Abstract
Background
Peroxisome proliferator-activated receptor γ (PPARγ), a nuclear transcription factor, modulates the expression/activity of G protein-coupled receptors (GPCRs), but its role in GPCR signaling is not clear. Increased GPCR kinase-2 (GRK-2) activity and receptor desensitization have been reported in hypertension.
Method
In this study we investigated the role of GRK-2 in PPARγ-mediated blood pressure regulation in hypertension. SHR or WKY rats were treated with GW1929, a selective PPARγ ligand (0.5 mg/kg/day), or vehicle for 2 months. Systolic blood pressure (tail cuff plethysmography), whole kidney perfusion (laser scanner) and renal vascular reactivity (isolated perfused kidney) was determined.
Results
GW1929 significantly reduced blood pressure (20 ± 1%) and increased renal perfusion (61 ± 3%) in SHR compared to WKY rats. Vasoconstriction to phenylephrine (100 μg) in the isolated perfused kidney was greater in SHRs (29 ± 1%) compared to WKY rats and this was abolished by GW1929. GW1929 enhanced acetylcholine-induced (30–300 μg) and sodium nitroprusside-induced vasodilatation in SHR by 46 ± 2% (p < 0.05) and 33 ± 2% (p < 0.05), respectively. Isoprenalin-induced (5–30 μg) vasodilatation was 43 ± 2% lower in SHR compared to WKY and GW1929 enhanced this vasodilatation by 55 ± 2%. In SHR kidney, GW1929 enhanced expression of PPARγ mRNA (34 ± 1%) but reduced that of GRK-2 (31 ± 3%).
Conclusion
We suggest that downregulation of PPARγ but upregulation of GRK-2 increases blood pressure and impaired renal vascular reactivity in SHR and that PPARγ-mediated improvement in hypertension may involve transcriptional regulation of GRK-2 function.
Key Words: Peroxisome proliferator-activated receptor γ, Hypertension, G protein-coupled receptor kinase-2, G protein-coupled receptor, Vascular reactivity, Renal perfusion
Introduction
G protein-coupled receptors (GPCRs) are regulated by multiple mechanisms that serve to adapt their signaling ability to a constantly changing environment. One of the most rapid mechanisms modulating agonist-activated receptors is phosphorylation by G protein-coupled receptor kinases (GRKs) resulting in uncoupling of the GPCR-mediated transduction process [1]. This phenomenon termed desensitization involves several processes including phosphorylation, sequestration/internalization and degradation of receptor protein [1]. Seven distinct GRKs (GRK1–7) have been identified to date [2]. GRKs 1 and 7 belong to the rhodopsin family, GRKs 2 and 3 belong to β-adrenergic receptor kinase (βARK) family and GRKs 4, 5 and 6 belong to the GRK4 family [1,2,3,4]. The phosphorylation of GPCRs, including β-adrenergic receptors, leads to the binding of a member or members of the arrestin family and uncoupling of the receptors from the G protein complex decreasing its functional response.
Because of the lack of drug acting directly on GRKs, the physiological function of GRK-2 (βARK) is still largely unknown. The role of GRK-2 in the pathology of hypertension has long been postulated and special emphasis was given to its function in cardiac development and its role in regulating cardiac β-adrenergic response. The fundamental role of GRK-2 in cardiac development was confirmed in 1996 [5]. Accordingly, transgenic mice overexpressing either βARK1 or a peptide inhibitor of βARK1 in the heart demonstrated that the activity of the kinase significantly contributes to the regulation of myocardial contractility [6]. Similarly, Eckhart et al. [7] showed that vascular overexpression of GRK-2 attenuated β-agonist-stimulated vasodilatation. This observation was corroborated by recent works suggesting that embryonic developmental abnormalities following cardiac-specific ablation of GRK-2 may be attributable to the extracardiac effect of GRK-2 [8]. Increased expression and activity of GRK-2 have been reported in both hypertensive subjects [9, 10] and in animal models of hypertension [11]. Penela et al. [12, 13] showed in an aortic smooth muscle cell line that physiological vasoconstrictors markedly enhanced GRK-2 promoter activity resulting in increased GRK-2 mRNA expression suggesting that the expression of GRK-2 is strongly controlled at the transcriptional level.
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors that belong to the nuclear receptor superfamily and consist of three isoforms named PPARα, PPARδ (or β), and PPARγ. PPARγ is expressed in adipose tissue, endothelial cells, and vascular smooth muscle cells [14]. The role of PPARγ in vascular function has been studied extensively in diabetics [15] as well as in nondiabetics [16] and convincing evidence emerged that PPARγ activation contributes to vascular function [17].
Involvement of PPARγ in modulating renal vascular function is less well characterized. Previously, we have shown that PPARγ ligand mediated improvement in renal vascular reactivity in a disease model like glycerol-induced acute renal failure [18, 19]. Similarly, Efrati et al. [20] reported PPARγ ligand-mediated improved renal vascular function and suggest that PPARγ effect in the kidney extends beyond glycemic and lipidemic control. This effect is probably via transcriptional regulation of vasoactive components such as catecholamine (β-adrenergic receptor), angiotensin II (AT1 receptor), and endothelin-1 (ETA receptor) that works as GPCRs [21]. PPARγ ligands may also potentiate β-adrenergic vasodilatation via activation of Raf/MEK/ERK1/2 signaling pathways [22] where activated ERK1/2 reduced GRK-2 activity [23, 24] and, therefore, plays an important role in GPCR signaling cascade. Although the connection between PPARγ and GRK-2 has never been examined the possibility of PPARγ influencing GRK-2 was never excluded either. At the same time, numerous studies demonstrated the possibility that PPARγ transcriptionally regulates the regulators of G protein signaling (RGS) molecules [25, 26] opening up the possibility of PPARγ-dependent regulation of GRK-2 (also an RGS family of protein). In this study, we have tested the hypothesis that PPARγ ligand-mediated improvement in blood pressure and renal vascular reactivity may be attributed to a transcriptional effect of PPARγ on GRK-2 expression. We, therefore, activated PPARγ in hypertensive rats to examine the association of improvement in blood pressure/renal vascular reactivity with that of GRK-2 expression.
Materials and Methods
Male (300–350 g, Harlan) WKY and SHR rats were divided into groups: control and treatment (n = 6–8 rats/group). Treatment group received 0.5 mg/kg of GW1929, an orally active PPARγ ligand, for 2 months while control rats received an equal amount of vehicle (saline). Rats were housed in controlled environment with a 12-hour light and dark cycle and free access to food and water. Systolic blood pressure (SBP) was measured before and 2 months after the treatment period using tail cuff plethysmography (Apollo 179; IITC, Woodland Hills, Calif., USA).
Whole Kidney Perfusion
At the end of the treatment, rats were anesthetized with pentobarbital (40 mg/kg, i.p.) and whole kidney perfusion was determined using a laser scanner (Moor Instrument, Axminster, UK). A mid-ventral incision approximately 5 cm long was then made and the abdomen was kept open using a clamp. Both right and left kidneys were identified and cleaned gently with a saline-soaked cotton bud to separate them from any surrounding tissues. A laser scanner was positioned directly over a set area covering both kidneys and programmed using the software program attached to the laser scanner camera. Three repetitive scans were performed and the mean value determined for each kidney. Renal perfusion was expressed as perfusion units per unit area of kidney.
Rat Isolated Perfused Kidney Preparation
Immediately after determination of whole kidney perfusion, isolated perfused kidney was used to measure renal vascular responses to vasoconstrictors and vasodilators. While still under anesthesia, the left renal artery was cannulated through the abdominal aorta and the kidney was perfused by means of a peristaltic pump (model 7515-10, Masterflex cartridge pump, Cole Parmer Instrument, Vernon Hills, Ill., USA) with warmed (37°C), oxygenated (95% O2 and 5% CO2) Krebs-Henseleit buffer composed of 118 mM NaCl, 25 mM NaHCO3, 1.9 CaCl2, 1.19 MgSO4, 4.75 KCl, 1.19 KH2PO4, and 11.1 mM dextrose (pH 7.2). The flow of perfusate was adjusted in each kidney to obtain a perfusion pressure of 80–120 mm Hg. Mean flow rate was maintained within 8.5–13 ml/min. The inferior vena cava was cut to allow the flow of perfusate and the kidney was removed from the surrounding fat and placed in an organ chamber prewarmed at 37°C. The perfusion pressure was monitored constantly by means of a transducer (Transbridge, model TBM-4, World Precision Instrument, Sarasota, Fla., USA) which was connected to a computer by a signal manifold (model DI-720 and DI-205, DataQ Instruments). Because flow was maintained at a constant rate, change in perfusion pressure was used as an index of renal vascular resistance, an increase in perfusion pressure indicating vasoconstriction while a reduction indicates vasodilatation. To determine vasodilator responses baseline perfusion pressure was elevated with phenylephrine (PE; 100 μM) in the buffer before adding the vasodilator agents. All responses were measured as the differences between the minimum pressure and the peak responses (for vasoconstriction) or the difference between the maximum pressure with PE (100 μg) and the lowest response (for vasodilatation). Renal vascular response to PE (100 μg), acetylcholine (Ach; 30–300 μg), sodium nitroprusside (SNP; 5–30 μg), and isoprenaline (5–30 μg) was determined.
GRK-2 and PPARγ Gene Expression by RT-PCR
Whole kidney homogenates were prepared by homogenizing kidney tissues in RIPA buffer containing protease inhibitor (SC 24948; Santa Cruz Biotechnology, Santa Cruz, Calif., USA). The homogenate was processed to isolate total RNA using the RNeasy Mini kit from Qiagen (Valencia, Calif., USA) following the manufacturer's instructions. Total RNA was quantified and 100 ng of the RNA was reverse transcribed to synthesize cDNA using ReactionReady first strand cDNA synthesis kit (Cat. No. C-03) from Super Array Bioscience (Frederick, Md., USA). The cDNA was then amplified using SYBR Green (Cat. No. PA-011) real-time PCR master mix from SA Bioscience in Bio-Rad iCycler real-time PCR equipment. Rat GRK-2 (Cat. No. PPR45759A) and PPAR-γ (PPR47599A) specific primer sets were used (SA Bioscience) together with a primer set for GAPDH (PPR06557A) as the housekeeping gene for standardization. Each PCR amplification was performed in triplicate wells following PCR conditions prescribed for the SYBR Green PCR master mix by the manufacturer: 95°C for 15 min followed by 40 cycles of 95°C for 30 s, 55°C for 30 s and 72°C for 30 s. A melting curve analysis was performed immediately after PCR cycles to assure that there were no primer dimers. NegativePCR controls including omission of reverse transcriptase or omissionof cDNA or primers were used to validate each batch of template before use. Individual data were adjusted with GAPDH value and expressed as ΔΔct.
Statistical Analysis
Response to agonists was expressed as peak change in perfusion pressure from their basal values. Data were presented as mean ± SEM and comparisons were made within each group and between groups using ANOVA and Student's t test for significant differences. ANOVA was also used to determine differences in blood pressure and whole kidney perfusion values that were compared between groups while gene expression values were expressed as fold change. In all cases p ≤ 0.05 was considered significant.
Results
Effects of PPARγ Induction on SBP and Whole Kidney Perfusion
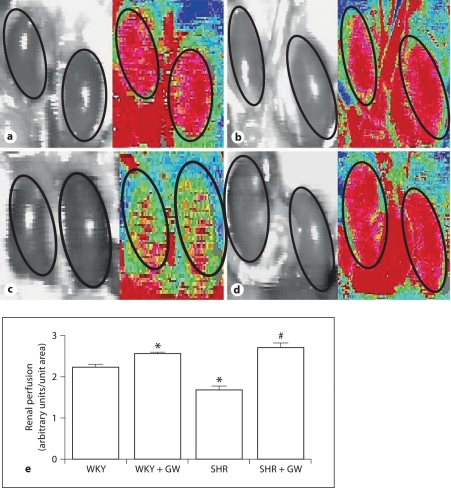
Figure 1 illustrates that baseline SBP in SHR rats was 116 ± 7% higher than that of the WKY rats (WKY: 81 ± 11 mm Hg; SHR: 176 ± 6 mm Hg). After a study period of 2 months, SBP in untreated SHR rose to 201 ± 6 mm Hg but was reduced by 20 ± 1% in GW1929-treated rats (p < 0.05). Similarly, whole kidney perfusion (fig. 2) was significantly lower in SHR rats compared to that of WKY rats (WKY: 2.22 ± 0.07 perfusion units/unit area; SHR: 1.7 ± 0.08 perfusion units/unit area). PPARγ induction by GW1929 enhanced whole kidney perfusion in both WKY and SHR rats by 15 ± 0.5% (p < 0.05) and 61 ± 3% (p < 0.01), respectively.
Fig. 1.
SBP in WKY and SHR treated with GW1929 (0.5 mg/kg; 2 months) or vehicle before and after treatment. Values are mean ± SEM (n = 6–8 rats/group). * p < 0.05 versus WKY; # p < 0.05 versus SHR.
Fig. 2.
Representative scan (a–d) and quantification of whole kidney perfusion (e) measured by laser scanner in WKY (a), WKY + GW1929 (b), SHR (c) and SHR + GW1929 (d). Rats were treated with GW1929 (0.5 mg/kg) or their vehicle for 2 months. Values are mean ± SEM (n = 6–8 rats/group). * p < 0.05 versus WKY; # p < 0.05 versus SHR.
Effect of GW1929 on Renal Vasoconstriction
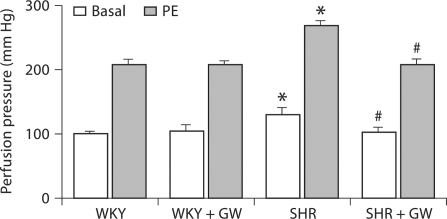
Basal perfusion pressure in the kidneys isolated from SHR rats was 29 ± 2% (p < 0.05) higher than that in WKY rats (WKY: 100 ± 4 mm Hg; SHR: 129 ± 11 mm Hg) (fig. 3), whereas in the GW1929-treated SHR group (102 ± 8 mm Hg) it was similar to that of WKY rats. The addition of PE (100 μg) in the perfusate increased perfusion pressure in the kidneys from all groups. This vasoconstriction was 29 ± 1% (p < 0.05) higher in kidneys from the SHR than WKY rats (WKY: 208 ± 7 mm Hg; SHR: 268 ± 7 mm Hg). On the contrary, this enhancement in vasoconstrictor response was blunted in GW1929-treated SHR by 29 ± 7% (p < 0.05) compared to that in vehicle-treated SHR.
Fig. 3.
Vascular response to PE (100 μg) determined in kidneys from WKY and SHR rats treated with GW1929 (0.5 mg/kg; 2 months) or vehicle. Values are mean ± SEM (n = 6–8 rats/group). * p < 0.05 versus WKY; # p < 0.05 versus SHR without heparin.
Effect of GW1929 on Endothelium-Dependent and Endothelium-Independent Renal Vasodilatation
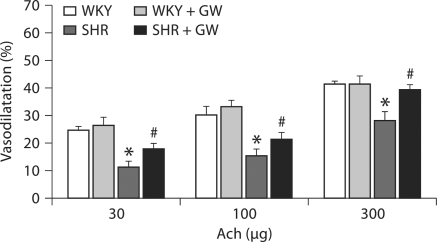
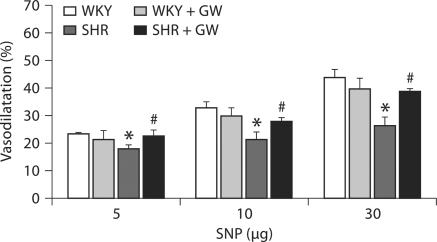
We tested both endothelium-dependent (Ach) and endothelium-independent (SNP) vasodilatation in the isolated kidney from WKY and SHR rats with or without PPARγ induction. Ach-induced vasodilatation was reduced by 46 ± 2% (p < 0.05) in SHR kidney compared to those from WKY rats (WKY: 24–55%; SHR: 12–18%) (fig. 4). GW1929 prevented this fall in Ach vasodilatation by maintaining vasodilatation to a level that is similar to that in WKY rats. Similarly, SNP vasodilatation was attenuated in kidney of SHR rats by 33 ± 2% (p < 0.05) compared to that of WKY rats (WKY: 23–44%; SHR: 18–26%) and GW1929 prevented this fall in SNP dilatation to an extent similar to that of WKY rats (fig. 5).
Fig. 4.
Vascular response to Ach (30–300 μg) determined in the PE (150 μg/l)-preconstricted isolated perfused kidneys from WKY and SHR rats treated with GW1929 (0.5 mg/kg; 2 months) or vehicle. Values are mean ± SEM (n = 6–8 rats/group). * p < 0.05 versus WKY; # p < 0.05 versus SHR.
Fig. 5.
Vascular response to SNP (3–30 μg) determined in PE (150 μg/l)-preconstricted isolated perfused kidney from WKY and SHR rats treated with GW1929 (0.5 mg/kg; 2 months) or vehicle. Values are mean ± SEM (n = 6–8 rats/group). * p < 0.05 versus WKY; # p < 0.05 versus SHR.
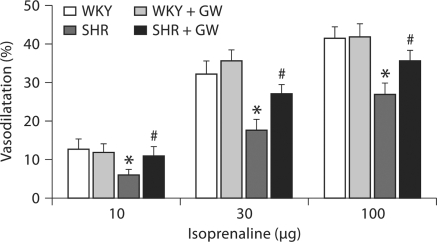
Effect of GW1929 on β-Adrenergic Stimulation-Mediated Renal Vasodilatation
We examined the vasodilator responses to isoprenaline as a representative of β-adrenergic stimulation where GRK-2 has been shown to have a profound regulatory effect. Isoprenaline-induced vasodilatation (fig. 6) was significantly lower (43 ± 2%; p < 0.02) in kidneys from SHR rats than in WKY rats (WKY: 13–42%; SHR: 6–27%). PPARγ ligand GW1929 enhanced vasodilatation in SHR by 55 ± 2% (p < 0.01) to a level similar to that of WKY rats.
Fig. 6.
Vascular response to isoprenaline (10–100 μg) determined in PE (150 μg/l)-preconstricted isolated perfused kidneys from WKY and SHR rats treated with GW1929 (0.5 mg/kg; 2 months) or vehicle. Values are mean ± SEM (n = 6–8 rats/group). * p < 0.05 versus WKY; # p < 0.05 versus SHR.
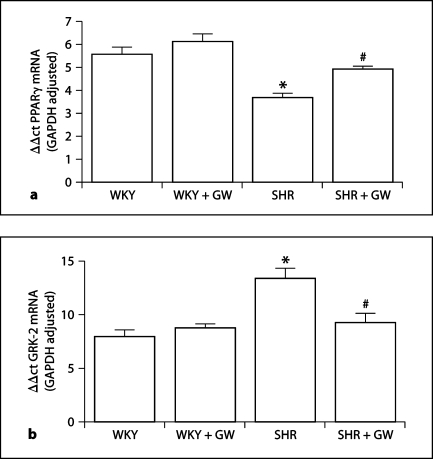
Effect of GW1929 on PPARγ and GRK-2 Gene Expression in the Kidney
RT-PCR results showed that PPARγ mRNA was 34 ± 2% (p < 0.05) lower (fig. 7a) and GRK-2 mRNA was 69 ± 6% (p < 0.05) higher (fig. 7b) in kidneys from SHR rats compared to kidneys from WKY rats. GW1929 significantly enhanced PPARγ mRNA expression (34 ± 1%) but attenuated GRK-2 mRNA expression (31 ± 3%) in SHR but not in WKY rats translating into a 2.4-fold increase in PPARγ gene expression in SHR after GW1929 treatment but a 17.8-fold reduction in GRK-2 expression in SHR.
Fig. 7.
Real-time PCR analysis of PPARγ (a) and GRK-2 (b) mRNA in total RNA isolated from WKY and SHR kidneys treated with GW1929 (0.5 mg/kg; 2 months) or vehicle. Values are mean ± SEM (n = 6–8 rats/group). * p < 0.05 versus WKY; # p < 0.05 versus SHR.
Discussion
We have shown in this study that GW1929, an orally active PPARγ ligand, prevented the increase in SBP in SHR. GW1929 treatment also enhanced whole kidney perfusion in hypertensive rats confirming the role of PPARγ in cardiovascular regulation. The most interesting observation from this study was the relationship observed between PPARγ and GRK-2, which suggests that PPARγ-mediated improvement in vascular function may involve the GRK-2 gene. This observation was accompanied by attenuation of vasoconstrictor response to PE but enhancement of both endothelium-dependent and endothelium-independent vasodilatation in hypertensive rats.
PPARγ is expressed in both endothelial cells and vascular smooth muscle cells making it possible that this receptor plays a role in regulating vascular tone and blood pressure[14,27,28,29,30]. Indeed, PPARγ agonists including thiozolidinediones lower blood pressure in experimental hypertension, an effect that is at least partially independent of their insulin-sensitizing effect [31,32,33,34]. Similarly, in transgenic hypertensive mice, rosiglitazone improved relaxation of carotid arteries to Ach suggesting that the blood pressure-lowering effect of thiozolidinediones may be due to their direct impact on blood vessels [35]. To date, the underlying mechanisms are still under investigation. PPARγ activation may regulate blood pressure via modulation of endothelial vasoactive factors such as endothelin-1, prostacyclin, and nitric oxide. PPARγ may also directly reduce vascular smooth muscle cell tone through downregulation of angiotensin II receptor 1 [36]. It is also hypothesized that at baseline PPARγ suppresses gene expression in vascular cells to contribute to the maintenance of normal blood pressure [37]. Therefore, a reduced PPARγ may lead to a hypertensive phenotype similar to what we observed in this study. Interestingly, a majority of the vasoactive components that seem to be influenced by PPARγ mediate their effects through GPCR and, therefore, GRK-dependent regulation may also contribute to vascular tone. Conversely, PPARγ-mediated vascular regulation may also be involved in regulating GRK activity.
The mechanism by which PPARγ ligands provide renoprotection is not totally clear at present. Identification of functional PPARγ receptors in renal glomerular and tubular tissues besides vascular tissues has raised the possibility that both systemic and vascular responses are involved in PPARγ-mediated renal protection [38, 39]. A considerable number of animal and human studies have shown that all PPARγ ligands are capable of producing small but significant changes in blood pressure level and their actions such as improvement in endothelial function, attenuation of sympathetic overactivity, or reduction of intracellular calcium content in vascular smooth muscle cells could be responsible for this effect [40]. Since renal perfusion is a direct reflection of afferent arteriolar pressure or SBP, a reduced SBP would automatically translate into an enhanced renal perfusion and this was a crucial observation in this study. Our data is in agreement with earlier animal [41, 42] and human studies [43,44,45] that showed a significant blood pressure reduction and renoprotection by PPARγ ligands.
In this study, we proposed another modality for PPARγ-mediated improvement in renal vascular function: PPARγ activation inhibits GRK-2. The role of GRK-2 in hypertension has been studied extensively because of the observation of increased GRK-2 protein and mRNA expression in hypertension and its role in attenuating β-adrenergic vasodilator response. Experimental studies also show that vascular overexpression of GRK-2 in transgenic mice attenuates β-adrenergic receptor signaling and increased resting blood pressure [7]. At the same time, Ramos-Ruiz et al. [13] showed that physiological vasoconstrictors increased GRK-2 promoter activity of the GRK-2 gene transfected in human aortic smooth muscle cell line suggesting a role for GRK-2 in vascular function. It has been shown earlier that PPARγ can modulate the expression and activity of major endogenous vasoconstrictors such as angiotensin II, endothelin-1, thromboxane as well as their GPCRs. Therefore, it is possible that PPARγ may also have a transcriptional influence on GRKs that are the principal regulators of GPCRs. There is no evidence so far that PPARγ may have a direct influence on GRK-2 gene expression, although indirectly it may. PPARγ ligands have been shown to activate Raf-1/MEK/ERK 1/2 pathway while activated ERK1/2 has been linked to enhanced GPCR-mediated response. Interestingly, several reports confirmed that ERK1/2 phosphorylates GRK-2 leading to a reduced GRK-2 activity and downregulation of GRK-2 protein expression [23, 24]. In this study, we have shown that PPARγ-mediated improvement in blood pressure and vascular reactivity may be a direct influence of GRK-2 inhibition. This notion is based on our observation that reduced PPARγ expression in hypertension is associated with increased GRK-2 gene expression, which suggests that PPARγ may have a negative regulation on GRK-2 gene. Therefore, PPARγ activation will reduce GRK-2 and this may be the mechanism for PPARγ ligand-mediated reduction in blood pressure and improvement in vascular reactivity in the hypertensive rats. This hypothesis is further strengthened by our observation that PPARγ activation is associated with a reduced GRK-2 gene expression. These observations led to the possibility that PPARγ transcriptionally regulates GRK-2 gene expression. This notion is underscored by the fact that all the GRKs belong to the RGS family of proteins. Therefore, the presence of a common RGS protein binding domain [46] in GRK-2 gene raised the possibility of regulation by PPARγ since PPARγ has been shown to regulate some other RGS (RGS-2) during adipocyte differentiation [26]. Our findings of reduced GRK-2 mRNA in GW1929-treated SHR comply with this notion and suggest that PPARγ has a transcriptional effect on GRK-2 gene.
In summary, these results suggest that downregulation of PPARγ coupled with upregulation of GRK-2 may be responsible for hypertension and PPARγ ligand-mediated reduction in blood pressure and improved vascular function in SHR may involve transcriptional regulation of GRK-2.
Acknowledgements
This study was supported by National Institutes of Health grants HL03674 and HL59884 and American Heart Association grant BGIA 017369.
References
- 1.Ferguson SS. Evolving concepts in G-protein coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 2.Penn RB, Pronin AN, Benovic JL. Regulation of G-protein coupled receptor kinases. Trends Cardiovasc Med. 2000;10:81–89. doi: 10.1016/s1050-1738(00)00053-0. [DOI] [PubMed] [Google Scholar]
- 3.Sallese M, Mariggio S, Collodel G, et al. G protein coupled receptor kinase GRK4: molecular analysis of the four isoforms and ultrastructural localization in spermatozoa and germinal cells. J Biol Chem. 1997;272:10188–10195. doi: 10.1074/jbc.272.15.10188. [DOI] [PubMed] [Google Scholar]
- 4.Premont RT, Macrae AD, Stoffel RH, et al. Characterization of the G protein coupled receptor kinase GRK4. Identification of four splice variants. J Biol Chem. 1997;271:6403–6410. doi: 10.1074/jbc.271.11.6403. [DOI] [PubMed] [Google Scholar]
- 5.Jabber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, Ross J, Lefkowitz RJ, Caron MG, Giros B. Essential role of β-adrenergic receptor kinases 1 in cardiac development and function. Proc Natl Acad Sci USA. 1996;93:12974–12979. doi: 10.1073/pnas.93.23.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koch WJ, Rockman HA, Samama P, Hamilton R, Bond R, Milano CA, Lefkowitz R. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 7.Eckhart AD, Ozaki T, Tevaearai H, Rockman HA, Koch WJ. Vascular-targeted overexpression of G protein-coupled receptor kinase-2 in transgenic mice attenuates β-adrenergic receptor signaling and increases resting blood pressure. Mol Pharmacol. 2002;61:749–758. doi: 10.1124/mol.61.4.749. [DOI] [PubMed] [Google Scholar]
- 8.Matkovich SJ, Diwan A, Klanke JL, Hammer DJ, Marreez Y, Odley AM, Brunskill EW, Koch WJ, Schwartz RJ, Dorn GW. Cardiac-specific ablation of G-protein receptor kinase 2 redefines its role in heart development and beta-adrenergic signaling. Circ Res. 2006;99:996–1003. doi: 10.1161/01.RES.0000247932.71270.2c. [DOI] [PubMed] [Google Scholar]
- 9.Gros R, Benovic JL, Tan CM, Feldman RD. G-protein coupled receptor kinase activity is increased in hypertension. J Clin Invest. 1997;99:2087–2093. doi: 10.1172/JCI119381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gros R, Tan CM, Chorazyezewski J, Kelvin DJ, Benovic JL, Feldman RD. G-protein coupled receptor kinase expression in hypertension. Clin Pharmacol Ther. 1999;65:545–551. doi: 10.1016/S0009-9236(99)70074-3. [DOI] [PubMed] [Google Scholar]
- 11.Gros R, Chorazyezewski J, Meek MD, Benovic JL, Ferguson SS, Feldman RD. G-protein coupled receptor kinase activity in hypertension: increased vascular and lymphocyte G-protein receptor kinase protein expression. Hypertension. 2000;35:38–42. doi: 10.1161/01.hyp.35.1.38. [DOI] [PubMed] [Google Scholar]
- 12.Penela P, Murga C, Ribas C, Tutor AS, Peregrin S, Mayor F. Mechanisms of regulation of G protein coupled receptor kinases (GRKs) and cardiovascular diseases. Cardiovasc Res. 2006;69:46–56. doi: 10.1016/j.cardiores.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Ramos-Ruiz R, Penela P, Penn RB, Mayor F., Jr Analysis of the human G protein coupled receptor kinase 2 (GRK-2) gene promoter: regulation by signal transduction systems in aortic smooth muscle cells. Circulation. 2000;101:2083–2089. doi: 10.1161/01.cir.101.17.2083. [DOI] [PubMed] [Google Scholar]
- 14.Marx N, Bourcier T, Sukhova GK, Libby P, Plutzky J. PPARgamma activation in human endothelial cells increases plasminogen activator inhibitor type-1 expression: PPARgamma as a potential mediator in vascular disease. Arterioscler Thromb Vasc Biol. 1999;19:546–551. doi: 10.1161/01.atv.19.3.546. [DOI] [PubMed] [Google Scholar]
- 15.Vinik A, Parson H, Ullal J. The role of PPARs in the microvascular dysfunction in diabetes. Vascul Pharmacol. 2006;45:54–64. doi: 10.1016/j.vph.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Panunti B, Fonseca V. Effects of PPAR gamma agonists on cardiovascular function in obese, non-diabetic patients. Vascul Pharmacol. 2006;45:29–35. doi: 10.1016/j.vph.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Blaschke F, Spanheimer R, Khan M, Law RE. Vascular effects of TZDs: new implications. Vascul Pharmacol. 2006;45:3–18. doi: 10.1016/j.vph.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Yousefipour Z, Hercule H, Truong L, Oyekan A, Newaz M. Ciglitazone, a peroxisome proliferator-activated receptor gamma inducer, ameliorates renal preglomerular production and activity of angiotensin II and thromboxane A2 in glycerol-induced acute renal failure. J Pharmacol Exp Ther. 2007;322:461–468. doi: 10.1124/jpet.107.122473. [DOI] [PubMed] [Google Scholar]
- 19.Newaz M, Yousefipour Z, Oyekan A. Role of PPAR-gamma on the pathogenesis and vascular changes in glycerol-induced acute renal failure. Pharmacol Res. 2006;54:234–240. doi: 10.1016/j.phrs.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 20.Efrati S, Berman S, Ilgiveav E, Averbukh Z, Weissgarten J. PPARγ activation inhibits angiotensin-II synthesis, apoptosis, and proliferation of mesangial cells from spontaneously hypertensive rats. Nephron Exp Nephrol. 2007;106:107–112. doi: 10.1159/000104834. [DOI] [PubMed] [Google Scholar]
- 21.Feldman RD, Gros R. Impaired vasodilator function in hypertension. The role of alterations in receptor-G protein coupling. Trends Cardiovasc Med. 1998;8:297–305. doi: 10.1016/s1050-1738(98)00022-x. [DOI] [PubMed] [Google Scholar]
- 22.Huang WC, Chio CC, Chi KH, Wu HM, Lin WW. Superoxide anion-dependent Raf/MEK/ERK activation by peroxisome proliferator activated receptor gamma agonist 15-deoxy-delta(12,14)-prostaglandin J(2), ciglitazone, and GW1929. Exp Cell Res. 2002;277:192–200. doi: 10.1006/excr.2002.5546. [DOI] [PubMed] [Google Scholar]
- 23.Florza A, Penela P, Sarmago S, Mayor F. MAPK dependent degradation of G protein coupled receptor kinase 2. J Biol Chem. 2003;278:29164–29173. doi: 10.1074/jbc.M304314200. [DOI] [PubMed] [Google Scholar]
- 24.Pitcher JA, Tesmer JJ, Freeman JL, et al. Feedback inhibition of G-protein coupled receptor kinase 2 activity by extracellular signal regulated kinases. J Biol Chem. 1999;274:34531–34534. doi: 10.1074/jbc.274.49.34531. [DOI] [PubMed] [Google Scholar]
- 25.Harris IS, Treskov I, Rowley MW, Heximer S, Kaltenbronn K, Finck BN, Gross RW, Kelly DP, Blumer KJ, Muslin AJ. G-protein signaling participates in the development of diabetic cardiomyopathy. Diabetes. 2004;53:3082–3090. doi: 10.2337/diabetes.53.12.3082. [DOI] [PubMed] [Google Scholar]
- 26.Nishizuka M, Honda K, Tsuchiya T, Nishihara T, Imagawa M. RGS2 promotes adipocyte differentiation in the presence of ligand for peroxisome proliferator activated receptor γ. J Biol Chem. 2001;276:29625–29627. doi: 10.1074/jbc.C100272200. [DOI] [PubMed] [Google Scholar]
- 27.Iijima K, Yoshizumi M, Ako J, Eto M, Kim S, Hashimoto M, Sugimoto N, Liang YO, Sudoh N, Toba K, Ouchi Y. Expression of peroxisome proliferaor activated receptor gamma in rat aortic smooth muscle cells. Biochem Biophys Res Commun. 1998;247:353–356. doi: 10.1006/bbrc.1998.8794. [DOI] [PubMed] [Google Scholar]
- 28.Law RE, Goetze S, Xi XP, Jackson S, Kawano Y, Demer L, Fishbein MC, Meehan WP, Hsueh WA. Expression and function of PPARgamma in rat and human vascular smooth muscle cells. Circulation. 2000;101:1311–1318. doi: 10.1161/01.cir.101.11.1311. [DOI] [PubMed] [Google Scholar]
- 29.Marx N, Schonbeck U, Lazar MA, Libby P, Plutzky J. Peroxisome proliferator-activated receptor gamma activators inhibit gene expression and migration in human vascular smooth muscle cells. Circ Res. 1998;83:1097–1103. doi: 10.1161/01.res.83.11.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satoh H, Tsukamoto K, Hashimoto Y, Hashimoto N, Togo M, Hara M, Maekawa H, Isoo N, Kimura S, Watanabe T. Thiazolidinediones suppress endothelin-1 secretion from bovine vascular endothelial cells: a new possible role of PPARgamma on vascular endothelial function. Biochem Biophys Res Commun. 1999;254:757–763. doi: 10.1006/bbrc.1998.0126. [DOI] [PubMed] [Google Scholar]
- 31.Willson TM, Lambert MH, Kliewer SA. Peroxisome proliferator activated receptor gamma and metabolic disease. Annu Rev Biochem. 2001;70:341–367. doi: 10.1146/annurev.biochem.70.1.341. [DOI] [PubMed] [Google Scholar]
- 32.Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic diseases. Trends Pharmacol Sci. 2005;26:244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:995–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 34.Chetty VT, Sharma AM. Can PPARgamma agonists have a role in the management of obesity related hypertension? Vascul Pharmacol. 2006;45:46–53. doi: 10.1016/j.vph.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. PPARγ agonists rosiglitazone improves vascular function and lowers blood pressure in hypertensive transgenic mice. Hypertension. 2004;43:661–666. doi: 10.1161/01.HYP.0000116303.71408.c2. [DOI] [PubMed] [Google Scholar]
- 36.Takeda K, Ichiki T, Tokunou T, Funakoshi Y, Iino N, Hirano K, Kanaide H, Takeshita A. Peroxisome proliferator-activated receptor gamma activators downregulate angiotensin II type 1 receptor in vascular smooth muscle cells. Circulation. 2000;102:1834–1839. doi: 10.1161/01.cir.102.15.1834. [DOI] [PubMed] [Google Scholar]
- 37.Duan SZ, Usher MG, Mortensen RM. Peroxisome proliferator-activated receptor γ-mediated effects in the vasculature. Circ Res. 2008;102:283–294. doi: 10.1161/CIRCRESAHA.107.164384. [DOI] [PubMed] [Google Scholar]
- 38.Guan Y, Zhang Y, Davis L, Breyer MD. Expression of peroxisome proliferator-activated receptors in urinary tract of rabbits and humans. Am J Physiol. 1997;273:F1013–F1022. doi: 10.1152/ajprenal.1997.273.6.F1013. [DOI] [PubMed] [Google Scholar]
- 39.Yang T, Michele DE, Park J, et al. Expression of peroxisomal proliferator-activated receptors and retinoid X receptors in the kidney. Am J Physiol. 1999;277:F966–F973. doi: 10.1152/ajprenal.1999.277.6.F966. [DOI] [PubMed] [Google Scholar]
- 40.Sarafidis PA, Lasaridis AN. Actions of PPAR agonists explaining a possible blood pressure lowering effect. Am J Hypertens. 2006;19:646–653. doi: 10.1016/j.amjhyper.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 41.Buckingham RE, Al-Barazanji KA, Toseland CD, et al. Peroxisome proliferator-activated receptor-gamma agonist, rosiglitazone, protects against nephropathy and pancreatic islet abnormalities in Zucker fatty rats. Diabetes. 1998;47:1326–1334. doi: 10.2337/diab.47.8.1326. [DOI] [PubMed] [Google Scholar]
- 42.Fujiwara K, Hayashi K, Ozawa Y, et al. Renal protective effect of troglitazone in Wistar fatty rats. Metabolism. 2000;49:1361–1364. doi: 10.1053/meta.2000.9509. [DOI] [PubMed] [Google Scholar]
- 43.Sironi AM, Vichi S, Gastaldelli A, et al. Effects of troglitazone on insulin action and cardiovascular risk factors in patients with non-insulin-dependent diabetes. Clin Pharmacol Ther. 1997;62:194–202. doi: 10.1016/S0009-9236(97)90068-0. [DOI] [PubMed] [Google Scholar]
- 44.Bakris G, Viberti G, Weston WM, et al. Rosiglitazone reduces urinary albumin excretion in type 2 diabetes. J Hum Hypertens. 2003;17:7–12. doi: 10.1038/sj.jhh.1001444. [DOI] [PubMed] [Google Scholar]
- 45.Sarafidis PA, Lasaridis AN, Nilsson PM, et al. The effect of rosiglitazone on urine albumin excretion in patients with type 2 diabetes mellitus and hypertension. Am J Hypertens. 2005;18:227–234. doi: 10.1016/j.amjhyper.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 46.Carman CV, Parent JL, Day PW, Pronin AN, Sternweis PM, Wedegaertner PB, Gilman AG, Benovic JL, Kozasa T. Selective regulation of Gαq by an RGS domain in the G protein coupled receptor kinase GRK-2. J Biol Chem. 1999;274:34483–34492. doi: 10.1074/jbc.274.48.34483. [DOI] [PubMed] [Google Scholar]