Abstract
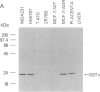
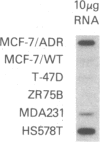
The development of multidrug resistance in MCF7 human breast cancer cells is associated with overexpression of P-glycoprotein, changes in activities of several detoxication enzymes, and loss of hormone sensitivity and estrogen receptors (ERs). We have cloned the cDNA for one of the drug-detoxifying enzymes overexpressed in multidrug-resistant MCF7 cells (AdrR MCF7), the anionic isozyme of glutathione S-transferase (GST pi). Hybridization with this GST pi cDNA, GST pi-1, demonstrated that increased GST pi activity in AdrR MCF7 cells is associated with overexpression but not with amplification of the gene. We mapped the GST pi gene to human chromosome 11q13 by in situ hybridization. Since multidrug resistance and GST pi overexpression are associated with the loss of ERs in AdrR MCF7 cells, we examined several other breast cancer cell lines that were not selected for drug resistance. In each of these cell lines we found an inverse association between GST pi expression and ER content. We also examined RNA from 21 primary breast cancers and found a similar association between GST pi expression and ER content in vivo. GST pi mRNA content in 11 ER-positive tumors (less than or equal to 10 fmol/mg of protein) was significantly different from the GST pi content of 10 ER-negative tumors (P = 0.002; Mann-Whitney Wilcoxon test for two independent samples). The finding of similar patterns of expression of a drug-detoxifying enzyme and of ERs in vitro as well as in vivo suggests that ER-negative breast cancer cells may have greater protection against antineoplastic agents conferred by GST pi than ER-positive tumors.
Full text
PDF

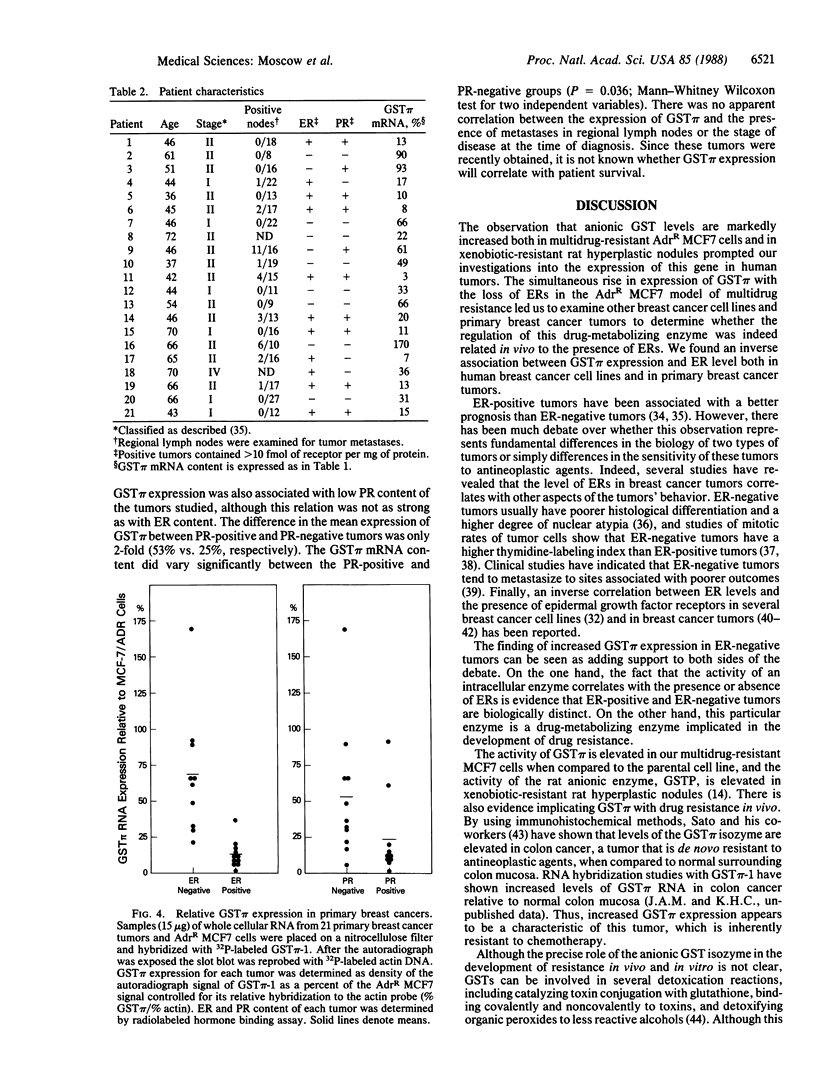

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Lidereau R., Theillet C., Callahan R. Reduction to homozygosity of genes on chromosome 11 in human breast neoplasia. Science. 1987 Oct 9;238(4824):185–188. doi: 10.1126/science.3659909. [DOI] [PubMed] [Google Scholar]
- Batist G., Tulpule A., Sinha B. K., Katki A. G., Myers C. E., Cowan K. H. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. J Biol Chem. 1986 Nov 25;261(33):15544–15549. [PubMed] [Google Scholar]
- Beck W. T., Mueller T. J., Tanzer L. R. Altered surface membrane glycoproteins in Vinca alkaloid-resistant human leukemic lymphoblasts. Cancer Res. 1979 Jun;39(6 Pt 1):2070–2076. [PubMed] [Google Scholar]
- Biedler J. L., Riehm H. Cellular resistance to actinomycin D in Chinese hamster cells in vitro: cross-resistance, radioautographic, and cytogenetic studies. Cancer Res. 1970 Apr;30(4):1174–1184. [PubMed] [Google Scholar]
- Buller A. L., Clapper M. L., Tew K. D. Glutathione S-transferases in nitrogen mustard-resistant and -sensitive cell lines. Mol Pharmacol. 1987 Jun;31(6):575–578. [PubMed] [Google Scholar]
- Chang J. C., Wergowske G. Correlation of estrogen receptors and response to chemotherapy of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) in advanced breast cancer. Cancer. 1981 Dec 1;48(11):2503–2506. doi: 10.1002/1097-0142(19811201)48:11<2503::aid-cncr2820481126>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Cowan K. H., Batist G., Tulpule A., Sinha B. K., Myers C. E. Similar biochemical changes associated with multidrug resistance in human breast cancer cells and carcinogen-induced resistance to xenobiotics in rats. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9328–9332. doi: 10.1073/pnas.83.24.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson N. E., Gelmann E. P., Lippman M. E., Dickson R. B. Epidermal growth factor receptor gene expression in estrogen receptor-positive and negative human breast cancer cell lines. Mol Endocrinol. 1987 Mar;1(3):216–223. doi: 10.1210/mend-1-3-216. [DOI] [PubMed] [Google Scholar]
- Evans C. G., Bodell W. J., Tokuda K., Doane-Setzer P., Smith M. T. Glutathione and related enzymes in rat brain tumor cell resistance to 1,3-bis(2-chloroethyl)-1-nitrosourea and nitrogen mustard. Cancer Res. 1987 May 15;47(10):2525–2530. [PubMed] [Google Scholar]
- Fairchild C. R., Ivy S. P., Kao-Shan C. S., Whang-Peng J., Rosen N., Israel M. A., Melera P. W., Cowan K. H., Goldsmith M. E. Isolation of amplified and overexpressed DNA sequences from adriamycin-resistant human breast cancer cells. Cancer Res. 1987 Oct 1;47(19):5141–5148. [PubMed] [Google Scholar]
- Fairchild C. R., Ivy S. P., Rushmore T., Lee G., Koo P., Goldsmith M. E., Myers C. E., Farber E., Cowan K. H. Carcinogen-induced mdr overexpression is associated with xenobiotic resistance in rat preneoplastic liver nodules and hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7701–7705. doi: 10.1073/pnas.84.21.7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber E. Cellular biochemistry of the stepwise development of cancer with chemicals: G. H. A. Clowes memorial lecture. Cancer Res. 1984 Dec;44(12 Pt 1):5463–5474. [PubMed] [Google Scholar]
- Farber E., Parker S., Gruenstein M. The resistance of putative premalignant liver cell populations, hyperplastic nodules, to the acute cytotoxic effects of some hepatocarcinogens. Cancer Res. 1976 Nov;36(11 Pt 1):3879–3887. [PubMed] [Google Scholar]
- Fojo A., Akiyama S., Gottesman M. M., Pastan I. Reduced drug accumulation in multiply drug-resistant human KB carcinoma cell lines. Cancer Res. 1985 Jul;45(7):3002–3007. [PubMed] [Google Scholar]
- Gros P., Ben Neriah Y. B., Croop J. M., Housman D. E. Isolation and expression of a complementary DNA that confers multidrug resistance. Nature. 1986 Oct 23;323(6090):728–731. doi: 10.1038/323728a0. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Jakoby W. B. The glutathione S-transferases: a group of multifunctional detoxification proteins. Adv Enzymol Relat Areas Mol Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976 Nov 11;455(1):152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Kartner N., Riordan J. R., Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983 Sep 23;221(4617):1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- Kessel D., Corbett T. Correlations between anthracycline resistance, drug accumulation and membrane glycoprotein patterns in solid tumors of mice. Cancer Lett. 1985 Sep 15;28(2):187–193. doi: 10.1016/0304-3835(85)90074-6. [DOI] [PubMed] [Google Scholar]
- Kodate C., Fukushi A., Narita T., Kudo H., Soma Y., Sato K. Human placental form of glutathione S-transferase (GST-pi) as a new immunohistochemical marker for human colonic carcinoma. Jpn J Cancer Res. 1986 Mar;77(3):226–229. [PubMed] [Google Scholar]
- Ling V., Thompson L. H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol. 1974 Feb;83(1):103–116. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- Manoharan T. H., Puchalski R. B., Burgess J. A., Pickett C. B., Fahl W. E. Promoter-glutathione S-transferase Ya cDNA hybrid genes. Expression and conferred resistance to an alkylating molecule in mammalian cells. J Biol Chem. 1987 Mar 15;262(8):3739–3745. [PubMed] [Google Scholar]
- Meyer J. S., Friedman E., McCrate M. M., Bauer W. C. Prediction of early course of breast carcinoma by thymidine labeling. Cancer. 1983 May 15;51(10):1879–1886. doi: 10.1002/1097-0142(19830515)51:10<1879::aid-cncr2820511021>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Meyer J. S., Lee J. Y. Relationships of S-phase fraction of breast carcinoma in relapse to duration of remission, estrogen receptor content, therapeutic responsiveness, and duration of survival. Cancer Res. 1980 Jun;40(6):1890–1896. [PubMed] [Google Scholar]
- Meyer J. S., McDivitt R. W., Stone K. R., Prey M. U., Bauer W. C. Practical breast carcinoma cell kinetics: review and update. Breast Cancer Res Treat. 1984;4(2):79–88. doi: 10.1007/BF01806389. [DOI] [PubMed] [Google Scholar]
- Meyers M. B., Biedler J. L. Increased synthesis of a low molecular weight protein in vincristine-resistant cells. Biochem Biophys Res Commun. 1981 Mar 16;99(1):228–235. doi: 10.1016/0006-291x(81)91736-8. [DOI] [PubMed] [Google Scholar]
- Peterson R. H., Meyers M. B., Spengler B. A., Biedler J. L. Alteration of plasma membrane glycopeptides and gangliosides of Chinese hamster cells accompanying development of resistance to daunorubicin and vincristine. Cancer Res. 1983 Jan;43(1):222–228. [PubMed] [Google Scholar]
- Pérez R., Pascual M., Macías A., Lage A. Epidermal growth factor receptors in human breast cancer. Breast Cancer Res Treat. 1984;4(3):189–193. doi: 10.1007/BF01806484. [DOI] [PubMed] [Google Scholar]
- Rushmore T. H., Sharma R. N., Roomi M. W., Harris L., Satoh K., Sato K., Murray R. K., Farber E. Identification of a characteristic cytosolic polypeptide of rat preneoplastic hepatocyte nodules as placental glutathione S-transferase. Biochem Biophys Res Commun. 1987 Feb 27;143(1):98–103. doi: 10.1016/0006-291x(87)90635-8. [DOI] [PubMed] [Google Scholar]
- Sainsbury J. R., Farndon J. R., Needham G. K., Malcolm A. J., Harris A. L. Epidermal-growth-factor receptor status as predictor of early recurrence of and death from breast cancer. Lancet. 1987 Jun 20;1(8547):1398–1402. doi: 10.1016/s0140-6736(87)90593-9. [DOI] [PubMed] [Google Scholar]
- Sainsbury J. R., Farndon J. R., Sherbet G. V., Harris A. L. Epidermal-growth-factor receptors and oestrogen receptors in human breast cancer. Lancet. 1985 Feb 16;1(8425):364–366. doi: 10.1016/s0140-6736(85)91385-6. [DOI] [PubMed] [Google Scholar]
- Satoh K., Kitahara A., Soma Y., Inaba Y., Hatayama I., Sato K. Purification, induction, and distribution of placental glutathione transferase: a new marker enzyme for preneoplastic cells in the rat chemical hepatocarcinogenesis. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3964–3968. doi: 10.1073/pnas.82.12.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried J. M., Tritton T. R., Sartorelli A. C. Comparison of anthracycline concentrations in S180 cell lines of varying sensitivity. Eur J Cancer Clin Oncol. 1983 Aug;19(8):1133–1141. doi: 10.1016/0277-5379(83)90039-1. [DOI] [PubMed] [Google Scholar]
- Sirotnak F. M., Yang C. H., Mines L. S., Oribé E., Biedler J. L. Markedly altered membrane transport and intracellular binding of vincristine in multidrug-resistant Chinese hamster cells selected for resistance to vinca alkaloids. J Cell Physiol. 1986 Feb;126(2):266–274. doi: 10.1002/jcp.1041260217. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stewart J. F., King R. J., Sexton S. A., Millis R. R., Rubens R. D., Hayward J. L. Oestrogen receptors, sites of metastatic disease and survival in recurrent breast cancer. Eur J Cancer. 1981 Apr;17(4):449–453. doi: 10.1016/0014-2964(81)90254-1. [DOI] [PubMed] [Google Scholar]
- Suguoka Y., Kano T., Okuda A., Sakai M., Kitagawa T., Muramatsu M. Cloning and the nucleotide sequence of rat glutathione S-transferase P cDNA. Nucleic Acids Res. 1985 Sep 11;13(17):6049–6057. doi: 10.1093/nar/13.17.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Board P. Glutathione-S-transferase gene mapped to chromosome 11 is GST3 not GST1. Somat Cell Mol Genet. 1984 May;10(3):319–320. doi: 10.1007/BF01535254. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson S. S., Huber B. E., Sorrell S., Fojo A., Pastan I., Gottesman M. M. Expression of the multidrug-resistant gene in hepatocarcinogenesis and regenerating rat liver. Science. 1987 May 29;236(4805):1120–1122. doi: 10.1126/science.3576227. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Cardarelli C., Gottesman M. M., Pastan I. Expression of a full-length cDNA for the human "MDR1" gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc Natl Acad Sci U S A. 1987 May;84(9):3004–3008. doi: 10.1073/pnas.84.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Bliek A. M., Van der Velde-Koerts T., Ling V., Borst P. Overexpression and amplification of five genes in a multidrug-resistant Chinese hamster ovary cell line. Mol Cell Biol. 1986 May;6(5):1671–1678. doi: 10.1128/mcb.6.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. L., Tew K. D. Increased glutathione-S-transferase activity in a cell line with acquired resistance to nitrogen mustards. Cancer Treat Rep. 1985 Jun;69(6):677–682. [PubMed] [Google Scholar]
- de Bruijn M. H., Van der Bliek A. M., Biedler J. L., Borst P. Differential amplification and disproportionate expression of five genes in three multidrug-resistant Chinese hamster lung cell lines. Mol Cell Biol. 1986 Dec;6(12):4717–4722. doi: 10.1128/mcb.6.12.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]