeranyl di-P; HMBPP, hydroxymethylbutenyl 4-di-P; IPP, isopentenyl di-P; MVA, mevalonic acid; NDGA, nordihydroguaiaretic acid; PA, phaseic acid; Pyr, piruvate. Involved enzymes: AAO-MoCo, abscisic aldehyde oxidase or MoCo sulfurase; βCH, β-carotene hydroxylase; βCHY and βCRTR, β-ring hydroxylases; εCHY, ε-ring hydroxylase; ABA 8′-hydroxylase (Hordeum vulgare, HvABA8'OH); CRTISO, carotenoid isomerase; βCRTR, β-ring hydrolase; DXPS, DXP synthase; GGPS, geranylgeranyl diphosphate synthase; HDR, HMBPP reductase; βLCY, lycopene β-cyclase; εLCY, lycopene ε-cyclase; NCED, 9-cis-epoxycarotenoid dioxigenase (AtNCED1–9; maize, VP14; tomato, NOT); NXS, neoxanthin synthase; PDS, phytoene desaturase; PSY, phytoene synthase; SDR, member of short-chain dehydrogenases/reductases family; VDE, violoxanthin de-epoxidase; XISO, xanthophyll cis-isomerase (predicted); ZDS, ξ-carotene desaturase; ZEP, zeaxanthin epoxidase.
The 9-cis-epoxycarotenoid dioxygenase (NCED) catalyzes the oxidative cleavage of the 9-cis-violoxanthin or 9-cis-neoxanthin, synthesized from all-trans-violoxanthin to produce 9-cis-xanthoxin.25 It is suggested that 9-cis-neoxanthin might be the major substrate in vivo of NCED to produce cis-xantosin, the first cytoplasmic precursor for the catalytic conversion to ABA.17 NCED expression in response to environmental stresses is so rapid that NCED activity is considered the rate-limiting step in ABA biosynthesis. On the other hand, AtCCD1 enzyme catalyzes the oxidative cleavage of the 9,10 (9′,10′) double bonds of carotenoid substrates as β-carotene.26 The NCED activity is inhibited by nordihydroguaiaretic acid (NDGA), abamine AbM; competitive inhibitor;27 and AbM-SG (more potent competitive inhibitor than AbM to reduce ABA accumulation28). NCED genes are not regulated by ABA, which indicates that ABA does not have a positive feedback effect on NCED gene expression.29 Although NCED genes have been characterized in several species, limited data are available about their expression in seeds.8,16 Overexpression of LeNCED gene in tomato leads to higher ABA level in seeds, increasing seed dormancy and induced expression of bean PvNCED1 in imbibed tobacco seeds delays seed germination.30 Differential expression of AtNCED members in different tissues and subcellular localization was found,31 suggesting that a dynamic mobility of ABA precursors and/or ABA to the target sites despite the developing seeds themselves being capable of synthesizing ABA. Similar conclusions were made in the case of AAO family.32,33 Molecular genetic analyses indicate that different members of AtNCED family play distinct roles in the regulation of ABA synthesis during seed development and germination, AtNCED3 (mainly expressed in the base of seed), AtNCED5 and AtNCED6 (both expressed throughout the seed) and AtNCED9, contribute to expression in developing seeds with high levels of AtNCED6 present at an early stage.31 Transcripts of several members of AtNCED family are present in dry seeds.31 AtNCED6 gene is expressed specifically in immature endosperm, and AtNCED9 gene is abundantly expressed in the embryo and endosperm during seed development, playing a major role in ABA synthesis during last steps of zygotic embryogenesis and germination.34 The nced6 and nced9 mutants show reduced ABA contents in dry seeds and radicle emergence of these mutants seeds is not affected by paclobutrazol. Together, the results of Lefebvre et al.34 suggest that cis-xanthosin synthesis is a prerequisite for the induction of seed dormancy. The nced3 mutant was identified in a screen for enhanced germination on hypertonic media,35 concluding these authors that AtNCED3 is involved in germination under hyperosmotic conditions. On the other hand, reduced seed dormancy was only observed in the nced6 nced9 double mutant. Each member of NCED family seems to play a particular role in seeds. Overexpression of bean PvNCED1 in tobacco30 and AtNCED3 in Arabidopsis36 displays an increased ABA levels in seeds and extended seed dormancy. Similar results were also obtained by the overexpression of ABI genes in Arabidopsis (reviewed in ref. 16). Interestingly, unlike other plants with overexpressed NCEDs, prolonged delay of seed germination is the only ABA-related phenotypic effect in the GINCED1 transgenic lines.37
However, ABA levels may not only be controlled by NCED because overexpression of zeaxanthin epoxidase (ZEP) in tobacco resulted in increased ABA levels in mature seed and greater seed dormancy.38 Also, since ABA4 does not convert trans-violoxanthin to its cis isomer, an unknown isomerase is still one of the missing links to the biosynthetic pathway.39 The 9-cis-xanthosin formation is now considered to be the most important regulatory step in ABA biosynthesis.30 The cleavage product 9-cis-xanthosin is further processed in the cytosol to the biologically active cis-configuration ABA, via ABA aldehyde, and involving the short-chain dehydrogenase/reductase (SDR)40 and ABA aldehyde oxidase (AAO; a molybdenum cofactor-requiring enzyme) activities,16,39 respectively. AtSDR1 gene is expressed at low levels in seeds and developing embryos and may contribute to maternally derived ABA synthesis.41 However, reporter gene analysis in Arabidopsis showed strong AtSDR1 expression in seed funiculus and at the junction of pedicels and young siliques.41 Taking into account the results from Cheng's group,41 a possibility exists that in addition to ABA, xanthosin might also be supplied to reproductive organs by vegetative tissues to be further converted into ABA. Since AAO requires the sulfurylated form of a molybdenum cofactor (MoCo) for its activity, mutants defective in MoCo., sulfurase (MOSU) (v.e. AtABA3) also result in ABA deficiency.18,42 Finally, the expression of genes encoding ZEP, SDR, AAO, NCED and MOSU are regulated in organ- and/or stress-specific manners, and theirs transcripts or ABA levels are reduced or eliminated in most mutant allels reflecting the positive feedback of ABA biosynthesis (reviewed in ref. 18).
The committed steps in ABA catabolism are categorized into two types of reactions: hydroxylation and conjugation.8 Normally, the ABA is converted into a compound hormonally inactive and unestable (i.e. 8′-hydroxy ABA) through the intervention of the ABA 8′-hydroxylase, which is a cytochrome P450 monooxygenase (P450),43 whose family has four members in Arabidopsis (AtCYP707A1–4;44). Alternatively, ABA can also be hydroxylated at position C-7′. Recently, an ABA 9′-hydroxylation pathway has been identified.45 The 8′-hydroxy ABA isomerizes spontaneously to phaseic acid (PA) and is further catabolized to dihydrophaseic acid (DPA) by an unknown soluble reductase enzyme.43 Both PA and DPA as ABA can be conjugated to compounds of low molecular weight (i.e. UDP-D-glucose to ABA by means a glycosyltransferase activity).8,46 The decrease in ABA during both barley and Arabidopsis seed imbibition is associated with increase in PA.47,48
It is likely that the ABA hydroxylation is involved in seed dormancy. In recent reports, Arabidopsis aldehyde-oxidase3 (AAO3) was shown to be localized abundantly in vascular tissues of roots, hypocotyls and leaves, indicating that the vascular tissue is an important site of ABA biosynthesis in vegetative tissues.49 On the other hand, AAO3 is scarcely expressed at early phases of seed development and AAO3-mRNA is present in dry viable seeds.50 However, Seo's works32,33 were the first to demonstrate the AtAAO3 expression in seeds. Moreover, the authors concluded that AtAAO3 is the AAO that plays a major role in ABA biosynthesis in Arabidopsis seeds as well as in leaves.33 In addition, genes for the short-chain alcohol dehydrogenase/reductase (AtABA2) and AAO3 are also expressed in the vascular tissues of the embryo during the mid-maturation stage. On the other hand, genetic evidence demonstrated that overexpressing ABA2 in Arabidopsis transgenic plants leads to seed germination delay, and elevated levels of both ABA and dormancy.51 ABA biosynthesis and degradation in Arabidopsis seeds is localized in the embryo as well as in the endosperm.34,52 Thus, CYP707A1 and CYP707A2 genes have been shown, respectively, to play roles in the reduction of ABA content in the embryo at mid-maturation and in both the embryo and endosperm during late maturation.52 The high abundance of CYP707A2-mRNA in the dry seeds, and its transient expression pattern during early imbibition (6 h), suggests that ABA degradation in seeds is mainly achieved by the CYP707A2 isoform.44,53 CYP707A2 is a single copy gene that displays only subtle phenotypes during other developmental stages outside the seed which makes it ideal for genetic manipulation. The CYP707A2-mRNA is localized in the radicle tip and the micropylar endosperm during early imbibition, suggesting that the ABA degradation is mediated by the CYP707A2 enzyme expressed in these tissues. It is speculated that endosperm weakening is delayed in the Arabidopsis cyp707a2 mutant due to impaired ABA degradation and that is, at least in part, the reason for the higher ABA sensitivity of the cyp707a2 endosperm rupture. By contrast, CYP707A1 is hardly expressed during zygotic embryogenesis.44 In short, ABA 8′-hydroxylase family plays a prominent role in regulating endogenous ABA levels during seed development and germination in A. thaliana.52,53 In non dormant Hodeum vulgare seeds, it was demonstrated that HvABA8'OH-1 was expressed strongly and uniformly through the coleorhiza in the region of the primary root tip. These authors conclude that the coleorhiza may be the pivotal tissue in determining whether or not germination occurs.47 Moreover, HvNCED2 is responsible for a significant increase in ABA levels during grain development, whereas HvCYP707A1 is responsible for a rapid subsequent decrease in ABA levels.
The cyp707a2 mutant accumulates more than sixfold ABA content in dry and imbibed seeds and these exhibits hyperdormancy.44 On the other hand, the cyp707a1 mutant accumulated ABA to higher levels in dry seed than cyp707a2 and CYP707A1 was expressed predominantly in the vascular tissue in the embryo during mid-maturation and was downregulated during late-maturation.47 Likewise, these authors demonstrated that both cyp707a mutants exhibited enhanced seed dormancy. Taken together, it is suggested that expression of CYP707As genes, and mainly HvCYP707A1,54 is controlled by environmental cues or GA,7,44,55,56 and has also been found to be the major mechanism regulating ABA catabolism in the seeds of bean57 and barley.53 Finally, the glucose-induced delay of seed germination is a consequence of an increase in the expression of ABA biosynthesis genes (v.e. AtABA2 and AtNCED3)58 or suppression of ABA catabolism genes (v.e. OsABA8ox2 and OsABA8ox3)59 by glucose signaling.
Is Rhizosphere ABA Affecting Plant Growth and Seed Dormancy?
Plant hormones present in soils are believed to play a significant role in root growth and development. However, other functions are not discarded (i.e., rhizosphere microorganisms development, seed-bank physiology, etc.). Microorganisms are considered the primary sources of biologically active substances in soil, although plants may also contribute to the soil pool through root exudation, especially under non-transpiring conditions. At present, there is increasing interest in studies of microorganisms producing phytohormones and hormone-like substances, which determine the formation and development of relationships within natural communities (reviewed in refs. 60 and 61). The ability of plant-associated microorganisms to synthesize some phytohormones is widely known.62–64 Soil microorganisms can either break down65 or produce phytohormones, including ABA.60,66,67 Direct ABA synthesis (via MVA) is largely found in phytopathogenic fungi.67 Microbial communities in soil, particularly the rhizosphere (v.e. soil in which the proliferation of microorganisms is induced by the presence of plants roots) (Fig. 2), possess great potential to produce a vast range of metabolites that may affect plant growth directly after being taken up by the plant, or indirectly by modifying the soil environment.68,69 Rhizosphere bacteria confers beneficial effects for the plants such as increased growth or toleration of abiotic stress.70
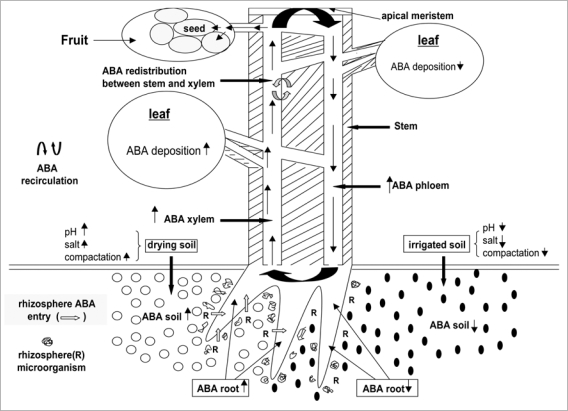
Figure 2.
Presence of ABA in rhizosphere, their entry in roots and distribution toward leaves and seeds (ref. 80).
Soil ABA is necessary to maintain an ABA equilibrium between root and rhizosphere. Moreover, ABA is known to be involved in plant-pathogenic fungi interactions, as the level of ABA in the plant determines its susceptibility to phytopathogenic microorganisms.71,72 Several explanations for this phenomenon have been proposed: plant stimulation of fungal ABA biosynthesis, stimulation of plant ABA biosynthesis by pathogenic fungi, and suppression of metabolic activity of the plant host. Many phytopathogenic fungi synthesize and excrete ABA into the medium.71,73 There are researchers who believe that the ability of phytopathogens to synthesize ABA may be viewed as a factor of pathogenicity in plant infections.73 Notable ABA synthesis and accumulation in roots can be observed in hemiparasites such as Rhinanthus minor. They release ABA in substantial amounts to rhizosphere solution.74
Scarce data exist on ABA levels in soil. However, it is well known that the root is equipped with all the enzymes and precursors that synthesize ABA. A mathematical model predicts that most of the ABA synthesised in the root would move to the soil solution unless the soil water contained at least 1.0 nM ABA at a slightly acid pH.75 Likewise, simulation also predicted that at pH 5.5 in rhizosphere ABA synthesized in roots under stress would be released into xylem rather than into the soil. The Slovik's group postulated that: (1) ABA is likely to be present in the rhizosphere in the low nanomolar range to prevent dramatic loss of ABA from roots; and (2) in most of the cases, the ABA in the soil solution had a concentration that allowed plants to maintain an equilibrium between external and internal ABA. Taken together, the above predicted data indicates that soil ABA concentrations in the low nanomolar range maintain this equilibrium and prevent dramatic ABA loss to the rhizosphere. Hartung et al.76 found in a study with different soils that the ABA in rhizosphere solution ranged from 0.6–2.8 nM. This range, predicted with computer simulations, is required in soils in order to prevent ABA release from the root hair zones of plant roots. The highest concentrations of ABA around 4 nM were detected in acid soils where ABA degradation by rhizosphere microorganisms is weak.76
By contrast, plants growing under alkaline conditions would become ABA deficient, unless a sufficiently high ABA concentration was present in the rhizosphere solution to re-establish equilibrium conditions.75,77 Nevertheless, anatomical apoplastic barriers such as Casparian bands in the exodermis could retard ABA loss and uptake.77–79 ABA retention by roots causes alkalinity tolerance in species which contain these bands, but only in fertilized soils.77 Legumes suffer severe ABA loss into the alkaline surroundings. The absence of the ABA equilibrium between root and soil could disturb the root-to-shoot signaling processes. In drying soils the ABA concentration increases probably because water is removed. It is not clear how much of this external ABA will be taken up under these conditions because water and solute movement in the soil will slow dramatically as it dries. Localized soil drying around the roots will also restrict the uptake of phytohormones in the soil solution. ABA can be accumulated in fruit and seeds in drying soil because these are relatively alkaline compartments.80 Recently, it has been shown that growth-promoting rhizobacteria have an impact on ABA flows in plants. Thus, increased amounts of ABA was detected in the shoots of lettuce that were treated with the cytokinin-producing bacterium Bacillus subtilis.63 These authors concluded that locally high cytokinin concentrations induced ABA biosynthesis in the roots and this ABA would be loaded quickly to the xylem vessels. Auxin-producing rhizobacteria should also be able to affect ABA flows. Since IAA is known to induce the ethylene biosynthesis, an impact of auxin-producing rhizobacteria on ABA production and flows may be expected as previously was demonstrated with rhizobacterium Variovovax paradoxus, an ABA synthetizing microorganism.66,81 On the other hand, exogenous ABA supply to plants can promote rather than inhibit plant growth, perhaps by limiting shoot ethylene production (reviewed in ref. 82). Moreover, microorganisms that are able to degrade ABA in the rhizosphere should be able to influence ABA flows. Whether it is possible for such soil microorganisms to influence ABA signaling in plants remains to be shown.
Finally, the impact of soil conditions and rhizosphere microorganisms on ABA signaling in seeds are far to be known. That is, although the presence of ABA in the rhizosphere appears to be beyond doubt, there is no evidences that the soil seed banks may be affected by this and other phytohormones. There are no data about the possible effects of environmental parameters on both rizhosphere ABA levels and the behavior soil seed banks. Possible influences of rizhosphere ABA on seed dormancy maintenance and seed germination can be of great interest, and therefore should be studied. Likewise, although ABA accumulates in all seed tissues, either as a result of biosynthesis in the seed itself or translocation from the mother plant through the phloem, it is far to know if rizhosphere constitutes a source of ABA. A better knowledge of rizhosphere ABA and its transport toward the seed (sink) would be valuable for the understanding of ABA action in seeds. However, the identification of an ABA transporter is still an enigma.
ABA is Not the Only Signal in Establishment and Dormancy Release
The seed is the organ by which angiosperms disperse and propagate and assures the survival and perpetuation of the mother plant.83 To survive in a particular environment, plants have developed mechanisms that regulate seed germination to coincide with the most appropriate season of the year. One mechanism for proper timing of seed germination is seed dormancy, a genetically and environmentally determined process.1–4,9–11,84 That is, seed dormancy prevents the adverse environmental conditions, maximizes the competitive advantages and ensures the establishement of the mother plant.85,86 Dormancy is the most important altered trait during domestication of wild species87 and its function is similar between different species. An appropriate balance between dormancy and germination is a desirable trait for the crop industry since too much dormancy can lead to non-uniform germination while too little makes seeds germinate early (pre-harvest sprouting).54,88 Pre-harvest sprouting, which is very important in cold and humid environments, reduces grain quality and viability and is one of the most significant losses to industry.85,88
On the other hand, it is still unclear whether all higher plants have a common molecular mechanism for a trait very well preserved traitsuch as seed dormancy. Seed dormancy appears at the end of the seed maturation, in which the cell cycle ceases, molecular dependence from the mother plant disappears, water content decreases, storage products are synthesized, abscisic acid (ABA) is accumulated (i.e., high ABA to GA ratio89), and primary seed dormancy is established,4,8–11,90,91. There is a growing body of scientific evidence that the ABA content in the seed must be lowered in order for dormancy to be broken, and that the germination potential of a given seed is determined, at least in part, by hormones ABA and GA.1,10,92 Thus, the loss of dormancy of many seeds is directly related to the increase in sensitivity to GA91,93–96 and the ABA/GA ratio is important in the maintenance and loss of seed dormancy.89 The mutants with altered ABA/GA ratio seem to prove it. The high levels of ABA in imbibited A. thaliana ecotype Cvi, which is strongly dormant,97 decrease when the dormancy is broken.91 The dormancy state of A. thaliana Cvi accession depends of balance between biosynthesis and catabolism of GA and ABA; that is to say, it depends on the endogenous levels of both bio-active hormones.89,91 The Cadman's group suggests that the key genes to lead to seed germination or dormancy belong to families NCED and CYP707A. Taking together, a dynamic balance between synthesis and catabolism of these two antagonistic hormones controls the equilibrium between dormancy and germination processes by regulating signaling pathways that modify seed sensitivity to the ambient germination environment (reviewed in refs. 98–100). Recently, an interesting study was published on the alterations in metabolism of ABA and GA induced by after-ripening in dormant barley seeds.101 However, a crosstalk between ABA and GA with other signals (v.e. ethylene,98,102–104 reactive oxygen species (ROS),10,59,104 sugars such as glucose,10,59 nitrate105 or calcium-binding protein like calreticulin106), must be taken into account to understand the triggering of seed dormancy process.
ABA Production in Seeds and -Omics Involved in Dormancy Signaling
Seed dormancy is a sufficiently complex process to be controlled by a single endogenous or exogenous factor. There are increasing molecular and genetic evidences that indicate strongly that ABA is central to the establisment and maintenance of both primary1,9–11,96 and secondary107,108 dormancy. Thus, ABA deficiencies during seed development are associated with the absence of primary dormancy of mature seed, whereas overexpression of ABA synthesis genes increases the ABA content and seed dormancy.10,48,96,109,110 On the other hand, endogenous ABA levels and expression pattern of genes involved in its biosynthesis change drastically during seed development in response to developmental and environmental cues.9,16 In addition, the ABA seems to avoid the abortion of the seed and promotes the growth of embryo during zygotic embryogenesis.41,111 An increase in seed abortion in the pea lh-2 mutant indicates that GAs is essential for normal seed development. The lh-2 mutation was shown to be a single base substitution in the ent-kaurene oxidase gene.110 This experiment with lh-2 mutant is one of many that demonstrate the negative correlation ABA/GAs during seed development. During seed development, ABA is synthesized in tissues of different origins conditioning its physiological action. Thus, the ABA accumulated during mid-maturation is of maternal origin, is related to FUS3 and LEC and involved in the inhibition of precocious germination and processes of seed maturation.10,90,111
The ABA de novo synthesized during late-maturation is derived from the zygotic tissues and is essential for desiccation tolerance and induction and maintenance of durable seed dormancy10 (Fig. 3). This synthesized ABA is partially accumulated in dry seed and decreases with seed imbibition. However, the relationship between the ABA content in mature dry seed and the dormancy degree is not yet clear. That is, while the ABA is important to start the dormancy, high ABA levels are not need to maintain it.9 Endogenous ABA in imbibed seed is maintained at a given level that is correlated with the germination potential of the seed. Thus, de novo synthesis of ABA during the imbibition of dormant mature seeds contributes to dormancy maintenance.9,85,91,112 In fact, the degree of seed dormancy is correlated with endogenous ABA levels in imbibed seeds rather than in dry seeds in various species, such as Arabidopsis,91 lettuce,55 barley,47 and tobacco (Nicotiana plumbaginifolia).112 By contrast, those seeds have been subjected to an effective treatment for the dormancy rupture (v.e. after-ripening or stratification), still synthesize ABA; but ABA degradation generally predominates over its biosynthesis8,9,91 (Fig. 3). Several studies have shown that the catabolic removal of ABA is essential for the transition dormancy-germination. The increase in ABA catabolism (i.e., oxidation or conjugation;45) is associated with completion of seed dormancy of barley, Pseudotsuga menziesii, Cupressus nootkatensis and yellow-cedar.47,93,113,114 Thus, mutant seeds deficient in ABA 8′-hydroxilase show increased levels of dormancy.48 The breaking of dormancy by the most-chilling (cold stratification) is because the cold increased the ABA catabolism.9 Interestingly, seed dormancy can be eliminated by smoke,88 and this elimination is accompanied in Nicotiana attenuata seeds by a decrease of 8 times in the ABA content.115
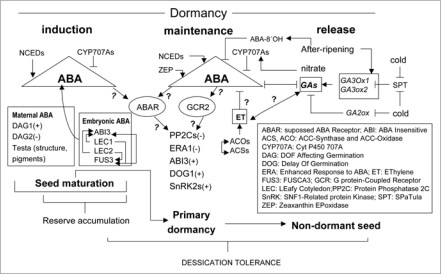
Figure 3.
Possible regulation of seed dormancy status by ABA and its interaction with GAs (cross-talk) and environmental factors (mainly, cold and after-ripening). ABA, ET and GAs means relative hormone levels due to action of theirs anabolic and catabolic enzymes. Promotive and repressive effects are shown by arrows and bars, respectively. Interrogation symbol indicates the absence of data to confirm the effect (refs. 2, 10, 187 and 188).
ABA-like genes appear to have some function in the control of seed dormancy. This feature is supported by studies carried out with orthologue genes (i.e., Vp1 ortologue of ABI3).1,9,96 Although the intensity of Vp1 expression and the degree of seed dormancy seems to be related, more data are needed to confirm it.116 Interestingly, the dormancy state is characterized by the transcription of genes with large presence of ABRE sequences to which transcription factors TFs-like (i.e., bZIP) bind to regulate the seed dormancy.1 Transcriptomic studies have demonstrated the existence in dormant seeds of groups of genes that have very plentiful expression (reviewed in ref. 117). This expression is scantily related to environmental conditions, demonstrating that the dormancy process has its own signaling.89,118 During seed development, environmental factors can significantly influence on the content and sensitivity to ABA of mature seed and alter their dormancy.86,96,119–122 Several mRNAs present in the dormant stage are also found in dry and imbibited seeds, and these transcrips are consistent with those regulated by ABA or environmental stress.89 They have been identified in A. thaliana 30 genes which expression during dormancy phase was higher than under after-ripening status; these genes can be strong candidates to regulate the seed dormancy.118 Within this group of 30 genes are included phosphatases (i.e. ABI1 and ABI2), TFs (ABI3, ABI4 and ABI5) and genes with unknown functions (i.e. (reviewed in ref. 117) DOG1).12,123 Recently, was evidenced a relationship between changes in chromatine structure and transcriptional control of seed dormancy.124
Despite studies of both Cadman and Liu's groups, is currently unknown what is the mechanism by which increases the transcription of genes involved in seed dormancy.10 Likewise, it was not demonstrated that alterations detected in the dormant state are concomitantly related to transcriptional and translational activities.2 However, the post-transcriptional seems to be envolved; at least for some genes.125,126 The delay in breaking of seed dormancy induced by exogenous ABA is associated to regulation of translation at the level of initiation and elongation factors;106 this fact suggests that the dormant status regulates the ability of seed translation. Chibani et al. (2006) show that de novo synthesized proteins during imbibition are very different in dormant and non-dormant seeds.127 Moreover, they also demonstrated that although ABA inhibits germination in non-dormant seeds, does not inhibit translation. Likewise, it was observed that the transcriptomes from after-ripened treated with ABA seeds were more similar to after-ripened non-treated ones than to those dormant seeds.127–129 Only the seeds capable of breaking the dormancy can adquire the ability to reprogram the pattern of protein synthesis during imbibition, allowing completion of the germination process.127 All results discussed until now, together to those of Müller et al. (2006),130 suggest that exogenous ABA inhibits germination by a route different from the one of dormancy.
Seed Dormancy in Woody Species: Searching Genes ABA Regulated
Genetic evidences have shown the involvement of three Arabidopsis Ser/Thr protein phosphatases 2C (AtPP2C), named ABI1, ABI2 and PP2CA, as negative regulators of ABA signaling.131,132 Whereas ABI1 and ABI2 are key regulators in seeds,133 PP2CA does not appear.134 However, from the work of Wu et al.135 in which is demonstrated that ABI1 overexpression does not affect the ABA-signaling pathway, a controversy with regard the role of ABI1 has been created. On the other hand, ABI3, ABI4 and ABI5 encode different types of transcription factors known to act as positive regulators of ABA-mediated regulation of seed development, germination and early seedling growth.10,136
One of the woody species most studied is beechnuts (Fagus silvatica). Their seeds have a dormancy maintained by ABA and eliminated by a long stratification (8 weeks) in water at 4°C.137 Exogenous GA3 proved to be also efficient in breaking the dormancy and could be substituted for cold treatment. These treatments regulate the expression of some dormancy-related genes (v.e. FsPk1 and FsPK2,138 FsERF1,137,139 and FsPP2C1 and FsPP2C2.140,141 The expression of FsPP2C1, a functional PP2C from beechnuts, is: (1) specifically induced in seeds upon ABA treatment but not by drought stress, while low temperatures or GAs treatment decrease the level of transcripts; (2) negatively correlated with seed germination; (3) abolished by treatments that break seed dormancy; (4) upregulated by ABA and its expression is correlated with the level of seed dormancy; and (5) seed exclusive.142 Taking togheter all results, FsPP2C1 is a strong candidate to be a negative regulator of ABA signaling in seeds.142,143 Likewise, the constitutive expression of FsPP2C1 confers ABA insensitivity in seeds and, consequently, a reduced degree of seed dormancy.140 Therefore, the negative regulation of ABA signaling by FsPP2C1 is a factor that contributes to promote the transition from dormancy to germination during early weeks of stratification. Moreover, FsPP2C2 gene was also isolated and characterized in Fagus silvatica seeds.144 Contrary to FsPP2C1,142 it is probably that ABA regulate FsPP2C2 expression in a Ca+2-dependent way, which feature is described for the first time in seeds.144 On the other hand, in Arabidopsis plants overexpressing FsPP2C2 was demonstrated:141 (1) an enhanced sensitivity to ABA and a deeper degree of seed dormancy compared to WT seeds; (2) transgenic lines of 35S:FsPP2C2 contain reduced levels of GA associated with altered expression of GA20ox and GA3ox genes; and (3) FsPP2C2 is localized in the nucleus only in the presence of ABA and strongly supports its involvement in ABA signaling through possibily a reduction of GA biosynthesis, probably affecting GA3-oxidase activity. The above results indicate the existence of potential cross-talk between ABA signaling and GA biosynthesis with a role of FsPP2C2 as a positive regulator of ABA signaling by inhibiting GA biosynthesis. By contrast, HAB1, a protein phosphatase type-2C, seems to play as a negative regulator of ABA signaling.145 More recently, a proteomic approach was used to analyze mechanisms of dormancy breaking in beechnuts seeds and the participation of ABA and GAs in this process.106 Most of the ABA-responsive proteins are involved in protein destination, energy metabolism and development. Finally, CnABI3, an ABI3/VP1 gene homologue, was cloned from yellow cedar, a conifer that produces seeds that are deeply dormant at maturity.146 CnABI3 was synthesized exclusively in megagametophyte and embryo of dormant mature and warm stratified seeds, but decline during subsequent moist chilling, a treatment effective in breaking dormancy.
How Many ABA Receptors?
Background.
Several biochemical and genetic approaches have permitted the characterization of numerous components envolved in ABA biosynthesis and signaling.8,10,110,123,147 However, until the last decade there was a failure in the approach to the identification and characterization of ABA receptors. That is, although proteins that bind ABA have been identified, no strong evidence has been presented to link them to physiological effects of ABA in vivo. At present, four supposed ABA receptors exist in A. thaliana: the nuclear flowering-time protein FCA,148 the plastid-associated Mg-chelatase H subunit (CHLH),149 a protein originally identified as a membrane-bound G-protein-coupled receptor (GCR2)124 and, recently, two novel G-protein coupled receptors (GPCRs), GPCR-type G proteins (GTG) 1 and GTG2.150 This variety of cellular sites for potential ABA perception may be a way of explaining the complexity of its signaling and suggests that multiple receptors exist for ABA. Moreover, biochemical and electrophysiological studies provide evidences for both intracellular (i.e., CHCL and FCA) and extracellular (i.e., GCR2, GTG1 and GTG2) perception of the ABA.150–152 The most important implications is that ABA acts simultaneously and independently at multiple sites in the cell and evokes different responses at each site.153
CHLH (magnesium protoporphyrin-IX CHeLatase H subunit).
On the year 2002, a protein (putative ABA receptor; ABAR) with affinity by ABA and involved in stomatal signaling, was isolated from Vicia faba.154 El gen CHLH was identified in Arabidopsis as a homologue of ABAR.149 ABAR/CHLH encode for the subunit H of Mg-protoporphyrin IX chelatase, an enzyme located in chloroplasts and key component in both chlorophyll biosynthesis and plastid-to-nucleus signaling. ABAR is a single-copy gene in the A. thaliana genome, is highly conserved in higher plants and shares high sequence similarities to its homologues in bacteria. It is said that CHLH perceives the ABA signal since in seed germination: (1) ABAR binds specifically to ABA; (2) transgenic downregulation of ABAR expression results in a decline in the number of ABA-binding sites and leads to ABA-insensitive phenotypes; (3) ABAR-overexpressing plants have ABA-hypersensitive phenotypes with a elevated number of ABA-binding sites; (4) a LOF mutation in ABAR results in an immature embryo; and (5) a chlh mutant that downregulates both ABAR expression and ABA-binding activity is an ABA-insensitive mutant like the post-transcriptional gene-silencing RNAi or antisense mutants.149 CHLH-mRNA is also present in seeds,149 and abar-1 seeds are deficient in lipid and mature protein bodies, indicating a possible alteration of late embryonic development.123 In short, CHLH has been proposed to be an ABA receptor involved in mediating cellular responses to ABA during seed development (Fig. 3), germination and post-germination growth and stomatal movement.149 However, the CHLH-ABA binding assays used were similar to those of the paper retracted on July 14 2008,147 and therefore further experiments are required to validate the role of CHLH.155
FCA (flowering time control protein A).
Although ABA-binding protein FCA is predominantly localized to the nucleus, it shares sequence homology at the C-terminus with ABAP1, an in vitro ABA-binding protein that is associated with the plasma-membrane of barley aleurone cells.156 According to the Razem group, ABAP1 is an ABA-inducible protein, and posseses: (1) high affinity for 3H-(+)-ABA; (2) saturation kinetic and specificity for S-(+)-ABA; and (3) the ability to bind to both natural precursors of ABA i.e., (+)-ABA aldehyde and (+)-ABA alcohol.156 However, because the difficulty of purifying ABAP1, and the same happens with CHLH-ABA binding, the published results of ABAP1-ABA binding are subjected to criticisms.156 Like ABAR/CHCL, FCA binds ABA with an interaction that is stereospecific (i.e., FCA binds (+)-ABA but not two of the non-active ABA analogues (−)-ABA and trans-(+)-ABA) and follows receptor kinetics.148 The FY protein, a RNA 3′-end processing factor, is required to FCA funtion157 and ABA disrupts the FCA-FY interaction in vitro and FCA function in vivo.148 Through the use of mutants abi-1 and abi-2, it was demonstrated that FCA and ABI1 proteins are involved in distinct ABA responses.148 Seeds of mutant fca-1 were used to examine the posible role of FCA in germination. Because none of the fca-1 seeds germinated in the presence of ABA, the authors concluded that FCA is not required for Arabidopsis seed germination. FCA is not also required for stomatal opening. Taking one thing with another, FCA has been proposed to be an ABA receptor, which controls ABA-mediated RNA metabolism and flowering.148
GCR (G-protein-coupled receptor).
In contrast to the ABA intracellular perception, several experiments had suggested that extracellular perception is critical to achieve ABA functions. That is, ABA receptors localized at cellular periphery must be necessary to recognize external ABA. At the cell surface (i.e., plasma-membrane), the ABA signal was recently proposed to be perceived by GCR2, which acts as an extracellular-ABA receptor and controls all the major responses mediated by ABA (Fig. 3), including seed germination.124 Thus, the T-DNA insertional mutations of GCR2 causes expression of ABA inducible genes and ABA insensitivity in seeds germination. GCR2 specifically binds with high affinity to natural occurring ABA, but not to the physiologically inactive isomer (trans-ABA). Moreover, GCRC2 interacts physically with GPA1, the only Arabidopsis Gα subunit of trimeric G-protein, and the binding of ABA to GCR2 disrupts the GCR2-GPA1 interaction.124 On the other hand, it was also shown that LOF gcr2 exhibits all known ABA defects, and overexpression of GCR2 shows an ABA-hypersensitive phenotype.158 These authors suggest that GCR2 and GCR2-likes genes (i.e., GCL1 and GCL2) share partial functional redundance,158 being this fact recently demostrated.159 Likewise, it was also proved that GCR2, GCL1 and GCL2, the three only members of GCR2 family in Arabidopsis, are not required for ABA response in seed germination,159 thus discarding that GCR2 functions as an ABA receptor in this process.124 Therefore, because it was not discovered any morphological or conditional phenotypes in all gcr2 mutants, the exact role of GCR2 in plants remains unknown and possibility that GCR2 is an ABA receptor is at present debatable.158 However, it was previously demonstrated that the overexpression of GCR1 abolished seed dormancy160 and that GCR1 interacts specifically with GPA1 suggesting that GCR1 is a component of an ABA perception and signaling complex.161 Interestingly, it was also suggested that GCR2 may actually be a member of the bacterial lanthionine synthetase (LanC),158 and define a new type of “non-classical” ABA-signaling G-protein-coupled receptor (GPCR) required for ABA perception.162
GT1 and GT2 (GPCR-type G-proteins).
G-proteins are involved in several fundamental growth and developmental processes in higher plants (reviewed in ref. 163). Phenotypic analysis of null mutants were used to demonstrate that G-proteins also modulate the seed germination.164 GPCR-like proteins exist in plants.165 In the year 2009, two proteins named GTG1 and GTG2 (GPCR-type G-proteins 1 and 2), were characterized.150 Both GTG1 and GTG2 proteins: (1) are topologycally similar to GPCRs but with classic GTP-binding/GTPase activity; (2) interact with GPA1; (3) have highly specific ABA binding; (4) posses two different conformations, GTP-bound and GDP-bound; (5) have dependence of the efficiency of ABA-binding on their conformation; (6) are consistent with their proposed role in ABA signaling, Arabidopsis gtg1/gtg2 double mutants show typical hyposensitivity to ABA, including reduced seed dormancy; and (7) no effect of ABA on expression of GTG transcripts, indicating that the role of GTG proteins in ABA signaling is posttranslational.150 However, these authors report that ABA-binding experiments were carried out with purified protein; but only 1% of the purified GTP proteins were capable of binding ABA.
News, Views and Controversies
The approach of the study of hormone receptors is highly complex. Thus, although it is relatively feasible to isolate and characterize molecules that bind to a hormonal ligand, it is not easy to prove that some of them fulfil the conditions that must have a receptor. Between the end of the second millenium and the beginning of the third, the receptors of several plant hormones have been characterized. Likewise, mutants with an impediment in the hormone-receptor binding were also isolated.166 By contrast, the speed to get the ABA receptor was rapid but very slippery, and some approaches have ended in failure and others that seemed correct, still need to be strongly confirmed. The use of sequence analysis and bioinformatics approaches appears to be the main weaknesses of developped protocols to confirm a true ABA receptor. To confirm these criticisms, we will refer to very recent manuscripts that do not agree with certain publications listed in section 6.1. In the last two years: (2) Risk et al. (2008)167 found no evidence to indicate that FCA, a RNA-binding protein reported by Razem et al. (2006) as an ABA receptor,148 is an ABA receptor; (3) Jones and Sussman (2009) propose that “A ligand binds to its cognate receptor reversibly, saturably, selectively, and with a stoichiometry of one or more molecules of ligand per molecule protein and the binding is usually heat sensitive and affected by proteases”;168 (3) Risk et al. (2009)167 showed that the putative extracellular ABA receptor GCR2, a protein originally identified by Liu et al. (2007) as a membrane-bound G-protein coupled receptors,124 does not bind ABA;155 other researchers have also questioned some results of Liu's work;162,169,170 (4) McCourt and Creelman (2008) published an interesting review with a title no less interesting and subliminally intentional: “The ABA receptors—we report you decide”;171 and finally, (5) Pandey et al. (2009),150 with the isolation and characterization of GTG1 and GTG2, novel GPCR proteins, may be closer to the coveted ABA receptor(s). However, some experimental criticisms (i.e., protein purification and ABA-binding experiments) still cast a shadow over the protocole carried out by the Pandey group.
ABA Mutants as Key to Breakthrough
Notable progresses are currently being carried out on dormancy at the transcriptomic,14,89,118,129 proteomic127 and metabolomic172 levels. ABA-mutants with alterations in the degree of seed dormancy and germination provide special tools to approach to the understanding of the mechanisms of dormancy. The first participants loci in the dormancy of A. thaliana seeds were identified in the last years of the twentieth century through mutations affecting the ABA biosynthesis and signaling (reviewed in ref. 94). At present, although there are genes that are specifically expressed in dormant seeds, it has not yet been convincingly shown that the products of these genes directly affect inhibiting the germination process or how ABA is involved in it. Some characterized ABA mutants for building-up the ABA signaling pathway related to the seed dormancy appears in an updated Table 1.
Table 1.
Selected ABA synthesis/catabolism and response genes involved in moduling seed dormancy
| Species | Gene/locus | Protein | Mutant/transgenic line | Effects on dormancy and ABA sensitivity | References |
| Synthesis/Catabolism | |||||
| Arabidopsis | ABA1 | Zeaxanthin epoxidase (ZEP) | aba1 | Reduced | 173–175 |
| ABA2 | Short-chain dehydrogenasereductase (AB-SDR) | aba2 | Reduced | 40, 41, 176 | |
| ABA3 | Molybdenum cofactor sulfurase (MCS) | aba3 | Reduced | 42, 173 | |
| AtNCED6 AtNCED9 | 9-cis Epoxycarotenoid dioxygenase (NCED) | Atnced6/Atnced9 double mutant | Reduced | 31, 34 | |
| AAO3 | Aldehyde oxidase 3 | Aao3-1 | Slightly reduced | 33 | |
| CYP707A2 | ABA 8′-hydroxylase | cyp707a2-1 cyp707a2-2 | Enhanced | 48 | |
| CYP707A1 | ABA 8′-hydroxylase | cyp707a1 | Enhanced | 52 | |
| Zea mays | VP14 | 9-cis Epoxycarotenoid dioxygenase (NCED) | vp14 | Vivipary, Reduced | 177 |
| Nicotiana plumbaginifolia | NpABA1 | Zeaxanthin epoxidase (ZEP) | Npaba1 | Reduced | 111 |
| NpABA2 | Npaba2 | Reduced | 16 | ||
| Lycopersicum esculentum | NOT | 9-cis Epoxycarotenoid dioxygenase (NCED) | not | Reduced | 178 |
| Response | |||||
| Arabidopsis | ABI1 | PP2CSer/Thr protein phosphatase | abi1-1 | Reduced; ABA insensitive | 179 |
| ABI2 | PP2CSer/Thr protein phosphatase | abi2-1 | Reduced; ABA insensitive | 180 | |
| ABI3 | TF specific seeds, B3 domain | abi3 | Reduced; ABA insensitive | 98, 181 | |
| ABI4 | TF specific seeds, DREB subfamily A-3 of ERF/APETALA TF | abi4 | Normal; ABA insensitive | 173, 182 | |
| ABI5 | TF specific seeds, bZIP | abi5 | Normal; ABA insensitive | 183, 184 | |
| ERA1 | Farnesyl transferase | era1 | Enhanced | 179 | |
| ERA3 | Farnesyl transferase | era3 | Enhanced | 174 | |
| AHG1 | Putative protein phosphatase 2C (PP2C) | ahg1-1 | Enhanced | 126 | |
| SAD1 | Sm-like snRNP protein | sad1 | Enhanced | 177 | |
| MARD1 | Zinc-finger protein (TF?) | mard1 | Reduced; ABA insensitive | 185 | |
| Zea mays | VP1 | TF specific seeds, B3 domain | vp1 | Vivipary or reduced dormancy; ABA insensitive | 186 |
TF, transcription factor.
Conclusions
The ABA is a well-known hormone that participates in the induction and maintenance of seed dormancy. A negative correlation ABA/GAs during seed development was demonstrated. However, the role of ET in this ABA/GAs cross-talk, if it has one, is not clarified. ET, together with GAs, antagonize ABA actions during dormancy induction/termination and germination. In recent years, progress has been made, but a significant number of gaps in ABA signaling still need to be filled. Perhaps the existence of genetic redundancy in plants is one of the culprits. Isolation and characterization of genes in the synthesis and deactivation pathways of ABA were and are key tools to decipher the spatial compartimentalization of ABA and its participation in different kinds of seed dormancy. Besides, the application of molecular genetic tools and the large-scale transcriptome and proteome technologies newly available to seed biology will be powerful aids in developing our understanding of the exact roles of each phytohormone in seed dormancy. Currently, it is unknown the role of DELLA and basic helix-loop-helix (HLH) proteins, negative regulators of GAs signaling, in seed dormancy. Likewise, no information is available about the alleged action of ET on both GAs regulators. On the other hand, as updated in this review, the existing puzzle on ABA receptors is, at present, indecipherable. A high percentage of failure lies in the methodology used to check the binding and specificity in the ABA-receptor complex. It would be interesting to use measures of free-energy to check the binding ABA-ligand, and make sure that the ligand (v.e. a protein) has not undergone changes throughout the experimental procedure. Finally, deep experimentation on the ABA levels in the rhizosphere is needed, and its potential impact on seed dormancy in soil banks must be analyzed. We must not forget that the soil is the natural habitat of the seeds once dispersed from mother plant.
Acknowledgements
We thank our colleagues who kindly provided manuscripts for this overview. The authors apologize to those whose work could not be included in the space available. This work was supported by Ministerio de Educación y Ciencia (Spain; grant no. CGL2009-11425/BOS). We want to expressly thank Dr. Abelenda (CNB, CSIC, Madrid, Spain) for the help in the search of mutants of ABA.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article9902
References
- 1.Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. New Phytol. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 2.Holdsworth MJ, Finch-Savage WE, Grappin P, Job D. Post-genomics dissection of seed dormancy and germination. Trends Plant Sci. 2008;13:7–13. doi: 10.1016/j.tplants.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Donohue K, Dorn L, Griffith C, Kim E, Aguilera A, Polisetty CR, et al. The evolutionary ecology of seed germination of Arabidopsis thaliana: variable natural selection on germination timing. Evol Int Org J Evol. 2005;59:758–770. [PubMed] [Google Scholar]
- 4.Gutierrez L, Van Wuytswinkel O, Castelain M, Bellini C. Combined networks regulating seed maturation. Trends Plant Sci. 2007;12:294–300. doi: 10.1016/j.tplants.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Seo M, Hanada A, Kuwahara A, Endo A, Okamoto M, Yamauchi Y, et al. Regulation of hormone metabolism in Arabidopsis seeds: phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J. 2006;48:354–366. doi: 10.1111/j.1365-313X.2006.02881.x. [DOI] [PubMed] [Google Scholar]
- 6.Oh E, Yamaguchi S, Hu J, Yusuke J, Jung B, Paik I, et al. PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell. 2007;19:1192–1208. doi: 10.1105/tpc.107.050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toh S, Imamura A, Watanabe A, Nakabayashi K, Okamoto M, Jikumaru Y, et al. High temperature induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiol. 2008;146:1368–1385. doi: 10.1104/pp.107.113738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nambara E, Marion-Poll A. Abscisic acid biosynthesis and catabolism. Ann Rev Plant Biol. 2005;56:165–185. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- 9.Kermode AR. Role of abscisic acid in seed dormancy. J Plant Growth Regul. 2005;24:319–344. [Google Scholar]
- 10.Finkelstein RR, Reevers W, Ariizumi T, Steber C. Molecular aspects of seed dormancy. Ann Rev Plant Biol. 2008;59:387–415. doi: 10.1146/annurev.arplant.59.032607.092740. [DOI] [PubMed] [Google Scholar]
- 11.Bentsink L, Soppe WJJ. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol. 2008;179:33–54. doi: 10.1111/j.1469-8137.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 12.Himmelbach A, Yang Y, Grill E. Relay and control of abscisic acid signalling. Curr Opin Plant Biol. 2003;6:470–479. doi: 10.1016/s1369-5266(03)00090-6. [DOI] [PubMed] [Google Scholar]
- 13.Shinozaki K, Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. J Exp Bot. 2007;58:221–227. doi: 10.1093/jxb/erl164. [DOI] [PubMed] [Google Scholar]
- 14.Nakabayashi K, Okamoto M, Koshiba T, Kamiya Y, Nambara E. Genome-wide profiling of stored mRNA in Arabidopsis thaliana seed germination: epigenetic and genetic regulation of transcription in seed. Plant J. 2005;41:697–709. doi: 10.1111/j.1365-313X.2005.02337.x. [DOI] [PubMed] [Google Scholar]
- 15.Holman TJ, Jones PD, Russell L, Medhurst A, Ubeda Tomás S, Talloji P, et al. The N-end rule pathway promotes seed germination and establishment through removal of ABA sensitivity in Arabidopsis. Proc Natl Acad Sci USA. 2009;106:4549–4554. doi: 10.1073/pnas.0810280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo M, Koshiba T. Complex regulation of ABA biosynthesis in plants. Trends Plant Sci. 2002;7:41–48. doi: 10.1016/s1360-1385(01)02187-2. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz SH, Qin X, Zeevaart JA. Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes and enzymes. Plant Physiol. 2003;131:1591–1601. doi: 10.1104/pp.102.017921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong L, Zhu JK. Regulation of abscisic acid biosynthesis. Plant Physiol. 2003;133:26–36. doi: 10.1104/pp.103.025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marion-Poll A, Leung J. Abscisic acid synthesis, metabolism and signal transduction. In: Hedden P, Thomas SG, editors. Plant Hormone Signalling, Ann Plant Rev. Vol. 24. Oxford UK: Blackwell Publishers; 2006. pp. 1–35. [Google Scholar]
- 20.Fraser PD, Bramley PM. The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res. 2004;43:228–265. doi: 10.1016/j.plipres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Lange BM, Rujan T, Martin W, Croteau R. Isoprenoid biosynthesis: The evolution of two ancian and distinct pathways across genomes. Proc Natl Acad Sci USA. 2000;97:13172–13177. doi: 10.1073/pnas.240454797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichtebthaler HK, Schwender J, Disch A, Rohmer M. Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway. FEBS Letts. 1997;400:271–274. doi: 10.1016/s0014-5793(96)01404-4. [DOI] [PubMed] [Google Scholar]
- 23.Audran C, Liotenberg S, Gonneau M, et al. Localisation and expression of zeaxanthin epoxidase mRNA in Arabidopsis in response to drought stress and during seed development. Aust J Plant Physiol. 2001;28:1161–1173. [Google Scholar]
- 24.North HM, De Almeida A, Boutin JP, Frey A, To A, Botran L, et al. The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers. Plant J. 2007;50:810–824. doi: 10.1111/j.1365-313X.2007.03094.x. [DOI] [PubMed] [Google Scholar]
- 25.Chernys JT, Zeevaart JAD. Characterization of the 9-cis-epoxycarotenoid dioxygenase gene family and the regulation of abscisic acid biosynthesis in avocado. Plant Physiol. 2000;124:343–353. doi: 10.1104/pp.124.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt H, Kurtzer R, Elsenreich W, Schwab W. The carotenase AtCCD1 from A. thaliana is a dioxygenase. J Biol Chem. 2006;281:9845–9851. doi: 10.1074/jbc.M511668200. [DOI] [PubMed] [Google Scholar]
- 27.Han SY, Kitahata N, Sekimata K, Saito T, Kobayashi M, Nakashima K, et al. A novel inhibitor of 9-cisepoxycarotenoid dioxygenase in abscisic acid biosynthesis in higher plants. Plant Physiol. 2004;135:1574–1582. doi: 10.1104/pp.104.039511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitahata N, Han SY, Noji N, Saito T, Kobayashi M, Nakano T, et al. A 9-cis-epoxycarotenoid dioxygenase inhibitor for use in the elucidation of abscisic acid action mechanisms. Bioorg Med Chemi. 2006;14:5555–5561. doi: 10.1016/j.bmc.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 29.Iuchi S, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K. A stress-inducible gene for 9-cis-epoxycarotenoid dioxygenase involved in abscisic acid biosynthesis under water stress in drought-tolerant cowpea. Plant Physiol. 2000;123:553–562. doi: 10.1104/pp.123.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin X, Zeevaart JAD. Overexpression of a 9-cis-epoxycarotenoid dioxygenase gene in Nicotiana plumbaginifolia increases abscisic acid and phaseic acid levels and enhances drought tolerance. Plant Physiol. 2002;128:544–551. doi: 10.1104/pp.010663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, et al. Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J. 2003;35:44–56. doi: 10.1046/j.1365-313x.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- 32.Koiwai H, Nakaminami K, Seo M, Mitsuhashi W, Toyomasu T, Koshiba T. Tissue-specific localization of an abscisic acid biosynthetic enzyme, AAO3, in Arabidopsis. Plant Physiol. 2004;134:158–165. doi: 10.1104/pp.103.036970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo M, Aoki H, Koiwai H, Kamiya Y, Nambara E, Koshiba T. Comparative studies on the Arabidopsis aldehyde oxidase (AAO) gene family revealed a major role of AAO3 in ABA biosynthesis in seeds. Plant Cell Physiol. 2004;45:1694–1703. doi: 10.1093/pcp/pch198. [DOI] [PubMed] [Google Scholar]
- 34.Lefebvre V, North H, Frey A, Sotta B, Seo M, Okamoto M, et al. Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J. 2006;45:309–319. doi: 10.1111/j.1365-313X.2005.02622.x. [DOI] [PubMed] [Google Scholar]
- 35.Ruggiero B, Koiwa H, Manabe Y, Quist TM, Inan G, Saccardo F, et al. Uncoupling the effects of ABA on plant growth and water relations: analysis of sto1/nced3, an ABA-deficient but salt-tolerant mutant in Arabidopsis. Plant Physiol. 2004;136:3134–3147. doi: 10.1104/pp.104.046169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, et al. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001;27:325–333. doi: 10.1046/j.1365-313x.2001.01096.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhu C, Kauder F, Römer S, Sandmann G. Cloning of two individual cDNAs encoding 9-cis-epoxycarotenoid dioxygenase from Gentiana lutea, their tissue-specific expression and physiological effect in transgenic tobacco. J Plant Physiol. 2007;164:195–204. doi: 10.1016/j.jplph.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Frey A, Audran C, Marin E, Sotta B, Marion-Poll A. Engineering seed dormancy by the modification of zeaxanthin epoxidase gene expression. Plant Mol Biol. 1999;39:1267–1274. doi: 10.1023/a:1006145025631. [DOI] [PubMed] [Google Scholar]
- 39.Seo M, Peeters AJ, Koiwai H, Oritani T, Marion-Poll A, Zeevaart JA, et al. The Arabidopsis aldehyde oxidase 3 (AAO3) gene product catalyzes the final step in abscisic acid biosynthesis in leaves. Proc Natl Acad Sci USA. 2000;97:12908–12913. doi: 10.1073/pnas.220426197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.González-Guzmán M, Apostolova N, Bellés JM, Barrero JM, Piqueras P, Ponce MR, et al. The short-chain alcohol dehydrogenase ABA2 catalyzes the conversion of xanthoxin to abscisic aldehyde. Plant Cell. 2002;14:1833–1846. doi: 10.1105/tpc.002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng WH, Endo A, Zhou L, Penney J, Chen HC, Arroyo A, et al. A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell. 2002;14:2723–2743. doi: 10.1105/tpc.006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong L, Ishitani M, Lee H, Zhu JK. The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell. 2001;13:2063–2083. doi: 10.1105/TPC.010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaharia LI, Walker-Simmon MK, Nicolás C, Abrams SR. Chemistry of abscisic acid, abscisic acid catabolites and analogs. J Plant Growth Regul. 2005;24:274–284. [Google Scholar]
- 44.Saito S, Hirai N, Matsumoto C, Ohigashi H, Ohta D, Sakata K, et al. Arabidopsis CYP707As encode (+)-abscisic acid 8′-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiol. 2004;134:1439–1449. doi: 10.1104/pp.103.037614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou R, Cutler AJ, Ambrose SJ, Galka MM, Nelson KM, Squires TM, et al. A new abscisic acid catabolic pathway. Plant Physiol. 2004;134:361–369. doi: 10.1104/pp.103.030734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim EK, Doucet CJ, Hou B, Jackson RG, Abrams SR, Bowles DJ. Resolution of (+)-abscisic acid using an Arabidopsis glycosyltransferase. Tetrahedron: Asymmetry. 2005;16:143–147. [Google Scholar]
- 47.Jacobsen JV, Pearce DW, Poole AT, Pharis RP, Mander LN. Abscisic acid, phaseic acid and gibberellin contents associated with dormancy and germination in barley. Physiol Plant. 2002;115:428–441. doi: 10.1034/j.1399-3054.2002.1150313.x. [DOI] [PubMed] [Google Scholar]
- 48.Kushiro T, Okamoto M, Nakabayashi K, Yamagishi K, Kitamura S, Asami T, et al. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: key enzymes in ABA catabolism. EMBO J. 2004;23:1647–1656. doi: 10.1038/sj.emboj.7600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koiwai H, Nakaminami K, Seo M, Mitsuhashi W, Toyomasu T, Koshiba T. Tissue-specific localization of an abscisic acid biosynthetic enzyme, AAO3, in Arabidopsis. Plant Physiol. 2004;134:1697–1707. doi: 10.1104/pp.103.036970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.González-Guzmán M, Abia D, Salinas J, Serrano R, Rodríguez PR. Two new alleles of the abscisic aldehide oxidase 3 gene reveal its role in abscisic acid biosynthesis in seeds. Plant Physiol. 2004;135:325–333. doi: 10.1104/pp.103.036590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin PC, Hwang SG, Endo A, Okamoto M, Koshiba T, Cheng WH. Ectopic expression of ABSCISIC ACID 2/GLUCOSE INSENSITIVE 1 in Arabidopsis promotes seed dormancy and stress tolerance. Plant Physiol. 2007;143:745–758. doi: 10.1104/pp.106.084103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okamoto M, Kuwahara A, Seo M, Kushiro T, Asami T, Hirai N, et al. CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol. 2006;141:97–107. doi: 10.1104/pp.106.079475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Millar AA, Jacobsen JV, Ross JJ, Helliwell CA, Poole AT, Scofield G, et al. Seed dormancy and ABA metabolism in Arabidopsis and barley: the role of ABA 8′-hydroxylase. Plant J. 2006;45:942–954. doi: 10.1111/j.1365-313X.2006.02659.x. [DOI] [PubMed] [Google Scholar]
- 54.Chono M, Honda I, Shinoda S, Kushiro T, Kamiya Y, Nambara E, et al. Field studies on the regulation of abscisic acid content and germinability during grain development of barley: molecular and chemical analysis of pre-harveswt sprouting. J Exp Bot. 2006;57:2421–2434. doi: 10.1093/jxb/erj215. [DOI] [PubMed] [Google Scholar]
- 55.Gonai T, Kawahara S, Tougou M, Satoh S, Hashiba T, Hirai N, et al. Abscisic acid in the thermoinhibition of lettuce seed germination and enhancement of its catabolism by gibberellin. J Exp Bot. 2004;55:111–118. doi: 10.1093/jxb/erh023. [DOI] [PubMed] [Google Scholar]
- 56.Yamaguchi S, Ogawa M, Kuwahara A, Hanada A, Kamiya Y, Yamaguchi S. Activation of gibberellin biosynthesis and response pathways by low temperatura during imbibition of Arabidopsis thaliana seeds. Plant Cell. 2004;16:367–378. doi: 10.1105/tpc.018143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang SH, Zeevaart JA. Expression of ABA 8′-hydroxylases in relation to leaf water relations and seed development in bean. Plant J. 2006;47:675–686. doi: 10.1111/j.1365-313X.2006.02815.x. [DOI] [PubMed] [Google Scholar]
- 58.Chen Y, Xie H, Liang J, Zhang J. The regulator of G protein signaling proteins involved in sugar and abscisic acid signaling in Arabidopsis seed germination. Plant Physiol. 2006;140:302–310. doi: 10.1104/pp.105.069872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu G, Ye N, Zhang J. Glucose-induced delay of seed germination in rice is mediated by the suppression of ABA catabolism rather than an enhancement of ABA biosynthesis. Plant Cell Physiol. 2009;50:644–651. doi: 10.1093/pcp/pcp022. [DOI] [PubMed] [Google Scholar]
- 60.Tsavkelova EA, Klimova SYu, Cherdyntseva TA, Netrusov AI. Hormones and hormone-like substances of microorganisms: A review. Appl Biochem Microbiol. 2006;42:229–235. [PubMed] [Google Scholar]
- 61.Boeiro L, Perrig D, Masciarelli O, Penna C, Cassán F, Luna U. Phytohormones production by three strains of Bradyrhizobium japonicum and possible physiological and technological implications. Appl Microbiol Biotechnol. 2007;74:874–880. doi: 10.1007/s00253-006-0731-9. [DOI] [PubMed] [Google Scholar]
- 62.Costacurta A, Vanderleyden J. Synthesis of phytohormones by plant-associated bacteria. Crit Rev Microbiol. 1995;21:1–18. doi: 10.3109/10408419509113531. [DOI] [PubMed] [Google Scholar]
- 63.Arkhipova TN, Veselov SU, Melentiev AI, Martynenko EV, Kudoyarova GR. Ability of bacterium Bacillis subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil. 2005;272:201–209. [Google Scholar]
- 64.Frugier F, Kosuta S, Murray JD, Crespi M, Szczyglowski K. Cytoquinin: secret agent of symbiosis. Trends Plant Sci. 2008;13:115–120. doi: 10.1016/j.tplants.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Belimov AA, Dodd IC, Hontzeas N, Theobald JC, Safronova VI, Davies WJ. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytol. 2009;181:413–423. doi: 10.1111/j.1469-8137.2008.02657.x. [DOI] [PubMed] [Google Scholar]
- 66.Forchetti G, Masciarelli O, Alemano S, Alvarez D, Abdala G. Endophytic bacteria in sunflower (Helianthus annuus L.): isolation, characterization and production of jasmonates and ABA in culture medium. Appl Microbiol Biotechnol. 2007;76:1145–1152. doi: 10.1007/s00253-007-1077-7. [DOI] [PubMed] [Google Scholar]
- 67.Hirai N, Yoshida R, Todoroki Y, Ohigshi H. Biosynthesis of ABA by non-mevalonate pathway in plants and by the mevalonate pathway in fungi. Bios Biotechnol Biochem. 2000;64:1448–1454. doi: 10.1271/bbb.64.1448. [DOI] [PubMed] [Google Scholar]
- 68.Dey R, Pal KK, Bhatt DM, Chauhan SM. Growth promotion and yield enhancement of peanut (Arachis hypogea L.) by application of plant growth-promoting rhizobacteria. Microbiol Res. 2004;159:371–394. doi: 10.1016/j.micres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 69.Frankenberger WT, Arshad M. Phytohormones in Soils. New York USA: Marcel Dekker; 1995. [Google Scholar]
- 70.Yang J, Kloepper JW, Ryu Ch-M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009;14:1–4. doi: 10.1016/j.tplants.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 71.Audenaert K, De Meyer G, Hofte M. Abscisic Acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid-dependent signaling mechanisms. Plant Physiol. 2002;128:491–501. doi: 10.1104/pp.010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mauch-Mani B, Mauch F. The role of abscisic acid in plant-pathogen interactions. Curr Opin Plant Biol. 2005;8:409–414. doi: 10.1016/j.pbi.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 73.Janitor A. Growth of mycelia of phytopathogenic fungi after application of abscisic acid in vitro conditions. Plant Protect Sci. 2002;38:94–97. [Google Scholar]
- 74.Jiang F, Jeschke WD, Hartung W. ABA flows from Hordeum vulgare to the hemiparasite Rhinanthus minor and the influence of infection on host and parasite ABA relations. J Exp Bot. 2004;55:2323–2329. doi: 10.1093/jxb/erh240. [DOI] [PubMed] [Google Scholar]
- 75.Slovik S, Daeter W, Hartung W. Compartmental distribution and redistribution of ABA in roots as influenced by environmental changes in the rhizosphere. A biomathematical model. J Exp Bot. 1995;46:881–894. [Google Scholar]
- 76.Hartung W, Sauter A, Turner NC, Fillery I, Heilmeier H. ABA in soils: what is its function and which factors and mechanisms influence its concentration? Plant Soil. 1996;184:105–110. [Google Scholar]
- 77.Degenhardt B, Gimmler H, Hose E, Hartung W. Effect of alkaline and saline substrates on ABA contents, distribution and transport in plant roots. Plant Soil. 2000;225:83–94. [Google Scholar]
- 78.Freundl E, Steudle E, Hartung W. Apoplastic transport of abscisic acid across maize roots. The role of the exodermis. Planta. 2000;210:222–231. doi: 10.1007/PL00008129. [DOI] [PubMed] [Google Scholar]
- 79.Hartung W, Sauter A, Hose E. ABA in the xylem: where does it come from, where does it go to? J Exp Bot. 2002;53:27–32. [PubMed] [Google Scholar]
- 80.Sauter A, Davies WJ, Hartung W. The long-distance ABA signal in the droughted plant: the fate of the hormone on its way from root to shoot. J Exp Bot. 2001;52:1991–1997. doi: 10.1093/jexbot/52.363.1991. [DOI] [PubMed] [Google Scholar]
- 81.Jiang F, Hartung W. Long-distance signalling of ABA: the factors regulating the intensity of the ABA signal. J Exp Bot. 2008;59:37–43. doi: 10.1093/jxb/erm127. [DOI] [PubMed] [Google Scholar]
- 82.Sharp RE. Interaction with ethylene: changing views on the role of ABA in root and shoot growth responses to water stress. Plant Cell Environ. 2002;25:211–222. doi: 10.1046/j.1365-3040.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- 83.Bewley JD. Seed germination and dormancy. Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bentsink L, Soppe W, Koornneef M. Genetics aspects of seed dormancy. In: Bradford K, Nonogaki H, editors. Seed Development, Dormancy and Germination. Oxford, UK: Blackwell Publishing; 2007. pp. 113–132. [Google Scholar]
- 85.Kermode AR. Developmental traits. Germination. In: Klee H, Christou P, editors. Handbook of plant biotechnology. UK: John Wiley & Sons; 2004. pp. 673–713. [Google Scholar]
- 86.Fenner M, Thompson K. The ecology of seeds. Cambridge RU: Cambridge University Press; 2005. p. 250. [Google Scholar]
- 87.Vaughan DA, Balázs E, Heslop-Harrison JS. From Crop Domestication to Super-domestication. Ann Bot. 2007;100:893–901. doi: 10.1093/aob/mcm224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gubler F, Millar AA, Jacobsen JV. Dormancy release, ABA and pre-harvest sprouting. Curr Opin Plant Biol. 2005;8:183–187. doi: 10.1016/j.pbi.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 89.Cadman CS, Toorop PE, Hilhorst HW, Finch-Savage WE. Gene expression profiles of Arabidopsis Cvi seeds during dormancy cycling indicate a common underlying dormancy control mechanism. Plant J. 2006;46:805–822. doi: 10.1111/j.1365-313X.2006.02738.x. [DOI] [PubMed] [Google Scholar]
- 90.Raz V, Bergervoet JH, Koornneef M. Sequential steps for developmental arrest in Arabidopsis seeds. Development. 2001;128:243–252. doi: 10.1242/dev.128.2.243. [DOI] [PubMed] [Google Scholar]
- 91.Ali-Rachedi S, Bouinot D, Wagner MH, Bonnet M, Sotta B, Grappin P, et al. Changes in endogenous abscisic acid levels during dormancy release and maintenance of mature seeds: studies with the Cape Verde Islands ecotype, the dormant model of Arabidopsis thaliana. Planta. 2004;219:479–488. doi: 10.1007/s00425-004-1251-4. [DOI] [PubMed] [Google Scholar]
- 92.Yazaki J, Kikuchi S. The genomic view of genes responsive to the antagonistic phytohormones, abscisisc acid and gibberellin. Vit Horm. 2005;72:1–30. doi: 10.1016/S0083-6729(05)72001-X. [DOI] [PubMed] [Google Scholar]
- 93.Corbineau F, Bianco J, Garello G, Côme D. Breakage of Pseudotsuga menziesii seed dormancy by cold treatment as related to changes in seed ABA sensitivity and ABA levels. Physiol Plant. 2002;114:313–319. doi: 10.1034/j.1399-3054.2002.1140218.x. [DOI] [PubMed] [Google Scholar]
- 94.Koornneef M, Bentsink L, Hilhorst H. Seed dormancy and germination. Curr Opin Plant Biol. 2002;5:33–36. doi: 10.1016/s1369-5266(01)00219-9. [DOI] [PubMed] [Google Scholar]
- 95.Chiwocha SD, Cutler AJ, Abrams SR, Ambrose SJ, Yang J, Ross ARS, et al. The etr1-2 mutation in Arabidopsis thaliana affects the abscisic acid, auxin, cytokinin and gibberellin metabolic pathways during maintenance of seed dormancy, moist-chilling and germination. Plant J. 2005;42:35–48. doi: 10.1111/j.1365-313X.2005.02359.x. [DOI] [PubMed] [Google Scholar]
- 96.Kucera B, Cohn MA, Leubner-Metzger G. Plant hormone interactions during seed dormancy release and germination. Seed Sci Res. 2005;15:281–307. [Google Scholar]
- 97.Alonso-Blanco C, Bentsink L, Hanhart CJ, Blankestijn-de Vries H, Koornneef M. Analysis of natural allelic variation at seed dormancy loci of Arabidopsis thaliana. Genetics. 2003;64:711–729. doi: 10.1093/genetics/164.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brady SM, McCourt P. Hormone cross-talk in seed dormancy. J Plant Growth Regul. 2003;22:25–31. [Google Scholar]
- 99.Yamaguchi S, Nambara N. Seed development and germination. In: Hedden P, Thomas SG, editors. Plant Hormone Signaling. 9600 Garsington Road, Oxford OX4 2DQ UK: Blackwell Publishing Ltd; 2006. pp. 311–338. [Google Scholar]
- 100.Yamaguchi S, Kamiya Y, Nambara N. Regulation of ABA and GA levels during seed development and germination in Arabidopsis. In: Bradford K, Nonogaki H, editors. Seed Development, Dormancy and Germination. 9600 Garsington Road, Oxford OX4 2DQ UK: Blackwell Publishing Ltd; 2007. pp. 224–247. [Google Scholar]
- 101.Gubler F, Hughes T, Waterhouse P, Jacobsen J. Regulation of dormancy in barley by blue light and after-ripening: effects of abscisic acid and gibberellin metaboloism. Plant Physiol. 2008;147:886–896. doi: 10.1104/pp.107.115469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dugardeyn J, Vandenbussche F, Van Der Straeten D. To grow or not grow: what can we learn on ethylenegibberellin cross-talk by in silico gene expression analysis? J Exp Bot. 2008;59:1–16. doi: 10.1093/jxb/erm349. [DOI] [PubMed] [Google Scholar]
- 103.Matilla AJ, Matilla-Vázquez MA. Involvement of ethylene in seed physiology. Plant Sci. 2008;175:87–97. [Google Scholar]
- 104.Cao X, Costa LM, Biderre-Petit C, Kbhaya B, Dey N, Perez P, et al. Abscisic acid and stress signals induce Viviparous 1 expression in seed and vegetative tissues of maiz. Plant Physiol. 2007;143:720–731. doi: 10.1104/pp.106.091454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alboresi A, Gestin C, Leydecker M-T, Bedu M, Meyer C, Truong HN. Nitrate, a signal relieving seed dormancy in Arabidopsis. Plant Cell Environm. 2005;28:500–512. doi: 10.1111/j.1365-3040.2005.01292.x. [DOI] [PubMed] [Google Scholar]
- 106.Pawlowski TA. Proteomic of european beech (Fagus silvatica L.) seed dormancy breaking: influence of abscisic acid and gibberellic acids. Proteomics. 2007;114:482–490. doi: 10.1002/pmic.200600912. [DOI] [PubMed] [Google Scholar]
- 107.Leymarie J, Bruneaux E, Gibot-Leclerc S, Corbineau F. Identification of transcripts potentially involved in barley seed germination and dormancy using cDNA-AFLP. J Exp Bot. 2007;58:425–437. doi: 10.1093/jxb/erl211. [DOI] [PubMed] [Google Scholar]
- 108.Leymarie J, Robayo-Romero ME, Gendreau E, Benech-Arnold RL, Corbineau F. Involvement of ABA in induction of secondary dormancy in barley (Hordeum vulgare L.) seeds. Plant Cell Physiol. 2008;49:1830–1838. doi: 10.1093/pcp/pcn164. [DOI] [PubMed] [Google Scholar]
- 109.Nambara E, Marion-Poll A. ABA action and interactions in seeds. Trends Plant Sci. 2003;8:195–244. doi: 10.1016/S1360-1385(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 110.Finkelstein RR. The role of hormones during seed development and germination. In: Davies PJ, editor. Plant Hormones-Biosynthesis, signal transduction, action! Dordrecht: Kluwer Academic; 2004. pp. 513–537. [Google Scholar]
- 111.Frey A, Godin B, Bonnet M, Sotta B, Marion-Poll A. Maternal synthesis of abscisic acid controls seed development and yield in Nicotiana plumbaginifolia. Planta. 2004;218:958–964. doi: 10.1007/s00425-003-1180-7. [DOI] [PubMed] [Google Scholar]
- 112.Grappin P, Bouinot D, Sotta B, Miginiac E, Jullien M. Control of seed dormancy in Nicotiana plumbaginifolia: postimbibition abscisic acid synthesis imposes dormancy maintenance. Planta. 2000;210:279–285. doi: 10.1007/PL00008135. [DOI] [PubMed] [Google Scholar]
- 113.Schmitz N, Abrams SR, Kermode AR. Changes in abscisic acid content and embryo sensitivity to (+)-abscisic acid during termination of dormancy of yellow-cedar seeds. J Exp Bot. 2000;51:1159–1162. doi: 10.1093/jexbot/51.347.1159. [DOI] [PubMed] [Google Scholar]
- 114.Schmitz N, Abrams SR, Kermode AR. Changes in ABA turnover and sensitivity that accompany dormancy termination of yellow-cedar (Chamaecyparis nootkatensis) seeds. J Exp Bot. 2002;53:89–101. [PubMed] [Google Scholar]
- 115.Schwachtje J, Baldwin IT. Smoke exposure alters endogenous gibberellin and abscisic acid pools and gibberellin sensitivity while eliciting germination in the post-fire annual, Nicotiana attenuate. Seed Sci Res. 2004;14:51–60. [Google Scholar]
- 116.Baumbusch LO, Hughes DW, Galau GA, Jakobse KS. LEC1, FUS3, ABI3 and Em expression reveals no correlation with dormancy in Arabidopsis. J Exp Bot. 2004;55:77–87. doi: 10.1093/jxb/erh014. [DOI] [PubMed] [Google Scholar]
- 117.Bentsink L, Koornneef M. Seed dormancy and germinatino. The Arabidopsis Book BioOne ASPB. 2008:1–18. doi: 10.1199/tab.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Finch-Savage WE, Cadman CS, Toorop PE, Lynn JR, Hilhorst HW. Seed dormancy release in Arabidopsis Cvi by dry after-ripening, low temperature, nitrate and light shows common quantitative patterns of gene expression directed by environmentally specific sensing. Plant J. 2007;51:60–78. doi: 10.1111/j.1365-313X.2007.03118.x. [DOI] [PubMed] [Google Scholar]
- 119.Benech-Arnold RL, Sánchez RA, Forcella F, Kruk BC, Ghersa CM. Enviromental control of dormancy in weed seed banks in soil. Field Crops Res. 2000;67:105–122. [Google Scholar]
- 120.Baskin JM, Baskin CC. A classification system for seed dormancy. Seed Sci Res. 2004;14:1–16. [Google Scholar]
- 121.Battla D, Benech-Arnold RL. A predictive model for dormancy loss in Polygonum aviculare L. seeds based on changes in population hydrotime parameters. Seed Sci Res. 2004;14:277–286. [Google Scholar]
- 122.Oh E, Yamaguchi S, Hu J, Yusuke J, Jung B, Inyup Paik I, et al. PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell. 2007;19:1192–1208. doi: 10.1105/tpc.107.050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Finkelstein RR, Srinivas SL, Gampala SSL, Rock CD. Abscisic acid signaling in seeds and seedlings. Plant Cell. 2002;14:15–45. doi: 10.1105/tpc.010441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu X, Yue Y, Li B, Nie Y, Li W, Wu WH, Ma L. A G protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid. Science. 2007;315:1712–1716. doi: 10.1126/science.1135882. [DOI] [PubMed] [Google Scholar]
- 125.Bove J, Lucus P, Godin B, Ogé L, Jullien M, Grappin P. Gene expression analysis by cDNA-AFLP highlights a set of new signalling networks and translational control during seed dormancy breaking in N. plumbaginifoloia. Plant Mol Biol. 2005;57:593–612. doi: 10.1007/s11103-005-0953-8. [DOI] [PubMed] [Google Scholar]
- 126.Nishimura N, Yoshida T, Kitahata N, Asami T, Shinozaki K, Hirayama T. ABA-Hypersensitive Germination 1 encodes a protein phosphatase 2C, an essential component of abscisic acid signalling in Arabidopsis seed. Plant J. 2007;50:935–949. doi: 10.1111/j.1365-313X.2007.03107.x. [DOI] [PubMed] [Google Scholar]
- 127.Chibani K, Ali-Rachedi S, Job C, Job D, Jullien M, Grappin P. Proteomic analysis of seed dormancy in Arabidopsis. Plant Physiol. 2006;142:1493–1510. doi: 10.1104/pp.106.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Carrera E, Holman T, Medhurst A, Peer W, Schmuths H, Footitt S, et al. Gene expression profiling reveals defined functions of the ABC transporter COMATOSE late in phase II of germination. Plant Physiol. 2007;143:1669–1679. doi: 10.1104/pp.107.096057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carrera E, Holman T, Medhurst A, Dietrich D, Footitt S, Theodoulou FL, et al. Seed after-ripening is a discrete developmental pathway associated with specific gene networks in Arabidopsis. Plant J. 2008;53:214–224. doi: 10.1111/j.1365-313X.2007.03331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Müller K, Tintelnot S, Leubner-Metzger G. Endosperm-limited Brassicaceae seed germination: Abscisic acid inhibits embryo-induced endosperm weakening of Lepidium sativum (cress) and endosperm rupture of cress and Arabidopsis thaliana. Plant Cell Physiol. 2006;47:864–877. doi: 10.1093/pcp/pcj059. [DOI] [PubMed] [Google Scholar]
- 131.Merlot S, Gosti F, Guerrier D, Vavasseur A, Giraudat J. The ABI1 and ABA2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid pathway. Plant J. 2001;25:295–303. doi: 10.1046/j.1365-313x.2001.00965.x. [DOI] [PubMed] [Google Scholar]
- 132.Khun JM, Boisson-Dernier A, Dizon MB, Maktabi MH, Schroeder JI. The protein phosphatase AtPP2CA negatively regulates abscisic acid signal transduction in Arabidopsis, and effects of abh1 on AtPP2CA mRNA. Plant Physiol. 2006;140:127–139. doi: 10.1104/pp.105.070318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rodríguez PL. ABI2, a second protein phosphatase 2C (PP2C) function in higher plants. Plant Mol Biol. 1998;38:919–927. doi: 10.1023/a:1006054607850. [DOI] [PubMed] [Google Scholar]
- 134.Tahtiharju S, Palva T. Antisense inhibition of protein phosphatase 2C accelerates cold acclimation in A. thaliana. Plant J. 2001;26:461–470. doi: 10.1046/j.1365-313x.2001.01048.x. [DOI] [PubMed] [Google Scholar]
- 135.Wu Y, Sánchez JP, López-Molina L, Himmelbach A, Grill E, Chua NH. The abi1-1 mutation bloks ABA downstream cADPR action. Plant J. 2003;34:307–315. doi: 10.1046/j.1365-313x.2003.01721.x. [DOI] [PubMed] [Google Scholar]
- 136.Arroyo A, Bossi F, Finkelstein RR, Leon P. Three gene that affect sugar sensing (abscisic acid insensitive 4, abscisic acid insensitive 5, and constitutive triple response 1) are differentially regulated by glucose in Arabisopsis. Plant Physiol. 2003;133:231–242. doi: 10.1104/pp.103.021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mortensen LC, Rodriguez D, Nicolás G, Eriksen EN, Nicolás C. Decline in a seed-specific ABA responsive glycine rich protein (GRPF1) mRNA may reflect the release of seed dormancy in Fagus silvatica during moist prechilling. Seed Sci Res. 2004;14:27–34. [Google Scholar]
- 138.Lorenzo O, Rodríguez D, Nicolás C, Nicolás G. Characterization and expression of two protein kinase and an EIN3-like genes, which are regulated by ABA and GA3 in dormant Fagus silvatica seeds. In: Black M, Bradford KJ, Vázquez-Ramos J, editors. Seed Biology: Advances and applications. Wallingford: CAB International; 2000. pp. 329–340. [Google Scholar]
- 139.Jiménez JA, Rodríguez D, Calvo AP, Mortensen LCh, Nicolás G, Nicolás C. Expresión of a transcription factor (FsERF1) involved in ethylene signallling during the breaking of dormancy in Fagus silvatica seeds. Physiol Plant. 2005;125:373–380. [Google Scholar]
- 140.González-García MP, Rodríguez D, Nicolás C, Rodríguez PL, Nicolás G, Lorenzo O. Negative regulation of abscisic acid signaling by the Fagus silvatica FsPP2C1 plays a role in seed dormancy regulation and promotion of seed germination. Plant Physiol. 2003;133:135–144. doi: 10.1104/pp.103.025569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Reyes D, Rodríguez D, González-García MP, Lorenzo O, Nicolás G, García-Martínez JL, et al. Overexpression of a protein phosphatase 2C from beech seeds in Arabisopsis shows phenotypes related to abscisic acid responses and gibberellin biosynthesis. Plant Physiol. 2006;141:1414–1424. doi: 10.1104/pp.106.084681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lorenzo O, Rodríguez D, Nicolás G, Rodríguez PL, Nicolás C. A new protein phosphatase 2C (FsPP2c1) induced by abscisic acid is specifically expressed in dormant beechnut seeds. Plant Physiol. 2001;125:1949–1956. doi: 10.1104/pp.125.4.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lorenzo O, Nicolás C, Nicolás G, Rodríguez D. Characterization of a dual plant protein kinase (FsPK1) upregulated by abscisic acid and calcium and specifically expressed in dormant seeds of Fagus sylvatica L. Seed Sci Res. 2003;13:261–271. [Google Scholar]
- 144.Lorenzo O, Nicolás C, Nicolás G, Rodríguez D. Molecular cloning of a functional protein phosphatase 2C (FsPP2C2) with unusual features and synergistically upregulated by ABA and calcium in dormant seeds of Fagus sylvatica. Physiol Plant. 2002;114:482–490. doi: 10.1034/j.1399-3054.2002.1140318.x. [DOI] [PubMed] [Google Scholar]
- 145.Saez A, Apostolova N, Gonzalez-Guzman M, Gonzalez-Garcia MP, Nicolas C, Lorenzo O, et al. Gain-of-function and loss-of-function phenotypes of the protein phsophatases 2C HAB1 reveal its role as a negative regulator of abscisic acid signalling. Plant J. 2004;37:354–369. doi: 10.1046/j.1365-313x.2003.01966.x. [DOI] [PubMed] [Google Scholar]
- 146.Zeng Y, Raimondi N, Kermode AR. Role of an ABI3 homologue in dormancy maintenance of yellow-cedar seeds and in the activation of storage protein and Em gene promoters. Plant Mol Biol. 2003;51:39–49. doi: 10.1023/a:1020762304937. [DOI] [PubMed] [Google Scholar]
- 147.Razem FA, Baron K, Hill RD. Turning on gibberellin and abscisic acid signaling. Curr Opin Plant Sci. 2006;9:454–459. doi: 10.1016/j.pbi.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 148.Razem FA, El-Kereamy A, Abrams SR, Hill RD. The RNA-binding protein FCA is an abscisic acid receptor. Nature. 2006;439:290–294. doi: 10.1038/nature04373. [DOI] [PubMed] [Google Scholar]
- 149.Shen YY, Wang XF, Wu FQ, Du SY, Cao Z, Shang Y, et al. The Mg-chelatase H subunit is an abscisic acid receptor. Nature. 2006;443:823–826. doi: 10.1038/nature05176. [DOI] [PubMed] [Google Scholar]
- 150.Pandey S, Nelson DC, Assmann SM. Two novel GPCR-type G proteins are abscisic acid receptors in Arabidopsis. Cell. 2009;136:136–148. doi: 10.1016/j.cell.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 151.Christmann A, Moes D, Himmerlbach A, Yang Y, Tang Y, Grill E. Integration of abscisic acid signalling into plant responses. Plant Biol. 2006;8:314–325. doi: 10.1055/s-2006-924120. [DOI] [PubMed] [Google Scholar]
- 152.Verslues PE, Zhu J-KN. New developments in abscisic acid perception and metabolism. Curr Opin Plant Biol. 2007;10:447–452. doi: 10.1016/j.pbi.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 153.Hirayama T, Shinozaki K. Perception and transduction of abscisic acid signals: key to the function of the versatile plant hormone ABA. Trend Plant Sci. 2007;12:343–351. doi: 10.1016/j.tplants.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 154.Zhang DP, Wu ZY, Li XY, Zhao ZX. Purification and identification of a 42-kilodalton abscisic acid-specific-binding protein from epidermis of broad bean leaves. Plant Physiol. 2002;128:714–725. doi: 10.1104/pp.010531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Risk JM, Day CL, Macknight RC. Re-evaluation of abscisic acid (ABA) binding assays shows that GCR2 does not bind ABA. Plant Physiol. 2009;150:6–11. doi: 10.1104/pp.109.135749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Razem FA, Luo M, Liu JH, Abrams SR, Hill RD. Purification and characterization of a barley aleurone abscisic acid-binding protein. J Biol Chem. 2004;279:9922–9929. doi: 10.1074/jbc.M311064200. [DOI] [PubMed] [Google Scholar]
- 157.Simpson GG, Dijkwel PP, Quesada V, Henderson I, Dean C. FY is an RNA 3′end-processing factor that interacts with FCA to control the Arabisopsis floral transition. Cell. 2003;113:777–787. doi: 10.1016/s0092-8674(03)00425-2. [DOI] [PubMed] [Google Scholar]
- 158.Gao Y, Zeng Q, Guo J, Cheng J, Ellis BE, Chen J-G. Genetic characterization reveals no role for the reported ABA receptor, GCR2, in ABA control of seed germination and early seedling development in Arabisopsis. Plant J. 2007;52:1001–1013. doi: 10.1111/j.1365-313X.2007.03291.x. [DOI] [PubMed] [Google Scholar]
- 159.Guo J, Zeng Q, Emami M, Ellis BE, Chen J-G. The GCR2 gene family is not required for ABA control of seed germination and early seedling development in Arabidopsis. PLoS ONE. 2008;3:2982. doi: 10.1371/journal.pone.0002982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Colucci G, Apone F, Alyeshmerni N, Chalmers D, Chrispeels MJ. GCR1, the putative Arabidopsis G protein-coupled receptor gene is cell cycle-regulated, and its overexpression abolished seed dormancy and shortens time to flowering. Proc Natl Acad Sci USA. 2002;99:4736–4741. doi: 10.1073/pnas.072087699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pandey S, Assmann SM. The Arabidopsis putative G protein-coupled receptor GCR1 interacts with the G protein α subunit GPA1 and regulates abscisic acid signaling. Plant Cell. 2004;16:1616–1632. doi: 10.1105/tpc.020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Illingworth CJ, Parkes KE, Snell CR, Mullineaux PM, Reynolds CA. Criteria for confirming sequence periodicity identified by Fourier transform analysis: Applicaation to GCRC2, a candidate plant GPCR? Biophys Chem. 2008;133:28–35. doi: 10.1016/j.bpc.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 163.Assmann SM. Plant G proteins, phytohormones and plasticity: three questions and a speculation. Sci STKE. 2004;264:20. doi: 10.1126/stke.2642004re20. [DOI] [PubMed] [Google Scholar]
- 164.Pandey S, Chen JG, Jones AM, Assmann SM. G-protein complex mutants are hypersensitive to abscisic acid regulation of germination and postgermination development. Plant Physiol. 2006;141:243–256. doi: 10.1104/pp.106.079038. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 165.Gookin TE, Kim J, Assmann SM. Whole proteome identification of plant candidate G-protein coupled receptors in Arabidopsis, rice and poplar: computational prediction and in-vivo protein coupling. Gen Biol. 2008;8:120. doi: 10.1186/gb-2008-9-7-r120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chow B, McCourt P. Plant Hormone receptors: perception is everything. Genes Dev. 2009;20:1998–2008. doi: 10.1101/gad.1432806. [DOI] [PubMed] [Google Scholar]
- 167.Risck JM, Macknight RC, Day CL. FCA does not bind abscisic acid. Nature. 2008;456:5–6. doi: 10.1038/nature07646. [DOI] [PubMed] [Google Scholar]
- 168.Jones AM, Sussman MR. A binding resolution. Plant Physiol. 2009;150:3–5. doi: 10.1104/pp.109.136606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gao Y, Zeng Q, Guo J, Cheng J, Ellis BE, Chen J-G. Genetic characterization reveals no role for the reported ABA receptor, GCR2, in ABA control of seed germination and early seedling development in Arabisopsis. Plant J. 2007;52:1001–1013. doi: 10.1111/j.1365-313X.2007.03291.x. [DOI] [PubMed] [Google Scholar]
- 170.Guo J, Zeng Q, Emami M, Ellis BE, Chen J-G. The GCR2 gene family is not required for ABA control of seed germination and early seedling development in Arabidopsis. PLoS ONE. 2008;3:2982. doi: 10.1371/journal.pone.0002982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.McCourt P, Creelman R. The ABA receptors—we report you decide. Curr Opin Plant Biol. 2008;11:474–478. doi: 10.1016/j.pbi.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 172.Fait A, Angelovici R, Less H, Ohad I, Urbanczyk-Wochniak E, Fernie AR, et al. Arabidopsis seed development and germination is associated with temporally distinct metabolic switches. Plant Physiol. 2006;142:839–854. doi: 10.1104/pp.106.086694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Arenas-Huertero F, Arroyo A, Zhou L, Sheen J, Leon P. Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev. 2000;14:2085–2096. [PMC free article] [PubMed] [Google Scholar]
- 174.Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P. Regulation of abscisicacid signaling by the ethylene response pathway in Arabidopsis. Plant Cell. 2000;12:1117–1126. doi: 10.1105/tpc.12.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xiong L, Lee H, Ishitani M, Zhu JK. Regulation of osmotic stress-responsive gene expression by the LOS6/ABA1 locus in Arabidopsis. J Biol Chem. 2002;277:8588–8596. doi: 10.1074/jbc.M109275200. [DOI] [PubMed] [Google Scholar]
- 176.Rook F, Corke F, Card R, Munz G, Smith C, Bevan MW. Impaired sucrose-induction mutants reveal the modulation of sugar-induced starch biosynthetic gene expression by abscisic acid signalling. Plant J. 2001;26:421–433. doi: 10.1046/j.1365-313x.2001.2641043.x. [DOI] [PubMed] [Google Scholar]
- 177.White CN, Proebsting WM, Hedden P, Rivin CJ. Gibberellins and seed development in maize I. Evidence that gibberellin/abscisic acid balance governs germination versus maturation pathways. Plant Physiol. 2000;122:1081–1088. doi: 10.1104/pp.122.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Thompson AJ, Jackson AC, Symonds RC, Mulholland BJ, Dadswell AR, Blake PS, et al. Ectopic expression of a tomato 9-cis-epoxycarotenoiddioxygenase gene causes over-productionof abscisic acid. Plant J. 2000;23:363–374. doi: 10.1046/j.1365-313x.2000.00789.x. [DOI] [PubMed] [Google Scholar]
- 179.Beaudoin N, Serizet C, Gosti F, Giraudat J. Interactions between abscisic acid and ethylene signaling cascades. Plant Cell. 2000;12:1103–1115. doi: 10.1105/tpc.12.7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nambara E, Suzuki M, Abrams S, McCarty DR, Kamiya Y, McCourt P. A screen for genes that function in abscisic acid signaling in Arabidopsis thaliana. Genetics. 2002;161:1247–1255. doi: 10.1093/genetics/161.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mazzella MA, Arana MV, Staneloni RJ, Perelman S, Rodriguez Batiller MJ, Muschietti J, et al. Phytocrome control of the Arabidopsis transcriptome anticipates seedling exposure to light. Plant Cell. 2005;17:2507–2516. doi: 10.1105/tpc.105.034322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Acevedo-Hernandez GJ, León P, Herrera-Estrella LR. Sugar and ABA responsiveness of a minimal RBCS light responsive unit is mediated by direct binding of ABI4. Plant J. 2005;43:506–519. doi: 10.1111/j.1365-313X.2005.02468.x. [DOI] [PubMed] [Google Scholar]
- 183.Finkelstein RR, Lynch TJ. The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell. 2000;12:599–609. doi: 10.1105/tpc.12.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Laby RJ, Kincaid MS, Kim D, Gibson SI. The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J. 2000;23:587–596. doi: 10.1046/j.1365-313x.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- 185.He YH, Gan SS. A novel zinc-finger protein with a proline-rich domain mediates ABA-regulated seed dormancy in Arabidopsis. Plant Mol Biol. 2004;54:1–9. doi: 10.1023/B:PLAN.0000028730.10834.e3. [DOI] [PubMed] [Google Scholar]
- 186.Young TE, Gallie DR. Programmed cell death during endosperm development. Plant Mol Biol. 2000;44:283–301. doi: 10.1023/a:1026588408152. [DOI] [PubMed] [Google Scholar]
- 187.Iglesias-Fernández R, Angel Matilla AJ. Afterripening alters the gene expression pattern of oxidases involved in the ethylene and gibberellin pathways during the early imbibition of Sisymbrium officinale L. seeds. J Exp Bot. 2009;60:1645–1665. doi: 10.1093/jxb/erp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Matakiadis T, Alboresi A, Jikumaru Y, Tatematsu K, Pichon O, Renou JP, et al. The Arabidopsis abscisic acid catabolic gene CYP707A2 plays a key role in nitrate control of seed dormancy. Plant Physiol. 2009;149:949–960. doi: 10.1104/pp.108.126938. [DOI] [PMC free article] [PubMed] [Google Scholar]