Abstract
Polybrominated diphenyl ethers (PBDEs), a group of flame retardants comprising 209 congeners, have become widespread environmental pollutants. High levels of PBDEs have been detected in human tissues, particularly in North America, and body burden is especially high in infants and toddlers because of exposure through breast milk and house dust. Increasing evidence, provided by animal studies, suggests that PBDEs are developmental neurotoxicants, although the underlying mechanisms are still unknown. Various PBDEs have been reported to cause oxidative stress and to induce apoptotic cell death in several cell types. In the present study, we investigated the comparative neurotoxicity in mouse cerebellar granule neurons of five brominated diphenyl ether (BDE) congeners, chosen among the most commonly found at the highest levels in human tissues. All BDE congener tested (BDE-47, BDE-99, BDE-100, BDE-153, and BDE-209) decreased cell viability and induced apoptotic cell death, which was prevented by antioxidants. They also caused oxidative stress, as indicated by an increase in reactive oxygen species and in lipid peroxidation. For all end points measured, the potency ranking of the congeners was BDE-100 > BDE-47 > BDE-99 > BDE-153 >> BDE-209. Measurement of BDE congener levels in neurons after exposure to different concentrations showed a significant accumulation in cells, which followed the same relative ranking. The findings suggest that all BDE congeners tested exhibit the same general mode of action (induction of oxidative stress–mediated apoptosis) and that the ability of each isomer to elicit such effects is dependent upon their accumulation in neurons, particularly in the microsomal fraction and the mitochondria.
Keywords: PBDEs, neurotoxicity, oxidative stress, cellular accumulation
Polybrominated diphenyl ethers (PBDEs) are an important group of flame retardants, widely used in a variety of consumer products. Since PBDEs are additive flame retardants and are not chemically bonded to the polymer product, they leach out into the environment (Alaee et al., 2003). Like other halogenated compounds, PBDEs have become ubiquitous persistent pollutants; they have been detected in outdoor air, sediments, sludge, soil, water; in indoor air and house dust; in several food commodities; and in birds, marine species, fish, and terrestrial, including domestic animals (Darnerud et al., 2001; de Wit, 2002; Gill et al., 2004; Hites, 2004; Law et al., 2006; Schecter et al., 2004). PBDEs have also been detected in human blood, adipose tissue, and breast milk (Furst, 2006; Petreas et al., 2003; Schecter et al., 2003, 2005; Sjodin et al., 2003). In contrast to other halogenated compounds, such as polychlorinated biphenyls, dioxins, or furans, whose levels in biota and in human tissues have been decreasing over the past three decades, levels of PBDEs have significantly increased (Schecter et al., 2005; Thomsen et al., 2002).
There are 209 possible PBDE congeners. PBDEs have been marketed as one of three mixtures known as pentabrominated diphenyl ether (BDE), octaBDE, and decaBDE. In recent years, pentaBDE and octaBDE have been banned in the European Union and in several states in the United States and are no longer produced in these countries. DecaBDE (BDE-209) remains the most widely used PBDE globally, and it is still produced in Europe and the United States, although some states (e.g., Maine, Washington) or countries (e.g., Sweden) have banned this compound as well. The PBDE congeners found most commonly and at the highest levels in biota and human tissues are BDE-47, a tetraBDE; BDE-99 and BDE-100, two pentaBDEs; BDE-153 and BDE-154, two hexaBDEs; and BDE-209, decaBDE (Costa and Giordano, 2007; Costa et al., 2008).
Levels of PBDEs in human tissues in North America have been consistently found to be one to two orders of magnitude higher than in Asia or Europe (Lorber, 2008; Schecter et al., 2005). Particularly, alarming is the fact that body burden is highest in infants (because of exposure through breast milk) and in toddlers (because of exposure through house dust and the diet) (Allen et al., 2007; Schecter et al., 2003, 2005; Wilford et al., 2005).
The high body burden of PBDEs present in infants and toddlers has raised concern for their potential developmental toxicity and particularly for their developmental neurotoxicity (Birnbaum and Staskal, 2004; Branchi et al., 2003; Costa and Giordano, 2007; Costa et al., 2008; Eriksson et al., 2001; McDonald, 2005). Animal studies in rodents have provided evidence that exposure to different PBDEs (e.g., BDE-47, BDE-99, BDE-153, BDE-209, the pentaBDE mixture DE-71) during the prenataland/or postnatal periods causes long-term behavioral abnormalities, particularly in the domains of motor activity and cognition (Branchi et al., 2002; Dufault et al., 2005; Gee and Moser, 2008; Kuriyama et al., 2004; Rice et al., 2007; Viberg et al., 2003a,b).
The exact mechanisms of PBDEs’ developmental neurotoxicity are still elusive, although two general, and not mutually exclusive modes of action are emerging: one indirect related to the effects of PBDEs on thyroid hormones and the other one involving possible direct effects of PBDEs on the developing brain (Costa and Giordano, 2007). In vitro studies have shown that certain PBDEs can cause cell death by an apoptotic mechanism. For example, BDE-99 causes apoptotic cell death in human astrocytomas (Madia et al., 2004), DE-71 in cerebellar granule cells (Giordano et al., 2008), and BDE-47 in hippocampal neurons and human neuroblastoma cells (He et al., 2008a,b). There is also evidence that PBDE-induced apoptotic cell death may be mediated by oxidative stress, as shown e.g., for BDE-47 (He et al., 2008a,b; Shao et al., 2008) and DE-71 (Giordano et al., 2008).
Purpose of the present study was to carry out a comparative assessment of five PBDE congeners, chosen among those most often found in biota and human tissues at the highest concentrations, with regard to their ability to induce apoptotic cell death and oxidative stress in mouse cerebellar granule neurons (CGNs). The results indicate that all PBDE congeners appear to share a similar mode of action and that their relative neurotoxicity is associated with the ability to cross the cell membrane and to accumulate in cells, particularly in mitochondria.
MATERIALS AND METHODS
Materials.
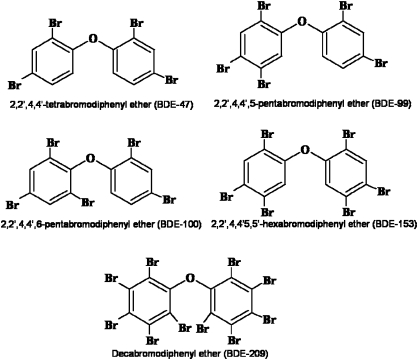
The PBDE congeners, 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47), 2,2′,4,4′,5-pentabromodiphenyl ether (BDE-99), 2,2′,4,4′,6-pentabromodiphenyl ether (BDE-100), 2,2′,4,4′,5,5′-hexabromodiphenyl ether (BDE-153), and 2,2′,3,3′,4,4′,5,5′,6,6′-deca-bromodiphenyl ether (BDE-209) were obtained from AccuStandard, Inc. (New Haven, CT). Manufacturer’s certification indicated 99.9% purity. Their structures are shown in Figure 1. Poly-D-lysine, potassium chloride, papain, DNase, magnesium chloride, 1-beta-d-arabinofuranosyl-cytosine cytarabine (Ara-C), glutathione ethyl ester (GSHEE), dimethyl sulfoxide (DMSO), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma Chemical Co. (St. Louis, MO). Neurobasal-A medium, B27 supplement, B27 supplement minus antioxidant, glutamax, gentamicin, fungizone, Hanks’ balanced salt solution (HBSS), (4-(2-hydroxyethyl)piperatine-1-ethanesulfonic acid) (HEPES), Hoechst 33342 trihydrochloride, 2,7′-dichlorofluorescein diacetate, and 10% SDS solution were from Invitrogen (Carlsbad, CA). Melatonin was from Tocris Cookson Inc. (Ellisville, MO). Tissue culture plates and nylon mesh filters were from BD Falcon (Franklin Lakes, NJ). The bicinchoninic acid protein assay kit was from Thermo Fisher Scientific (Rockford, IL), while the ELISA kit to measure PBDEs was from Abraxis (Warminster, PA). Glass coverslips were from Fisher Scientific (Federal Way, WA) and plastic coverslips from Nunc (Rochester, NY).
FIG. 1.
Chemical structures of BDE congeners used in the study.
CGN cultures.
Primary CGN cultures were prepared from 7-day-old mice sacrificed by decapitation, as previously described (Giordano et al., 2006). Briefly, the cerebella were dissected from the brain and the meninges were removed. Tissues were collected in HBSS supplemented with 10mM HEPES; cut into 1 mm3 pieces; and incubated in HBSS containing papain (1.5 mg/ml), DNase (40 μg/ml), and MgCl2 (10μM), for 30 min at 37°C for enzymatic dissociation. Tissues were centrifuged at 200 × g for 5 min at 4°C, the papain solution was removed, and the tissues were washed twice in HBSS with 10mM HEPES. Tissues were further dissociated by trituration in complete Neurobasal-A media containing B27 minus antioxidant (2%), glutamax (2mM), gentamicin (100 μg/ml), fungizone (1 μg/ml), and KCl (25mM). Cells were passed through a nylon mesh filter (40-μm pore size), centrifuged at 200 × g for 10 min at 4°C, gently resuspended in the complete media, and counted. Cells were seeded at a density of 0.6 × 106 cells/cm2 in plates coated with poly-D-lysine (0.3 mg/ml sterile tissue culture grade irrigation water at 37°C overnight). Neurons were allowed to attach for 1 h at 37°C in humidified 5% CO2/95% air, after which, they were washed with HBSS with 10mM HEPES and incubated in complete Neurobasal-A media. After 4 days, the original medium containing B27 supplement was removed and substituted with complete Neurobasal-A media containing B27 minus AO to provide an antioxidant-free primary CGN culture. Neurons were grown for a total of 10–12 days before experiments.
Cell treatments.
PBDE congeners were dissolved in DMSO to obtain a stock solution of 12.5mM and diluted to the desired concentrations in Neurobasal-A media containing B27 supplement minus AO, glutamax, gentamicin, fungizone, and KCl. Treatments for reactive oxygen species (ROS) assays were made with Locke’s buffer containing HEPES (10mM), KCl (25mM), CaCl2 (2.3mM), glucose (10mM), NaHCO3 (5mM), glutamax (2mM), gentamicine (100 μg/ml), MgCl2 (1.2mM), and NaCl (130mM). Since the PBDEs were dissolved in DMSO, which is cytotoxic at concentration above 0.8%, the maximum PBDE treatment concentration achievable was 50μM. GSHEE (2.5mM) and melatonin (200μM) were added to cell cultures 1 h prior to treatments with PBDEs.
Measurement of cell viability.
Cell viability was quantified colorimetrically using the metabolic dye MTT, as previously described (Giordano et al., 2006, 2008). Briefly, CGNs in 24-well plates were treated with PBDE for 24 h at 37°C. At the end of exposure, medium was removed and incubated with 500 μl per well of Locke’s buffer solution containing 4 μg/ml MTT for 30 min. MTT was then removed and reaction product dissolved in 0.25 ml DMSO per well. Absorbance was read at 570 nm, and the results expressed as percentage of viable cells relative to the DMSO-exposed controls.
Measurement of apoptosis.
To visualize nuclear morphology following 24-h PBDE exposure, cells were grown on plastic coverslips in 24-well plates and stained with Hoechst stain, as previously described (Giordano et al., 2007, 2008). After staining, the plastic coverslips were mounted on glass microscope slides and examined with Labophot-2 microscope (Nikon, Japan). Cells exhibiting either classical apoptotic bodies or shrunken rounded nuclei were scored as being apoptotic. Three fields across a diameter were photographed using a camera attached to a fluorescence microscope and counted manually using Image J software (http://rsbweb.nih.gov/ij/).
Assay of ROS formation.
ROS formation was determined by fluorescence using 2′,7′-dichlorofluorescein diacetate (DCFH2-DA), as previously described in detail (Giordano et al., 2006, 2007). DCFH2-DA is readily taken up by cells and is subsequently de-esterified to DCFH2, which can be oxidized to dichlorofluorescein (DCF) by hydrogen peroxide, peroxynitrite, and other ROS/reactive nitrogen species. Cells were first washed with Locke’s solution and then preincubated for 30 min at 37°C with DCFH2-DA (50 nmol/mg cell protein) in Locke’s solution. DCFH2 was added from a stock solution prepared in DMSO, which was also added to the blank. Cells were then washed with Locke’s solution to remove extracellular DCFH2-DA before treatments with PBDEs. After treatments (at 37°C), cells were then washed twice with Locke’s buffer and lysed with 0.1M KH2PO4 and 0.5% Triton X-100, pH 7.2, for 5 min. Cell lysates were then scraped from the dishes, and the supernatant was collected. The fluorescence was immediately read at 488 nm excitation and 525 nm emission, using SpectraMax 190 (Molecular Devices, Sunnyvale, CA). ROS formation was expressed as the amount of fluorescence per milligram of protein measured using the bicinchoninic acid protein assay kit.
Measurement of lipid peroxidation.
Lipid peroxidation, a major indicator of oxidative stress, was measured by the Thiobarbituric Acid Reactive Substances (TBARS) assay, with a kit from Cayman Chemical Company (Ann Arbor, MI) used according to manufacturer’s instructions, as described by Giordano et al. (2006). TBARS measure the changes in levels of malondialdehyde, a natural byproduct of lipid peroxidation, which upon binding to thiobarbituric acid forms a fluorescent red adduct. After exposure to PBDEs and wash out, cells were scraped with 1% SDS lysis buffer (pH 7.4) and aliquots were removed to determine protein content; 200 μg samples were used for determination of TBARS. butylhydroxytoluene (1μM), an antioxidant, was added to the lysis buffer to prevent sample oxidation, and cells were centrifuged at 3000 × g for 10 min to remove large particles.
Measurement of intracellular PBDE accumulation.
Intracellular levels of PBDE were measured using a magnetic particle immunoassay for PBDEs, which has been shown to exhibit high sensitivity and precision for detection of PBDEs in animal samples (Shelver et al., 2008). Cell cultures were grown in 50-mm glass bottom plates and exposed to 10nM, 100nM, 1μM, or 10μM of each BDE congener for 24 h. At the end of treatment, cells were washed twice with Locke’s buffer and lipids were extracted from cells by incubation with hexane-isopropanol (3:2) at 4°C to prevent evaporation. After 20 min, samples were transferred to glass tubes and dried using an argon stream and by allowing the solvent to evaporate overnight under the fume hood. NaOH was added to the plates and left on a shaker for 2 h to recover cell contents and measure protein concentrations using the bicinchoninic acid protein assay kit. For ELISA analysis, samples were resuspended in 500 μl of 50% methanol and 500 μl of the anti-PBDE–coupled magnetic particles were added to 250 μl of samples. After 20-min incubation at room temperature, 250 μl of antibody conjugate was added, vortexed, and incubated for another 20 min. The magnetic separator was used to separate and remove unbound anti-PBDE particles and conjugates. A color reagent was used to measure relative concentrations of lipid-bound PBDE by absorbance at 450 nm. Absorbance was converted to mass of lipid-bound PBDE from a calibration curve using standards provided by the kit, including solution containing 0, 0.012, 0.025, 0.05, 0.1, and 0.5 ppb of BDE-47. Data are expressed as concentration of PBDE (micromolar) in cells. The wet weight of cells was calculated by harvesting cells from a sister plate, followed by centrifugation to obtain a pellet, and weighing of the pellet, as described by Mundy et al. (2004).
Subcellular fractionation.
Subcellular fractionation of CGNs was performed as described by Cox and Emili (2006), with some modifications, to isolate the following fractions: cytosol, nuclei, mitochondria, and microsomes (a collection of endoplasmic recticulum, Golgi, intracellular vesicles, and plasma membranes). Briefly, CGNs were collected and homogenized in ice-cold lysis buffer containing 250mM sucrose, 50mM Tris-HCl (pH 7.4), 5mM MgCl2, 1mM dithiothreitol, and 1mM PMSF using a tight fitting Teflon pestle. The lysate was centrifuged in a bench-top centrifuge at 800 × g for 15 min; the supernatant served as source for cytosol, mitochondria, and microsomes. The pellet, which contains the nuclei, was rehomogenized in lysis buffer and centrifuged as above. Mitochondria were isolated from the crude cytoplasmic fraction by bench-top centrifugation at 6000 × g for 15 min. The mitochondrial pellet was washed and pelleted twice in lysis buffer. The cytosolic fraction was obtained after removal of the microsomal fraction by ultracentrifugation at 100,000 × g for 1 h.
Statistical analysis.
Data are expressed as the mean ± SE of the indicated number of independent experiments. Statistical analysis was performed using one-way or two-way ANOVA followed by Bonferroni test for multiple comparisons.
RESULTS
Effects of BDE Congeners on Cell Viability
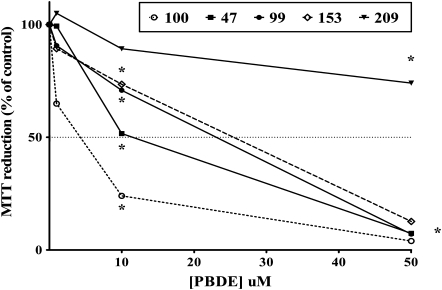
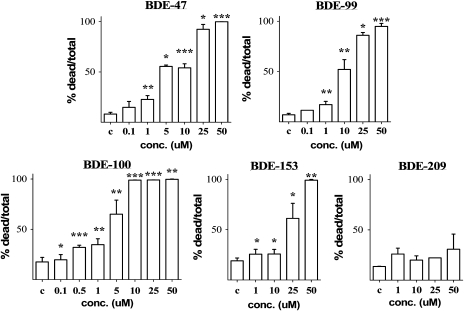
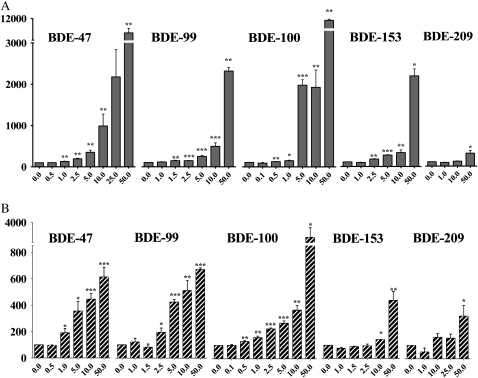
The effects of BDE congeners on cell viability (measured by the MTT assay) and their ability to induce apoptotic cell death in mouse CGNs were first investigated. Figure 2 shows the concentration-dependent effects of the five BDE congeners on cell viability, while Figure 3 shows their ability to cause apoptotic cell death. Values of IC50 and EC50 for each end points are indicated in Table 1. BDE-100 was the most potent congener causing an increase in the number of apoptotic cells at 100–500nM. BDE-47 was slightly less potent, followed by BDE-99 and BDE-153, while BDE-209 was the least toxic and did not cause any statistically significant apoptotic cell death at any of the concentration tested. We had previously confirmed the ability of PBDEs to induce apoptosis in mouse CGNs, by measuring DNA laddering following exposure to DE-71 (Giordano et al., 2008) and BDE-47 (Giordano and Costa, unpublished data). As previously reported for DE-71 (Giordano et al., 2008), cell death induced by PBDEs appears to be primarily of the apoptotic nature. Indeed, correlations between IC50 values in the MTT assay and EC50 values in the Hoechst assay provided a Pearson’s coefficient, r2, of 0.99 and a p value of 0.001.
FIG. 2.
Effect of PBDEs on cell viability in mouse CGNs, as determined by the MTT assay. Cell death in control cells was 5.4 ± 1.9%. Results are the mean (± SE) of three separate experiments for each congener, and the results are expressed as percent of control values. SEs are omitted for better clarity. Significantly different from control, *p < 0.05.
FIG. 3.
Concentration-dependent apoptosis induced by BDE congeners in mouse CGNs. Apoptosis was assessed by Hoechst staining. Concentrations are expressed as micromolar. Apoptotic cell death in control cells ranged from 7.3 to 17.3% as shown. Results are the mean (± SE) of three to five independent experiments. Significantly different from control, *p < 0.05, **p < 0.01, and ***p < 0.001.
TABLE 1.
Cytotoxicity of BDE Congeners in Mouse CGNs
| Cell viability | Apoptosis | |
| BDE congener | IC50 (μM) | EC50 (μM) |
| BDE-47 | 11.0 ± 2.0 | 9.6 ± 1.7 |
| BDE-99 | 15.7 ± 3.3 | 10.6 ± 2.0 |
| BDE-100 | 4.5 ± 0.3 | 4.3 ± 0.4 |
| BDE-153 | 19.9 ± 1.6 | 18.9 ± 3.5 |
| BDE-209 | > 50.0 | > 50.0 |
Note. Cell viability was measured by the MTT assay and apoptosis was assessed by Hoechst staining, as described in the “Materials and Methods” section. Values of IC50 and of EC50 were determined from concentration-response curves using three to seven concentrations of each BDE. Values are expressed as mean (± SE) of three to five independent experiments.
BDE Congeners Cause Oxidative Stress
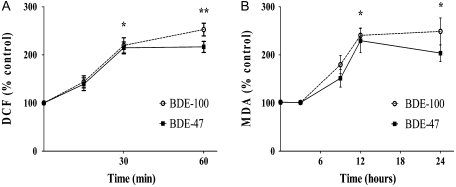
Since PBDEs have been reported to cause cytotoxicity by inducing oxidative stress, the relative levels of oxidative stress induced by each congener should correspond to their ranking in inducing cytotoxicity. Oxidative stress was assessed by measuring ROS formation and lipid peroxidation, as described in the “Materials and Methods” section. Time-course experiments were carried out with BDE-47 and BDE-100 (Fig. 4). These experiments indicated that the times of maximum response for ROS formation and lipid peroxidation were 60 min and 12 h, respectively (Fig. 4), and these time points were used for concentration-response experiments with all congeners. All congeners increased ROS formation (Fig. 5A), with the following relative order of potency: BDE-100 > BDE-47 > BDE-99 > BDE-153 > BDE-209. Similar effects and the same potency ranking were also observed with regard to lipid peroxidation (Fig. 5B). BDE-209 was consistently the least active congener causing only a limited degree of oxidative stress at the highest concentration tested (50μM).
FIG. 4.
Time course of ROS formation and of lipid peroxidation induced by BDE-100 and BDE-47 in mouse CGNs. Concentration of each BDE was 5μM. Results are the mean (± SE) of three independent experiments. Control are untreated cells. Significantly different from control, *p < 0.05 and **p < 0.01.
FIG. 5.
Concentration-dependent effects of BDE congeners on ROS formation (A) and on lipid peroxidation (B) in mouse CGNs. Incubation times were 60 min for ROS measurements and 12 h for lipid peroxidation (malondialdehyde measurements). Values are the mean (± SE) of five to eight independent experiments. Significantly different from control, *p < 0.05, **p < 0.01, and ***p < 0.001.
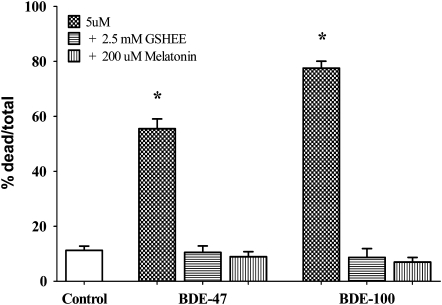
These results support the hypothesis that PBDE-induced neuronal cell death is due to the ability of these compounds to elicit oxidative stress. Further evidence is provided by the finding that two antioxidants, GSHEE and melatonin, completely antagonized apoptosis induced by BDE-100 and BDE-47 (Fig. 6).
FIG. 6.
Effect of antioxidants on apoptosis induced by BDE-47 and BDE-100 in mouse CGNs. Cells were pre-treated with GSHEE (2.5mM) or melatonin (200μM) for 1 h before addition of BDEs (each at 5μM). Results are the mean (± SE) of three independent experiments. Significantly different from control, *p < 0.01.
Accumulation of BDE Congeners in Cells
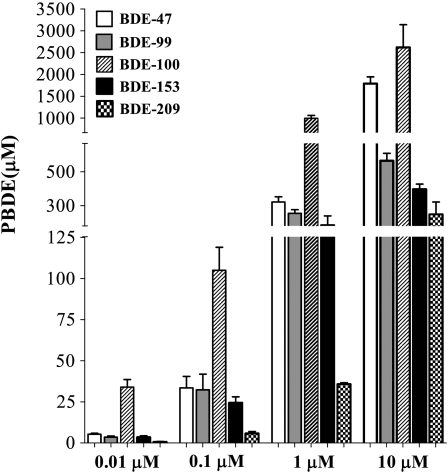
To determine whether the cytotoxicity of PBDEs was related to their ability to enter and concentrate in cells, levels of each congener were measured in mouse CGNs upon 24-h incubation with different concentrations of each BDE. As shown in Figure 7, incubation with 0.01, 0.1, 1.0, or 10μM of each congener led to a significant accumulation of BDEs in cells. There was a 25- to 3400-fold increase in the cellular concentration relative to applied concentration, and cellular accumulation, as percentage of the applied concentration, was higher at lower BDE concentration. Changes in treatment volume did not significantly affect BDE accumulation (not shown). BDE-100 showed the greatest accumulation, followed by BDE-47, BDE-99, and BDE-153. Accumulation of BDE-209 was the lowest. These findings suggest that accumulation of BDE congeners in cells is a major determinant of their relative cytotoxicity.
FIG. 7.
Accumulation of BDE congeners in mouse CGNs. Cells were exposed to BDEs (0.01, 0.1, 1.0, or 10μM) for 24 h, and BDE concentration was measured as described in the “Materials and Methods” section. Results are the mean (± SE) of three independent experiments. Two-way ANOVA indicated significant difference among concentrations, F(3,40) = 84.1; p < 0.001, and congeners, F(4,40) = 204.8; p < 0.0001.
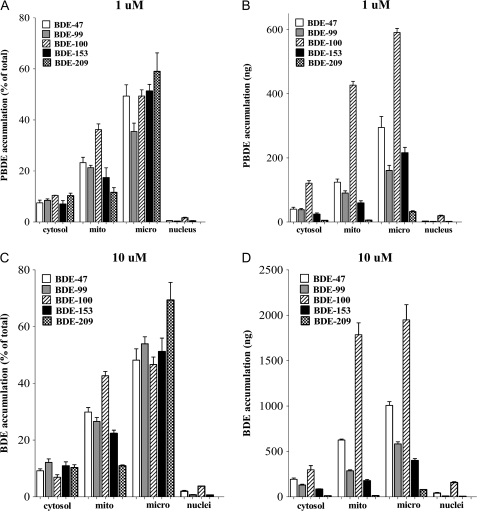
To ascertain the intracellular distribution of PBDEs, mouse CGNs were exposed for 24 h to each congener (1 or 10μM), and cellular fractions (cytosol, microsomes, nucleus, and mitochondria) were isolated according to Cox and Emili (2006). Figure 8 shows the results of these experiments in which data are expressed as percentage of BDE congener in each fraction (Figs. 8A and 8C) or as nanogram of BDE congener in each fraction (Figs. 8B and 8D). At both concentrations, for all congeners, the highest percentage of BDE was associated with the microsomal fraction, which is a collection of endoplasmic reticulum, Golgi apparatus, intracellular vesicles, and plasma membrane. The second highest fraction containing PBDEs was the mitochondria, while lower amounts were found in the cytosol and the nucleus (Fig. 8). Association of BDE congeners with the mitochondrial fraction is of particular interest, as it followed their relative ranking in inducing apoptotic cell death: BDE-100 > BDE-47 > BDE-99 > BDE-153 >> BDE-209. Thus, e.g., after incubation with 10μM PBDE, 43% of BDE-100 was in the mitochondrial fraction compared to 11% for BDE-209 (Fig. 8C).
FIG. 8.
Accumulation of BDE congeners in different cellular fractions of CGNs. Cells were exposed to 1 or 10μM BDEs for 24 h. At the end of exposure, subcellular fractionation was carried out as described in the “Materials and Methods” section. BDE levels in each fraction were measured as described in the “Materials and Methods” section. In (A) and (C), results are expressed as percent of the total amount recovered, while in (B) and (D), results are expressed as nanogram of BDE. Note the different scales in (B) and (D). Results represent the mean (± SE) of three separate experiments. Mito = mitochodria; micro = microsomal fraction.
DISCUSSION
This study directly compared the neurotoxicity in mouse CGNs of the five most environmentally and biologically relevant BDE congeners. The results confirmed previous observations with BDE-47, BDE-99, and the pentaBDE mixtures DE-71 that PBDEs decrease cell viability by causing apoptotic cell death (Giordano et al., 2008; He et al., 2008a,b; Madia et al., 2004; Shao et al., 2008). The present study also extends these observation to BDE-100 and BDE-153, suggesting that the ability of inducing apoptosis is common to most PBDEs, and shows that BDE-100 is the most potent of all tested congeners, with an IC50 of ∼4μM.
Previous studies had suggested that PBDE-induced cytotoxicity and apoptosis were mediated by oxidative stress (Giordano et al., 2008; He et al., 2008a,b; Reistad et al., 2006). The present study confirms and extends these findings, showing that all BDE congeners tested are capable of inducing oxidative stress, as evidenced by an increase in ROS and in lipid peroxidation. As antioxidants were able to completely antagonize apoptosis induced by PBDEs (Fig. 6), this study also substantiates the hypothesis that PBDE-induced oxidative stress is causally related to the induction of apoptotic cell death. In addition, the five congeners tested in this study induced cytotoxicity and apoptosis in the order BDE-100 > BDE-47 > BDE-99 > BDE-153 >> BDE-209, and the same order was preserved for their ability to induce ROS formation and lipid peroxidation.
BDE-209, the decaBDE congener, appeared to be the least potent of all compounds as it caused minimal cytotoxicity and only slight increases in oxidative stress at the highest concentration tested (50μM). BDE-209 had been reported to cause apoptotic cell death in human hepatoma Hep G2 cells (Hu et al., 2007). However, this effect was seen only at relatively high concentrations (50–100μM) and with long incubation times (48 h). Similarly, the same authors reported that 25–100μM BDE-209 increased ROS formation in the same cells but only after long incubation times (5–15 h) (Hu et al., 2007). The minor differences between hepatoma cells and CGNs may be explained by the potential ability of liver cells to metabolize BDE-209 to lower, more biologically active congeners, or alternatively, by a differential accumulation of BDE-209 in the two cell types.
Indeed, an important aspect of the present study is the measurement of the accumulation of each BDE congener in CGNs, upon exposure of cells to different concentrations (0.01–10μM) of PBDEs. All BDE congeners appeared to accumulate in CGNs, and the concentrations associated with cells were 25- to 3400-fold higher than the applied concentrations (Fig. 7). BDE-100 showed the highest accumulation in CGNs, followed by BDE-47, BDE-99, BDE-153, and BDE-209. This order is the same identified for induction of oxidative stress and apoptosis, suggesting that cellular accumulation of PBDEs is a major determinant of their toxicity.
Mundy et al. (2004) examined accumulation of 14C-BDE-47 in rat neocortical neurons. They found that exposure to 0.01–3.0μM BDE-47 for 60 min resulted in a 100-fold magnification of the applied concentration. We utilized a similar range of concentrations and found a higher accumulation of BDE-47 in CGNs, most likely because of the longer incubation time (24 vs. 1 h). Differently from Mundy et al. (2004), however, we did not observe any significant difference in the 24-h accumulation of BDE congeners when exposure volumes were modified (not shown). Kodavanti et al. (2005) measured accumulation of 14C-labeled BDE-47, BDE-99, and BDE-153 in rat CGNs over a 60-min period. They found that the percent accumulation of these BDE congeners increased with time at all concentration tested (0.67–30.67μM) and that the accumulation was linear. Similarly to what we found (Fig. 7), they reported that cellular accumulation was higher at lower BDE concentrations. Kodavanti et al. (2005) also found that the accumulation of BDE-47 was higher than that of BDE-99 and of BDE-153, consistent with our findings, and that the biological effect investigated (translocation of protein kinase C) was highly correlated with accumulation of PBDEs in cells.
Another important finding of this study is provided by the subcellular fractionation experiments in which levels of BDE congeners associated with different cellular fractions were quantified. Of particular interest was the relative distribution of BDEs in the two fractions containing the highest amounts of BDEs, i.e., the microsomal and the mitochondrial fractions. Almost 43% of BDE-100 was associated with mitochondria, followed by BDE-47 (30%), BDE-99 (27%), BDE-153 (22%), and BDE-209 (11%). This order follows that previously seen for PBDE-induced apoptotic cell death, suggesting that accumulation of PBDEs in mitochondria, which would elicit oxidative stress and activate the apoptotic pathway cascade, is an important step in this process. Levels of PBDEs associated with the microsomal fraction were the highest for all congeners and were actually highest for the least toxic congener, BDE-209. Low and comparable levels of BDE congeners (7–12%) were found in the cytosol, while the lowest levels were found in the nucleus, where they ranged from 3.8% (BDE-100) to 0.01% (BDE-209) of the total BDE recovered.
The mechanisms by which BDEs gain access to CGNs and by which they elicit oxidative stress are still elusive. As said, we observed relative potency in the order of BDE-100 > BDE-47 > BDE-99 > BDE-153 > BDE-209 for reduction in cell viability, induction of apoptosis, ROS formation, lipid peroxidation, cellular accumulation, and accumulation in mitochondria. A number of quantitative structure-activity relationship (QSAR) models for PBDE congeners have been established. For example, the QSAR model established by Harju et al. (2007) concluded that PBDE congeners with lower bromination, with bromines in ortho-position, without bromines in the meta and para positions, and with an uneven distribution of bromines on the different phenyl rings have the highest activity as androgen antagonists. Congeners that were tested in the present study appear to follow this general rule observed for PBDE congeners in relationship to their androgen receptor antagonist and estrogen receptor agonist activities (Hamers et al., 2006; Harju et al., 2007).
BDE-209 displayed the least biological activity in vitro and the least cellular accumulation; despite having four bromine at ortho-positions, the BDE-209 molecule is bulky (having 10 bromines) and has a symmetrical configuration. Moderate cellular accumulation and toxicity observed for BDE-153 may be accounted for by the presence of two ortho substitutions and two meta substitutions, by structural symmetry, and by medium size (six bromines). Comparison between BDE-100, BDE-47, and BDE-99 is less straightforward. BDE-47 had been suggested as the most biologically active, given its small size (four bromines) and low degree of bromination (U.S. Environmental Protection Agency, 2008). Our findings clearly indicate that BDE-100 was the most toxic, because of its higher cellular accumulation, despite having five bromines. BDE-100 has three ortho substitutions and an asymmetrical conformation, while BDE-47 is symmetrical. It may be concluded that ortho substitution is a stronger contributor of PBDE cellular accumulation and toxicity than the degree of bromination. Furthermore, while BDE-99 and BDE-100 are both asymmetrical and have five bromines, BDE-99 displayed lower cellular accumulation and toxicity, perhaps for the presence of a meta substitution. Overall, the relative cytotoxicity among the five congeners tested in this study suggests that cell accumulation and hence, toxicity, is first related first to the number of ortho substitution, second to the number of bromines, third to the number of meta bromines, and finally to conformational symmetry.
In conclusion, results of this study indicate that several BDE congeners are capable of causing apoptotic cell death of CGNs and that this effect is most likely mediated by their ability to induce oxidative stress. All BDE congeners accumulated in CGNs, and their toxicity appeared to be strongly associated with their ability to accumulate in cells, particularly in mitochondria. Thus, the pentabrominated BDE-100, which showed the highest cellular accumulation, was the most toxic of all congener tested, while the decabrominated BDE-209 displayed the least toxicity. Of relevance is also that cellular accumulation was relatively more pronounced at low nanomolar concentrations, which have environmental significance (Lorber, 2008).
FUNDING
National Institute of Environmental Health Sciences (P30ES07033).
References
- Alaee M, Arias P, Sjodin A, Bergman A. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ. Int. 2003;29:683–689. doi: 10.1016/S0160-4120(03)00121-1. [DOI] [PubMed] [Google Scholar]
- Allen JG, McClean MD, Stapleton HM, Nelson JW, Webster TF. Personal exposure to polybrominated dipehenyl ethers (PBDEs) in residential indoor air. Environ. Sci. Technol. 2007;41:4574–4579. doi: 10.1021/es0703170. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ. Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I, Alleva E, Costa LG. Effects of perinatal exposure to a polybrominated diphenyl ether (PBDE 99) on mouse neurobehavioral development. Neurotoxicology. 2002;23:375–384. doi: 10.1016/s0161-813x(02)00078-5. [DOI] [PubMed] [Google Scholar]
- Branchi I, Capone F, Alleva E, Costa LG. Polybrominated diphenyl ethers: neurobehavioral effects following developmental exposure. Neurotoxicology. 2003;24:449–462. doi: 10.1016/S0161-813X(03)00020-2. [DOI] [PubMed] [Google Scholar]
- Costa LG, Giordano G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology. 2007;28:1047–1067. doi: 10.1016/j.neuro.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Giordano G, Tagliaferri S, Caglieri A, Mutti A. Polybrominated diphenyl ether (PBDE) flame retardants: environmental contamination, human body burden and potential adverse health effects. Acta Biomed. 2008;79:172–183. [PubMed] [Google Scholar]
- Cox B, Emili A. Tissue subcellular fractionation and protein extraction for use in mass-spectrometry-based proteomics. Nat. Protoc. 2006;1:1872–1878. doi: 10.1038/nprot.2006.273. [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Eriksen GS, Johannesson T, Larsen PB, Viluksela M. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ. Health Perspect. 2001;109(Suppl. 1):49–68. doi: 10.1289/ehp.01109s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- Dufault C, Poles G, Driscoll LL. Brief postnatal PBDE exposure alters learning and cholinergic modulation of attention in rats. Toxicol. Sci. 2005;88:172–180. doi: 10.1093/toxsci/kfi285. [DOI] [PubMed] [Google Scholar]
- Eriksson P, Jakobson E, Fredriksson A. Brominated flame retardants: a novel class of developmental neurotoxicants in our environment? Environ. Health Perspect. 2001;109:903–908. doi: 10.1289/ehp.01109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst P. Dioxins, polychlorinated biphenyls and other halogenated compounds in human milk. Mol. Nutr. Food Res. 2006;50:922–933. doi: 10.1002/mnfr.200600008. [DOI] [PubMed] [Google Scholar]
- Gee JR, Moser VC. Acute postnatal exposure to brominated diphenylether 47 delays neuromotor ontogeny and alters motor activity in mice. Neurotoxicol. Teratol. 2008;30:79–87. doi: 10.1016/j.ntt.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Gill U, Chu I, Ryan JJ, Feeley M. Polybrominated diphenyl ethers: human tissue levels and toxicology. Rev. Environ. Contam. Toxicol. 2004;183:55–97. doi: 10.1007/978-1-4419-9100-3_3. [DOI] [PubMed] [Google Scholar]
- Giordano G, Kavanagh TJ, Costa LG. Neurotoxicity of a polybrominated diphenyl ether mixture (DE-71) in mouse neurons and astrocytes is modulated by intracellular glutathione levels. Toxicol. Appl. Pharmacol. 2008;232:161–168. doi: 10.1016/j.taap.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano G, White CC, McConnachie LA, Fernandez C, Kavanagh TJ, Costa LG. Neurotoxicity of domoic acid in cerebellar granule neurons in a genetic model of glutathione deficiency. Mol. Pharmacol. 2006;70:2116–2126. doi: 10.1124/mol.106.027748. [DOI] [PubMed] [Google Scholar]
- Giordano G, White CC, Mohar I, Kavanagh TJ, Costa LG. Glutathione levels modulate domoic acid-induced apoptosis in mouse cerebellar granule cells. Toxicol. Sci. 2007;100:433–444. doi: 10.1093/toxsci/kfm236. [DOI] [PubMed] [Google Scholar]
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MH, Andersson PL, Legler J, Brouwer A. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol. Sci. 2006;92:157–173. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- Harju M, Hamers T, Kamstra JH, Sonneveld E, Boon JP, Tysklind M, Andersson PL. Quantitative structure-activity relationship modeling on in vitro endocrine effects and metabolic stability involving 26 selected brominated flame retardants. Environ. Toxicol. Chem. 2007;26:816–826. doi: 10.1897/06-308r.1. [DOI] [PubMed] [Google Scholar]
- He P, He W, Wang A, Xia T, Xu B, Zhang M, Chen X. PBDE-47-induced oxidative stress, DNA damage and apoptosis in primary cultured rat hippocampal neurons. Neurotoxicology. 2008b;29:124–129. doi: 10.1016/j.neuro.2007.10.002. [DOI] [PubMed] [Google Scholar]
- He W, He P, Wang A, Xia T, Xu B, Chen X. Effects of PBDE-47 on cytotoxicity and genotoxicity in human neuroblastoma cells in vitro. Mutat. Res. 2008a;649:62–70. doi: 10.1016/j.mrgentox.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ. Sci. Technol. 2004;38:945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Xu Y, Hu DC, Hui Y, Yang FX. Apoptosis induction on human hepatoma cells Hep G2 of decabrominated diphenyl ether (PBDE-209) Toxicol. Lett. 2007;171:19–28. doi: 10.1016/j.toxlet.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Ward TR, Ludewig G, Robertson LW, Birnbaum LS. Polybrominated diphenyl ether (PBDE) effects in rat neuronal cultures: 14C-PBDE accumulation, biological effects, and structure-activity relationships. Toxicol. Sci. 2005;88:181–192. doi: 10.1093/toxsci/kfi289. [DOI] [PubMed] [Google Scholar]
- Kuriyama SN, Talsness CE, Chaboud I. Sex-dependent behavioral changes in rat offspring after in utero exposure to a single low dose of PBDE 47. Organohalogen Compd. 2004;66:3893–3900. [Google Scholar]
- Law RJ, Allchin CR, de Boer J, Covaci A, Herzke D, Lepom P, Morris S, Tronczynski J, de Wit CA. Levels and trends of brominated flame retardants in the European environment. Chemosphere. 2006;64:187–208. doi: 10.1016/j.chemosphere.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Lorber M. Exposure of Americans to polybrominated diphenyl ethers. J. Expos. Sci. Environ. Epidemiol. 2008;18:2–19. doi: 10.1038/sj.jes.7500572. [DOI] [PubMed] [Google Scholar]
- Madia F, Giordano G, Fattori V, Vitalone A, Branchi I, Capone F, Costa LG. Differential in vitro neurotoxicity of the flame retardant PBDE-99 and of the PCB Aroclor 1254 in human astrocytoma cells. Toxicol. Lett. 2004;154:11–21. doi: 10.1016/j.toxlet.2004.06.013. [DOI] [PubMed] [Google Scholar]
- McDonald TA. Polybrominated diphenylether levels among United States residents: Daily intake and risk of harm to the developing brain and reproductive organs. Integr. Environ. Assess. Manag. 2005;1:343–354. [PubMed] [Google Scholar]
- Mundy WR, Freudenrich TM, Crofton KM, DeVito MJ. Accumulation of PBDE-47 in primary cultures of rat neocortical cells. Toxicol. Sci. 2004;82:164–169. doi: 10.1093/toxsci/kfh239. [DOI] [PubMed] [Google Scholar]
- Petreas M, She J, Brown FR, Winkler J, Windham G, Rogers E, Zhao G, Bhatia R, Charles MJ. High body burdens of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) in California women. Environ. Health Perspect. 2003;111:1175–1179. doi: 10.1289/ehp.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reistad T, Fonnum F, Mariussen E. Neurotoxicity of the pentabrominated diphenyl ether mixture, DE-71, and hexabromocyclododecane (HBCD) in rat cerebellar granule cells in vitro. Arch. Toxicol. 2006;80:785–796. doi: 10.1007/s00204-006-0099-8. [DOI] [PubMed] [Google Scholar]
- Rice DC, Reeve EA, Herlihy A, Zoeller RT, Thompson WD, Markowski VP. Developmental delays and locomotor activity in the C57BL6/J mouse following neonatal exposure to the fully brominated PBDE, decabromodiphenyl ether. Neurotoxicol. Teratol. 2007;29:511–520. doi: 10.1016/j.ntt.2007.03.061. [DOI] [PubMed] [Google Scholar]
- Schecter A, Papke O, Tung KC, Joseph J, Harris TR, Dahlgren J. Polybrominated diphenyl ether flame retardants in the U.S. population: current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. J. Occup. Environ. Med. 2005;47:199–211. doi: 10.1097/01.jom.0000158704.27536.d2. [DOI] [PubMed] [Google Scholar]
- Schecter A, Papke O, Tung KC, Staskal D, Birnbaum L. Polybrominated diphenyl ethers contamination of United States food. Environ. Sci. Technol. 2004;38:5306–5311. doi: 10.1021/es0490830. [DOI] [PubMed] [Google Scholar]
- Schecter A, Pavuk M, Papke O, Ryan JJ, Birnbaum L, Rosen R. Polybrominated diphenyl ethers (PBDEs) in U.S. mothers’ milk. Environ. Health Perspect. 2003;111:1723–1729. doi: 10.1289/ehp.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J, Eckert ML, Lee LEJ, Gallagher EP. Comparative oxygen radical formation and toxicity of BDE 47 in rainbow trout cell lines. Mar. Environ. Res. 2008;66:7–8. doi: 10.1016/j.marenvres.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelver WL, Parrotta CD, Slavecki R, Li QX, Ikonomou MG, Barcelo D, Lacorte S, Rubio FM. Development of a magnetic particle immunoassay for polybrominated diphenyl ethers and application to environmental and food matrices. Chemosphere. 2008;73(Suppl.):S18–S23. doi: 10.1016/j.chemosphere.2007.01.088. [DOI] [PubMed] [Google Scholar]
- Sjodin A, Patterson DG, Jr, Bergman A. A review on human exposure to brominated flame retardants—particularly polybrominated diphenyl ethers. Environ. Int. 2003;29:829–839. doi: 10.1016/S0160-4120(03)00108-9. [DOI] [PubMed] [Google Scholar]
- Thomsen C, Lundanes E, Becher G. Brominated flame retardants in archived serum samples from Norway: a study of temporal trends and role of age. Environ. Sci. Technol. 2002;36:1414–1418. doi: 10.1021/es0102282. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Toxicological Review of 2,2’,4,4’-Tetrabromodiphenyl Ether (BDE-47) 2008. Integrated Risk Information System (IRIS). Available at: http://www.epa.gov/ncea/iris/toxreviews/1010-tr.pdf. [Google Scholar]
- Viberg H, Fredriksson A, Erikson P. Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behavior, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicol. Appl. Pharmacol. 2003a;192:95–106. doi: 10.1016/s0041-008x(03)00217-5. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Jakobsson E, Orn U, Erikson P. Neurobehavioral derangements in adult mice receiving decabrominated diphenyl ether (PBDE 209) during a defined period of neonatal brain development. Toxicol. Sci. 2003b;76:112–120. doi: 10.1093/toxsci/kfg210. [DOI] [PubMed] [Google Scholar]
- Wilford BH, Shoeib M, Harner T, Zhu J, Jones KC. Polybrominated diphenyl ethers in indoor dust in Ottawa, Canada: implications for sources and exposure. Environ. Sci. Technol. 2005;39:7027–7035. doi: 10.1021/es050759g. [DOI] [PubMed] [Google Scholar]