Abstract
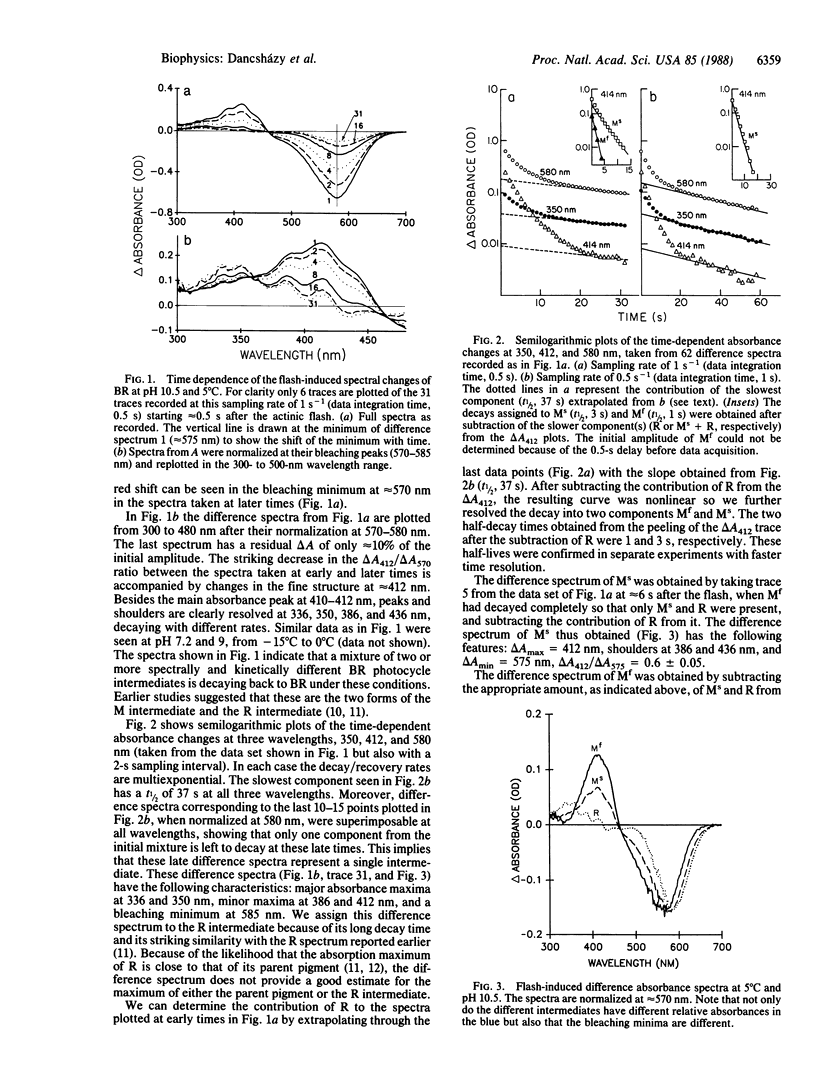
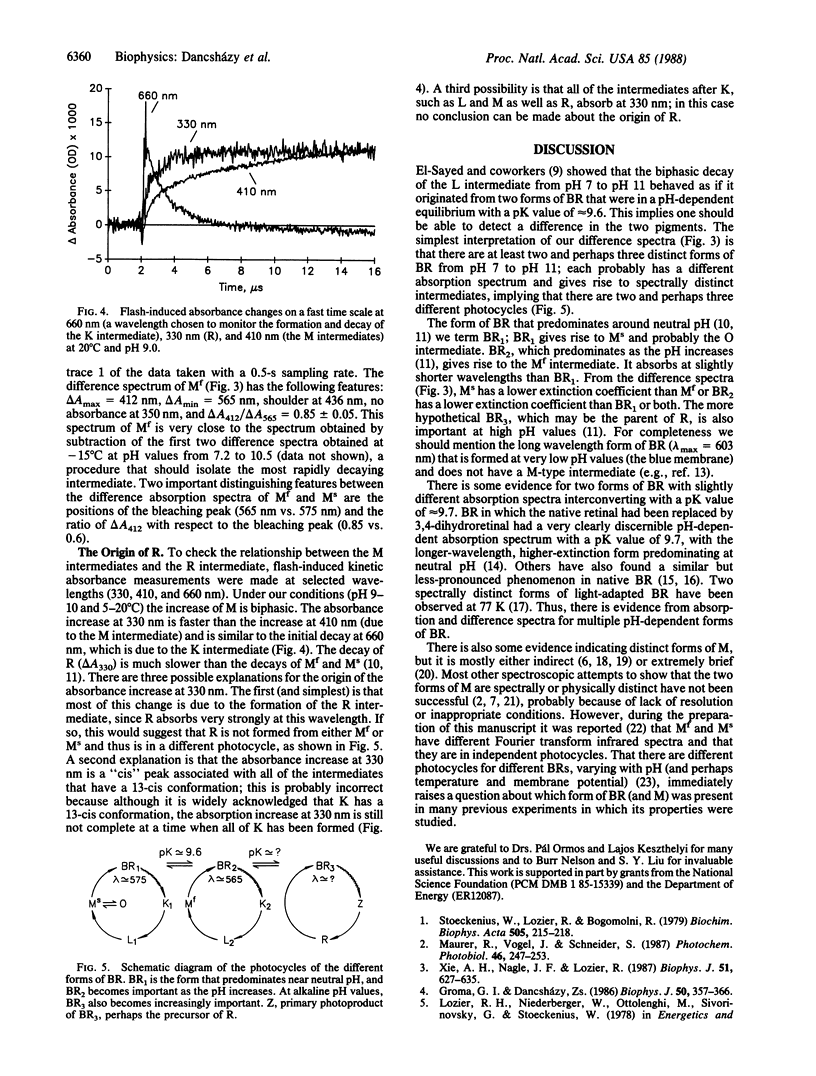
Time-resolved, flash-induced difference absorbance spectra (300-700 nm) at pH 10.5 and 5°C for the bacteriorhodopsin photocycle fast and slow decaying forms of the M intermediate (Mf and Ms, respectively) and R intermediate are reported. The main distinguishing features are as follows: For Mf, ΔAmax = 412 nm, a shoulder at 436 nm, no absorbance change at 350 nm; ΔAmin = 565 nm; ΔA412/ΔA565 = 0.85. For Ms, ΔAmax = 412 nm, a shoulder at 386 nm; ΔAmin = 575 nm; ΔA412/ΔA575 = 0.6. For R, ΔAmax = 336 and 350 nm (double peak), smaller peaks at 386 and 412 nm; ΔAmin = 585 nm; ΔA350/ΔA585 = 0.2. The different difference spectra for Mf and Ms provide direct evidence that these species, initially identified by their kinetics, are physically distinct. With fast transient absorption spectroscopy, it was shown that R may form very fast, perhaps faster than the L intermediate decays. On the basis of the different bleaching peaks for Mf and Ms, we propose that Mf and Ms are in independent photocycles formed from slightly different forms of bacteriorhodopsin. R may also be in a different photocycle. The different forms of bacteriorhodopsin are probably in dynamic equilibrium with their ratios, controlled by pH and temperature.
Keywords: purple membrane, M intermediate, R intermediate, difference spectra, Halobacterium halobium
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang C. H., Chen J. G., Govindjee R., Ebrey T. Cation binding by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1985 Jan;82(2):396–400. doi: 10.1073/pnas.82.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H., Pande C., Callender R. H., Ebrey T. G. A detailed resonance Raman study of the M412 intermediate in the bacteriorhodopsin photocycle. Photochem Photobiol. 1985 Apr;41(4):467–470. doi: 10.1111/j.1751-1097.1985.tb03513.x. [DOI] [PubMed] [Google Scholar]
- Groma G. I., Dancshazy Z. How Many M Forms are there in the Bacteriorhodopsin Photocycle? Biophys J. 1986 Aug;50(2):357–366. doi: 10.1016/S0006-3495(86)83469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groma G. I., Helgerson S. L., Wolber P. K., Beece D., Dancsházy Z., Keszthelyi L., Stoeckenius W. Coupling between the bacteriorhodopsin photocycle and the protonmotive force in Halobacterium halobium cell envelope vesicles. II. Quantitation and preliminary modeling of the M----bR reactions. Biophys J. 1984 May;45(5):985–992. doi: 10.1016/S0006-3495(84)84243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamoto J. H., Dupuis P., El-Sayed M. A. On the protein (tyrosine)-chromophore (protonated Schiff base) coupling in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7083–7087. doi: 10.1073/pnas.81.22.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess B., Kuschmitz D. The photochemical reaction of the 412 nm chromophore of bacteriorhodopsin. FEBS Lett. 1977 Feb 15;74(1):29–24. doi: 10.1016/0014-5793(77)80743-6. [DOI] [PubMed] [Google Scholar]
- Li Q., Govindjee R., Ebrey T. G. A correlation between proton pumping and the bacteriorhodopsin photocycle. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7079–7082. doi: 10.1073/pnas.81.22.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccio D. D., Cassim J. Y. Interpretations of the effects of pH on the spectra of purple membrane. J Mol Biol. 1979 Dec 15;135(3):595–609. doi: 10.1016/0022-2836(79)90166-9. [DOI] [PubMed] [Google Scholar]
- Ohno K., Govindjee R., Ebrey T. G. Blue light effect on proton pumping by bacteriorhodopsin. Biophys J. 1983 Aug;43(2):251–254. doi: 10.1016/S0006-3495(83)84347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer P. L., Sherman W. V. Alkaline quenching of bacteriorhodopsin tryptophanyl fluorescence: evidence for aqueous accessibility or a hydrogen-bonded chain. Photochem Photobiol. 1985 Nov;42(5):541–547. doi: 10.1111/j.1751-1097.1985.tb01607.x. [DOI] [PubMed] [Google Scholar]
- Parodi L. A., Lozier R. H., Bhattacharjee S. M., Nagle J. F. Testing kinetic models for the bacteriorhodopsin photocycle--II. Inclusion of an O to M backreaction. Photochem Photobiol. 1984 Oct;40(4):501–506. doi: 10.1111/j.1751-1097.1984.tb04624.x. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Tokunaga F., Ebrey T. The blue membrane: the 3-dehydroretinal-based artificial pigment of the purple membrane. Biochemistry. 1978 May 16;17(10):1915–1922. doi: 10.1021/bi00603a018. [DOI] [PubMed] [Google Scholar]
- Xie A. H., Nagle J. F., Lozier R. H. Flash spectroscopy of purple membrane. Biophys J. 1987 Apr;51(4):627–635. doi: 10.1016/S0006-3495(87)83387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


