Summary
Considering the regulatory complexities of PR action throughout the female reproductive axis and mammary gland, we generated a mouse model that enables conditional ablation of PR function in a spatiotemporal specific manner. Exon 2 of the murine PR gene was floxed to generate a conditional PR allele (PRflox) in mice. Crossing the PRflox/flox mouse with the ZP3-cre transgenic demonstrated that the PRflox allele recombines to a PR null allele (PRd). Mice homozygous for the recombined null PR allele (PRd/d) exhibit uterine, ovarian, and mammary gland defects that phenocopy those of our previously described PR Knockout (PRKO) model. Therefore this conditional mouse model for PR ablation represents an invaluable resource with which to further define in a developmental and/or reproductive stage-specific manner the individual and integrative roles of distinct PR populations resident in multiple progesterone-responsive target sites.
Keywords: Progesterone Receptor, Mouse, Conditional Knockout, Reproduction, Mammary Gland
Global abrogation of progesterone receptor (PR) expression in the mouse disclosed the essential role of PR mediated signaling in the female reproductive tract and mammary gland (Lydon et al., 1995). While studies on the PR knockout (PRKO) mouse underscored the overall importance of PR as a pleiotropic coordinator of numerous physiological systems required for normal reproductive function in the female, selective functional ablation of the PR isoforms (PR-A and PR-B) dissected the individual contributions of these receptor subtypes in reproductive and mammary gland biology (Mulac-Jericevic et al., 2003; Mulac-Jericevic et al., 2000). In addition to generating global and isoform specific PRKOs, the murine PR locus has also been engineered (through “knockin” gene-targeting strategies) to express the lacZ reporter (Ismail et al., 2002), cre recombinase (Mukherjee et al., 2006b; Soyal et al., 2005), and reverse tetracycline transactivator (rtTA) (Mukherjee et al., 2007) under the control of the endogenous PR promoter. These second generation PR mouse models have been instrumental in rapidly expanding our understanding of PR action in vivo at the morphological, cellular, and molecular level (Ismail et al., 2002; Lee et al., 2006; Mukherjee et al., 2006a).
In keeping with PR’s pleiotropic effects in female reproductive biology, studies with the PRlacz reporter mouse (along with immunohistochemical approaches) have revealed a complex spatiotemporal expression profile for PR in many target tissues (Ismail et al., 2002; Tibbetts et al., 1998). Depending on the target tissue, PR’s expression profile has been shown to change during development and/or with reproductive state. Importantly, application of reciprocal transplantation techniques has shown that PR populations in distinct cellular compartments within complex target tissues (i.e. the uterus) exhibit different functional properties when exposed to hormone (Kurita et al., 1998). Collectively, these findings suggest that PR not only coordinates the functional activities of numerous target tissues within the reproductive axis that ensure normal female fecundity but also orchestrates signaling cross-talk between different cellular compartments within a given target tissue.
Although the aforementioned mouse models have afforded much needed insight into PR action in vivo, these models are not designed to allow study of PR function in a single target tissue or select cell-lineage. To address this deficiency, we report here on the generation of a new PR mouse model which carries a conditional PR null allele (PRflox allele) to enable restricted spatiotemporal cre mediated ablation of PR expression in vivo.
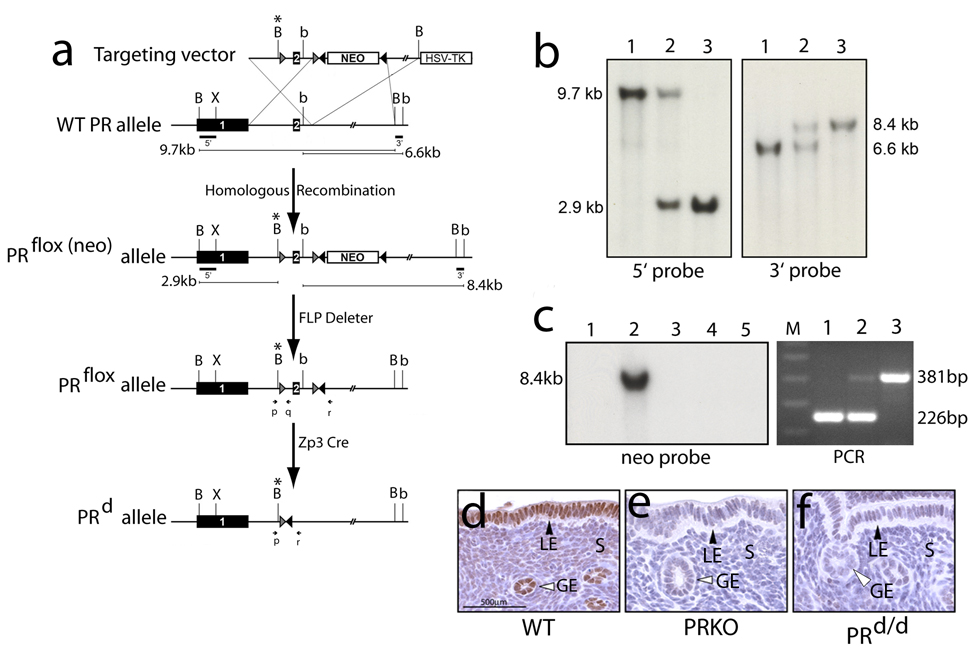
The PRflox allele was engineered by flanking (or “floxing”) exon 2 of the murine PR gene with a loxP site (exon 2 of the PR gene encodes the first zinc finger of the receptor’s DNA binding domain (DBD)). Following the gene-targeting strategy described in Fig. 1a (and in the Materials & Methods section), screening 576 murine embryonic stem (ES) cell clones yielded 11 ES clones that were positive for the correctly targeted PRflox(neo) mutation. Using standard procedures (Lydon et al., 1995), seven of these PRflox(neo) positive ES cell clones were chosen to generate high-quality chimeric male mice. Four of these mouse lines transmitted the PRflox(neo) mutation through the germline. Southern analysis (and PCR analysis (data not shown)) confirmed that loxP sites flanked both sides of exon 2 (Fig. 1b). Three of these mouse lines were crossed with the FLPeR (or FLP deleter) mouse (Farley et al., 2000) to remove the FRT-PGKNeo-FRT cassette (as judged by Southern blot analysis using a specific neo probe (Fig. 1c)). To validate the PRflox allele as a conditional allele for the PRKO phenotype, PRflox/flox mice were crossed with ZP3-Cre mice (Lewandoski et al., 1997) to delete PR expression in the PRflox/flox: ZP3-Cre bigenic (termed PRd/d hereon). Immunohistochemistry for PR expression confirmed the absence of PR expression in the uterus of the PRd/d mouse ((Fig. 1d–f); abrogation of PR expression in other target tissues (i.e. mammary gland) was also observed (data not shown)). Its important to note that mice heterozygous or homozygous for the PRflox(neo) or PRflox allele phenocopied WT mice (data not shown).
FIG. 1.
Generation of the PRflox/flox and PRd/d mouse. (a) Schematic shows the gene-targeting strategy and relevant mouse crosses to generate mice carrying the PRflox/flox and PRd allele. The targeting vector contains 7.9kb of PR homologous sequence in which the 5’ and 3’ arms of homology are 2.9kb and 5kb respectively. Within the 5’arm, exon 2 is engineered to be flanked by loxP sites (gray triangles) in which the 5’ loxP site also contains a novel Bam HI site (B*). The FRTNEOFRT cassette (NEO) is inserted in intron 2 in which the neomycin resistance gene (neo) is flanked by direct FRT repeats (black triangles). The HSV-TK gene is attached to the 3’ end of the targeting vector to allow negative selection. Homologous recombination with the PR allele in ES cells results in the generation of the PRflox(neo) targeted allele in which exon 2 is floxed and the FRTNEOFRT cassette is inserted in intron 2. Mice carrying the PRflox(neo) targeted allele were crossed with FLP deleter mice to remove the FRTNEOFRT cassette to generate mice harboring the PRflox allele in which exon 2 is floxed but phenotypically normal. The PRflox mouse was crossed with the Zp3 Cre mouse to excise exon 2. Following cre-mediated excision, a single loxP and FRT site remain in the intron of the PRd allele. For experiments described here, mice heterozygous for the PRd allele were crossed to generate mice homozygous for the PRd allele (PRd/d mice). Bam HI, Bgl II, and Xho I are denoted by B, b, and X respectively. The positions of the PCR primers used to identify mice carrying the WT and PRd allele are indicated by p, q, and r. (b) Southern analysis of mouse genomic DNA digested with Bam HI and hybridized with the 5’ probe yields a 9.7kb band for WT mice, a 9.7 kb and 2.9kb band for PRflox(neo)/+ mice, and a single 2.9kb for PRflox(neo)/flox(neo) mice (lanes 1, 2, and 3 respectively). Southern analysis using the 3’ probe yields a single 6.6kb hybridizing band for WT mice (lane 1), a 6.6kb and 8.4kb band for PRflox(neo)/+ mouse (lane 2), and a single 8.4kb hybridization band for PRflox(neo)/flox(neo) mice (lane 3). (c) Left panel shows a Southern analysis of genomic DNA digested with Bgl II and hybridized with the neo probe (see Materials & Methods). As expected, a hybridizing band is not detected in WT genomic DNA (lane 1); however, an 8.4kb band is detected in genomic DNA from the PRflox(neo)/+ mouse (lane 2). Lanes 3, 4, and 5 correspond to genomic DNA isolated from representative mice from three separate lines of PRflox/+ mice in which the FRTNEOFRT cassette is deleted following a cross with FLP deleter mice. The right panel shows a typical PCR result (using the primers p, q, and r (Fig 1a)) for the WT, PRd/+, and PRd/d genotype (lanes 1–3 respectively). The 226bp PCR band denotes the WT allele whereas the 381 bp band indicates the PRd allele; lane M signifies the marker lane. (d–f) show representative uterine sections stained for PR immunoreactivity from 10 week-old WT, PRKO, and PRd/d mice respectively. Note the absence of PR expression in the luminal epithelial (LE), glandular epithelial (GE), and stromal (S) compartments in the uterus of the PRKO and PRd/d mouse.
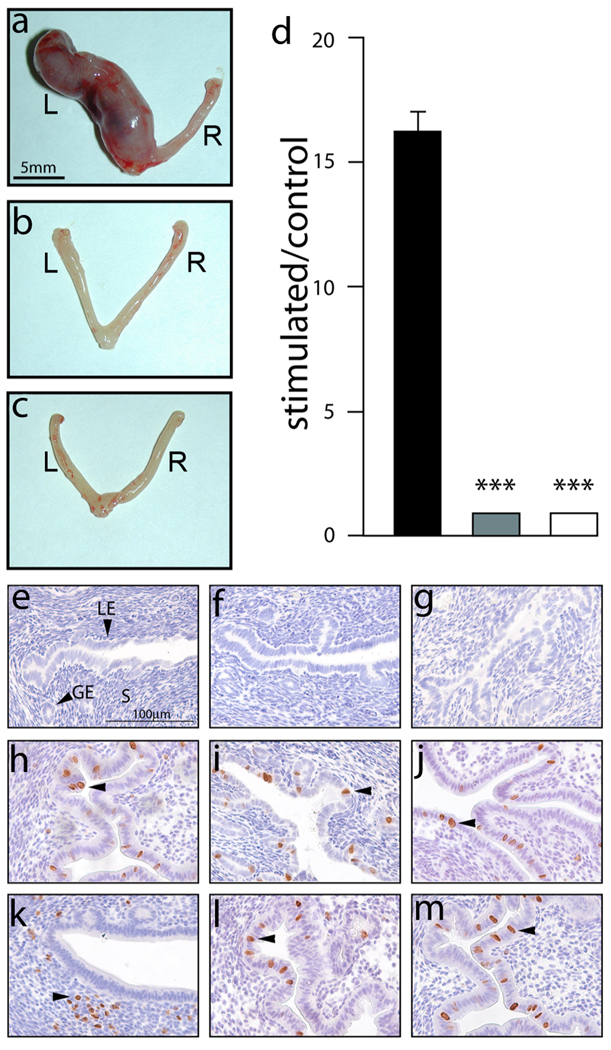
A number of murine genetic models have reinforced and significantly extended our understanding of uterine PR action in the mouse (Lee et al., 2006; Lee et al., 2007; Lydon et al., 1995; Mukherjee et al., 2006a; Mulac-Jericevic et al., 2003; Mulac-Jericevic et al., 2000). Apart from establishing a pivotal role for uterine PR in the development of the receptive endometrium for embryo implantation and subsequent decidualization of the uterine stroma to form decidua, these studies have also disclosed the cellular and molecular mechanisms that underlie PR action in these uterine processes. Using a previously described protocol to elicit an artificial decidual response in the murine uterine horn (Lydon et al., 1995) (also see Material & Methods), the WT stimulated left (L) uterine horn shows clear evidence of a full decidual reaction (Fig. 2a; as expected, the unstimulated right horn fails to show this morphological response). However, a decidual response is not induced in the stimulated horn in the case of the similarly treated PRKO and PRd/d uterus (Fig. 2b–d). Furthermore, a number of mouse studies have demonstrated that P4 administration effectively blocks the mitogenic effects of E2 in the luminal epithelial compartment of the uterus (Chen et al., 2005; Pan et al., 2006; Tong and Pollard, 1999; Zhu and Pollard, 2007). Indeed reciprocal transplantation experiments have suggested that the stromal derived PR population is responsible for countering E2 proliferative effects in the uterine cellular compartment (Kurita et al., 1998). Following ovariectomy, the uterus of the WT, PRd/d and PRKO mouse do not display cells undergoing proliferation (Fig. 2e–g respectively). In response to acute E2 exposure (see Methods), uteri from all three genotypes show an equivalent number of luminal epithelial cells undergoing proliferation (as judged by the incorporation of BrdU (Fig. 2h–j)). In response to E2P4 co-administration, proliferation is suppressed in the luminal epithelial compartment of the WT uterus whereas a subset of subepithelial stromal cells score positive for BrdU incorporation (Fig. 2k). In contrast, absence of PR in the PRd/d and PRKO uterus results in a failure of P4 induced repression of E2 induced luminal epithelial proliferation when exposed to E2P4 (Fig. 2l and m respectively); also, P4 directed stromal proliferation is absent in these uteri. Therefore, absence of PR expression in the PRKO and PRd/d uterus results in a block in both P4-induced suppression of E2-induced luminal epithelial proliferation and a defect in the redirection of the proliferative stimulus to the subepithelial stromal compartment (Fig. 2e–m).
FIG. 2.
Uterine defects in the PRd/d mouse. (a) At the macro level, a decidual reaction is clearly evident in the left (L) uterine horn of the hormone-treated WT mouse following the decidual stimulus (Materials & Methods); a decidual reaction was not detected in the unstimulated right (R) or control horn. (b–c) show that similarly treated PRKO and PRd/d uteri respectively do not exhibit a decidual response in the mechanically stimulated left horn. (d) Histogram displays the average ratio of wet weights of stimulated horn over control horn (± SEM) for WT (black column); PRKO (grey column); and PRd/d (white column) mouse groups. ***, P < 0.001, one-way ANOVA followed by Tukey’s post-hoc multiple range test (N=4 per genotype). (e–g) show representative uterine sections stained for BrdU incorporation from sesame oil treated ovariectomized WT, PRKO, and PRd/d mice respectively. Note the absence of BrdU positive cells in the luminal epithelium (LE), glandular epithelium (GE), and stromal (S) compartments of the WT, PRKO, and PRd/d uterus. H, I, and J show representative uterine sections stained for BrdU incorporation from E2 treated WT, PRKO, and PRd/d mice respectively. Arrowheads indicate the position of BrdU positive cells in the luminal epithelial compartment of uteri from all three genotypes. (k–m) show representative uterine sections stained for BrdU incorporation from E2P4 treated WT, PRKO, and PRd/d mice. Note that BrdU positive cells are absent in the luminal epithelial compartment of the E2P4 treated WT uterus whereas a subset of stromal cells are positive for BrdU incorporation (arrowhead). However, only the luminal epithelial compartment is positive for BrdU incorporation in the E2P4 treated PRKO and PRd/d uterus (arrowhead in (l) and (m) respectively). Scale bar in (e) applies to (f–m).
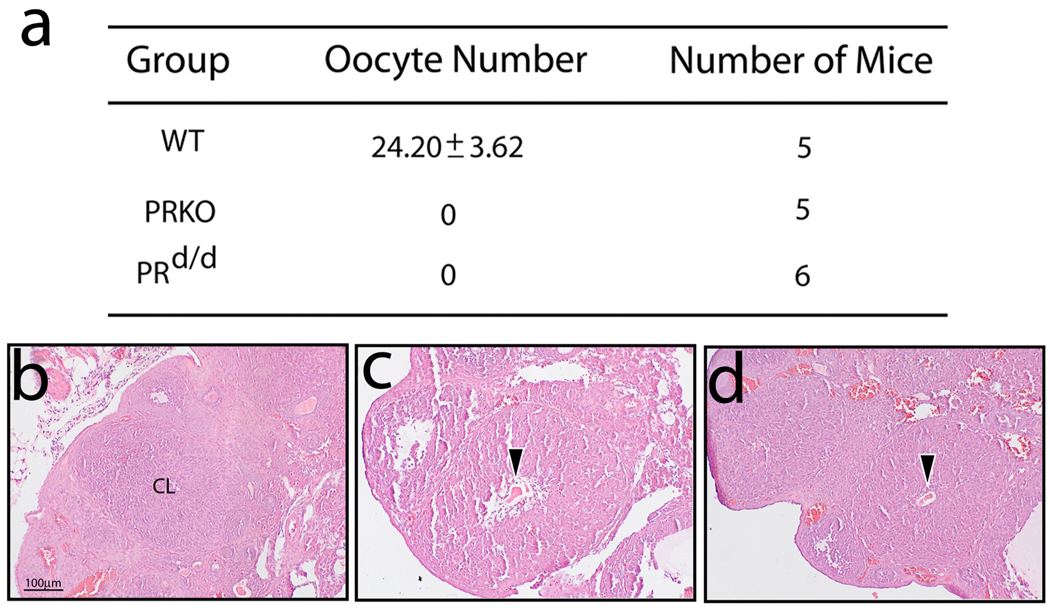
Intraovarian PRs were shown to be critical for gonadotropin-induced follicular rupture in the ovulating mouse (Lydon et al., 1995; Robker and Richards, 2000; Robker et al., 2000). Studies on the PRKO ovary revealed that absence of luteinizing hormone (LH) or hCG induced PR expression in the mural granulosa cells of the preovulatory follicle resulted in a block in follicular wall rupture with subsequent oocyte entrapment in the recently formed corpus luteum (CL). Based on these studies, the intrinsic ovarian function of PR is necessary for LH induced follicular wall breakdown (and exudation of the oocyte) but not for subsequent LH induced differentiation of granulosa cells to luteal cells. Using an established gonadotropin hormone treatment paradigm (see Materials & Methods), the PRd/d ovary displays the typical PRKO ovarian phenotype which consists of entrapped oocytes in CLs following 24h of hCG exposure (Fig. 3).
FIG. 3.
Ovulation impairment in the gonadotropin treated PRd/d mouse. (a) Table summarizes the average number of oocytes (± SEM) retrieved per mouse following gonadotropin treatment (see Materials & Methods). Note that the WT ovary yields a significant number of oocytes whereas oocytes are absent from the similarly treated PRKO and PRd/d ovary. (b) Representative section (stained with H&E) of a WT ovary following gonadotropin treatment. Note the absence of intraovarian oocytes but the presence of a corpus luteum (CL). (c) and (d) show representative sections of an ovary derived from similarly treated PRKO and PRd/d mice respectively. Arrowhead indicates the position of an entrapped oocyte within CLs in both sections. Scale bar in (b) applies to (c) and (d) (N=5 per genotype).
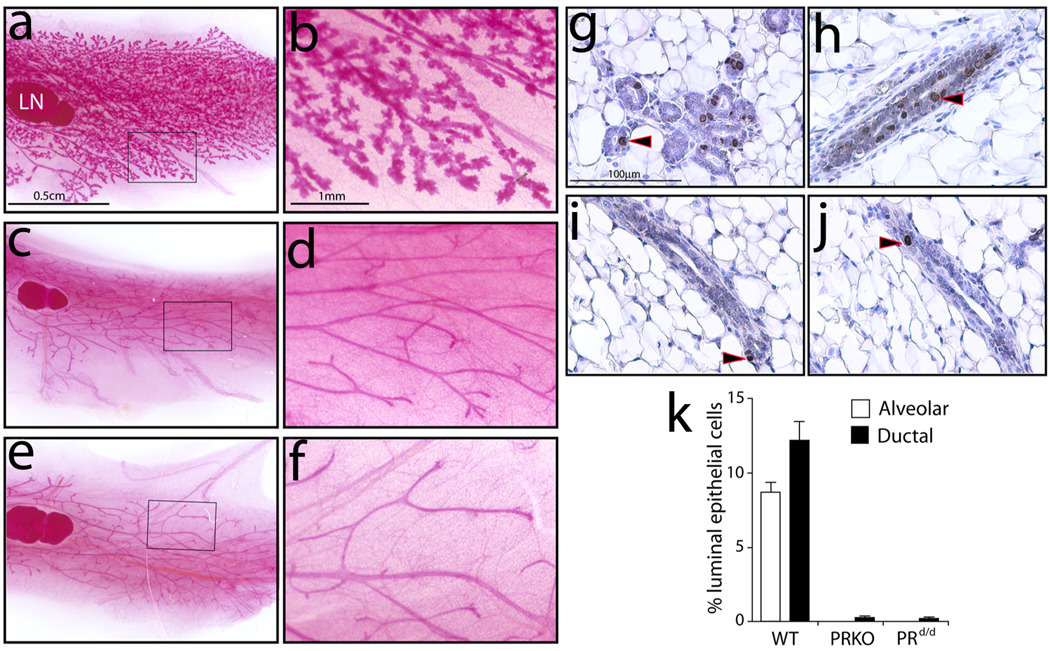
While not essential for mammary gland ductal elongation and simple bifurcation that occurs during puberty, PR is indispensable for hormone-induced mammary ductal side-branching and alveologenesis that manifests during pregnancy (Brisken et al., 1998; Lydon et al., 1995). The inability of the PRKO mammary gland to elicit these morphological changes results from an impairment in its luminal epithelial compartment to undergo proliferation when exposed to pregnancy hormones. Identical to the PRKO mammary gland, the E2P4 treated PRd/d mammary gland fails to undergo ductal side-branching and alveologenesis as observed for the similarly treated WT gland (Fig. 4a–f). As expected, ductal elongation and simple dichotomous branching is not compromised in the pubertal PRd/d mammary gland (data not shown). Immunohistochemical studies clearly show that the underlying cause of the PRKO and PRd/d mammary phenotype is the absence of a significant proliferative response in the presence of E2P4 hormone (Fig. 4g–k).
FIG. 4.
A block in ductal-side branching and alveologenesis in the mammary gland of the hormone-treated PRd/d mouse. (a) Whole-mount of a mammary gland obtained from a WT mouse previously treated with E2P4 for three weeks; (b) higher magnification image of a region of this tissue. Note the widespread presence of extensive ductal side-branching and alveologenesis. (c), (d) and (e), (f), show low and high magnification images of mammary tissue derived from the similarly treated PRKO and PRd/d mouse respectively. Note the absence of ductal side branches and alveolar bodies in the PRKO and PRd/d mammary glands. (g) and (h) show numerous luminal epithelial cells scoring positive (arrowhead) in the alveolar and ductal compartment of the E2P4 treated WT mammary gland respectively. (i) and (j) show a single cell registering positive for BrdU incorporation (arrowhead) in the ductal compartment of the similarly treated PRKO and PRd/d mammary gland respectively. (k) A histogram displaying the percentage of BrdU positive luminal epithelial cells in the mammary gland of the hormone treated WT, PRKO, and PRd/d mouse. Scale bar in (a) and (b) applies to (c), (e), and (d), (f), respectively; LN denotes lymph node (a structural reference point).
For over a decade, experimental mouse genetics has afforded unprecedented insight into PR action throughout the female reproductive axis as well as in mammary morphogenesis and tumorigenesis. During the course of these studies, these animal models have also highlighted unexpected roles for PR signaling in a myriad of physiological processes ranging from vascular injury to male behavior (Karas et al., 2001; Phelps et al., 1998; Schneider et al., 2005; Schneider et al., 2003). Apart from abrogation of PR function, the murine PR allele has also been engineered to enable the endogenous PR promoter to direct expression of the lacZ reporter, Cre recombinase, and rtTA in the PRlacZ, PRcre, and PRrtTA knockin mouse respectively (Ismail et al., 2002; Mukherjee et al., 2007; Mukherjee et al., 2006b; Soyal et al., 2005). While the PRlacZ reporter mouse underscored the dynamic and spatial complexity of PR expression in vivo (Ismail et al., 2002), the PRcre knockin mouse model has proven to be both a powerful and versatile mouse platform with which to selectively ablate (or activate) gene function specifically in PR positive cell-lineages (Daikoku et al., 2008; Jeong et al., 2008; Kim et al., 2008; Kurihara et al., 2007; Lee et al., 2006; Lee et al., 2007; Mukherjee et al., 2006a).
Given the growing need to dissect the multifunctional roles of PR in female reproductive biology, we have reengineered the PR locus to generate a conditional PR mouse model (the PRflox/flox mouse) that allows for tissue (or cell-type) specific and/or developmental stage-specific abrogation of PR expression in vivo. Crossing with the ZP3-Cre transgenic (Lewandoski et al., 1997), we demonstrate that the conditional PRflox allele in the mouse converts to a null allele (PRd allele) following cre-mediated excision of exon 2 of the PR gene which results in ablation of PR expression. Phenotyping the PRd/d mouse revealed significant impairments in uterine, ovarian, and mammary gland function that were previously observed in the PRKO mouse. Importantly, our PRd/d mouse represents a significant improvement in genetic design on a previously reported mouse model in which exon 1 of the PR gene was floxed with loxP sites. In this mouse model by Hashimoto-Partyka (Hashimoto-Partyka et al., 2006), the 5’ loxP site was inserted into the uncharacterized 5’ regulatory region of the mouse PR gene rather than in an intron. Such a design leaves open the possibility that the modified 5’ region may alter the transcriptional activity of the floxed PR allele compared to the corresponding WT version.
We believe the PRflox/flox mouse described here will be an invaluable resource with which to functionally parse PR’s recognized pleiotropic effects in female reproductive biology. Apart from being able to investigate PR function in an individual target tissue in an otherwise normal mouse, the PRflox/flox mouse will enable study of PR function in a particular cell-lineage within a responsive tissue. This capability will be particularly useful when defining the individual functional roles of epithelial and stromal PRs in the uterus or dissecting the selective functions of hypothalamic and pituitary derived PRs in the hypothalamic-pituitary axis. Developmental or reproductive stage-specific abrogation of PR will be particularly useful in determining the role of endometrial and mammary gland derived PR during later stages of pregnancy and at parturition.
In sum, our PRflox/flox mouse represents an effective means to conditionally ablate PR function within select cell-lineages in the mouse in a spatiotemporal manner. Crossing with appropriate cre expressing transgenics, the PRflox/flox mouse promises to provide critical insight into the necessity, interaction, or redundancy of individual PR populations in multiple cell-lineages within the female reproductive tract and mammary gland.
METHODS
Gene Targeting and Generation of the PRflox/flox and PRd/d Mouse Models
A previously cloned 8kb 129Sv derived murine genomic DNA fragment containing intron 1 (1.5kb), exon 2 (152 bp) and 6.4bp of intron 2 of the PR gene (Lydon et al., 1995)was used as a PCR template to provide specified homologous sequences for the construction of the gene-targeting vector shown in Figure 1a. Briefly, a 1.2 kb fragment of intron 1 was PCR amplified with PfuUltra™ High-Fidelity DNA polymerase (Stratagene Inc., La Jolla, CA; (catalog #600380)) using the primers: 5-TTAATTAAGGTACCGCGGGGGCTTGTGGAG and 5’-GGCGCGCCGGATCCAAGTGCTGGAAACAGAAGC. The resultant amplicon was cloned into Pac I and Asc I sites (underlined in the primers) of the pFRT-LoxP targeting vector (provided by Dr. James Martin, Institute of Biosciences & Technology, Texas A&M Health Science Center, Houston, Texas). A Bam HI site was introduced (bold in primer) to allow verification (by Southern analysis) of the presence of the 5’ loxP site flanking exon 2 (see Fig. 1a). An 1.7kb genomic fragment containing 0.2kb of intron 1, exon 2 (152 bp) and 1.3kb of intron 2 was PCR amplified with PfuUltra™ High-Fidelity DNA polymerase using primers: 5 ’-GGCCGGCCAGCTGGGAATTCATGAG and 5’-GGTACCAGTGAACTCAGCTCCTTG and cloned between the two loxP sites of the pFRT-LoxP construct using Fse I and Kpn I restriction sites (underlined in primers). The 3’ arm was generated by subcloning a 5kb genomic fragment of intron 2, obtained by digestion with restriction endonuclease ApaL 1 (made blunt) and Bam HI into the Xho I (made blunt) and Bam HI sites of the pFRT-LoxP targeting vector. The insertion of the 3’ arm positioned this homologous sequence between the positive selection marker FRT-PGKNeo-FRT cassette and the herpes simplex virus thymidine kinase (HSV-TK) negative selection marker. The transcriptional orientation of the HSV-TK gene was opposite to both the PGKNeo and PR genes.
The targeting vector was linearized with Pac I, purified, and electroporated into AB2.2 murine embryonic stem (ES) cells by the Darwin Transgenic Mouse Core Facility at Baylor College of Medicine. Correct gene targeting events were identified by Southern analysis using 5’ and 3’ DNA probes located outside the region of homology contained within the targeting construct (Fig. 1a). In the case of the WT allele, a single 9.7kb hybridizing band is visualized following Bam HI digestion of genomic DNA when hybridized with the 5’ probe by Southern analysis. In the case of the targeted PRfloxNeo allele, a 2.9kb hybridizing band is detected by Southern analysis due to the insertion of a novel Bam HI restriction site engineered at the loxP site 5’ to exon 2 (Fig. 1a). Wild type genomic DNA digested with Bgl II and probed with the 3’ probe by Southern analysis yields a single 6.6kb hybridizing band. In the case of the targeted PRfloxNeo allele, an 8.4kb hybridizing band is visualized with the 3’ probe. Only high quality male chimeras obtained from targeted ES cell clones were crossed with C57BL/6 albino mice to generate a number of PRfloxNeo/+ mouse lines. To remove the FRT-PGKNeo-FRT cassette, PRfloxNeo/+ mice were crossed with FLPeR (Flipper (FLP-1) or FLP deleter) mice (Farley et al., 2000) (The Jackson Laboratory, Bar Harbor, ME (129S4/SvJaeSor-Gt(ROSA)26Sortml(FLP1)Dym/J (stock number: 003946)). Absence of the FRT-PGKNeo-FRT cassette was verified by Southern blot analysis in which genomic DNA was digested with Bgl II and hybridized with a neo probe (digestion of the PGKNEObpA cassette with XbaI and PstI yielded a 630bp neo probe for Southern analysis). In the case of genomic DNA derived from the PRfloxNeo/+ mouse an 8.4kb hybridizing band is predicted to be detected whereas absence of this band in the PRflox/+ mouse indicates successful excision of the FRT-PGKNeo-FRT cassette. To allow in vivo functional validation of the resultant PRflox allele, mice homozygous for the PRflox allele were crossed with the ZP3 Cre transgenic mouse to generate the PRd/d mouse in which exon 2 is excised and PR expression is abrogated. The ZP3 Cre transgenic mouse (Lewandoski et al., 1997), in which cre expression is driven by the murine zona pellucida 3 regulatory region, was obtained from The Jackson Laboratory (B6.FVB-Tg(Zp3-cre)3Mrt/J (stock number: 003394)). To identify mice positive for the PRd allele, PCR analysis was used to genotype DNA extracts from tail biopsies (30 cycles for 94°C for 1’, 55°C for 1’, and 72°C for 2’). To detect the PRd allele, primers p, q, and r were used to amplify a 226bp amplicon from the WT allele and a 381bp amplicon from the PRd allele. Primer p (5 ’-GTATGTTTATGGTCCTAGGAGCTGGG-3’) is located in intron 1 and positioned 5’ to the first loxP site. Also located in intron 1, primer q (5’-TGCTAAAGGTCTCCTCATGTAATTGGG-3’) is positioned between the 5’ loxP site and exon 2. Primer r (5’-ATATTTATGACTTTGAGACTTG-3’) is located in intron 2 and 3’ to loxP/FRT site.
Mice and Hormone Treatments
Induction of the artificial decidual response in a single uterine horn of the mouse has been previously described (Lydon et al., 1995). Briefly, each ovariectomized mouse was treated with 3 daily subcutaneous (s.c.) injections of 100ng of estradiol (E2). Following 2 days of rest, mice were coadministered a daily injection of 1mg of progesterone (P4) and 6.5 ng of E2 for 3 days. Six hours after the third E2P4 injection, the left uterine horn of each mouse was mechanically stimulated by gently scratching the anti-mesometrial lumen with a burred needle. The right or contralateral horn was not stimulated and served as the control for each mouse. Following mechanical stimulation, mice received a daily s.c. injection of 1mg of P4 and 6.5ng of E2 for 5 additional days before sacrifice. Following sacrifice, wet weights of the stimulated left and control right uterine horns of each mouse were individually recorded. The ratio of wet weight of stimulated left horn over unstimulated right horn per mouse was calculated per genotype. Results per genotype were recorded as the average mean ratio of stimulated horn/unstimulated horn ± standard error of the mean (SEM).
For uterine proliferation studies, ovariectomized mice at 8 weeks-of-age received by s.c. injection either 1ug E2 alone or 1ug E2 plus 1mg P4 in 100ul of sesame oil before sacrifice 48h later (untreated controls received 100ul of sesame oil).
To elicit superovulation, 3-week-old mice were intraperitoneally (IP) administered 5 International Units (IU) of pregnant mare’s serum gonadotropin (PMSG; VWR Scientific, West Chester, PA). After 48 h of PMSG exposure, mice received an IP injection of 5IU of human chorionic gonadotropin (hCG or Pregnyl; Organon International, Roseland, NJ) before sacrifice 24h later. Oocytes were flushed and counted using a Nikon SMZ-2B StereoZoom microscope.
To induce mammary ductal side-branching and alveologenesis within a 3 week period, 10 week-old mice received subcutaneously in the intrascapular region a customized matrix-driven delivery pellet (1/8” diameter) designed to deliver 1ug E2 and 1mg P4 daily for 21 days (Innovative Research of America, Sarasota, FL).
For proliferation studies in the uterus and mammary gland, all mice received an IP injection of 5-bromo-2-deoxyuridine (BrdU (0.1ml (3mg/ml)/10g body weight); Amersham Biosciences Inc., Piscataway, NJ) two hours prior to sacrifice to allow scoring of cells in S phase of the cell-cycle by immunohistochemical methods (see below).
Mice were housed in a temperature controlled (22±2°C) animal facility at Baylor College of Medicine with a 12-h light, 12-h dark photocycle, and provided water and rodent chow meal (Purina Mills, LLC, St. Louis, MO) ad libitum. All mice were treated humanely in accordance with Baylor College of Medicine guidelines and Institutional Animal Care and Use Committee directives for the care and use of animals.
Immunohistochemical Analysis and Mammary Gland Whole-Mount Analysis
For immunohistochemical analysis, tissues were fixed overnight in 4% paraformaldehyde. Immunohistochemistry for PR expression and BrdU incorporation was performed as previously described (Lydon et al., 1999). The rabbit anti-human PR polyclonal antibody was purchased from Dako Corporation, Carpinteria, CA (catalog #A0098; 1/100 dilution). A biotinylated conjugated antibody to BrdU was used to detect BrdU incorporation (BD Biosciences, San Diego, CA; (catalog #550803; 1/10 dilution)). Carmine red staining of inguinal (#4) mammary gland whole mounts (including hematoxylin and eosin (H&E) staining of sections thereof) was followed as previously reported (Mukherjee et al., 2006a). Images were obtained with an Axioplan 2 microscope (Carl Zeiss, Jena, Germany). Captured digital images were initially processed with MetaVue Software 4.6r9 (Universal Imaging, Downington, PA) before final image composites were assembled with Photoshop CS4 (Adobe Systems, Inc., San Jose, CA).
ACKNOWLEDGMENTS
We thank both Jie Han for her technical assistance and the Darwin Transgenic Core Facility, Baylor College of Medicine for its gene-targeting services. We gratefully acknowledge Dr. James F. Martin at the Institute of Biosciences and Technology, Texas A&B University, Houston, Texas, for kindly providing the pFRT-LoxP vector construct. These studies were funded from the following National Institutes of Health grants: HD057873, HD042311 and CA077530 (to JJ, FJD and JPL respectively).
Research Support: National Institutes of Health grants HD057873, HD042311 and CA077530 (to JJ, FJD and JPL respectively)
LITERATURE CITED
- Brisken C, Park S, Vass T, Lydon JP, O'Malley BW, Weinberg RA. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci U S A. 1998;95:5076–5081. doi: 10.1073/pnas.95.9.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Pan H, Zhu L, Deng Y, Pollard JW. Progesterone inhibits the estrogen-induced phosphoinositide 3-kinase-->AKT-->GSK-3beta-->cyclin D1-->pRB pathway to block uterine epithelial cell proliferation. Mol Endocrinol. 2005;19:1978–1990. doi: 10.1210/me.2004-0274. [DOI] [PubMed] [Google Scholar]
- Daikoku T, Hirota Y, Tranguch S, Joshi AR, DeMayo FJ, Lydon JP, Ellenson LH, Dey SK. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res. 2008;68:5619–5627. doi: 10.1158/0008-5472.CAN-08-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley FW, Soriano P, Steffen LS, Dymecki SM. Widespread recombinase expression using FLPeR (flipper) mice. Genesis. 2000;28:106–110. [PubMed] [Google Scholar]
- Hashimoto-Partyka MK, Lydon JP, Iruela-Arispe ML. Generation of a mouse for conditional excision of progesterone receptor. Genesis. 2006;44:391–395. doi: 10.1002/dvg.20227. [DOI] [PubMed] [Google Scholar]
- Ismail PM, Li J, DeMayo FJ, O'Malley BW, Lydon JP. A novel lacZ reporter mouse reveals complex regulation of the progesterone receptor promoter during mammary gland development. Mol Endocrinol. 2002;16:2475–2489. doi: 10.1210/me.2002-0169. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Lee HS, Franco HL, Broaddus RR, Taketo MM, Tsai SY, Lydon JP, Demayo FJ. beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene. 2008 doi: 10.1038/onc.2008.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas RH, van Eickels M, Lydon JP, Roddy S, Kwoun M, Aronovitz M, Baur WE, Conneely O, O'Malley BW, Mendelsohn ME. A complex role for the progesterone receptor in the response to vascular injury. J Clin Invest. 2001;108:611–618. doi: 10.1172/JCI11374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Sato M, Li Q, Lydon JP, DeMayo FJ, Bagchi IC, Bagchi MK. Peroxisome proliferator-activated receptor gamma is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice. Mol Cell Biol. 2008;28:1770–1782. doi: 10.1128/MCB.01556-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3:e102. doi: 10.1371/journal.pgen.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Young P, Brody JR, Lydon JP, O'Malley BW, Cunha GR. Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology. 1998;139:4708–4713. doi: 10.1210/endo.139.11.6317. [DOI] [PubMed] [Google Scholar]
- Lee K, Jeong J, Kwak I, Yu CT, Lanske B, Soegiarto DW, Toftgard R, Tsai MJ, Tsai S, Lydon JP, DeMayo FJ. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet. 2006;38:1204–1209. doi: 10.1038/ng1874. [DOI] [PubMed] [Google Scholar]
- Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP, DeMayo FJ. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27:5468–5478. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandoski M, Wassarman KM, Martin GR. Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr Biol. 1997;7:148–151. doi: 10.1016/s0960-9822(06)00059-5. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptors exhibit pleiotropic reproductive abnormalities. Genes and Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Lydon JP, Ge G, Kittrell FS, Medina D, O'Malley BW. Murine mammary gland carcinogenesis is critically dependent on progesterone receptor function. Cancer Research. 1999;59:4276–4284. [PubMed] [Google Scholar]
- Mukherjee A, Soyal SM, Fernandez-Valdivia R, DeMayo FJ, Lydon JP. Targeting reverse tetracycline-dependent transactivator to murine mammary epithelial cells that express the progesterone receptor. Genesis. 2007;45:639–646. doi: 10.1002/dvg.20336. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Soyal SM, Fernandez-Valdivia R, Gehin M, Chambon P, DeMayo FJ, Lydon JP, O'Malley BW. Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Mol Cell Biol. 2006a;26:6571–6583. doi: 10.1128/MCB.00654-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Soyal SM, Wheeler DA, Fernandez-Valdivia R, Nguyen J, DeMayo FJ, Lydon JP. Targeting iCre expression to murine progesterone receptor cell-lineages using bacterial artificial chromosome transgenesis. Genesis. 2006b;44:601–610. doi: 10.1002/dvg.20257. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003;100:9744–9749. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- Pan H, Deng Y, Pollard JW. Progesterone blocks estrogen-induced DNA synthesis through the inhibition of replication licensing. Proc Natl Acad Sci U S A. 2006;103:14021–14026. doi: 10.1073/pnas.0601271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps SM, Lydon JP, O'Malley BW, Crews D. Regulation of male sexual behavior by progesterone receptor, sexual experience, and androgen. Horm Behav. 1998;34:294–302. doi: 10.1006/hbeh.1998.1485. [DOI] [PubMed] [Google Scholar]
- Robker RL, Richards JS. In: Progesterone: lessons from the progesterone receptor knockout. In ovulation: evolving scientific and clinical concepts. Adashi Eli Y., editor. New York: Springer-Verlag; 2000. pp. 121–129. [Google Scholar]
- Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW, Richards JS. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci U S A. 2000;97:4689–4694. doi: 10.1073/pnas.080073497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Burgess C, Sleiter NC, DonCarlos LL, Lydon JP, O'Malley B, Levine JE. Enhanced sexual behaviors and androgen receptor immunoreactivity in the male progesterone receptor knockout mouse. Endocrinology. 2005;146:4340–4348. doi: 10.1210/en.2005-0490. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Stone MK, Wynne-Edwards KE, Horton TH, Lydon J, O'Malley B, Levine JE. Progesterone receptors mediate male aggression toward infants. Proc Natl Acad Sci U S A. 2003;100:2951–2956. doi: 10.1073/pnas.0130100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41:58–66. doi: 10.1002/gene.20098. [DOI] [PubMed] [Google Scholar]
- Tibbetts TA, Mendoza-Meneses M, O'Malley BW, Conneely OM. Mutual and intercompartmental regulation of estrogen receptor and progesterone receptor expression in the mouse uterus. Biol Reprod. 1998;59:1143–1152. doi: 10.1095/biolreprod59.5.1143. [DOI] [PubMed] [Google Scholar]
- Tong W, Pollard JW. Progesterone inhibits estrogen-induced cyclin D1 and cdk4 nuclear translocation, cyclin E- and cyclin A-cdk2 kinase activation, and cell proliferation in uterine epithelial cells in mice. Mol Cell Biol. 1999;19:2251–2264. doi: 10.1128/mcb.19.3.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Pollard JW. Estradiol-17beta regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signaling. Proc Natl Acad Sci U S A. 2007;104:15847–15851. doi: 10.1073/pnas.0705749104. [DOI] [PMC free article] [PubMed] [Google Scholar]