Abstract
Francisella tularensis induces apoptosis within macrophages but the temporal and spatial modulation through activation of caspase-1, caspase-3, and the anti-apoptosis nuclear transcription factor B (NF-κB) is not known. Whether escape of the bacteria into the cytosol is sufficient and/or essential for activation of NF-κB is not known. Our results show that F. tularensis subsp. novicida induces sustained nuclear translocation of NF-κB at early time points after infection of human monocytes derived macrophages (hMDMs). The sustained nuclear translocation of NF-κB is defective in the iglC mutant that fails to escape into the cytosol of macrophages. Nuclear translocation of NF-κB by the wild type strain is abolished upon treatment with the NF-κB inhibitor caffein acid phenyl ester. While the wild type strain triggers caspase-3 and caspase-1 activation by 6 h post-infection the iglC mutant is defective in triggering both caspases. In hMDMs treated with the apoptosis-inducing agent, staurosporin, there is an induction of cell death in the iglC mutant-infected macrophages despite reduced frequency of caspase-1 and caspase-3 activity. The wt-infected macrophages are resistant to cell death-induced agent. We conclude that although caspase-1 and capsase-3 are triggered within F. tularensis-infected hMDMs during early stages of infection, cell death is delayed, which is correlated with simultaneous activation of NF-κB.
Keywords: tularemia, iglC, cell death
1. Introduction
Francisella tularensis is a gram-negative intracellular bacterium and the etiological agent of tularemia. There are four closely related subspecies of F. tularensis (tularensis, holarctica, mediasiatica and novicida), and subspecies tularensis is the most virulent to humans [1, 2]. F. tularensis subsp. novicida is attenuated in humans but causes disease in mice similar to that caused by subsp. tularensis, and is an attractive model to study pathogenesis of tularemia [3]. Relatively little is known about intracellular trafficking of F. tularensis. The F. tularensis-containing phagosome (FCP) of subsp tularensis, holarctica, and novicida matures to a late endosome-like phagosome that has a limited fusion to the lysosomes [1, 3, 4]. The FCP acquires the proton vATPase pump and is acidified within 15 min of its biogenesis, and the acidification is essential for disruption of the FCP and escape of F. tularensis into the macrophage cytosol, similar to L. monocytogenes [1, 5]. The Francisella pathogenicity island (FPI) gene iglC, and its regulator MglA are essential for bacterial escape into the cytosol of hMDMs, since mutants in both genes are defective in escape into the cytosol and their phagosomes fuse to lysosomes [1, 6].
Several bacterial pathogens induce apoptosis in mammalian cells by activating specific components of the apoptotic pathways [7]. Caspases play essential roles in apoptotic cell death [8]. Two major apoptotic pathways, designated the extrinsic and intrinsic pathways, mediate the activation of apoptotic caspases in response to various extra- and intracellular apoptotic stimuli [8]. The two main apoptotic pathways converge on the activation of caspase-3 [9]. The expression of many anti-apoptotic genes is regulated by the nuclear transcription factor kappa-B (NF-κB) that triggers the expression of two families of genes involved in inflammation and anti-apoptosis [10]. In activated macrophages, the ability of Francisella to escape the phagosome and replicate in the cytosol correlates with the activation of the inflammasome, which contains the host proteins ASC and caspase-1 [1, 11]. Inflammasome activation is critical for innate host defense and leads to the induction of cell death in infected cells and the concomitant release of the proinflammatory cytokines IL-1β and IL-18 [1, 11, 12]. F. tularensis LVS induces apoptosis in the J774A.1 murine macrophage cell line through a pathway partly resembling the intrinsic apoptotic pathway and is not thought to involve caspase 1, caspase 8, Bcl-2, or Bid [1, 13, 14].
The transcription factor NF-κB plays a crucial role in regulation of apoptosis by triggering expression of various anti-apoptotic genes [15]. NF-κB represents a family of homo- and heterodimer transcription factors, and the p65/p50 heterodimer is the most predominant active complex in mammalian cells [15]. In resting cells, NF-κB proteins are predominantly sequestered in the cytoplasm by the NF-κB inhibitory proteins (IkBs) [16]. The IκB kinase mediates phosphorylation of IκBs, followed by ubiquitination and proteosomal degradation, which is crucial to the activation and nuclear translocation of NF-κB [16]. Inhibition of NF-κB results in a differential effect on the levels of pro- and anti-inflammatory cytokine production in response to infection by the LVS strain of F. tularensis [1, 17, 18]. Inhibition of PI3K/Akt results in suppression of F. tularensis subsp novicida–induced cytokine production through the inhibition of NF-κB [19].
In this study, we examined the temporal and spatial modulation of F. tularensis induced apoptosis through activation of caspase-1, caspase-3, and the anti-apoptosis nuclear transcription factor B (NF-κB), and the role of phagosomal escape in modulation of these cellular processes at various stages of infection. Our data show that F. tularensis subsp. novicida induces sustained nuclear translocation of NF-κB, initiated at early time points after infection of hMDMs, which correlates with down-regulation of cell death despite robust activation of the executioner caspase-1 and caspase-3, tipping the balance in favour of survival of the infected cells till termination of intracellular proliferation.
2. Material and methods
2.1. Bacteria and macrophages
The wild-type (wt) F. tularensis subsp novicida strain U112 and it isogenic iglC mutant has been described previously [20]. The tetracyclin resistant plasmid pKK214 encodes gfp was introduced to the F. tularensis strains. Legionella pneumophila strain AA100 expressing GFP has been grown as described previously [21]. To prepare hMDMs, peripheral blood monocytes were isolated from healthy volunteers with no history of tularemia or Legionnaires’ disease and hMDMs were prepared as we described previously. Obtaining blood was approved by the institutional IRB with a consent form according to standard federal laws.
2.2. Confocal microscopy
In all confocal microscopy experiments, the cells were infected with bacteria at a MOI of 10 for 1 h, followed by 3x washing and 1 h of gentamicin treatment (50 µg ml−1) to remove extracellular bacteria. All experiments were done in triplicate and a minimum of 100 cells per sample were examined from different coverslips. On average, 8–15 0.2 µm serial z sections of each image were captured and stored for further analyses, using Adobe Photoshop 6.0 (Adobe Photoshop).
2.3. Apoptosis resistance of F. tularensis infected cells
To examine the sensitivity of F. tularensis-infected hMDMs to staurosporin-induced apoptosis, hMDMs were infected with the wt-GFP or the mutant strain iglC-GFP and either left untreated or treated with Staurosporin (50 nM) (Sigma, St Louis, MO). Treatment with staurosporine was carried out for 4 h starting at 8 h after infection. Control uninfected monolayers were either left untreated or treated with the above mentioned staurosporine for the same time periods. To examine the sensitivity of F. tularensis-infected hMDMs cells to CAPE-induced apoptosis, hMDMs were treated with 20 µg/ml CAPE for 30 min prior to infection as previously described [22].
2.4. Nuclear translocation of the p65 subunit of NF-κB in F. tularensis-infected cells
In all experiments, uninfected untreated cells, E. coli LPS-treated cells (1 µg ml−1 for 20 min) and L. pneumophla AA100 infected cells (Sigma, St Louis, MO) were used as a negative and positive controls, respectively, for nuclear translocation of the p65 subunit of NF-κB. To examine nuclear translocation of the p65 subunit in F. tularensis-infected cells, monolayers of hMDMs were infected with either wt-GFP or iglC-GFP and proceed by the protocol described previously [22].
2.5. Caspase 1 and Caspase 3 activation
To assess active caspase-1 or active caspase-3 staining by confocal microscopy, hMDMs on glass coverslips were infected with either wt-GFP or iglC-GFP strains at an MOI of 10 for 1, 6, and 24 h and proceed as described previously [22]. Briefly, macrophages were stained for 1 h with 6-carboxyfluorescein-YVAD fluoromethylketone (Immunochemistry Technologies) and caspase-3 inhibitor (BD, San Diego, CA) as recommended by the manufacturer. As a positive control for the hMDMs, macrophages were treated with 10 mM simvastatin (Calbiochem). For labeling of the caspase 3 positive cells, rabbit polyclonal antiactive caspase-3 antiserum (BD, San Diego, CA) and caspase-1 inhibitor were used (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h, and then incubated for 1 h with a goat anti-rabbit immunoglobulin G secondary antibody conjugated to Alexa red (Molecular Probes, Inc., Eugene, OR). Apoptotic nuclei were labled using an apoptosis detection kit (TUNEL) according to the manufacturer's instructions (Boehringer Mannheim Corporation, Indianapolis, IN).
3. Results
3.1. F. tularensis subsp novicida induces sustained nuclear translocation of NF-κB in hMDMs
In mammalian cells, the most predominant active complex of NF-κB is the p65/p50 heterodimer [16]. Although activation of NF-κB has been reported during F. tularensis LVS infection [17, 18], the temporal and spatial signaling mechanisms involved in modulation of macrophage survival and death are not well described. Thus, we examined whether F. tularensis triggered nuclear translocation of the p65 subunit of NF-κB in hMDMs. The iglC mutant was used as a control, since it does not escape from the phagosome [6]. That would allow us to decipher whether bacterial escape from the phagosome is essential for modulation of various cellular processes involved in survival of the infected host cell.
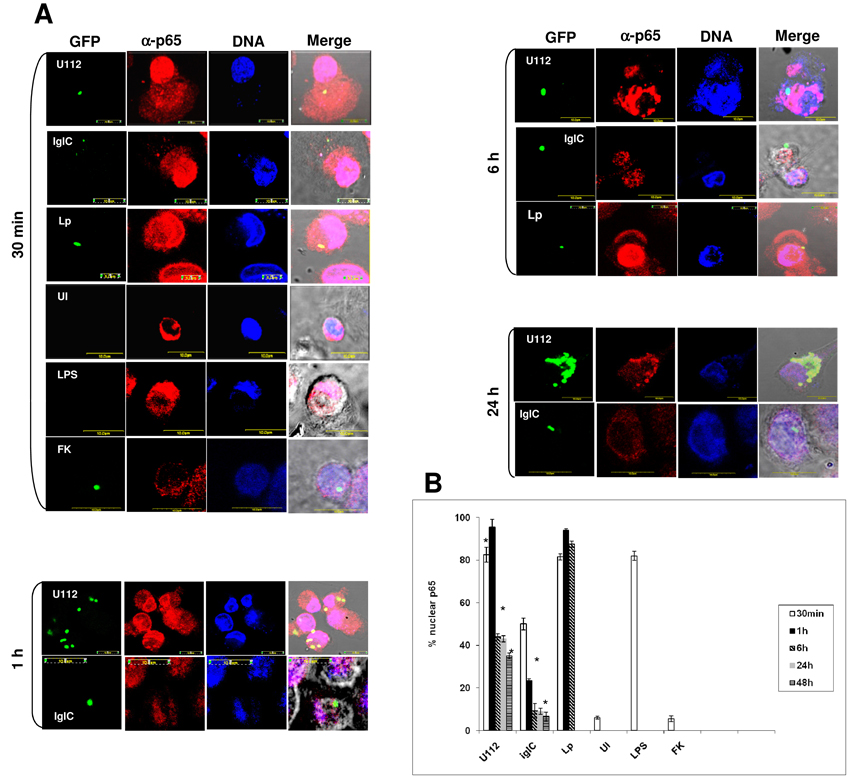
Using laser scanning confocal microscopy our results showed that NF-κB was translocated to the nuclei of only 7% of uninfected macrophages, while NF-κB was translocated to the nucleus in 85–95% of strain AA00 of L. pneumophila-infected hMDMs at all time points examined (Fig. 1A and B). The p65 subunit of NF-κB was translocated to the nucleus in ~85% of E. coli LPS-treated macrophages (Fig. 1A and B). By 1h after infection, 80–95% of the wt-infected macrophages showed nuclear translocation of NF-κB (Fig. 1 A and B). In contrast, only 25% of the iglC mutant-infected macrophages exhibited nuclear translocation of NF-κB, at 1h after infection (Fig. 1 A and B).
Fig. 1.
p65 nuclear translocation in F. tularensis-infected macrophages. (A) Representative confocal laser scanning microscopy images for nuclear translocation of the p65 subunit of NF-κB. Monolayers of hMDMs were infected with live or formalin-killed (FK) wt-GFP, iglC-GFP, AA100-GFP at moi of 10 for 1 h. At several time points after infection, the cells were labelled for the p65 subunit of NF-κB and nuclei. Uninfected monolayers and E. coli LPS-treated monolayers were included as negative and positive controls. Approximately 100 infected macrophages were analyzed from different coverslips. (B) Quantitative analysis of p65 nuclear translocation in F. tularensis-infected macrophages. The results are representative of three independent experiments and error bars represent standard deviations. The asterisks represent statistically significant difference.
By 6–48 h after infection, 35–45% of the wt strain-infected macrophages showed sustained nuclear translocation of NF-κB (Fig. 1A and B), but only 8–15% of the iglC mutant-infected macrophages showed nuclear translocation of NF-κB (Fig. 1 A and B). There was statistically significant difference between nuclear translocation of NF-κB by the wt strain and the iglC mutant strain at all time points during infection (Student t-test, p<0.001). We conclude that bacterial escape into the host cell cytosol by 60 min post-infection is essential for early nuclear translocation of NF-κB.
To examine whether F. tularensis subsp. novicida LPS is involved in F. tularensis induced nuclear translocation of NF-κB, formalin-killed bacteria were used for infection. At 30 min after infection, nuclear translocation of NF-κB was detected in only 5 % of hMDMs infected with the formalin-killed WT strain (Fig. 1 A and B), which was similar to uninfected hMDMs. These data may suggest that the F. tularensis subsp. novicida LPS is unlikely to be involved in the induction of nuclear translocation of NF-κB. However, it can’t be excluded that formalin-sensitive LPS components may be involved.
3.2. CAPE blocks sustained activation of NF-κB in human macrophages
We utilized caffeic acid phenethyl ester, which inhibits NF-κB activation in response to various stimuli, such as LPS to examine its effect on the infection by F. tularensis subsp. novicida [23]. We used L. pneumophila as a positive control, since CAPE does not inhibit nuclear translocation of NF-κB in L. pneumophila-infected macrophages [22]. Since CAPE blocks the LPS mediated activation of NF-κB, LPS-treated cells in presence of CAPE were used as a control.
Our data showed that at 30 min after infection in the presence of CAPE, nuclear translocation of NF-κB was detected only in 20 % of the wt strain infected macrophages while 80% of strain AA100 of L. pneumophila infected hMDMs treated with CAPE exhibited nuclear translocation (Fig. 2 A and B).
Fig. 2.
CAPE blocks NF-κB activation human macrophages. (A) Representative confocal laser scanning microscopy images for nuclear translocation of the p65 subunit of NF-κB in CAPE treated cells. Monolayers of hMDMs were treated with 20 µg ml−1 of CAPE 30 min prior to infection. Cells were infected with the wt strain of F. tularensis subsp. novicida at a moi 10. The parental strain AA100 of L. pneumophila was used as positive control. Uninfected and E. coli LPS-treated (1 µg ml−1 for 20 min) monolayers were included as a negative and positive control respectively. (B) Quantitative analysis of p65 nuclear translocation in F. tularensis-infected macrophages in CAPE treated cells. The results are representative of three independent experiments and error bars represent standard deviations. The asterisks represent statistically significant difference.
By 1 and 6 h after infection, only ~10% of the wt strain-infected hMDMs that were treated with CAPE exhibited nuclear translocation of NF-κB (Student t-test, p<0.001) (Fig. 2 A and B). The data indicate CAPE blocks NF-κB activation by F. tularensis subsp. novicida, which is very distinct from L. pneumophila.
3.3. Modulation of apoptosis via nuclear translocation of NF-κB by F. tularensis subsp. novicida
It has been shown that F. tularensis induce apoptosis in murine macrophages [13, 24]. We examined the role of sustained nuclear translocation of NF-κB activation in modulation of apoptosis in F. tularensis-infected human macrophages. We used L. pneumophila as a control, since nuclear translocation of NF-κB in L. pneumophila-infected macrophages is required for resistance to apoptosis-inducing agents [22].
Our data showed that only 5–10% of untreated uninfected, wt-infected and iglC mutant-infected cells were TUNEL-positive by 1–6 h after infection (Fig. 3B). In CAPE treated cells, 23–40% of uninfected, wt-infected and iglC mutant-infected cells were TUNEL-positive, which was significantly different from untreated cells (Student t-test, p<0.01) (Fig. 3 A and B). As expected, CAPE did not enhance apoptosis in L. pneumophila-infected control cells [22]. Importantly, CAPE does not activate caspases directly.
Fig. 3.
The protection from cell death correlates with nuclear translocation of NF-κ B in F. tularensis-infected cells. (A) Representative confocal laser scanning microscopy images of apoptosis in CAPE treated cells 6h post-infection. Monolayers of hMDMs were either untreated or treated with 20 µg ml−1 of CAPE 30 min prior to infection. Cells were infected with the wt strain of F. tularensis subsp. novicida or with the iglC mutant strain at a moi 10. The parental strain AA100 of L. pneumophila was used as control. At 1, 6 and 24 h after infection, cell death was determined by TUNEL. (B) Quantitative analysis of cell death detected by TUNEL after CAPE treatment. Approximately 100 macrophages were analysed by confocal microscopy from different coverslips. The asterisks represent statistically significant difference.
By 24 h after infection, 70% of the untreated cells infected with the wt strain of F. tularensis were TUNEL-positive while 85% of CAPE-treated infected cells underwent apoptosis (Student t-test, p<0.01) (Fig. 3 A and B). In untreated cells infected with the iglC mutant 16% of the cells were TUNEL-positive. In contrast, in CAPE treated cells 45% of the iglC mutant-infected cells underwent cell death, similar to L. pneumophila. We conclude that sustained nuclear translocation of NF-κB by the wt strain contributes modestly but significantly to down-regulation of cell death in F. tularensis subsp. novicida infected human macrophages.
3.4. The FPI-encoded protein IglC plays an important role in cell death through caspase-1 activation
F. tularensis escapes from the phagosome into the cytosol of macrophages within 30–60 min of entry into the cells (See [1] for a recent review). F. tularensis activates the inflammasome though caspase-1, and the iglC mutant is defective in caspase-1 activation within mice macrophages [25]. It is not known whether bacterial escape by itself or subsequent replication within the cytosol after phagosomal escape of F. tularensis is essential for triggering caspase-1 activation within human macrophages. Therefore, we used single cell analyses to determine, simultaneously, the kinetics of activation of caspase-1 and apoptosis in hMDMs infected by the iglC mutant and compared it to the wt strain.
The data showed at 1 h after infection, the wt strain of F. tularensis subsp. novicida induced caspase-1 activation in 45% of the infected cells but there were no signs of cell death at that time point (Fig. 4 A and B). In contrast, the iglC mutant did not trigger caspase-1 activation at 1 h after infection (Student t-test, p<0.001) (Fig. 4 A and B).
Fig. 4.
F. tularensis induces caspase 1 activation in hMDMs. (A) Representative confocal laser scanning microscopy images of hMDMs infected with the wt strain or iglC mutant. The activation of caspase 1 (C1) and apoptosis (TUNEL) were determined at 1, 6 and 24 h post infection. (B) Quantitative analysis of C1 activation in C3 inhibited cells stained for cell death. Approximately 100 macrophages were analysed by confocal microscopy from different coverslips. The data are representative of three independent experiments and error bars represent standard deviations. The asterisks represent statistically significant difference.
By 6 h after infection with the wt strain, 60% of the cells were active for caspase-1 but only 20% of the cells were also TUNEL-positive; thus, positive for both TUNEL and caspase-1 (Fig. 4 A and B). At 6 h after infection only ~10 % of the cells infected with the iglC mutant exhibited caspase-1 activation (Fig. 4 A and B) (Student t-test, p< p<0.0002), and were also TUNEL-positive (Student t-test, p<0.001) (Fig. 4 A and B). Therefore, bacterial escape into the cytosol is essential for caspase-1 activation during early stages of the infection as also showed by Mariathasan et al. [11]
By 24 h after infection by the wt strain, 70% of the cells were positive for both caspase-1 and TUNEL (Fig. 4 A and B), but only 16% of the iglC mutant-infected cells induced caspase-1 activation and 10% were also TUNEL-positive (Fig. 4 A and B) (Student t-test, p<0.0002). Our results showed that F. tularensis subsp novicida induced activation of caspase-1 during early stages of infection of hMDMs which was associated with the induction of delayed cell death by 24 h. The FPI-encoded protein IglC plays an important role in cell death through caspase-1 activation. Cell death is likely to be mediated by multiple processes including caspase-1, caspase-3, and other caspases-independent processes.
3.5. Bacterial escape is essential for caspase-3 activation in F. tularensis infected hMDMs
Although caspase-3 has been shown to be triggered by F. tularensis within the J744.1 murine macrophage cell line [24], it is not known whether this occurs in primary cells and whether escape and replication of the bacteria in the cytosol is also required for triggering caspase-3 activation.
The data showed that at 1h after infection approximately 20% of the cells infected by the wt strain exhibited activation of caspase-3, which was significantly different from the iglC mutant or uninfected cells (Student t-test, p<0.001). Only a few cells were positive for TUNEL at this time point (Fig. 5 B).
Fig. 5.
F. tularensis induces caspase 3 activation in the late stage of infection of hMDMs. (A) Representative confocal laser scanning microscopy images of hMDMs infected with the wt strain or the iglC mutant. The activation of caspase 3 (C3) and TUNEL were determined at 1, 6 and 24 h post-infection. (B) Quantitative analysis of C3 activation in C1 inhibited cells stained for cell death. Approximately 100 macrophages were analysed by confocal microscopy from different coverslips. The data are representative of three independent experiments and error bars represent standard deviations. The asterisks represent statistically significant difference.
At 6 h post-infection, 46% of wt-infected hMDMs exhibited caspase-3 activation, which was significantly different from cells infected by the iglC mutant (13%) (Student t-test, p<0.0009). Only 20% of the wt strain-infected cells that exhibited caspase-3 activation became TUNEL-positive (Fig. 5 B). We conclude that bacterial escape into the cytosol is sufficient and essential for early activation of caspase-3 prior to bacterial proliferation within the cytosol. Importantly, despite caspase-3 activation, cell death is not triggered till later stages of the infection.
By 24 h after infection by the wt strain, a large number of the cells were lysed, and 80% of the remaining infected cells exhibited caspase-3 activation but only 45% were also TUNEL-positive (Fig. 5 A and B). In contrast, the cells infected with the iglC mutant strains exhibited minimal frequency of caspase-3 activation or apoptosis (Fig. 5 A and B).
We conclude that bacterial escape into the cytosol is essential and sufficient for the induction of caspase-3 activation during early stages of the infection. Although there is robust caspase-3 activation in F. tularensis infected hMDMs, cell death is largely delayed, suggesting potential anti-apoptotic signalling in the infected cells. The delayed cells death is likely mediated by caspases as well as caspases-independent processes.
3.6. Resistance of infected macrophages to apoptotic stimuli
Caspase-3 is the executioner of apoptosis and its activation signals the point of 'no return“ in the apoptotic pathways. Since many infected cells exhibited robust activation of caspase-3 and caspase-1 but were not apoptotic, the delay in cell death of F. tularensis-infected cells may be correlated with up-regulation of anti-apoptotic signalling.
At 6 h after infection, more then 70% of the wt strain-infected cells treated with 50 nM staurosporin (ST) showed activated caspase-3 and were also TUNEL-positive, which was significantly different from uninfected cells (Student t-test, p<0.01). At all time points after infection, less then 50% of the iglC mutant infected cells exhibited activation of caspase-3 and were also TUNEL-positive (Fig. 6 A and B). These data showed that F. tularensis-infected cells exhibited modest but significant resistance to potent external apoptotic stimuli, compared to uninfected cells, and this correlated with the activation of anti-apoptotic signalling.
Fig. 6.
Resistance to apoptosis-inducing agents. (A) Representative confocal microscopy images of hMDMs macrophages infected with either F. tularensis subsp. novicida wild type strain or iglC mutant strain. Staurosporin was used to induce artificially apoptosis of the macrophages. The cells were stained with both the TUNEL assay labelling (red) and the active Caspase-3 antibodies (blue). (B) Quantitative analysis of cell death in staurosporin treated cells. Approximately 100 macrophages were analysed by confocal microscopy from different coverslips. The asterisks represent statistically significant difference.
4. Discussion
Francisella tularensis is a highly contagious, facultative intracellular bacterium that causes tularaemia in humans and animals [1]. F. tularensis rapidly escape the phagosome and replicate in the cytosol of host cells [1]. It is not known whether escape of F. tularensis from the phagosome into the cytosol and subsequent intracellular proliferation are critical for innate host defence mechanisms such as modulation of apoptosis through caspases and NF-kβ. In addition, since F. tularensis triggers caspase-1 and caspase-3 activation along with apoptosis and activation of NF-κB [1], the temporal and spatial modulation of these cellular processes is not known. Therefore, our study is the first to determine all of these processes in the same system under the same experimental conditions. Our data clearly show that despite activation of caspase-1 and caspase-3 during early stages of the infection by F. tularensis, cell death is delayed, which is correlated with the activation of NF-κB.
Fine-tuning caspases activation along with modulation of pro-apoptotic, anti-apoptotic, as well as proinflammatory processes through temporal modulation of NF-κB is likely to be an important aspect of the pathogenic evolution of F. tularensis. Several intracellular pathogens, such as Legionella pneumophila, Rickettsia rickettsii and, Toxoplasma gondii inhibit apoptosis of their host cells by a mechanism that involves NF- κB activation [22, 26, 27]. F. tularensis subsp novicida mglA mutant displays enhanced NF-κB activation and enhanced proinflammatory cytokine production compared to cells infected with the wild type strain [28]. Our results have shown that F. tularensis subsp. novicida induces nuclear translocation of NF-κB at early time points after infection of hMDMs. The sustained nuclear translocation of NF-κB in F. tularensis infected macrophages is defective in the iglC mutant that fails to escape into the cytosol at the early time point of infection. Therefore, bacterial escape into the cytosol is required, and is sufficient, for nuclear translocation of NF-κB in F. tularensis-infected macrophages.
Some bacteria induce apoptosis in mammalian cells by activating specific components of the apoptotic pathways, including the activation of caspases. The cytosolic pathogens F. tularensis triggers activation of caspase-1 through the adaptors ASC or Ipaf [1, 11]. In turn, caspase-1 activation leads to the release of two potent proinflammatory cytokines, IL-1α and -18, and eventually leads to cell death [11]. Previous work has shown that processing of caspase-1 does not occur in macrophages infected with the mglA mutant of F. tularensis [12], which is defective in a global regulator involved in stress and metabolic responses as well as bacterial escape into the cytosol. Our data show that the iglC mutant is defective in triggering caspase-1, indicating that bacterial escape into the cytosol is sufficient and essential for activation of caspase-1.
Our data have shown that by 6 h post-infection, the wt strain-infected hMDMs exhibited caspase-3 activation, but the iglC mutant strain is defective in triggering caspase-3 activation. Therefore, bacterial escape into the cytosol is essential and sufficient for triggering caspase-3 activation. Despite robust caspase-3 activation, cell death is not apparent till late stages of the infection, concomitant with the termination of intracellular replication. Cell death is most likely mediated by multiple process including caspas-1, caspase-3, and caspases-independent processes.
Our studies show for the first time simultaneous modulation of caspase-1, caspase-3, and activation of NF-κB, and the delicate balance between them to maintain viability of the infected cell. It is remarkable that both caspase-1 and caspase-3 are activated during initial stages of the infection, yet viability of the infected cell is largely maintained, which is correlated with simultaneous activation of the anti-apoptotic transcription factor NF-κB.
Acknowledgements
YAK is supported by Public Health Service Awards R01AI43965 and R01AI069321 from NIH and by the commonwealth of Kentucky Research Challenge Trust Fund and by Ministry of Science Educatuion and Sports Republic of Croatia (Grant number: 062-0621273-0950).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Santic M, Khodor SA, Kwaik YA. Cell biology and molecular ecology of Francisella tularensis. Cell Microbiol. 2009 doi: 10.1111/j.1462-5822.2009.01400.x. [DOI] [PubMed] [Google Scholar]
- 2.Forsman M, Sandstrom G, Jaurin B. Identification of Francisella species and discrimination of type A and type B strains of F. tularensis by 16S rRNA analysis. Appl Environ Microbiol. 1990;56:949–955. doi: 10.1128/aem.56.4.949-955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santic M, Molmeret M, Klose KE, Abu Kwaik Y. Francisella tularensis travels a novel, twisted road within macrophages. Trends Microbiol. 2006;14:37–44. doi: 10.1016/j.tim.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Golovliov I, Baranov V, Krocova Z, Kovarova H, Sjostedt A. An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells. Infect Immun. 2003;71:5940–5950. doi: 10.1128/IAI.71.10.5940-5950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santic M, Asare R, Skrobonja I, Jones S, Abu Kwaik Y. Acquisition of the vacuolar ATPase proton pump and phagosome acidification are essential for escape of Francisella tularensis into the macrophage cytosol. Infect Immun. 2008;76:2671–2677. doi: 10.1128/IAI.00185-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonquist L, Lindgren H, Golovliov I, Guina T, Sjostedt A. MglA and Igl proteins contribute to the modulation of Francisella tularensis live vaccine strain-containing phagosomes in murine macrophages. Infect Immun. 2008;76:3502–3510. doi: 10.1128/IAI.00226-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navarre WW, Zychlinsky A. Pathogen-induced apoptosis of macrophages: a common end for different pathogenic strategies. Cell Microbiol. 2000;2:265–273. doi: 10.1046/j.1462-5822.2000.00056.x. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9:459–470. doi: 10.1016/s1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]
- 9.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 10.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 11.Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavrilin MA, Bouakl IJ, Knatz NL, Duncan MD, Hall MW, Gunn JS, Wewers MD. Internalization and phagosome escape required for Francisella to induce human monocyte IL-1beta processing and release. Proc Natl Acad Sci U S A. 2006;103:141–146. doi: 10.1073/pnas.0504271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai XH, Golovliov I, Sjostedt A. Francisella tularensis induces cytopathogenicity and apoptosis in murine macrophages via a mechanism that requires intracellular bacterial multiplication. Infect Immun. 2001;69:4691–4694. doi: 10.1128/IAI.69.7.4691-4694.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lauriano CM, Barker JR, Yoon SS, Nano FE, Arulanandam BP, Hassett DJ, Klose KE. MglA regulates transcription of virulence factors necessary for Francisella tularensis intraamoebae and intramacrophage survival. Proc Natl Acad Sci U S A. 2004;101:4246–4249. doi: 10.1073/pnas.0307690101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burstein E, Duckett CS. Dying for NF-kappaB? Control of cell death by transcriptional regulation of the apoptotic machinery. Curr Opin Cell Biol. 2003;15:732–737. doi: 10.1016/j.ceb.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 17.Katz J, Zhang P, Martin M, Vogel SN, Michalek SM. Toll-like receptor 2 is required for inflammatory responses to Francisella tularensis LVS. Infect Immun. 2006;74:2809–2816. doi: 10.1128/IAI.74.5.2809-2816.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Telepnev M, Golovliov I, Grundstrom T, Tarnvik A, Sjostedt A. Francisella tularensis inhibits Toll-like receptor-mediated activation of intracellular signalling and secretion of TNF-alpha and IL-1 from murine macrophages. Cell Microbiol. 2003;5:41–51. doi: 10.1046/j.1462-5822.2003.00251.x. [DOI] [PubMed] [Google Scholar]
- 19.Rajaram MV, Ganesan LP, Parsa KV, Butchar JP, Gunn JS, Tridandapani S. Akt/Protein kinase B modulates macrophage inflammatory response to Francisella infection and confers a survival advantage in mice. J Immunol. 2006;177:6317–6324. doi: 10.4049/jimmunol.177.9.6317. [DOI] [PubMed] [Google Scholar]
- 20.Lauriano CM, Barker JR, Nano FE, Arulanandam BP, Klose KE. Allelic exchange in Francisella tularensis using PCR products. FEMS Microbiol Lett. 2003;229:195–202. doi: 10.1016/S0378-1097(03)00820-6. [DOI] [PubMed] [Google Scholar]
- 21.Molmeret M, Santic M, Asare R, Carabeo RA, Abu Kwaik Y. Rapid escape of the dot/icm mutants of Legionella pneumophila into the cytosol of mammalian and protozoan cells. Infect Immun. 2007;75:3290–3304. doi: 10.1128/IAI.00292-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abu-Zant A, Jones S, Asare R, Suttles J, Price C, Graham J, Abu Kwaik Y. Anti-apoptotic signalling by the Dot/Icm secretion system of L. pneumophila. Cell Microbiol. 2007;9:246–264. doi: 10.1111/j.1462-5822.2006.00785.x. [DOI] [PubMed] [Google Scholar]
- 23.Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci U S A. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai XH, Golovliov I, Sjostedt A. Expression of IglC is necessary for intracellular growth and induction of apoptosis in murine macrophages by Francisella tularensis. Microb Pathog. 2004;37:225–230. doi: 10.1016/j.micpath.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 26.Clifton DR, Goss RA, Sahni SK, Antwerp DV, Baggs RB, Marder VJ, Silverman DJ, Sporn LA. NF-kB-dependent inhibition of apoptosis is essential for host cell survival during Rickettsia rickettsii infection. Proc.Natl.Acad.Sci.USA. 1998;95:4646–4651. doi: 10.1073/pnas.95.8.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molestina RE, Payne TM, Coppens I, Sinai AP. Activation of NF-kappaB by Toxoplasma gondii correlates with increased expression of antiapoptotic genes and localization of phosphorylated IkappaB to the parasitophorous vacuole membrane. J Cell Sci. 2003;116:4359–4371. doi: 10.1242/jcs.00683. [DOI] [PubMed] [Google Scholar]
- 28.Butchar JP, Rajaram MV, Ganesan LP, Parsa KV, Clay CD, Schlesinger LS, Tridandapani S. Francisella tularensis induces IL-23 production in human monocytes. J Immunol. 2007;178:4445–4454. doi: 10.4049/jimmunol.178.7.4445. [DOI] [PubMed] [Google Scholar]