Abstract
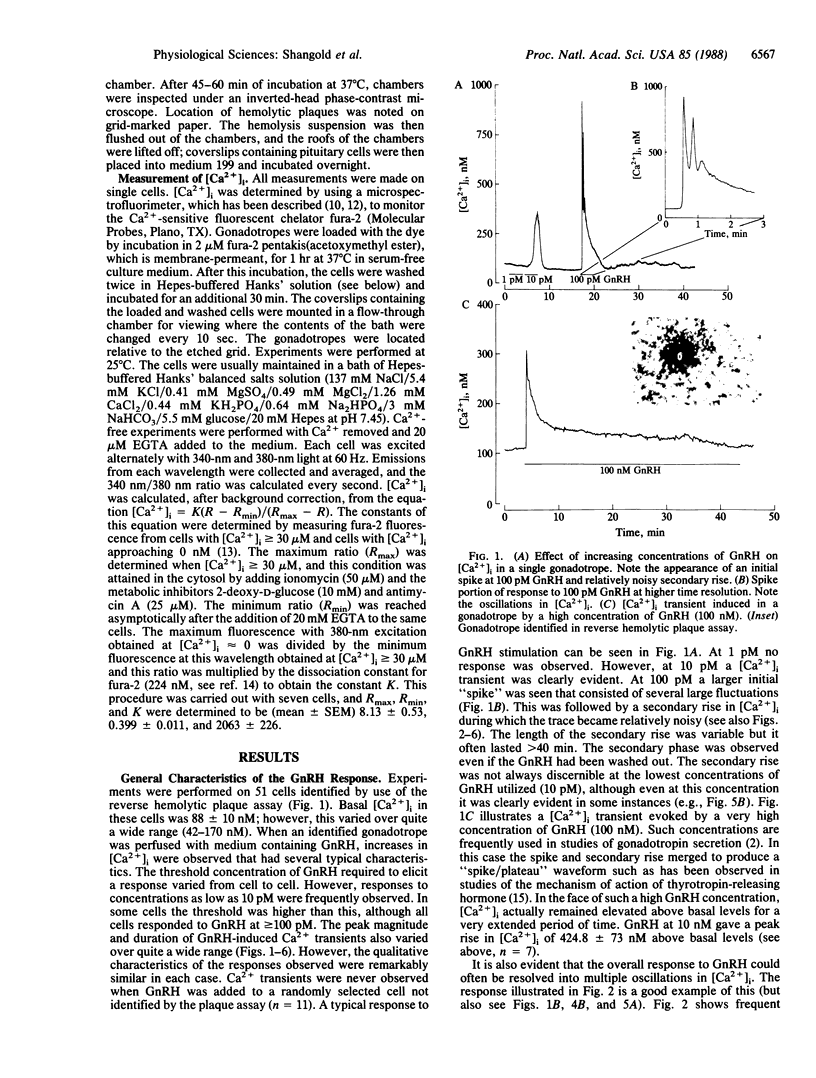
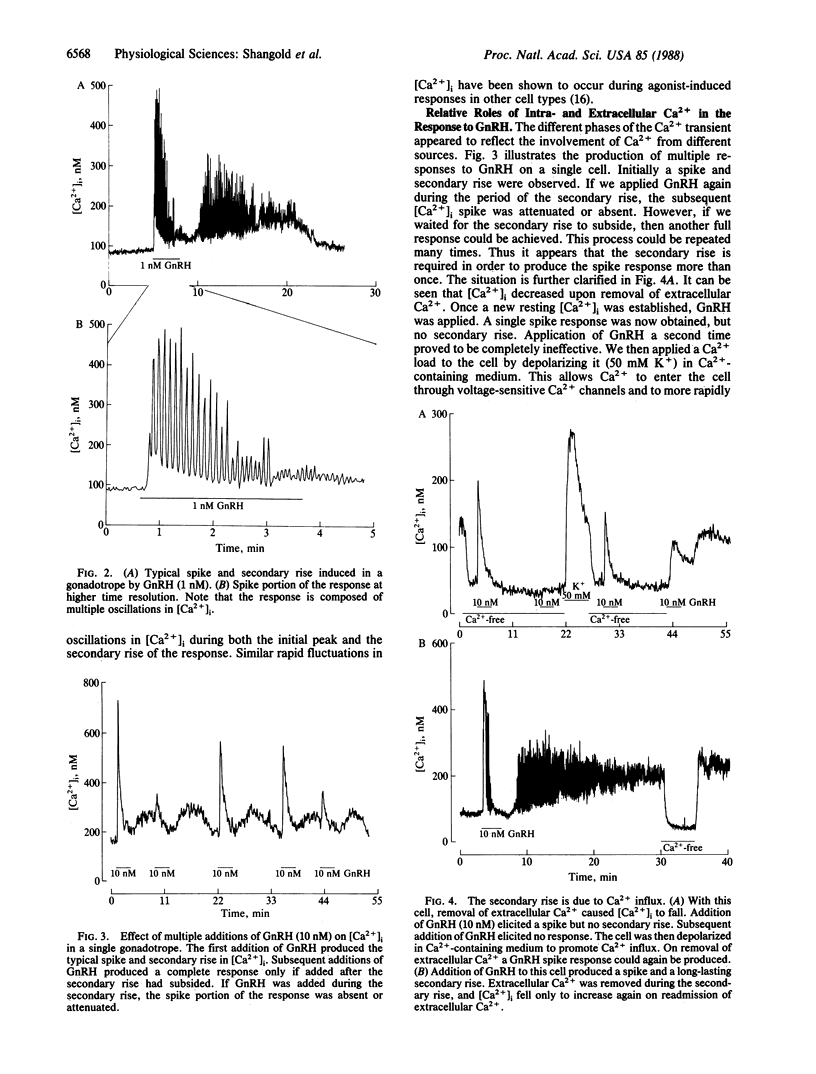
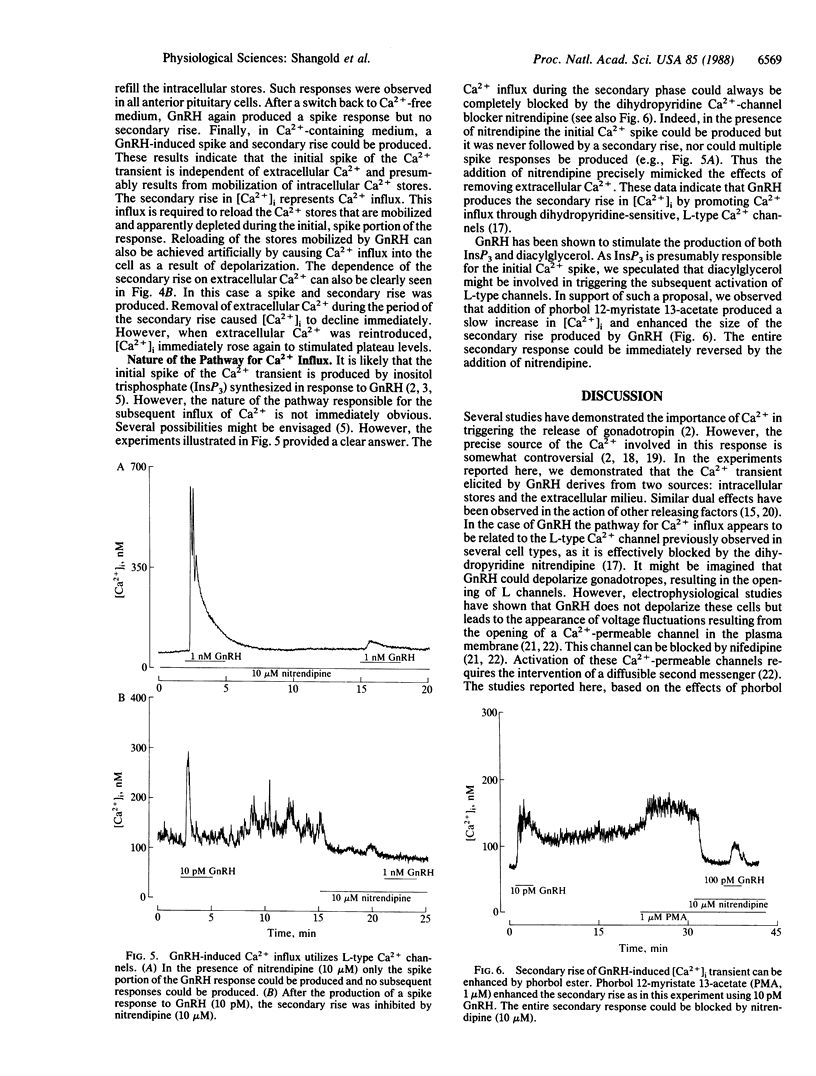
We examined the effects of gonadotropin-releasing hormone (GnRH) on the intracellular free Ca2+ concentration ([Ca2+]i) in single rat anterior pituitary gonadotropes identified by a reverse hemolytic plaque assay. Concentrations of GnRH greater than 10 pM elicited increases in [Ca2+]i in identified cells but not in others. In contrast, depolarization induced by 50 mM K+ increased [Ca2+]i in all cells. Ca2+ transients induced by GnRH exhibited a complex time course. After an initial rapid rise, the [Ca2+]i fell to near basal levels only to be followed by a secondary extended rise and fall. Analysis of the Ca2+ transients on a rapid time base revealed that responses frequently consisted of several rapid oscillations in [Ca2+]i. Removal of extracellular Ca2+ or addition of the dihydropyridine Ca2+-channel blocker nitrendipine completely blocked the secondary rise in [Ca2+]i but had no effect whatsoever on the initial spike. Nitrendipine also blocked 50 mM K+-induced increases in [Ca2+]i in identified gonadotropes. The secondary rise induced by GnRH could be enhanced by a phorbol ester in a nitrendipine-sensitive fashion. Multiple spike responses to GnRH stimulation of the same cell could only be obtained if subsequent Ca2+ influx was permitted either by allowing a secondary rise to occur or by producing a Ca2+ transient by depolarizing the cells with 50 mM K+. It therefore appears that the response to GnRH consists of an initial phase of Ca2+ mobilization, probably mediated by inositol trisphosphate, and a subsequent phase of Ca2+ influx through nitrendipine-sensitive Ca2+ channels that may be activated by protein kinase C. The relative roles of these phases in the control of gonadotropin secretion are discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert P. R., Tashjian A. H., Jr Thyrotropin-releasing hormone-induced spike and plateau in cytosolic free Ca2+ concentrations in pituitary cells. Relation to prolactin release. J Biol Chem. 1984 May 10;259(9):5827–5832. [PubMed] [Google Scholar]
- Andrews W. V., Conn P. M. Gonadotropin-releasing hormone stimulates mass changes in phosphoinositides and diacylglycerol accumulation in purified gonadotrope cell cultures. Endocrinology. 1986 Mar;118(3):1148–1158. doi: 10.1210/endo-118-3-1148. [DOI] [PubMed] [Google Scholar]
- Aquilano D. R., Dufau M. L. Functional and morphological studies on isolated Leydig cells: purification by centrifugal elutriation and metrizamide fractionation. Endocrinology. 1984 Feb;114(2):499–510. doi: 10.1210/endo-114-2-499. [DOI] [PubMed] [Google Scholar]
- Bates M. D., Conn P. M. Calcium mobilization in the pituitary gonadotrope: relative roles of intra- and extracellular sources. Endocrinology. 1984 Oct;115(4):1380–1385. doi: 10.1210/endo-115-4-1380. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. P., McCoy E. E., Graeter J., Tasaka K., Catt K. J. Participation of voltage-dependent calcium channels in the action of gonadotropin-releasing hormone. J Biol Chem. 1986 Jul 15;261(20):9105–9108. [PubMed] [Google Scholar]
- Clapper D. L., Conn P. M. Gonadotropin-releasing hormone stimulation of pituitary gonadotrope cells produces an increase in intracellular calcium. Biol Reprod. 1985 Mar;32(2):269–278. doi: 10.1095/biolreprod32.2.269. [DOI] [PubMed] [Google Scholar]
- Conn P. M., Huckle W. R., Andrews W. V., McArdle C. A. The molecular mechanism of action of gonadotropin releasing hormone (GnRH) in the pituitary. Recent Prog Horm Res. 1987;43:29–68. doi: 10.1016/b978-0-12-571143-2.50007-1. [DOI] [PubMed] [Google Scholar]
- Conn P. M., Rogers D. C., Seay S. G. Structure-function relationship of calcium ion channel antagonists at the pituitary gonadotrope. Endocrinology. 1983 Nov;113(5):1592–1595. doi: 10.1210/endo-113-5-1592. [DOI] [PubMed] [Google Scholar]
- Denef C., Hautekeete E., Dewals R., de Wolf A. Differential control of luteinizing hormone and follicle-stimulating hormone secretion by androgens in rat pituitary cells in culture: functional diversity of subpopulations separated by unit gravity sedimentation. Endocrinology. 1980 Mar;106(3):724–729. doi: 10.1210/endo-106-3-724. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Kiesel L., Catt K. J. Phosphatidic acid and the calcium-dependent actions of gonadotropin-releasing hormone in pituitary gonadotrophs. Arch Biochem Biophys. 1984 May 15;231(1):202–210. doi: 10.1016/0003-9861(84)90379-5. [DOI] [PubMed] [Google Scholar]
- Limor R., Ayalon D., Capponi A. M., Childs G. V., Naor Z. Cytosolic free calcium levels in cultured pituitary cells separated by centrifugal elutriation: effect of gonadotropin-releasing hormone. Endocrinology. 1987 Feb;120(2):497–503. doi: 10.1210/endo-120-2-497. [DOI] [PubMed] [Google Scholar]
- Lloyd J. M., Childs G. V. Differential storage and release of luteinizing hormone and follicle-releasing hormone from individual gonadotropes separated by centrifugal elutriation. Endocrinology. 1988 Apr;122(4):1282–1290. doi: 10.1210/endo-122-4-1282. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Waring D. W. Electrophysiological recordings from gonadotrophs. Evidence for Ca2+ channels mediated by gonadotrophin-releasing hormone. Neuroendocrinology. 1985 Sep;41(3):258–268. doi: 10.1159/000124186. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Waring D. W. Patch clamp recordings of single ion channel activation by gonadotrophin-releasing hormone in ovine pituitary gonadotrophs. Neuroendocrinology. 1986;43(2):205–219. doi: 10.1159/000124529. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Naor Z., Katikineni M., Loumaye E., Vela A. G., Dufau M. L., Catt K. J. Compartmentalization of luteinizing hormone pools: dynamics of gonadotropin releasing hormone action in superfused pituitary cells. Mol Cell Endocrinol. 1982 Jul;27(2):213–220. doi: 10.1016/0303-7207(82)90110-1. [DOI] [PubMed] [Google Scholar]
- Neher E. The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. J Physiol. 1988 Jan;395:193–214. doi: 10.1113/jphysiol.1988.sp016914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill J. D., Frawley L. S. Detection of hormone release from individual cells in mixed populations using a reverse hemolytic plaque assay. Endocrinology. 1983 Mar;112(3):1135–1137. doi: 10.1210/endo-112-3-1135. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Krafte D. S., Kass R. S. Voltage-dependent modulation of Ca channel current in heart cells by Bay K8644. J Gen Physiol. 1986 Sep;88(3):369–392. doi: 10.1085/jgp.88.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon M., Williams D. A., Fay F. S. A Ca2+-insensitive form of fura-2 associated with polymorphonuclear leukocytes. Assessment and accurate Ca2+ measurement. J Biol Chem. 1987 May 5;262(13):6308–6312. [PubMed] [Google Scholar]
- Smith C. E., Wakefield I., King J. A., Naor Z., Millar R. P., Davidson J. S. The initial phase of GnRH-stimulated LH release from pituitary cells is independent of calcium entry through voltage-gated channels. FEBS Lett. 1987 Dec 10;225(1-2):247–250. doi: 10.1016/0014-5793(87)81167-5. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- Veldhuis J. D., Evans W. S., Rogol A. D., Drake C. R., Thorner M. O., Merriam G. R., Johnson M. L. Intensified rates of venous sampling unmask the presence of spontaneous, high-frequency pulsations of luteinizing hormone in man. J Clin Endocrinol Metab. 1984 Jul;59(1):96–102. doi: 10.1210/jcem-59-1-96. [DOI] [PubMed] [Google Scholar]
- Waelbroeck M., Gillard M., Robberecht P., Christophe J. Kinetic studies of [3H]-N-methylscopolamine binding to muscarinic receptors in the rat central nervous system: evidence for the existence of three classes of binding sites. Mol Pharmacol. 1986 Oct;30(4):305–314. [PubMed] [Google Scholar]
- Winiger B. P., Wuarin F., Zahnd G. R., Wollheim C. B., Schlegel W. Single cell monitoring of cytosolic calcium reveals subtypes of rat lactotrophs with distinct responses to dopamine and thyrotropin-releasing hormone. Endocrinology. 1987 Dec;121(6):2222–2228. doi: 10.1210/endo-121-6-2222. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986 Feb 13;319(6054):600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Supattapone S., Wilson V. S., Snyder S. H. Characterization of inositol trisphosphate receptor binding in brain. Regulation by pH and calcium. J Biol Chem. 1987 Sep 5;262(25):12132–12136. [PubMed] [Google Scholar]



