Abstract
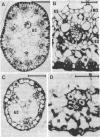
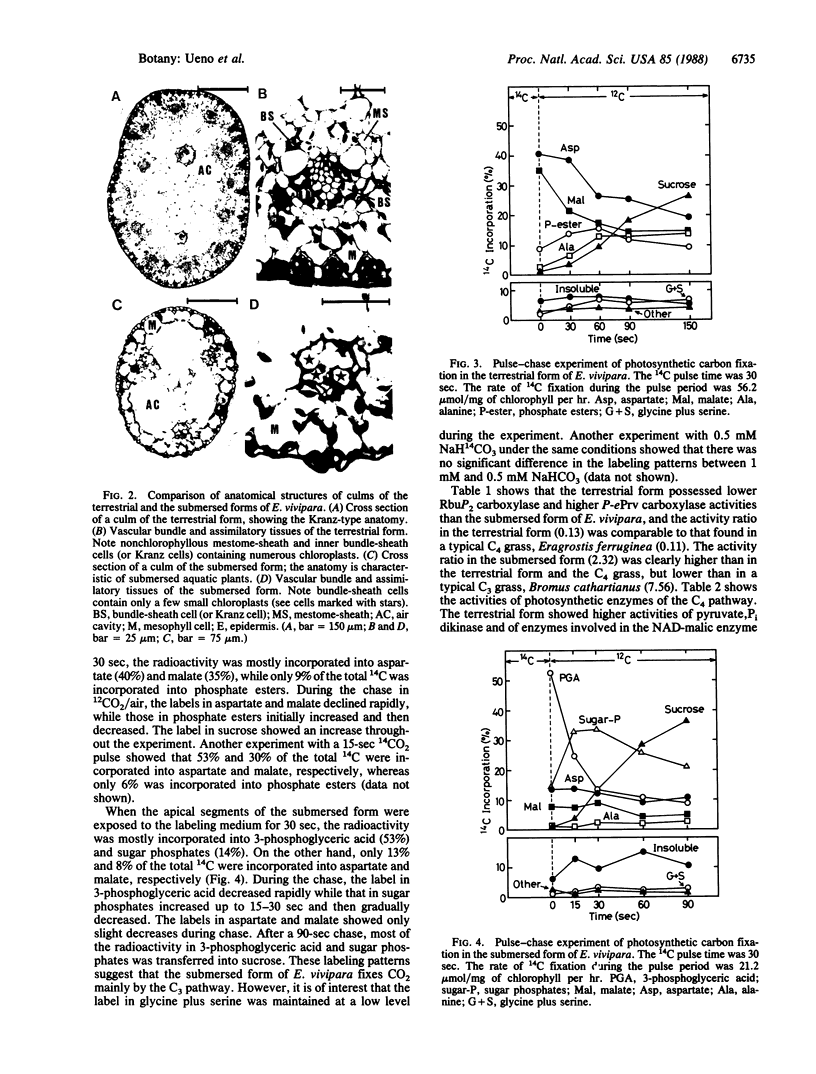
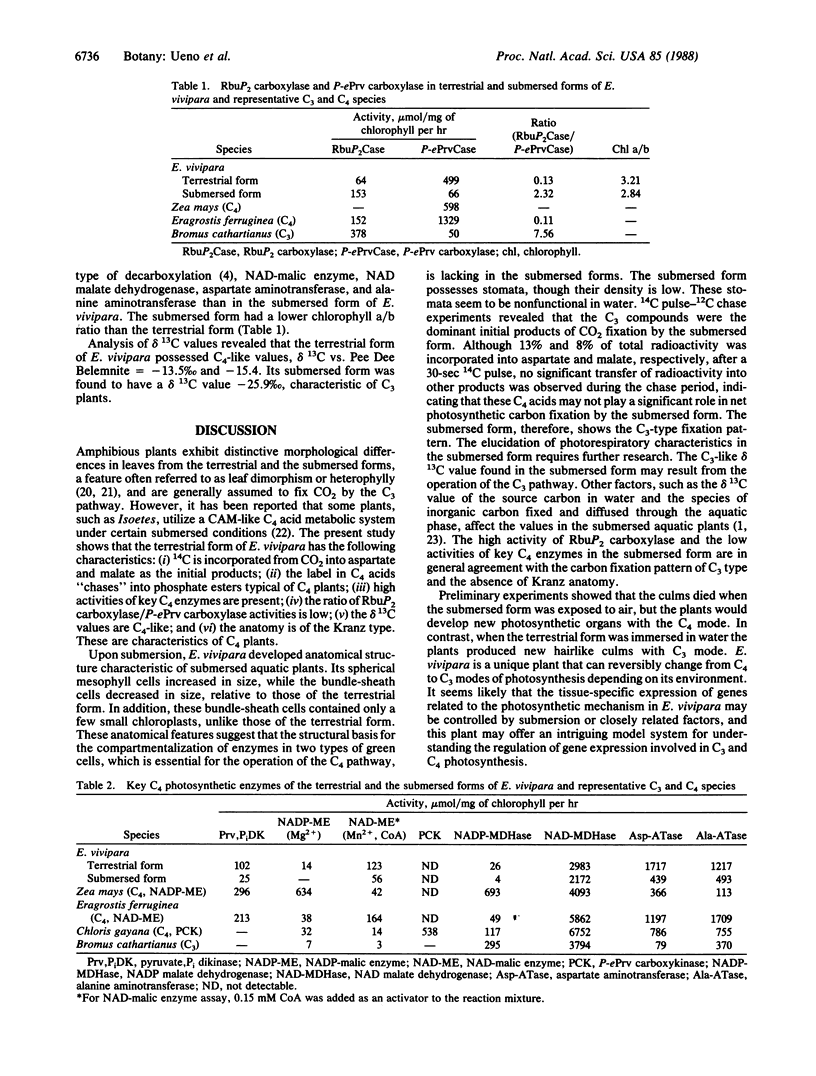
Eleocharis vivipara Link, a freshwater amphibious leafless plant belonging to the Cyperaceae can grow in both terrestrial and submersed aquatic conditions. Two forms of E. vivipara obtained from these contrasting environments were examined for the characteristics associated with C4 and C3 photosynthesis. In the terrestrial form (δ 13C values = -13.5 to -15.4‰, where ‰ is parts per thousand), the culms, which are photosynthetic organs, possess a Kranz-type anatomy typical of C4 plants, and well-developed bundle-sheath cells contain numerous large chloroplasts. In the submersed form (δ 13C value = -25.9‰), the culms possess anatomical features characteristic of submersed aquatic plants, and the reduced bundle-sheath cells contain only a few small chloroplasts. 14C pulse-12C chase experiments showed that the terrestrial form and the submersed form fix carbon by way of the C4 pathway, with aspartate (40%) and malate (35%) as the main primary products, and by way of the C3 pathway, with 3-phosphoglyceric acid (53%) and sugar phosphates (14%) as the main primary products, respectively. The terrestrial form showed photosynthetic enzyme activities typical of the NAD-malic enzyme-C4 subtype, whereas the submersed form showed decreased activities of key C4 enzymes and an increased ribulose 1,5-bisphosphate carboxylase (EC 4.1.1.39) activity. These data suggest that this species can differentiate into the C4 mode under terrestrial conditions and into the C3 mode under submersed conditions.
Keywords: C4 and C3 photosynthetic pathways, dimorphism, terrestrial and submersed aquatic environments
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Hatch M. D. Properties and mechanism of action of pyruvate, phosphate dikinase from leaves. Biochem J. 1969 Aug;114(1):117–125. doi: 10.1042/bj1140117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Mau S. L. Activity, location, and role of asparate aminotransferase and alanine aminotransferase isoenzymes in leaves with C4 pathway photosynthesis. Arch Biochem Biophys. 1973 May;156(1):195–206. doi: 10.1016/0003-9861(73)90357-3. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Tsuzuki M., Edwards G. E. Determination of NAD Malic Enzyme in Leaves of C(4) Plants : EFFECTS OF MALATE DEHYDROGENASE AND OTHER FACTORS. Plant Physiol. 1982 Feb;69(2):483–491. doi: 10.1104/pp.69.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. S., Hatch M. D. Properties and regulation of leaf nicotinamide-adenine dinucleotide phosphate-malate dehydrogenase and 'malic' enzyme in plants with the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1970 Sep;119(2):273–280. doi: 10.1042/bj1190273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley J. E., Busch G. Carbon Assimilation Characteristics of the Aquatic CAM Plant, Isoetes howellii. Plant Physiol. 1984 Oct;76(2):525–530. doi: 10.1104/pp.76.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. D-Ribulose-1,5-bisphosphate carboxylase-oxygenase. Improved methods for the activation and assay of catalytic activities. Anal Biochem. 1977 Mar;78(1):66–75. doi: 10.1016/0003-2697(77)90009-4. [DOI] [PubMed] [Google Scholar]
- Vu C. V., Allen L. H., Bowes G. Effects of Light and Elevated Atmospheric CO(2) on the Ribulose Bisphosphate Carboxylase Activity and Ribulose Bisphosphate Level of Soybean Leaves. Plant Physiol. 1983 Nov;73(3):729–734. doi: 10.1104/pp.73.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]