Abstract
In our recent paper in Plant Physiology and Biochemistry, we reported that the mRNAs encoding Arabidopsis thaliana cytosolic acyl-CoA-binding proteins, ACBP4 and ACBP5, but not ACBP6, are modulated by light/dark cycling. The pattern of circadian-regulated expression in ACBP4 and ACBP5 mRNAs resembles that of FAD7 which encodes omega-3-fatty acid desaturase, an enzyme involved in plastidial fatty acid biosynthesis. Recombinant ACBP4 and ACBP5 proteins were observed to bind oleoyl-CoA ester comparably better than recombinant ACBP6, suggesting that ACBP4 and ACBP5 are promising candidates in the trafficking of oleoyl-CoA from the plastids to the endoplasmic reticulum (ER) for the biosynthesis of non-plastidial membrane lipids. By western blot analyses using the ACBP4 and ACBP5-specific antibodies, we show herein that the levels of ACBP4 and ACBP5 proteins peak at the end of the light period, further demonstrating that they, like their corresponding mRNAs, are tightly controlled by light to satisfy demands of lipids in plant cells.
Key words: acyl-CoA-binding protein, ACBP4, ACBP5, lipid trafficking, phosphatidylcholine-binding
Introduction
Acyl-CoA-binding proteins (ACBPs) are conserved at the acyl-CoA-binding domain and possess the ability to bind long-chain acyl-CoA (LCA-CoA) esters.1 In Arabidopsis thaliana, six genes designated ACBP1 to ACBP6 have been identified to encode ACBPs.1 Among them, ACBP1 and ACBP2 are membrane-associated proteins,2–5 ACBP3 is extracellularly-targeted,6 and the remaining three (ACBP4, ACBP5 and ACBP6) are cytosolic proteins.7,8 The C-terminal ankyrin repeats in ACBP1 and ACBP2 and the kelch motifs in ACBP4 and ACBP5 have been reported to mediate protein-protein interaction.9–11 Given that both ACBP1-and ACBP2-overexpressing transgenic Arabidopsis showed improved tolerance to heavy metals (Pb or Cd) stress, we have suggested that these two plasma membrane-localized ACBPs may be involved in the repair of the phospholipid bilayer membrane following heavy metal stress.9,12
Recombinant ACBP4 and ACBP5 have been previously reported to preferentially bind oleoyl-CoA rather than palmitoyl-CoA or arachidonyl-CoA esters.13 Since these two larger cytosolic ACBPs (ACBP4 and ACBP5) resembles ACBP6 in binding palmitoyl-CoA and oleoyl-CoA esters in vitro,14 they are potential candidates for the shuttling of acyl-CoAs from the plastid to the ER for the biosynthesis of non-plastidial membrane lipids. We have also observed that recombinant ACBP6 binds phosphatidylcholine (PC) in vitro and that transgenic Arabidopsis overexpressing ACBP6 are altered in levels of PC and phosphatic acid and display an enhanced freezing tolerance phenotype.7 To further establish the functions of these three ACBPs in plant lipid metabolism, we have presented recent evidence that the levels of ACBP4 and ACBP5 (but not ACBP6) mRNAs increase in the light and are dampened-off upon darkness.15 This mirrors the expression pattern of the mRNA encoding FAD7, an omega-3-fatty acid desaturase involved in plastidial lipid metabolism.16 Taken together, we suggest that ACBP4 and ACBP5 likely function in oleoyl-CoA transfer between the chloroplasts and the ER.
Light-Regulated Expression of ACBP4 and ACBP5 Proteins
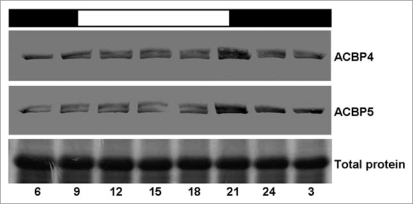
To further investigate the circadian-regulated expression of ACBP4 and ACBP5 mRNAs reported in Xiao et al. (2009),15 the expression levels of ACBP4 and ACBP5 proteins were measured by western blot analysis of total proteins from four-week-old rosettes of wild-type Arabidopsis (Col-0) collected at 3-h intervals. Protein extracts were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by western blot analysis using ACBP4-specific and ACBP5-specific polyclonal antibodies.8 Results from western blot analysis showed that both ACBP4 (molecular mass of 73.1 kDa; Fig. 1, upper) and ACBP5 (molecular mass of 71-kDa; Fig. 1, middle) increased during the light period (from 09:00 to 21:00), peaking at the end of the light period at 21:00. Subsequently, their expression declined in the dark (from 21:00 to 09:00) (Fig. 1). Also western blot analysis revealed the possibility of post-translational modification in ACBP4 and ACBP5 proteins during the light period, as suggested by the doubling of bands between 09:00–21:00. This preliminary observation requires further in-depth investigations to better understand the regulation of ACBP4 and ACBP5.
Figure 1.
Western blot analysis shows circadian-regulated expression of acBP4 and acBP5 in Arabidopsis grown under 12 h light/12 h dark cycles. Arabidopsis seeds were germinated and grown in a growth chamber under 12 h light [09:00 to 21:00]/12 h dark [21:00 to 09:00] cycles. Total protein was extracted from four-week-old rosettes harvested at 3-h intervals. The ACBP4- and ACBP5-specific antibodies were used in western blot analysis have been previously described.8 The cross-reacting 73.1-kDa ACBP4 and 71-kDa ACBP5 bands were detected using the Amplified Alkaline Phosphatase Assay Kit. Bottom, gel identically loaded and stained with Coomassie blue. Bottom numbering indicates time of day (24-h clock) and top white and black bars indicate light and dark periods, respectively.
Other Implications of ACBP4 and ACBP5 in Circadian Control
Our observations have revealed that cytosolic ACBP4 and ACBP5 are transcriptionally15 and translationally (reported herein) modulated by light in Arabidopsis rosettes. In plants, plastidial fatty acid biosynthesis is largely dependent on carbon fixation in the chloroplasts for the generation of acetyl-CoA17,18 and is controlled by light under a circadian rhythm.19 The production of acetyl-CoA oscillates daily within light/dark cycles, i.e., increases in the light and decreases in darkness.17,18 Consistently, some of the genes encoding proteins associated with plastidial fatty acid biosynthesis such as acetyl-CoA carboxylase (ACCase) in pea, and the chloroplast omega-3-fatty acid desaturase (FAD7) in Arabidopsis are transcriptionally controlled by light.16,20 It is still unclear how these genes are regulated to date. A recent study has revealed that the genes encoding the β-subunit of ACCase (accD) and a long-chain acyl-CoA synthetase are upregulated in transgenic Arabidopsis overexpressing soybean Dof proteins GmDof4 and GmDof11, respectively.21 Hence, the Dof family proteins, which are a large family of plant transcription factors involved in diverse processes such as light signals, defense responses as well as plant development,22 likely function in the transcriptional regulation of genes in plant lipid metabolism.
By analysis of the putative promoter sequences (c.a. 1.5-kb) obtained from the NCBI database (http://www.ncbi.nlm.nih.gov/), we have located some putative elements that may be relevant in the regulation of ACBP4 and ACBP5. In particular, four duplicated copies of the Dof element (WAAAG) occur in the ACBP4 and ACBP5 5′-flanking regions, suggesting that their circadian-regulated expression may also be subject to control by the conserved Dof family proteins in lipid metabolism. Furthermore, many other putative regulatory elements, particularly light-responsive elements and circadian rhythm-related elements have been identified too. For example, two circadian rhythm related elements (CAANNNNATC) are observed in the ACBP4 5′-flanking region, but are absent from the ACBP5 5′-flanking region. In contrast, other putative light-responsive elements that are present in both ACBP4 and ACBP5 5′-flanking regions include the AE-box (AGAAACAA), box I (TTTCAAA), box 4 (ATTAAT) and the ACE element (CTAACGTATT). The presence of these putative sequences in the 5′-flanking regions of ACBP4 and ACBP5 are consistent with our observations of circadian- and light-regulated expression of these two genes. Further analysis of these sequences need to be carried out to confirm their functionality in the regulation of ACBP4 and ACBP5 expression.
Acknowledgements
This work was supported by a Croucher Senior Research Fellowship (awarded to M.L.C.) and the University of Hong Kong (10208034 and 10208270). S.X. was supported by a postdoctoral fellowship from the University of Hong Kong and the University Grants Committee of the Hong Kong Special Administrative Region, China (Project No. AoE/B-07/99). Q.F.C. was supported by a postgraduate studentship from the University of Hong Kong.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9718
References
- 1.Xiao S, Chye ML. An Arabidopsis family of six acyl-CoA-binding proteins has three cytosolic members. Plant Physiol Biochem. 2009;47:479–484. doi: 10.1016/j.plaphy.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Chye ML. Arabidopsis cDNA encoding a membrane-associated protein with an acyl-CoA binding domain. Plant Mol Biol. 1998;38:827–838. doi: 10.1023/a:1006052108468. [DOI] [PubMed] [Google Scholar]
- 3.Chye ML, Huang BQ, Zee SY. Isolation of a gene encoding Arabidopsis membrane-associated acyl-CoA binding protein and immunolocalization of its gene product. Plant J. 1999;18:205–214. doi: 10.1046/j.1365-313x.1999.00443.x. [DOI] [PubMed] [Google Scholar]
- 4.Chye ML, Li HY, Yung MH. Single amino acid substitutions at the acyl-CoA-binding domain interrupt 14[C]palmitoyl-CoA binding of ACBP2, an Arabidopsis acyl-CoA-binding protein with ankyrin repeats. Plant Mol Biol. 2000;44:711–721. doi: 10.1023/a:1026524108095. [DOI] [PubMed] [Google Scholar]
- 5.Li HY, Chye ML. Membrane localization of Arabidopsis acyl-CoA binding protein ACBP2. Plant Mol Biol. 2003;51:483–492. doi: 10.1023/a:1022330304402. [DOI] [PubMed] [Google Scholar]
- 6.Leung KC, Li HY, Xiao S, Tse MH, Chye ML. Arabidopsis ACBP3 is an extracellularly targeted acyl-CoA-binding protein. Planta. 2006;223:871–881. doi: 10.1007/s00425-005-0139-2. [DOI] [PubMed] [Google Scholar]
- 7.Chen QF, Xiao S, Chye ML. Overexpression of the Arabidopsis 10-kilodalton acyl-CoA-binding protein ACBP6 enhances freezing tolerance. Plant Physiol. 2008;148:304–315. doi: 10.1104/pp.108.123331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao S, Li HY, Zhang JP, Chan SW, Chye ML. Arabidopsis acyl-CoA-binding proteins ACBP4 and ACBP5 are subcellularly localized to the cytosol and ACBP4 depletion affects membrane lipid composition. Plant Mol Biol. 2008;68:571–583. doi: 10.1007/s11103-008-9392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao W, Xiao S, Li HY, Tsao SW, Chye ML. Arabidopsis thaliana acyl-CoA-binding protein ACBP2 interacts with a heavy-metal-binding protein AtFP6. New Phytol. 2009;181:89–102. doi: 10.1111/j.1469-8137.2008.02631.x. [DOI] [PubMed] [Google Scholar]
- 10.Li HY, Chye ML. Arabidopsis acyl-CoA binding protein ACBP2 interacts with an ethylene-responsive element binding protein AtEBP via its ankyrin repeats. Plant Mol Biol. 2004;54:233–243. doi: 10.1023/B:PLAN.0000028790.75090.ab. [DOI] [PubMed] [Google Scholar]
- 11.Li HY, Xiao S, Chye ML. Ethylene- and pathogen-inducible Arabidopsis acyl-CoA-binding protein 4 interacts with an ethylene-responsive element binding protein. J Exp Bot. 2008;59:3997–4006. doi: 10.1093/jxb/ern241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao S, Gao W, Chen QF, Ramalingam S, Chye ML. Overexpression of membrane-associated acyl-CoA-binding protein ACBP1 enhances lead tolerance in Arabidopsis. Plant J. 2008;54:141–151. doi: 10.1111/j.1365-313X.2008.03402.x. [DOI] [PubMed] [Google Scholar]
- 13.Leung KC, Li HY, Mishra G, Chye ML. ACBP4 and ACBP5, novel Arabidopsis acyl-CoA-binding proteins with kelch motifs that bind oleoyl-CoA. Plant Mol Biol. 2004;55:297–309. doi: 10.1007/s11103-004-0642-z. [DOI] [PubMed] [Google Scholar]
- 14.Engeseth NJ, Pacovsky RS, Newman T, Ohlrogge JB. Characterization of an acyl-CoA-binding protein from Arabidopsis thaliana. Arch Biochem Biophys. 1996;331:55–62. doi: 10.1006/abbi.1996.0282. [DOI] [PubMed] [Google Scholar]
- 15.Xiao S, Chen QF, Chye ML. Light-regulated Arabidopsis ACBP4 and ACBP5 encode cytosolic acyl-CoA-binding proteins that bind phosphatidylcholine and oleoyl-CoA ester. Plant Physiol Biochem. 2009;47:926–933. doi: 10.1016/j.plaphy.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Nishiuchi T, Nakamura T, Abe T, Kodama H, Nishimur M, Iba K. Tissue-specific and light-responsive regulation of the promoter region of the Arabidopsis thaliana chloroplast omega-3 fatty acid desaturase gene (FAD7) Plant Mol Biol. 1995;29:599–609. doi: 10.1007/BF00020987. [DOI] [PubMed] [Google Scholar]
- 17.Buchanan BB. Role of light in the regulation of chloroplast enzymes. Annu Rev Plant Physiol. 1980;31:341–374. [Google Scholar]
- 18.Sasaki Y, Nagano Y. Plant acetyl-CoA carboxylase: structure, biosynthesis, regulation and gene manipulation for plant breeding. Biosci Biotechnol Biochem. 2004;68:1175–1184. doi: 10.1271/bbb.68.1175. [DOI] [PubMed] [Google Scholar]
- 19.Harmer SL, Hogenesch JB, Straume M, Chang HS, Han B, Zhu T, et al. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science. 2000;290:2110–2113. doi: 10.1126/science.290.5499.2110. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki Y, Kozaki A, Hatano M. Link between light and fatty acid synthesis: thioredoxin-linked reductive activation of plastidic acetyl-CoA carboxylase. Proc Natl Acad Sci USA. 1997;94:11096–11101. doi: 10.1073/pnas.94.20.11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang HW, Zhang B, Hao YJ, Huang J, Tian AG, Liao Y, et al. The soybean Dof-type transcription factor genes, GmDof4 and GmDof11, enhance lipid content in the seed of transgenic Arabidopsis plants. Plant J. 2007;52:716–729. doi: 10.1111/j.1365-313X.2007.03268.x. [DOI] [PubMed] [Google Scholar]
- 22.Yanagisawa S. The Dof family of plant transcription factors. Trends Plant Sci. 2000;7:555–560. doi: 10.1016/s1360-1385(02)02362-2. [DOI] [PubMed] [Google Scholar]