Abstract
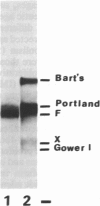
Tiazofurin (2-beta-D-ribofuranosyl-4-thiazole-carboxamide; NSC 286193), an antitumor carbon-linked nucleoside that inhibits IMP dehydrogenase (IMP:NAD+ oxidoreductase; EC 1.1.1.205) and depletes guanylate levels, can activate the erythroid differentiation program of K-562 human leukemia cells. Tiazofurin-mediated cell differentiation is a multistep process. The inducer initiates early (less than 6 hr) metabolic changes that precede commitment to differentiation; among these early changes are decreases in IMP dehydrogenase activity and in GTP concentration, as well as alterations in the expression of certain protooncogenes (c-Ki-ras). K-562 cells do express commitment-i.e., cells exhibit differentiation without tiazofurin. Guanosine was effective in preventing the action of tiazofurin, thus providing evidence that the guanine nucleotides are critically involved in tiazofurin-initiated differentiation. Activation of transcription of the erythroid-specific gene that encodes A gamma-globin is a late (48 hr) but striking effect of tiazofurin. Down-regulation of the c-ras gene appears to be part of the complex process associated with tiazofurin-induced erythroid differentiation and relates to the perturbations of GTP metabolism.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartha E., Oláh E., Szelényi J. G., Hollan S. R. Characterization of "fetal-type" acetylcholinesterase in hemin-treated K562 cell culture. Blood Cells. 1987;12(3):647–655. [PubMed] [Google Scholar]
- Bianchi Scarrà G. L., Romani M., Coviello D. A., Garrè C., Ravazzolo R., Vidali G., Ajmar F. Terminal erythroid differentiation in the K-562 cell line by 1-beta-D-arabinofuranosylcytosine: accompaniment by c-myc messenger RNA decrease. Cancer Res. 1986 Dec;46(12 Pt 1):6327–6332. [PubMed] [Google Scholar]
- Bloch A. Induced cell differentiation in cancer therapy. Cancer Treat Rep. 1984 Jan;68(1):199–205. [PubMed] [Google Scholar]
- Collins S. J., Groudine M. T. Rearrangement and amplification of c-abl sequences in the human chronic myelogenous leukemia cell line K-562. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4813–4817. doi: 10.1073/pnas.80.15.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney D. A., Jayaram H. N., Gebeyehu G., Betts C. R., Kelley J. A., Marquez V. E., Johns D. G. The conversion of 2-beta-D-ribofuranosylthiazole-4-carboxamide to an analogue of NAD with potent IMP dehydrogenase-inhibitory properties. Biochem Pharmacol. 1982 Jun 1;31(11):2133–2136. doi: 10.1016/0006-2952(82)90436-1. [DOI] [PubMed] [Google Scholar]
- Gambari R., del Senno L., Piva R., Barbieri R., Amelotti F., Bernardi F., Marchetti G., Citarella F., Tripodi M., Fantoni A. Human leukemia K562 cells: relationship between hemin-mediated erythroid induction, cell proliferation and expression of c-abl and c-myc oncogenes. Biochem Biophys Res Commun. 1984 Nov 30;125(1):90–96. doi: 10.1016/s0006-291x(84)80338-1. [DOI] [PubMed] [Google Scholar]
- Hughes S. M. Are guanine nucleotide binding proteins a distinct class of regulatory proteins? FEBS Lett. 1983 Nov 28;164(1):1–8. doi: 10.1016/0014-5793(83)80006-4. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Simon M. I., Teplow D. B., Robishaw J. D., Gilman A. G. Homologies between signal transducing G proteins and ras gene products. Science. 1984 Nov 16;226(4676):860–862. doi: 10.1126/science.6436980. [DOI] [PubMed] [Google Scholar]
- Ikegami T., Natsumeda Y., Weber G. Direct assay method for inosine 5'-monophosphate dehydrogenase activity. Anal Biochem. 1985 Oct;150(1):155–160. doi: 10.1016/0003-2697(85)90454-3. [DOI] [PubMed] [Google Scholar]
- Jackson R. C., Weber G., Morris H. P. IMP dehydrogenase, an enzyme linked with proliferation and malignancy. Nature. 1975 Jul 24;256(5515):331–333. doi: 10.1038/256331a0. [DOI] [PubMed] [Google Scholar]
- Jayaram H. N., Dion R. L., Glazer R. I., Johns D. G., Robins R. K., Srivastava P. C., Cooney D. A. Initial studies on the mechanism of action of a new oncolytic thiazole nucleoside, 2-beta-D-ribofuranosylthiazole-4-carboxamide (NSC 286193). Biochem Pharmacol. 1982 Jul 15;31(14):2371–2380. doi: 10.1016/0006-2952(82)90532-9. [DOI] [PubMed] [Google Scholar]
- Jayaram H. N., Smith A. L., Glazer R. I., Johns D. G., Cooney D. A. Studies on the mechanism of action of 2-beta-D-ribofuranosylthiazole-4-carboxamide (NSC 286193)--II. Relationship between dose level and biochemical effects in P388 leukemia in vivo. Biochem Pharmacol. 1982 Dec 1;31(23):3839–3845. doi: 10.1016/0006-2952(82)90300-8. [DOI] [PubMed] [Google Scholar]
- Kuttan R., Robins R. K., Saunders P. P. Inhibition of inosinate dehydrogenase by metabolites of 2-beta-D-ribofuranosyl thiazole-4-carboxamide. Biochem Biophys Res Commun. 1982 Aug;107(3):862–868. doi: 10.1016/0006-291x(82)90602-7. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lucas D. L., Robins R. K., Knight R. D., Wright D. G. Induced maturation of the human promyelocytic leukemia cell line, HL-60, by 2-beta-D-ribofuranosylselenazole-4-carboxamide. Biochem Biophys Res Commun. 1983 Sep 30;115(3):971–980. doi: 10.1016/s0006-291x(83)80030-8. [DOI] [PubMed] [Google Scholar]
- Lui M. S., Faderan M. A., Liepnieks J. J., Natsumeda Y., Olah E., Jayaram H. N., Weber G. Modulation of IMP dehydrogenase activity and guanylate metabolism by tiazofurin (2-beta-D-ribofuranosylthiazole-4-carboxamide). J Biol Chem. 1984 Apr 25;259(8):5078–5082. [PubMed] [Google Scholar]
- McCoy M. S., Bargmann C. I., Weinberg R. A. Human colon carcinoma Ki-ras2 oncogene and its corresponding proto-oncogene. Mol Cell Biol. 1984 Aug;4(8):1577–1582. doi: 10.1128/mcb.4.8.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Olah E., Lui M. S., Tzeng D. Y., Weber G. Phase and cell cycle specificity of pyrazofurin action. Cancer Res. 1980 Aug;40(8 Pt 1):2869–2875. [PubMed] [Google Scholar]
- Oláh E., Kremmer T., Boldizsár M. Potentiation of antimetabolite action by dibromodulcitol in cell culture. Adv Enzyme Regul. 1985;24:155–175. doi: 10.1016/0065-2571(85)90075-5. [DOI] [PubMed] [Google Scholar]
- Reiss M., Gamba-Vitalo C., Sartorelli A. C. Induction of tumor cell differentiation as a therapeutic approach: preclinical models for hematopoietic and solid neoplasms. Cancer Treat Rep. 1986 Jan;70(1):201–218. [PubMed] [Google Scholar]
- Rowley P. T., Ohlsson-Wilhelm B. M., Farley B. A., LaBella S. Inducers of erythroid differentiation in K562 human leukemia cells. Exp Hematol. 1981 Jan;9(1):32–37. [PubMed] [Google Scholar]
- Rutherford T. R., Weatherall D. J. Deficient heme synthesis as the cause of noninducibility of hemoglobin synthesis in a Friend erythroleukemia cell line. Cell. 1979 Feb;16(2):415–423. doi: 10.1016/0092-8674(79)90017-5. [DOI] [PubMed] [Google Scholar]
- Sachs L. Control of normal cell differentiation and the phenotypic reversion of malignancy in myeloid leukaemia. Nature. 1978 Aug 10;274(5671):535–539. doi: 10.1038/274535a0. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Papageorge A. G., Shih T. Y. Guanine nucleotide-binding activity as an assay for src protein of rat-derived murine sarcoma viruses. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5355–5359. doi: 10.1073/pnas.76.10.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloski J. A., Blair O. C., Sartorelli A. C. Alterations in glycoprotein synthesis and guanosine triphosphate levels associated with the differentiation of HL-60 leukemia cells produced by inhibitors of inosine 5'-phosphate dehydrogenase. Cancer Res. 1986 May;46(5):2314–2319. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricot G. J., Jayaram H. N., Nichols C. R., Pennington K., Lapis E., Weber G., Hoffman R. Hematological and biochemical action of tiazofurin (NSC 286193) in a case of refractory acute myeloid leukemia. Cancer Res. 1987 Sep 15;47(18):4988–4991. [PubMed] [Google Scholar]
- Varmus H. E. The molecular genetics of cellular oncogenes. Annu Rev Genet. 1984;18:553–612. doi: 10.1146/annurev.ge.18.120184.003005. [DOI] [PubMed] [Google Scholar]
- Weber G. Biochemical strategy of cancer cells and the design of chemotherapy: G. H. A. Clowes Memorial Lecture. Cancer Res. 1983 Aug;43(8):3466–3492. [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Willingham M. C., Pastan I., Shih T. Y., Scolnick E. M. Localization of the src gene product of the Harvey strain of MSV to plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1980 Apr;19(4):1005–1014. doi: 10.1016/0092-8674(80)90091-4. [DOI] [PubMed] [Google Scholar]