Abstract
Extensive studies during the last 30 years have led to considerable understanding of cellular and molecular control of hemoglobin switching. Cell biology studies in the 1970s defined the control of globin genes during erythroid differentiation and led to development of therapies for sickle cell disease. Molecular investigations of the last 20 years have delineated the two basic mechanisms that control globin gene activity during development—autonomous silencing and gene competition. Studies of hemoglobin switching have provided major insights on the control of gene loci by remote regulatory elements. Research in this field has an impact on understanding regulatory mechanisms in general and is of particular importance for eventual development of molecular cures for sickle cell disease and β thalassemia.
The first evidence for developmental changes in hemoglobin was obtained late in the 19th century when it was found that the hemoglobin of the newborn is alkali-resistant, while hemoglobin of the adult is alkali-sensitive. Differences in oxygen affinity between embryonic and adult blood were discovered in the beginning of the 20th century, while the first physical evidence that embryonic hemoglobin differs from adult hemoglobin was obtained in the 1930s. Switches in hemoglobin during development of humans and several animals were characterized in detail in the 1950s, when simple electrophoretic methods were introduced for hemoglobin analysis. In the 1950s and 1960s, various mutations that result in continuation of fetal hemoglobin in the adult (hereditary persistence of fetal hemoglobin) were characterized. In the 1950s and 1960s, research on hemoglobin switching was essentially based on description of hemoglobin phenotypes. Systematic mechanistic research was initiated in the 1970s with the introduction of hemopoietic cell cultures and recombinant DNA methods in the investigation of the phenomenon.
All animals that use hemoglobin for oxygen transport have different hemoglobin species during the early and latter stages of development [1]. In humans, two gene clusters direct the synthesis of hemoglobins: the α locus, which contains the embryonic ζ gene and the two adult α genes; and the β locus, which consists of the ε, Gγ, Aγ, δ, and β genes. Two globin gene switches occur during development: the embryonic to fetal globin switch, which coincides with the transition from embryonic (yolk sac) to definitive (fetal liver) hematopoiesis; and the fetal to adult switch, which occurs at the perinatal period. The switches from ε to γ and from γ to β globin gene expression are controlled exclusively at the transcriptional level [1]. The ζ to α switch is controlled predominantly at the transcriptional level, although posttranscriptional mechanisms also play a role [2]. Unlike humans, most species have only one switch, from embryonic to definitive globin expression occurring early in development. Expression of the γ genes during the fetal period is a rather recent event, which took place 35 to 55 million years ago during primate evolution [3].
The medical relevance of hemoglobin switching became apparent before any systematic experimentation started. The fact that fetal hemoglobin ameliorates the severity of sickle cell disease was appreciated in the late 1940s and the 1950s. The role of fetal hemoglobin in thalassemia was more difficult to understand. That the patients with Cooley’s anemia (severe β thalassemia) have alkaline-resistant, i.e., fetal hemoglobin, was first discovered by Italian hematologists in 1943. Many biochemical analyses of the hemoglobin of these patients followed and for about 15 years the presence of fetal hemoglobin in this condition remained a puzzle; there were even articles claiming that Cooley’s anemia is due to abnormal fetal hemoglobin! The pathophysiological role of fetal hemoglobin in thalassemia was finally delineated in the early 1960s. From various genetic and biochemical studies it was then realized that the production of fetal hemoglobin is the reason patients with severe β chain deficiency remain alive. It became obvious then that abundant synthesis of fetal hemoglobin can cure β thalassemia and sickle cell disease. The efforts of the last 30 to 40 years to understand the mechanisms that control hemoglobin switching have been influenced by the appreciation that this phenomenon is not only biologically interesting but also relevant to development of treatments and eventually a cure for thalassemia and sickle cell disease.
In this review, I will first summarize cell biological studies mostly done in the 1970s and 1980s and subsequently, I will discuss the most recent work on the molecular control of switching.
Cell biology of hemoglobin switching
The early question: switching of stem cell lineages or switching of programs within the same stem cell lineage?
It may be surprising that the cell biology work that eventually led to development of the current therapy of sickle cell disease with hydroxyurea started because of what could have been superficially thought to be a trivial question. In the early 1970s, immunochemical staining was introduced to visualize red cells containing fetal hemoglobin [4,5]. It was then found that the low amounts of fetal hemoglobin present in all adult individuals were restricted to a few red cells called F cells [4,5]. What is the origin of these cells? Why is fetal hemoglobin cellularly restricted? Several groups started examining this phenomenon and its relationship to hemoglobin switching. It was proposed that fetal to adult hemoglobin switching in humans is due to successions of two stem cell lineages that have discrete globin gene expression programs [6]: a fetal stem cell lineage committed to fetal globin formation is replaced around the perinatal period by an adult stem cell lineage committed to adult globin formation. According to this model, F cells of the adult represented the remnants of this fetal lineage that happened to exist in the adult bone marrow. This hypothesis was influenced from models explaining the embryonic to definitive hemoglobin switching in chicken and frogs, in which the changes in morphology, site of hematopoiesis, and globin gene expression coinciding with the transition from the embryonic to the definitive erythropoiesis, were best explained by the succession of two distinct stem cell lineages, one committed to embryonic and a second lineage committed to adult globin formation [7].
Our experiments on the cellular control of fetal to adult hemoglobin switching started in the early 1970s when clonal erythroid cultures were introduced in the analysis of hematopoiesis [8]. Colonies grown from a single erythroid progenitor could be analyzed for their hemoglobin phenotype using fluorescent anti-γ and anti-β antibodies, allowing us to identify, within each erythroid burst-forming unit (BFUe) colony, the types of hemoglobin produced in each subclone of the BFUe and in the cells composing the subclone. We then observed that abundant production of fetal hemoglobin was a characteristic of the BFUe cultures of normal adults [8]. These BFUe cultures also allowed us to directly test the hypothesis that a succession of a fetal stem cell lineage by the adult stem cell lineage is the cause of fetal to adult hemoglobin switching. If that was the case, the BFUe colonies should have been segregating into two categories—namely those containing fetal hemoglobin (derived from the fetal stem cell lineage) and those containing adult hemoglobin (derived from the adult stem cell lineage)—as the clonal model of globin gene switching would have predicted. The findings in the BFUe colonies were quite different: the majority of the colonies contained both cells that synthesized fetal globin and cells that synthesized adult globin [9], providing evidence that fetal and adult hemoglobins are produced by cells of the same lineage. These and similar findings in neonatal BFUe cultures suggested that hemoglobin switching does not represent a change in stem cell populations carrying fixed globin gene transcription programs, but a change in transcription programs taking place in the cells of the same stem cell lineage [9].
In an alternative approach we studied the blood of individuals with clonal hemopoietic cell disorders such as polycythemia vera or chronic myelogenous leukemia [10,11]. The clonal models of the γ to β switch predicted that in such cases, when all hematopoiesis is derived from the abnormal clone, the patient’s red cells should either be 100% F cells (if the mutation had occurred in an abnormal stem cell) or 100% non-F cells (if the mutation had occurred in normal stem cells). In contrast, both fetal and adult hemoglobin-producing red cells were present in the blood of patients with clonal hemopoietic cell disorders, as expected by the hypothesis that fetal and adult globin-producing cells are formed by the same stem cell lineage [10,11].
In the 1980s, experimental evidence that the γ to β globin gene switching occurs in the progeny of a single cell was obtained with the study of somatic cell hybrids [12]. Hybrids produced by fusing murine erythroleukemia (MEL) cells with human cells express human globins that faithfully reflect their human cell origin. Thus, when the human parental cells are adult erythroblasts, the hybrids produce exclusively human adult globin [13,14]; when the donor cells are human fetal erythroblasts, the hybrids express almost predominantly human γ globin and, after usually 20 to 40 weeks in culture, they switch to adult human globin production [12]. Because each hybrid is produced by a single cell, these results provided experimental proof that the γ to β switching can occur in the progeny of a single-cell lineage [12].
How cell biology led to development of therapies
Although attempts to induce fetal hemoglobin started in the 1960s, it was the cell biological studies done in the 1970s that led to development of therapies. Critical information in that regard was obtained from studies aimed at understanding how fetal hemoglobin is controlled in adult individuals.
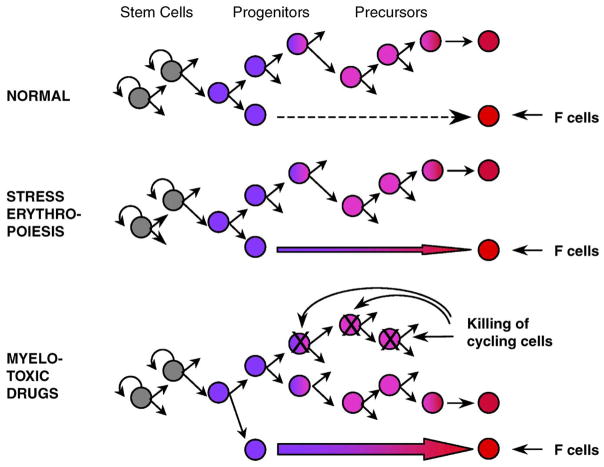
As mentioned earlier, the first clues on the control of fetal hemoglobin in the adult came from studies of clonal erythroid cell cultures showing that cells expressing fetal hemoglobin were being produced during the formation of the BFUe clones. Immunofluorescence staining showed that the erythroid bursts were composed of subclones producing fetal and adult hemoglobin or only adult hemoglobin. This finding suggested that cellular “decisions” about production or lack of production of fetal hemoglobin were taking place during the formation of erythroid bursts. Because the formation of an erythroid burst in culture is the outcome of a series of differentiation and maturation events, it was reasonable to postulate that “decisions” about fetal hemoglobin expression were in some way linked to differentiative events [9,15]. Other observations suggested that the frequency of the “decisions” to produce fetal globin (the frequency of fetal hemoglobin producing clones and subclones) was related to the degree of differentiation of progenitors from which the erythroid colonies derived [15]. These observations let us postulate a mechanistic relationship between expression of fetal hemoglobin and the process of differentiation in adult erythropoiesis. As differentiation advances, a change in globin gene transcription programs may occur, from a program allowing γ globin expression to one that supports only β globin expression. In the normal adult, the majority of erythroid cells mature normally and produce erythroblasts that synthesize only adult hemoglobin. Few progenitor cells become terminal prematurely, before the change in programs takes place, and their erythroblastic progeny produces fetal hemoglobin (Fig. 1). This hypothesis could explain several in vivo observations in humans and animal models, including the activation of fetal hemoglobin when erythropoiesis is stressed [16–19]: rapid erythroid regeneration stimulates production of fetal hemoglobin because it accelerates downstream differentiation, and thus induces the production of terminal cells that still possess the primitive fetal globin program (Fig. 1) [19]. Several experimental findings supported this concept. For example, treatment of baboons with high doses of erythropoietin resulted in the expected striking acceleration of erythropoiesis, which was associated with exuberant synthesis of fetal hemoglobin [20]. Careful analysis of in vivo erythropoiesis showed that induction of fetal hemoglobin in these animals was the result of a striking increase in fetal hemoglobin-containing CFUe colonies and erythroid clusters [21].
Figure 1.
Model of regulation of fetal hemoglobin production in adult individuals (see text). This model was instrumental for stimulating cell biology studies and experiments in primates [9,25,26] that led to the application of hydroxyurea treatment in sickle cell disease.
In 1982, DeSimone and colleagues [22] showed that 5-azacytidine induces synthesis of fetal hemoglobin in phlebotomized baboons. Nienhuis and colleagues at the National Institutes of Health expanded these observations by showing that administration of 5-azacytidine induces γ-mRNA synthesis in patients with β thalassemia [23]. Dover and colleagues at Johns Hopkins showed that 5-aza-cytidine induces fetal hemoglobin in patients with sickle cell disease [24]. Because it has been shown before that 5-azacytidine–induced DNA demethylation can activate silent genes, it was proposed that 5-azacytidine induces γ gene expression by demethylating the DNA of γ gene promoters [22–24]. We thought that induction of fetal hemoglobin by 5-azacytidine could also be explained by an alternative mechanism: this cytotoxic drug produces severe cytoreduction and we expected that a wave of erythroid regeneration will follow the cytoreduction. This post-cytoreduction regeneration could lead to fast downstream maturation of progenitors and increased production of F cells (Fig. 1). Indeed, this post-cytoreduction rapid erythroid regeneration was observed when baboons were treated with 5-azacytidine [25]. To test whether induction of fetal hemoglobin can indeed be achieved in the absence of DNA demethylation, baboons were treated with another cytotoxic drug, cytosine arabinoside, and a striking induction of fetal hemoglobin that coincided with the phase of post-cytoreduction erythroid regeneration was observed [26]. Similar results were obtained when baboons were treated with vinblastin [27] or when baboons [26] or rhesus monkeys [28] were treated with hydroxyurea. The least toxic of these compounds, hydroxyurea, was subsequently used for clinical trials [29–31].
Younger investigators in the field tend to ignore older literature (and some older investigators forget it) and think that introduction of hydroxyurea in the treatment of sickle cell disease was serendipitous; nothing is more erroneous. Hydroxyurea would not have been discovered if we just were getting drugs from our laboratory shelves to test whether they induce fetal hemoglobin, for the very simple reason that hydroxyurea does not induce fetal hemoglobin in clonal cultures when they are well controlled for maturation [32]. It was the cell biology work of the late 1970s and the prediction that the in vivo perturbation of the kinetics of erythropoiesis produced by hydroxyurea will induce fetal hemoglobin production, that led to the use of this compound in patients with sickle cell disease.
Molecular control of switching
Studies of mutants
When the era of recombinant DNA started in the mid 1970s, the globin field focused on delineation of the molecular pathology of hereditary persistence of fetal hemoglobin (HPFH) and the thalassemia syndromes. One of the first successes was the demonstration that the homozygous δβ thalassemia and the homozygous hereditary persistence of fetal hemoglobin were due to deletions that remove the δ and β genes but leave the Gγ and Aγ genes intact. A large body of molecular data was subsequently accumulated, particularly for the molecular pathology of the δβ thalassemia syndromes (reviewed in [1]); all this work helped delineate phenomenology but shed very little light on mechanisms of switching. Although hypotheses have been formulated, it is still unclear why the deletion in HPFH produces a phenotype characterized by abundant and pancellular synthesis of fetal hemoglobin in adult red cells and no disease in homozygotes, while the deletion in δβ thalassemia produces the phenotype of heterocellular and less abundant synthesis of fetal hemoglobin in the heterozygotes and disease of moderate severity in the homozygotes. “Imported” downstream enhancers juxtaposed, because of these deletions, to the γ genes have been proposed to explain the phenotype of deletional HPFH [33,34]. Results in transgenic mice are ambiguous. While some experiments using cosmid constructs support the hypothesis of imported enhancers [35,36], faithful reproduction of the deletional events in β locus yeast artificial chromosomes (YACs) and production of transgenic mice has failed to reproduce the phenotype of HPFH: the γ genes remain silent in the adult stage of erythropoiesis [37]. It is amazing that the first developmental mutant in man, identified 48 years ago and described in hundreds of publications, remains a mechanistic mystery.
Investigations of the nondeletional HPFHs, the so-called Gγ HPFH and Aγ HPFH mutants, have been more informative. The first clue came from Francis Collins and colleagues who, in 1983, found that a T-to-C substitution in position 175 of the Gγ promoter underlies the Gγ HPFH mutation they studied [38]. The second clue came from the study of the so-called Greek Aγ HPFH [39]. Collins and associates [40] and our group [41] simultaneously found a G-to-A transition in position −117 of the Aγ gene promoter. This mutation affected the CAAT box of the Aγ gene. It took quite a bit of persuasion to convince the editor of the journal to which the two Aγ HPFH articles were submitted, that a mutation of the Aγ gene promoter affecting the Aγ CAAT box should be responsible for the continuation of the Aγ chain synthesis in the adult life! Since then, this Aγ HPFH mutation and its phenotypical effects have been reproduced experimentally in transgenic mice [42]. Several other mutations of the Gγ and Aγ proximal promoters have been identified over the years (reviewed in [1]). These are the best-characterized developmental mutants in humans, but it is a measure of the difficulty of the delineation of the specific protein/DNA interactions that underlie hemoglobin switching that we still do not exactly know how these mutations affect γ globin gene transcription.
Discovery of the LCR
The 1980s were characterized by a considerable effort by several laboratories to identify proteins involved in the regulation of globin gene expression, with little success. Progress was achieved with studies of chromatin. Groudine et al. [43], in the early 1980s, applied DNAse I sensitivity assays (earlier developed by Weintraub and Groudine) to assess the status of the chromatin of the human β globin locus during development and observed that the switch from fetal to adult globin gene expression is accompanied by a switch in the DNAse I hypersensitive sites located in the γ and β promoters. Subsequently, Tuan et al. [44] and Groudine’s group [45] found that a series of DNAse I hypersensitive sites are located upstream of the ε globin gene. Two experiments, published in 1987, showed that this upstream region has regulatory relevance to the β locus. Groudine’s group, in collaboration with our group, examined the chromatin configuration of the human β locus in uninduced and induced MEL/human cell hybrids [46] and observed that formation of the upstream DNAse I hypersensitive region preceded the activation of the genes of the locus; it was proposed that the upstream region containing these hypersensitive sites (HSs) is responsible for activation of the locus. Therefore, this region was named locus activation region [46]. Grosveld’s group produced transgenic mice containing the upstream DNAse I HSs linked to the β globin gene [47]. Up to this experiment, investigators have produced transgenic mice carrying the β globin gene, but results were disappointing—only a few of the transgenes expressed β globin, the great majority were silent. In contrast, Grosveld’s group found that all their transgenics having the β gene linked to the upstream DNAse I HSs expressed the gene at levels as high as those of the endogenous murine β genes [47]. They attributed this effect to the upstream region that they called dominant control region. Both terminologies were used in the literature (and confused the field), until the term locus control region (LCR) was adapted in 1990 at The 7th Conference on Hemoglobin Switching.
Properties of the LCR
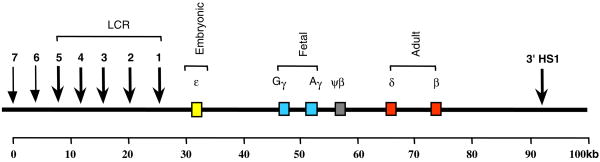
The LCR (Fig. 2) consists of 5 DNAse I H S located 6 to 20 kb upstream of the β globin gene. Each HS has a core sequence, approximately 250 nucleotides long, which is studded with motifs for transcriptional factors; motifs shared by the 5 HSs include NFE2 and GATA-1. HSs have specific functions. For example, HS5 (in humans) or HS4 (in chicken) function as insulators [48,49]. HS3 functions as an activator of ε and γ globin gene expression [50]. The LCR confers lineage-specific expression on the globin genes; it acts as the major enhancer of the β locus; it insulates the locus from surrounding inactive chromatin. On the basis of findings in transgenic mice, it was proposed that the LCR is responsible for opening the β locus chromatin domain [47]. However, deletion of the LCR of the endogenous murine β locus does not affect the formation of the DNAse I HSs in the downstream globin promoters although it reduces globin gene expression by 100-fold [51]; thus the chromatin of the locus remains open in spite of the absence of the upstream LCR [51]. In humans, LCR deletions produce β thalassemia characterized by complete absence of β gene expression (reviewed in [1]). In one well-characterized mutation, sequences of the LCR from HS2 to HS5 as well as sequences upstream of HS5 are deleted and the chromatin of the β locus becomes DNAse I insensitive [52]. Thus, mouse and human LCR deletions produce different effects on the downstream chromatin, but the reasons for these differences remain unknown.
Figure 2.
Diagram of the human β globin locus.
Following the description of the β globin locus LCR, several LCRs were described in other loci [53]. There appears to be a relationship between activation of the LCR and hematopoietic differentiation: the DNAse I HSs of the LCR are “on,” even in cells that have the characteristic of hematopoietic stem cells [54], and there is in vivo experimental evidence that the LCR is active in multipotent progenitor cells [55]. Experiments using somatic cell hybrids suggest that activation of the LCR in progenitor cells is required for normal LCR function [56].
How does the LCR function and activate the downstream globin genes? This is a question of general biological significance, and has been addressed with many experiments over the last 10 to 15 years. Various models have been proposed: a looping [57], a tracking [58], a facilitated tracking [59], and a linking [60] model. Models are contributory only when they are euristic and can be tested experimentally. The looping model fulfills the definition of an euristic model. It suggests that the HSs of the β globin LCR fold to form a complex, with the HS core elements forming an active site that binds transcription factors. This structure physically “loops” so that the LCR comes in close proximity to the appropriate gene. Close association with gene-proximal elements allows delivery of LCR-bound transcription factors and other coactivators that interact with the basal transcriptional apparatus.
The looping model has stimulated many studies that have enriched our knowledge on the interaction between genes and remote regulatory elements. For example, it has been shown that the LCR interacts with only one globin gene promoter at a time and that it “flip-flops” between two or more promoters, depending on the stage of development [61]. The looping model also stimulated investigators to introduce novel, imaginative techniques to investigate interactions between remote regulatory elements and the genes they control [62,63]. Such techniques have provided direct evidence for the interaction between the LCR and the downstream genes.
Mechanisms of switching
Discovery of the LCR had a major impact on the investigation of mechanisms of switching because it triggered many new experiments, including studies using transgenic mice. Today we have a general idea on how globin genes are “turned off” and how they are “turned on” during development, although we lag behind in understanding the specific biochemical reactions involved.
How are the globin genes turned off?
Studies in transgenic mice have shown that two mechanisms, gene competition and autonomous gene silencing account for the turning off of the γ genes during development.
Gene competition
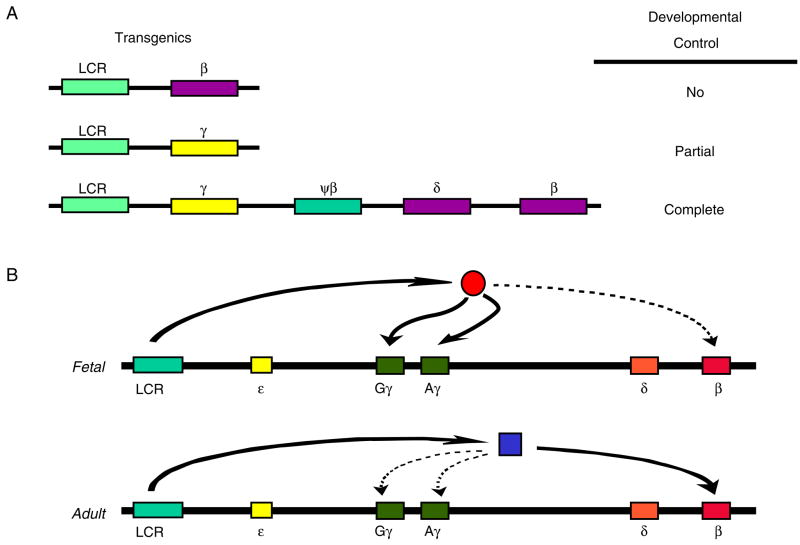
Evidence for a competitive mechanism of γ to β globin gene switching was derived from the experiments done in transgenic mice by Behringer et al. [64] and Enver et al. [65]. When the LCR was linked either to the γ gene alone or to the β globin gene alone these genes failed to display proper developmental regulation (Fig. 3A); thus, the β gene was active at all stages of development, and in the embryonic cells, β gene expression was as abundant as β gene expression in adult cells. The γ genes were expressed in the embryo and the fetus and, although at a much lower level, they were still also expressed in the adult. However, when the γ and the β genes were linked together in a construct also containing LCR sequences, developmental control was restored: β gene expression was restricted to the adult erythropoiesis while γ gene expression was restricted to the fetal erythropoiesis (Fig. 3A). These observations were explained by assuming that the γ and β genes compete for interaction with the LCR [65]. During the fetal stage of development, there is a preferential interaction between the γ genes and the LCR; the β globin genes are turned off competitively. On the other hand, the LCR interacts preferentially with the β gene during the adult stage of development, resulting in silencing of the γ globin genes (Fig. 3B). Extensive experimental evidence indicates that competition by the upstream ε and γ genes silences the β gene in embryonic erythropoiesis. As we will see later, competition by the β gene, but mainly autonomous silencing, are involved in turning off the γ gene in adult erythropoiesis.
Figure 3.
The competitive mechanism of hemoglobin switching. (A) This cartoon illustrates the initial experiments in transgenic mice that provided evidence in support of the competition mechanism of human fetal to adult globin gene switching [65]. Developmental control is lost when the human β or γ globin genes are linked individually to the LCR. However, control is fully restored when both the β and the γ genes in their normal chromosomal arrangement are linked to this dominant regulatory element; the γ gene is expressed only in the fetal stage and the β gene only in the adult stage. The best interpretation of these results is gene competition. As shown in (B), fetal and adult globin genes compete for the interaction of the LCR. The transcriptional environment favors the interaction between the LCR and the γ gene in the fetal stage, resulting in silencing of β gene expression. The opposite occurs in the adult stage. Plenty of experimental evidence indicates that gene competition is the mechanism of silencing of the β gene in the embryonic and fetal erythropoiesis.
Autonomous silencing
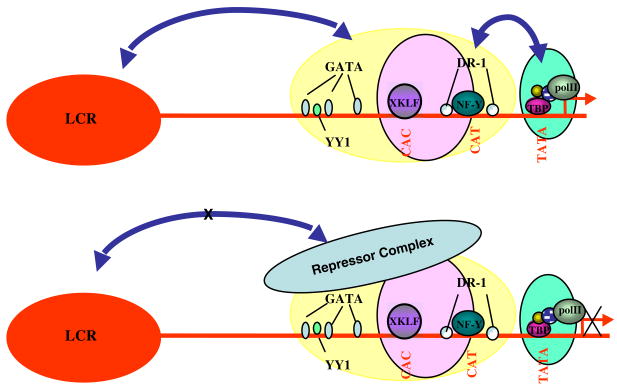
Transgenic mice carrying the embryonic human ε gene fail to express that gene at any stage of development. When, however, the ε gene is linked to the LCR, there is abundant ε expression, indicating that transcription of this gene is dependent on the presence of the LCR. Characteristically, however, expression is totally restricted in yolk sac erythropoiesis [66]. This finding led to the concept of autonomous silencing, whereby all the elements responsible for turning off gene expression are contained within the canonical gene or the adjacent sequences [66]. Several experiments have aimed to define elements of the ε gene promoter that contain these silencing sequences. One approach was to delete promoter sequences, produce transgenic mice, and test whether the deletions resulted in abolishment of the autonomous silencing and continuation of ε gene expression in the adult mice. With this approach, sequences participating in the silencing process were identified in both the proximal and the distal ε gene promoter [67,68]. Especially informative were studies in which specific motifs of the ε gene promoter were mutated to abolish protein binding and effects on ε gene silencing were assessed in transgenic mice. Thus, mutation of GATA-1, YY1, or SP1 sites in the upstream ε promoter abolished ε gene silencing [69], raising the possibility that globin gene silencing is combinatorial with several transcriptional factors participating in the formation of a silencing complex. The proximal ε gene promoter (as well as the γ promoter but not the other globin gene promoters) contain direct repeat sequences (DR1 box) located near the CAAT box. These DR elements bind a factor, which is identical to the orphan nuclear receptor COUP-TF [70]. Mutations of the DR elements that inhibit protein binding result in ε gene expression in the adult mice, indicating that the DR elements participate in ε gene silencing [70,71]. More recently a high molecular weight complex, named DRED, has been shown to bind to the DR element of the ε gene promoter [72]. The autonomous silencing of the ε globin gene thus appears to be a complex phenomenon that involves sequences in the proximal and distal promoter and participation of several DNA binding proteins. It is currently assumed that the silencing complex turns off ε gene expression in the definitive erythropoiesis by inhibiting the interaction between the ε gene and the LCR (Fig. 4).
Figure 4.
The autonomous silencing mechanism of hemoglobin switching. All sequences required for silencing are included in proximal to the gene regulatory elements. Silencing is autonomous, no competition by another globin gene is involved. The concept is illustrated by the autonomous silencing of the ε globin gene. Transcriptional factors bind on silencing sequences located in the proximal and the distal ε gene promoter and form a repressor complex that disrupts the interaction between the gene and the LCR, resulting in turning off of ε gene expression.
Initially there was debate whether competition [65] or autonomous silencing [73] account for turning off the γ globin gene, but it seems that both mechanisms are involved. Studies in transgenic mice have localized a silencing element in the −378 to −730 region of the upstream γ promoter [74]. Further evidence that this sequence contains a γ gene silencer has been recently obtained with studies of mice carrying mutant β locus YACs; when a β globin gene was placed upstream of the ε gene, it escaped developmental regulation and it was expressed in all stages of development. However, when the γ gene was placed in that location, it was expressed in the embryonic and the fetal stage but it was silenced in the adult stage. When the −378 to −730 region of the γ promoter was, however, deleted from that γ gene, the γ gene remained active in the adult, indicating that this −378 to −730 sequence contains elements that contribute to the autonomous silencing of the γ gene [75]. Recently a mutation in a G ATA site in the−378 to −730 sequence was found to produce the phenotype of hereditary persistence of fetal hemoglobin [76], further suggesting that this upstream region contributes to γ gene silencing in vivo. To delineate the sequences of the proximal γ gene promoter, which are involved in silencing, hybrid galago γ/human γ promoters were produced; in contrast to human the expression of the galago γ gene is restricted to the embryonic erythropoiesis and this restriction is controlled by sequences in the proximal promoter. Findings in transgenic mice carrying galago/human γ gene hybrid promoters suggest that the CACCC box is the main proximal γ gene promoter motif that participates in γ gene silencing [77].
Autonomous silencing is thus the principal mechanism that turns off the γ gene but evidence from human mutants suggests that competition by the β gene also contributes. Although alternative explanations have been proposed, the continuation of γ gene expression in the δβ thalassemia and the HPFH deletion mutants is best explained by the lack of competition by the deleted β globin genes. In addition to the δβ thalassemias, all but one β° thalassemia mutants due to β gene deletions are characterized by increased levels of fetal hemoglobin in adult heterozygotes [1].
How the globin genes are turned on during development? Gene order and proximity to the LCR
The sequential activation of ε, Gγ, Aγ, δ, and β globin genes during development reflects the order of their arrangement in the chromosome, raising the possibility that gene order determines the order of gene activation. The role of gene order has been shown with several experiments in transgenic mice. As mentioned earlier, whatever gene is placed next to the LCR, it is expressed first during development. Proximity to the LCR is apparently important because it increases the probability of interaction with that dominant regulatory element.
Stage specific transacting elements
For several years it was questioned whether transcriptional factors specific to globin genes do exist. The debate ended with the discovery of EKLF, a transcriptional factor that specifically interacts with the proximal CACCC box of the β but not the CACCC boxes of γ or the ε globin genes [78,79]. EKLF is necessary for β globin gene expression as the EKLF knockout mice and β thalassemias due to β gene CACCC box mutations have shown. EKLF also binds to DNAse I HSs of the LCR and it may function by increasing the probability of interaction between the LCR and the β globin gene promoter.
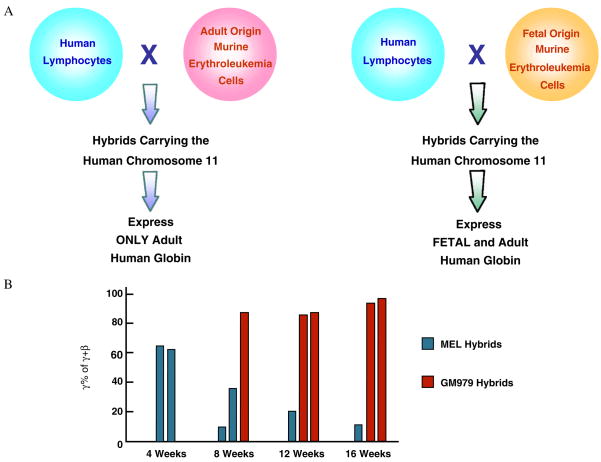
Several years of failing attempts to find a factor that activates the γ genes have raised doubts about the existence of such factors specific for γ globin gene. It can be argued that the γ genes are just activated by default: following the silencing of the ε gene, the LCR will interact with the next gene in the locus, the γ gene, and will turn it on; hence, there is no need for a special transcriptional environment to activate that gene. Results of experiments using cell fusion make this hypothesis unlikely. GM979 cell is a fetal-like murine erythroleukemia line that supports the synthesis of low levels of murine embryonic globins. Zitnik et al. [80] asked whether the transcriptional environment of this line can activate a silent human γ gene. They transferred, by cell fusion, the chromosome 11 from adult human lymphocytes into GM979 cells, as well as to control MEL cells, an adult erythroleukemia line. While only the β globin genes were activated when the human chromosome 11 was transferred into MEL cells, the γ (as well as the β) globin genes were activated when human chromosome 11 was transferred into the GM979 cells (Fig. 5A). This and a similar experiment using β locus YACs (Fig. 5B) [81] provide evidence for the existence of a fetal-like transcriptional environment that can transactivate the γ globin genes.
Figure 5.
Experimental evidence for the existence of γ gene-specific fetal transcriptional factor has been obtained from cell fusion experiments. (A) Results of transfer of lymphoid origin human chromosome 11 into adult or fetal-like murine erythroleukemic cells [80]. The silent human γ globin genes are activated in the fetal-like environment. (B) Results of transfer of a human β locus YAC into the adult or fetal-like murine erythroleukemia cells [81]. The γ globin gene of the β YAC fail to switch in the fetal environment.
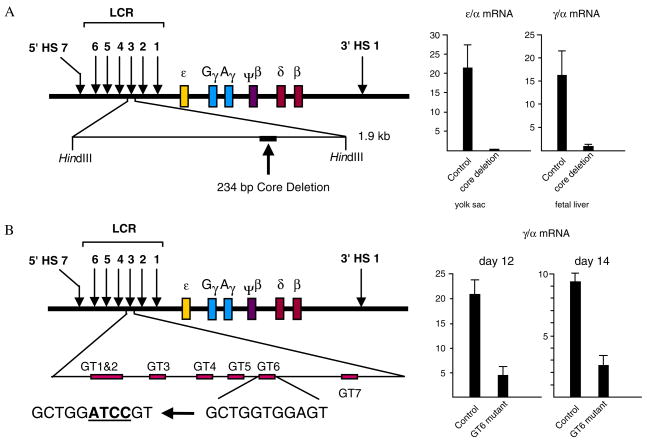
Sequences involved in γ gene activation are located both in the LCR and the γ gene promoter. Deletion of the core element of HS3 produces a distinct phenotype characterized by absence of ε gene expression in the yolk sac and absence of γ gene expression in the fetal liver stage of erythropoiesis (Fig. 6A), indicating that the HS3 core is required for γ gene activation [50]. The HS3 core is studded with transcriptional motifs, six of which, the GT motifs bind SP1/KLF type transcriptional factors. One of these motifs, GT6, shows differences in protein binding between fetal and adult globin expressing cells [82]. Abolishing protein binding by mutation of this motif strikingly affects γ gene expression in the fetal stage of erythropoiesis of γ locus YAC transgenic mice (Fig. 6B) [83]. Mutational analysis of the sequences of the γ gene promoter, on the other hand, indicates that the γ gene CACCC box is a principal determinant of γ gene activation [77]. Factors that interact with the γ gene CACCC box have been identified [84,85] but their in vivo role is still unclear. Our working hypothesis is that the CACCC box is involved, both in the silencing as well as in the activation of the γ globin gene. KLF-type transcriptional factors may bind both to the γ gene promoter and the HS3 of the LCR, thus facilitating the interaction between the γ gene and the LCR and enhancing γ gene transcription. As in the case of the ε gene, γ gene silencing occurs when a silencing complex disrupts the interaction between the γ gene promoter and the LCR.
Figure 6.
Sequences in the γ gene promoter as well as sequences of the LCR are involved in the activation of the γ globin gene. The diagrams illustrate the results of two experiments using β locus YAC transgenic mice. (A) When the core element of DNAse I hypersensitive site 3 (HS3) of the LCR is deleted, γ gene expression in fetal erythropoiesis is essentially abolished [50], providing evidence that this core sequence contains elements involved in γ gene activation. (B) Even mutations of a single motif of the core sequence of HS3 result in striking decrease of γ gene expression [83]. Experiments using β locus YAC transgenic mice have substantially increased our knowledge of the cis regulatory elements involved in globin gene switching.
Future challenges
An explosion of molecular biology studies during the last 20 years led to the recognition of the two basic mechanisms that control hemoglobin switching—gene silencing and gene competition. The challenge now is to delineate the specific biochemical processes involved in globin gene silencing, gene activation, and the interaction between globin genes and the upstream dominant regulatory element, the LCR. The hope is that eventually molecular biology research will contribute, as the cell biology of the 1970s and 1980s has done, to development of new approaches for induction of fetal hemoglobin in the patients with thalassemia and sickle cell disease.
Sickle cell disease and β thalassemia are unique among human genetic disorders in that nature has shown the way to treat them through production of fetal hemoglobin. The challenge to the field of cell and molecular biology of hemoglobin switching is to develop a cure for these diseases using the method discovered by nature.
References
- 1.Stamatoyannopoulos G, Grosveld F. Hemoglobin switching. In: Stamatoyannopoulos G, Majerus P, Perlmutter R, Varmus H, editors. The Molecular Basis of Blood Diseases. 3. Philadelphia: W.B. Saunders Publishing Co; 2001. pp. 135–182. Part II, Red Cells. [Google Scholar]
- 2.Liebhaber SA, Wang Z, Cash FE, Monks B, Russell JE. Developmental silencing of the embryonic ζ-globin gene: concerted action of the promoter and the 3′-flanking region combined with stage-specific silencing by the transcribed segment. Mol Cell Biol. 1996;16:2637–2646. doi: 10.1128/mcb.16.6.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomhon C, Zhu W, Millinoff D, Hayasaka K, Slighton JL, Goodman M, Gumucio DL. Evolution of a fetal expression pattern via cis changes near the γ globin gene. J Biol Chem. 1997;272:14062–14066. doi: 10.1074/jbc.272.22.14062. [DOI] [PubMed] [Google Scholar]
- 4.Boyer SH, Belding TK, Margolet L, Noyes AN. Fetal hemoglobin restriction to a few erythrocytes (F cells) in normal human adults. Science. 1975;188:361–363. doi: 10.1126/science.804182. [DOI] [PubMed] [Google Scholar]
- 5.Wood WG, Stamatoyannopoulos G, Lim G, Nute PE. F-cells in the adult: normal values and levels in individuals with hereditary and acquired elevations of Hb F. Blood. 1975;46:671–682. [PubMed] [Google Scholar]
- 6.Weatherall DJ, Clegg JB, Wood WG. A model for the persistence or reactivation of fetal haemoglobin production. Lancet. 1976;2:660–663. doi: 10.1016/s0140-6736(76)92469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ingram VM. Embryonic red cell formation. Nature. 1972;235:338–339. doi: 10.1038/235338a0. [DOI] [PubMed] [Google Scholar]
- 8.Papayannopoulou Th, Brice M, Stamatoyannopoulos G. Stimulation of fetal hemoglobin synthesis in bone marrow cultures from adult individuals. Proc Natl Acad Sci USA. 1976;73:2033–2037. doi: 10.1073/pnas.73.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papayannopoulou Th, Brice M, Stamatoyannopoulos G, Hemoglobin F. synthesis in vitro: evidence for control at the level of primitive erythroid stem cells. Proc Natl Acad Sci USA. 1977;74:2923–2927. doi: 10.1073/pnas.74.7.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papayannopoulou Th, Bunn HF, Stamatoyannopoulos G. Cellular distribution of hemoglobin F in a clonal hemopoietic stem-cell disorder. N Engl J Med. 1978;298:72–75. doi: 10.1056/NEJM197801122980203. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman R, Papayannopoulou Th, Landaw S, Wasserman L, DeMarsh QB, Stamatoyannopoulos G. Fetal hemoglobin in polycythemia vera. Cellular distribution in 50 unselected patients. Blood. 1979;53:1148–1155. [PubMed] [Google Scholar]
- 12.Papayannopoulou Th, Brice M, Stamatoyannopoulos G. Analysis of human hemoglobin switching in MEL x human fetal erythroid cell hybrids. Cell. 1986;46:469–476. doi: 10.1016/0092-8674(86)90667-7. [DOI] [PubMed] [Google Scholar]
- 13.Willing MC, Nienhuis AW, Anderson WF. Selective activation of human β- but not γ-globin gene in human fibroblast X mouse erythroleukaemia cell hybrids. Nature. 1979;277:534. doi: 10.1038/277534a0. [DOI] [PubMed] [Google Scholar]
- 14.Takegawa S, Brice M, Stamatoyannopoulos G, Papayannopoulou Th. Only adult hemoglobin is produced in fetal nonerythroid x MEL cell hybrids. Blood. 1986;68:1384–1388. [PubMed] [Google Scholar]
- 15.Stamatoyannopoulos G, Papayannopoulou Th. Fetal hemoglobin and the erythroid stem cell differentiation process. In: Stamatoyannopoulos G, Nienhuis AW, editors. Cellular and Molecular Regulation of Hemoglobin Switching. New York: Grune & Stratton; 1979. pp. 323–350. [Google Scholar]
- 16.Dover GJ, Boyer SH, Zinkham WH. Production of erythrocytes that contain fetal hemoglobin in anemia. J Clin Invest. 1979;63:173–176. doi: 10.1172/JCI109286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papayannopoulou Th, Vichinsky E, Stamatoyannopoulos G. Fetal Hb production during acute erythroid expansion. I. Observations in patients with transient erythroblastopenia and post-phlebotomy. Br J Haematol. 1980;44:535–546. doi: 10.1111/j.1365-2141.1980.tb08707.x. [DOI] [PubMed] [Google Scholar]
- 18.DeSimone J, Biel M, Heller P. Stimulation of fetal hemoglobin synthesis in baboons by hemolysis and hypoxia. Proc Natl Acad Sci USA. 1978;75:2937–2940. doi: 10.1073/pnas.75.6.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stamatoyannopoulos G, Veith R, Galanello R, Papayannopoulou Th. Hb F production instressed erythropoiesis: observations and kinetic models. Ann NY Acad Sci. 1985;445:188–197. doi: 10.1111/j.1749-6632.1985.tb17188.x. [DOI] [PubMed] [Google Scholar]
- 20.Al-Khatti A, Veith RW, Papayannopoulou Th, Fritsch EF, Goldwasser E, Stamatoyannopoulos G. Stimulation of fetal hemoglobin synthesis by erythropoietin in baboons. N Engl J Med. 1987;317:415–420. doi: 10.1056/NEJM198708133170704. [DOI] [PubMed] [Google Scholar]
- 21.Umemura T, Al-Khatti A, Papayannopoulou Th, Stamatoyannopoulos G. Fetal hemoglobin synthesis in vivo: direct evidence for control at the level of erythroid progenitors. Proc Natl Acad Sci USA. 1988;85:9278–9282. doi: 10.1073/pnas.85.23.9278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeSimone J, Heller P, Hall L, Zwiers D. 5-Azacytidine stimulates fetal hemoglobin synthesis in anemic baboons. Proc Natl Acad Sci USA. 1982;79:4428–4431. doi: 10.1073/pnas.79.14.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ley TJ, DeSimone J, Anagnou NP, et al. 5-Azacytidine selectively increases γ globin synthesis in a patient with β+ thalassemia. N Engl J Med. 1982;307:1469–1475. doi: 10.1056/NEJM198212093072401. [DOI] [PubMed] [Google Scholar]
- 24.Charache S, Dover G, Smith K, Talbot CC, Jr, Moyer M, Boyer S. Treatment of sickle cell anemia with 5-Azacytidine results in increased fetal hemoglobin production and is associated with non-random hypomethylation of DNA around the γ-δ-β globin gene complex. Proc Natl Acad Sci USA. 1983;80:4842–4846. doi: 10.1073/pnas.80.15.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torrealba de Ron AT, Papayannopoulou Th, Knapp MS, Fu MF, Knitter G, Stamatoyannopoulos G. Perturbations in the erythroid marrow progenitor cell pools may play a role in the augmentation of Hb F by 5 Azacytidine. Blood. 1984;63:201–210. [PubMed] [Google Scholar]
- 26.Papayannopoulou Th, Torrealba de Ron A, Veith R, Knitter G, Stamatoyannopoulos G. Arabinosylcytosine induces fetal hemoglobin in baboons by perturbing erythroid cell differentiation kinetics. Science. 1984;224:617–619. doi: 10.1126/science.6200940. [DOI] [PubMed] [Google Scholar]
- 27.Veith R, Papayannopoulou Th, Kurachi S, Stamatoyannopoulos G. Treatment of baboon with Vinblastine: insights into the mechanisms of pharmacologic stimulation of Hb F in the adult. Blood. 1985;66:456–459. [PubMed] [Google Scholar]
- 28.Letvin NL, Linch DC, Beardsley GP, McIntyre KW, Nathan DG. Augmentation of fetal-hemoglobin production in anemic monkeys by hydroxyurea. N Engl J Med. 1984;310:869–873. doi: 10.1056/NEJM198404053101401. [DOI] [PubMed] [Google Scholar]
- 29.Platt OS, Orkin SH, Dover G, Beardsley GP, Miller B, Nathan DG. Hydroxyurea enhances fetal hemoglobin production in sickle cell anemia. J Clin Invest. 1984;74:652–656. doi: 10.1172/JCI111464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veith R, Galanello R, Papayannopoulou Th, Stamatoyannopoulos G. Stimulation of F-cell production in Hb S patients treated with Ara-C or hydroxyurea. N Engl J Med. 1985;313:1571–1575. doi: 10.1056/NEJM198512193132503. [DOI] [PubMed] [Google Scholar]
- 31.Charache S, Dover GJ, Moyer MA, Moore JW. Hydroxyurea-induced augmentation of fetal hemoglobin production in patients with sickle cell anemia. Blood. 1987;69:109–116. [PubMed] [Google Scholar]
- 32.Galanello R, Stamatoyannopoulos G, Papayannopoulou Th. Mechanism of Hb F stimulation by S-stage compounds: in vitro studies with bone marrow cells exposed to 5-azacytidine, Ara-C or hydroxyurea. J Clin Invest. 1988;81:1209–1216. doi: 10.1172/JCI113437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feingold EA, Forget BG. The breakpoint of a large deletion causing hereditary persistence of fetal hemoglobin occurs within an erythroid DNA domain remote from the β-globin gene cluster. Blood. 1989;74:2178–2186. [PubMed] [Google Scholar]
- 34.Anagnou NP, Perez-Stable C, Gelinas R, et al. Sequences located 3′ to the breakpoint of the hereditary persistence of fetal hemoglobin-3 deletion exhibit enhancer activity and can modify the developmental expression of the human fetal Aγ globin gene in transgenic mice. J Biol Chem. 1995;270:1–8. doi: 10.1074/jbc.270.17.10256. [DOI] [PubMed] [Google Scholar]
- 35.Arcasoy MO, Romana M, Fabry ME, Skarpidi E, Nagel RL, Forget BG. High levels of human γ-globin gene expression in adult mice carrying a transgene of deletion-type hereditary persistence of fetal hemoglobin. Mol Cell Biol. 1997;17:2076–2089. doi: 10.1128/mcb.17.4.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katsantoni EZ, Langeveld A, Wai AW, et al. Persistent gamma-globin expression in adult transgenic mice is mediated by HPFH-2, HPFH-3, and HPFH-6 breakpoint sequences. Blood. 2003;102:3412–3419. doi: 10.1182/blood-2003-05-1681. [DOI] [PubMed] [Google Scholar]
- 37.Xiang P, Ye X, Han H, Stafford M, Stamatoyannopoulos G, Li Q. Juxtaposition of the HPFH2 enhancer is not sufficient to reactivate the γ-globin gene in adult erythropoiesis in βYAC transgenic mice. Blood. 2004;104:344a. doi: 10.1093/hmg/ddi337. [DOI] [PubMed] [Google Scholar]
- 38.Collins FS, Stoeckert CJ, Jr, Serjeant GR, Forget BG, Weissman SM. Gγβ+ hereditary persistence of fetal hemoglobin: cosmid cloning and identification of a specific mutation 5′ to the Gγ gene. Proc Natl Acad Sci USA. 1984;81:4894–4898. doi: 10.1073/pnas.81.15.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fessas Ph, Stamatoyannopoulos G. Hereditary persistence of fetal haemoglobin in Greece. A study and a comparison. Blood. 1964;24:223–240. [PubMed] [Google Scholar]
- 40.Collins FS, Metherall JE, Yamakawa M, Pan J, Weissman SM, Forget BM. A point mutation in the A-γ-gene promoter in Greek hereditary persistence of fetal haemoglobin. Nature. 1985;313:325–326. doi: 10.1038/313325a0. [DOI] [PubMed] [Google Scholar]
- 41.Gelinas R, Endlich B, Pfeiffer C, Yagi M, Stamatoyannopoulos G. G to A substitution in the distal CCAAT box of the Aγ-globin gene in Greek hereditary persistence of fetal haemoglobin. Nature. 1985;313:323–325. doi: 10.1038/313323a0. [DOI] [PubMed] [Google Scholar]
- 42.Peterson KR, Li Q, Clegg CH, et al. Use of YACs in studies of development: production of β-globin locus YAC mice carrying human globin developmental mutants. Proc Natl Acad Sci USA. 1995;92:5655–5659. doi: 10.1073/pnas.92.12.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Groudine M, Kohwi-Shigematsu T, Gelinas R, Stamatoyannopoulos G, Papayannopoulou Th. Human fetal to adult hemoglobin switching: changes in chromatin structure of the β-globin gene locus. Proc Natl Acad Sci USA. 1983;80:7551–7555. doi: 10.1073/pnas.80.24.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tuan D, Solomon W, Li Q, London IM. The “β-like-globin” gene domain in human erythroid cells. Proc Natl Acad Sci USA. 1985;82:6384. doi: 10.1073/pnas.82.19.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forrester WC, Thompson C, Elder JT, Groudine M. A developmentally stable chromatin structure in the human β-globin gene cluster. Proc Natl Acad Sci USA. 1986;83:1359. doi: 10.1073/pnas.83.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forrester WC, Takegawa S, Papayannopoulou Th, Stamatoyannopoulos G, Groudine M. Evidence for a locus activation region: the formation of developmentally stable hypersensitive sites in globin-expressing hybrids. Nucleic Acids Res. 1987;24:10159–10177. doi: 10.1093/nar/15.24.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grosveld F, van Assendelft GB, Greaves DR, Kollias G. Position-independent, high-level expression of the human β-globin gene in transgenic mice. Cell. 1987;51:975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- 48.Bell AC, West AG, Felsenfeld G. Insulators and boundaries: versatile regulatory elements in the eukaryotic genome. Science. 2001;291:447–450. doi: 10.1126/science.291.5503.447. [DOI] [PubMed] [Google Scholar]
- 49.Li Q, Zhang M, Han H, Rohde A, Stamatoyannopoulos G. Evidence that Dnase I hypersensitive site 5 of human beta-globin LCR functions as a chromosomal insulator in transgenic mice. Nucl Acids Res. 2002;30:2484–2491. doi: 10.1093/nar/30.11.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Navas PA, Peterson KR, Li Q, et al. Developmental specificity of the interaction between the locus control region and embryonic or fetal globin genes in transgenic mice with an HS3 core deletion. Mol Cell Biol. 1998;18:4188–4196. doi: 10.1128/mcb.18.7.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bender MA, Bulger M, Close J, Groudine M. Beta-globin gene switching and DNase I sensitivity of the endogenous beta-globin locus in mice do not require the locus control region. Mol Cell. 2000;5:387–393. doi: 10.1016/s1097-2765(00)80433-5. [DOI] [PubMed] [Google Scholar]
- 52.Forrester WC, Epner E, Driscoll MC, et al. A deletion of the human β-globin locus activation region (LAR) causes a major alteration in chromatin structure and replication across the entire β-globin locus. Genes Dev. 1990;4:1637–1649. doi: 10.1101/gad.4.10.1637. [DOI] [PubMed] [Google Scholar]
- 53.Li Q, Peterson KR, Fang X, Stamatoyannopoulos G. Locus control regions. Blood. 2002;100:3077–3086. doi: 10.1182/blood-2002-04-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jimenez G, Griffiths SD, Ford AM, Greaves MF, Enver T. Activation of the β-globin locus control region precedes commitment to the erythroid lineage. Proc Natl Acad Sci USA. 1992;89:10618–10622. doi: 10.1073/pnas.89.22.10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Papayannopoulou Th, Priestley GV, Rhode A, Peterson KR, Nakamoto B. Hemopoietic lineage commitment decisions: in vivo evidence from a transgenic mouse model harboring μLCR-βpro-LacZ as a transgene. Blood. 2000;95:1274–1282. [PubMed] [Google Scholar]
- 56.Vassilopoulos G, Navas PA, Skarpidi E, Peterson KR, Lowrey CH, Stamatoyannopoulos G. Correct function of the locus control region may require passage through a nonerythroid cellular environment. Blood. 1999;93:703–712. [PubMed] [Google Scholar]
- 57.Grosveld F. Activation by locus control regions? Curr Opin Genet Dev. 1999;9:152–157. doi: 10.1016/S0959-437X(99)80023-9. [DOI] [PubMed] [Google Scholar]
- 58.Tuan D, Kong S, Hu K. Transcription of the hypersensitive site HS2 enhancer in erythroid cells. Proc Natl Acad Sci USA. 1992;89:11219–11223. doi: 10.1073/pnas.89.23.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blackwood EM, Kadonaga JT. Going the distance: a current view of enhancer action. Science. 1998;281:61–63. doi: 10.1126/science.281.5373.60. [DOI] [PubMed] [Google Scholar]
- 60.Buler M, Groudine M. Looping versus linking: toward a model for long-distance gene activation. Genes Dev. 1999;13:2465–2477. doi: 10.1101/gad.13.19.2465. [DOI] [PubMed] [Google Scholar]
- 61.Wijgerde M, Grosveld F, Fraser P. Transcription complex stability and chromatin dynamics in vivo. Nature. 1995;377:209–213. doi: 10.1038/377209a0. [DOI] [PubMed] [Google Scholar]
- 62.Tolhuis B, Palstra RJ, Splinter E, Grosveld F, de Laat W. Looping and interaction between hypersensitive sites in active beta-globin locus. Mol Cell. 2002;10:1453–1465. doi: 10.1016/s1097-2765(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 63.Osborne C, Chakalova L, Brown KE, et al. Active genes dynamically colocalize to shared sites of ongoing transcription. Nat Genet. 2004;36:1065–1071. doi: 10.1038/ng1423. Epub 2004 Sep 07. [DOI] [PubMed] [Google Scholar]
- 64.Behringer RR, Ryan TM, Palmiter RD, Brinster RL, Townes TM. Human gamma- to beta-globin gene switching in transgenic mice. Genes Dev. 1998;4:380–390. doi: 10.1101/gad.4.3.380. [DOI] [PubMed] [Google Scholar]
- 65.Enver T, Raich N, Ebens AJ, Costantini F, Papayannopoulou Th, Stamatoyannopoulos G. Developmental regulation of human fetal-to-adult globin gene switching in transgenic mice. Nature. 1990;344:309–313. doi: 10.1038/344309a0. [DOI] [PubMed] [Google Scholar]
- 66.Raich N, Enver T, Nakamoto B, Josephson B, Papayannopoulou Th, Stamatoyannopoulos G. Autonomous developmental control of human embryonic globin gene switching in transgenic mice. Science. 1990;50:1147–1149. doi: 10.1126/science.2251502. [DOI] [PubMed] [Google Scholar]
- 67.Raich N, Papayannopoulou Th, Stamatoyannopoulos G, Enver T. Demonstration of a human ε globin gene silencer with studies in transgenic mice. Blood. 1992;79:861–864. [PubMed] [Google Scholar]
- 68.Li Q, Blau CA, Clegg CH, Stamatoyannopoulos G. Multiple ε promoter elements participate in the developmental control of ε globin genes in transgenic mice. J Biol Chem. 1998;273:17361–17367. doi: 10.1074/jbc.273.28.17361. [DOI] [PubMed] [Google Scholar]
- 69.Raich N, Clegg CH, Grofti J, Romeo P-H, Stamatoyannopoulos G. GATA1 and YY1 are developmental repressors of the human ε-globin gene. EMBO J. 1995;14:801–809. doi: 10.1002/j.1460-2075.1995.tb07058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Filipe A, Li Q, Deveaux S, et al. Regulation of embryonic/fetal globin genes by nuclear hormone receptors: a novel perspective on hemoglobin switching. EMBO J. 1999;18:687–697. doi: 10.1093/emboj/18.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanimoto K, Liu Q, Grosveld F, Bungert J, Engel JD. Context-dependent EKLF responsiveness defines the developmental specificity of the human epsilon-globin in erythroid cells of YAC transgenic mice. Genes Dev. 2000;14:2778–2794. doi: 10.1101/gad.822500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanabe O, Katsuoka F, Campbell AD, et al. An embryonic/fetal beta-type globin gene repressor contains a nuclear receptor TR2/TR4 heterodimer. EMBO J. 2002;21:3434–3442. doi: 10.1093/emboj/cdf340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dillon N, Grosveld F. Human gamma-globin genes silenced independently of other genes in the beta-globin locus. Nature. 1991;350:252–254. doi: 10.1038/350252a0. [DOI] [PubMed] [Google Scholar]
- 74.Stamatoyannopoulos G, Josephson B, Zhang J-W, Li Qiliang Developmental regulation of human γ-globin genes in transgenic mice. Mol Cell Biol. 1993;13:7636–7644. doi: 10.1128/mcb.13.12.7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harju S, Navas PA, Stamatoyannopoulos G, Peterson KR. Role of γ-globin gene silencing and chromatin sub-domain in globin gene switching. Blood Cells Mol Dis. 2003;331:145–146. [Google Scholar]
- 76.Luo HY, Mang D, Patrinos GP, et al. A mutation in a GATA-1 binding site 5′ to the Gγ-globin gene (nt −567, T>G) may be associated with increased levels of fetal hemoglobin. Blood. 2004;104:1452. [Google Scholar]
- 77.Li Q, Fang X, Han H, Stamatoyannopoulous G. The minimal promoter plays a major role in silencing of the galago γ-globin gene in adult erythropoiesis. Proc Natl Acad Sci USA. 2004;101:8096–8101. doi: 10.1073/pnas.0402594101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miller IJ, Bieker JJ. A novel, erythroid cell-specific murine transcription factor that binds to the CACCC element and is related to the Kruppel family of nuclear proteins. Mol Cell Biol. 1993;13:2776–2786. doi: 10.1128/mcb.13.5.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Donze D, Townes TM, Bieker JJ. Role of erythroid Kruppel-like factor in human gamma-beta-globin gene switching. J Biol Chem. 1995;270:1955–1959. doi: 10.1074/jbc.270.4.1955. [DOI] [PubMed] [Google Scholar]
- 80.Zitnik G, Hines P, Stamatoyannopoulos G, Papayannopoulou Th. Murine erythroleukemia cell line GM979 contains factors that can activate silent chromosomal human γ-globin genes. Proc Natl Acad Sci USA. 1991;88:2530–2534. doi: 10.1073/pnas.88.6.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peterson KR, Zitnik G, Huxley C, et al. Use of yeast artificial chromosomes (YACs) for studying control of gene expression: correct regulation of the genes of a human β-globin locus YAC following transfer to mouse erythroleukemia lines. Proc Natl Acad Sci USA. 1993;90:11207–11211. doi: 10.1073/pnas.90.23.11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ikuta T, Papayannopoulou Th, Stamatoyannopoulos G, Kan YW. Globin gene switching. In vivo protein-DNA interactions of the human β-globin locus in erythroid cells expressing the fetal or the adult globingene program. J Biol Chem. 1996;271:14082–14091. doi: 10.1074/jbc.271.24.14082. [DOI] [PubMed] [Google Scholar]
- 83.Navas PA, Swank RA, Yu M, Peterson KR, Stamatoyannopoulos G. Mutation of a transcriptional motif of a distant regulatory element reduces the expression of embryonic and fetal globin genes. Hum Mol Genet. 2003;12:2941–2948. doi: 10.1093/hmg/ddg319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Asano H, Li XS, Stamatoyannopoulos G. FKLF-2: A novel krüppel-like transcriptional factor that activates globin and other erythroid lineage genes. Blood. 2000;95:3578–3584. [PubMed] [Google Scholar]
- 85.Asano H, Li XS, Stamatoyannopoulos G. FKLF: a novel Krüppel-like factor that activates human embryonic and fetal β-like globin genes. Mol Cell Biol. 1999;19:3571–3579. doi: 10.1128/mcb.19.5.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]