Abstract
With the increasing prevalence of HIV-associated neurocognititve disorders (HAND), understanding the mechanisms by which HIV-1 induces neuro-inflammation and subsequent neuronal damage is important. The hallmark features of HIV-encephalitis, the pathological correlate of HIV-associated Dementia (HAD), are gliosis, oxidative stress, chemokine dysregulation, and neuronal damage/death. Since neurons are not infected by HIV-1, the current thinking is that these cells are damaged indirectly by pro-inflammatory chemokines released by activated glial cells. CXCL10 is a neurotoxic chemokine that is up-regulated in astroglia activated by HIV-1 Tat, IFN-γ, and TNF-α. In this study we have demonstrated that HIV-1 Tat increases CXCL10 expression in IFN-γ and TNF-α stimulated human astrocytes via NADPH oxidase. We have shown that the treatment of astrocytes with a mixture of Tat and cytokines leads to a respiratory burst that is abrogated by apocynin, an NADPH oxidase inhibitor. Pre-treatment of Tat, IFN-γ, and TNF-α stimulated astrocytes with apocynin also resulted in concomitant inhibition of CXCL10 expression. Additionally, apocynin was also able to reduce Tat and cytokine-mediated activation of the corresponding signaling molecules Erk1/2, Jnk, and Akt with a decrease in activation and nuclear translocation of NF-κB, important regulators of CXCL10 induction. Understanding the mechanisms involved in reducing both oxidative stress and the release of pro-inflammatory agents could lead to the development of therapeutics aimed at decreasing neuro-inflammation in patients suffering from HAD.
Keywords: NADPH Oxidase, Astrocytes, HIV-associated Dementia, CXCL10
Introduction
Shortly after infection, HIV-1 is able to penetrate the brain, eventually resulting in HIV-1 associated complications in the CNS (Kaul and Lipton 2006). Studies in the literature show that 60% of HIV-1 infected individuals have some form of neuropsychiatric impairment diagnosed by behavioral, cognitive, and motor abnormalities categorically classified as HIV-associated neurocognitive disorders (Giunta et al. 2006; Ozdener 2005). HIV associated dementia (HAD), the most severe form of HAND (Albright et al. 2003), is clinically characterized by motor and behavioral dysfunctions leading to seizures, coma, and death within six months of onset (Navia et al. 1986). HIV-encephalitis (HIVE), the pathological correlate of HAD, is characterized by widespread astrogliosis, oxidative stress, cytokine/chemokine dysregulation, and neuronal degeneration (Gonzalez-Scarano and Martin-Garcia 2005; Minagar et al. 2002; Navia et al. 1986). Since the severity of HAD/HIVE correlates better with the presence of activated glial cells rather than with the viral load in the brain, the current thinking about the disease is that the neuronal damage is an indirect consequence of pro-inflammatory cytokines and chemokines released by activated glial cells (Gonzalez-Scarano and Martin-Garcia 2005; Minagar et al. 2002).
Astroglia, the most numerous cell type within the brain, provide an important reservoir for the generation of inflammatory mediators in response to HIV-1 (Dong and Benveniste 2001; Minagar et al. 2002; Thompson et al. 2001). Once activated by the virus/viral proteins astrocytes undergo astrogliosis characterized by the release of several different cytokines and chemokines, including the neurotoxic chemokine, CXCL10. Increased levels of CXCL10 have been detected in the CSF and plasma of individuals with HIV-1 infection (Kolb et al. 1999). Additionally, brain tissue from patients with HAD reveal increased astrocyte expression of CXCL10 mRNA (Sui et al. 2004; Sui et al. 2006; van Marle et al. 2004) (Kolson and Pomerantz 1996; McArthur et al. 1993; Sanders et al. 1998). Levels of this neurotoxic chemokine are positively correlated with HAD progression (Kolb et al. 1999). In SHIV-infected macaque brains with lentiviral lesions, CXCL10 has been shown to be significantly up-regulated and is apoptotic to neurons (Sui et al. 2004). Furthermore, treatment of fetal neuronal cultures with exogenous CXCL10 induces neuronal apoptosis through the caspase-3 cascade (Sui et al. 2006). In addition to its synergistic induction by the pro-inflammatory cytokines IFN-γ and TNF-α, CXCL10 can also be induced by the HIV-1 viral proteins gp120, Nef, and Tat (Asensio et al. 2001; Kutsch et al. 2000; van Marle et al. 2004).
Although HIV-1 does not productively infect astrocytes, Tat is expressed in astrocytes in brain tissue derived from HAD patients (Dou et al. 2006; Gorry et al. 2003). Studies have demonstrated that Tat can activate several signaling pathways that lead to the dysregulation of cytokine/chemokine release in astrocytes (Dou et al. 2006; Wang et al. 2004). Furthermore, Tat, in the presence of IFN-γ and TNF-α, is able to enhance the expression of CXCL10 compared the cytokines themselves.
Recently, the role of oxidative stress in the regulation of cytokine/chemokine expression has garnered increased awareness. One possible mechanism by which oxidative stress can mediate its effect on protein expression is through intracellular signaling pathways culminating in the activation of critical transcription factors (Adler et al. 1999; Park et al. 2004; Wang et al. 1998). Interestingly, in HIVE oxidative stress markers have been found to co-localize with glial cells and neurons (Pocernich et al. 2005b). Several studies point to the effect of HIV-1 Tat in mediating oxidative stress in astrocytes (Blokhina et al. 2003; Nath 2002; Pocernich et al. 2005a; Pocernich et al. 2005b; Saha and Pahan 2007; Viviani et al. 2001) possibly leading to cell death (Firth et al. 2007; Muscoli et al. 2002; Viviani et al. 2001). Furthermore, it has also been demonstrated that HIV-1 induced oxidative stress in astrocytes can regulate target genes that are under the control of NF-κB, one of the essential transcription factors responsible for CXCL10 induction (Song et al. 2007).
One mechanism by which oxidative stress is able to impact signaling pathways and their corresponding transcription factors is through a respiratory burst orchestrated by the activation of NADPH oxidase (Adler et al. 1999; Park et al. 2004; Sundaresan et al. 1995; Turchan-Cholewo et al. 2009; Wang et al. 1998). NADPH oxidase, a multi-subunit membrane associated enzyme, is capable of producing superoxide (Babior 1999; Chanock et al. 1994; El-Benna et al. 2005; Raad et al. 2008). This enzyme consists of two membrane associated subunits, gp91phox and p22phox, and the cytosolic components p67phox, p47phox, p40phox, and the small GTPase Rac1/2 (Babior 1999; Chanock et al. 1994; El-Benna et al. 2005; Groemping and Rittinger 2005; Quinn and Gauss 2004; Vignais 2002). Once the cytosolic subunits have docked with the membrane associated subunits, the interaction between p67phox and gp91phox results in the transfer of electrons from NADPH to molecular oxygen, resulting in the production of superoxide (Babior 1999; El-Benna et al. 2005; Raad et al. 2008; Vignais 2002). The superoxide is subsequently converted to hydrogen peroxide, a critical redox signaling intermediate (Chanock et al. 1994; Li et al. 2006; Raad et al. 2008).
Based on recent findings linking NADPH oxidase activity to cytokine/chemokine production in microglia, macrophages, and astrocytes (Park et al. 2004; Turchan-Cholewo et al. 2009) we hypothesized that NADPH oxidase could have a role in CXCL10 induction in human astrocytes stimulated with HIV-1 Tat and the cytokines. In this study we have demonstrated that the treatment of astrocytes with a mixture of Tat and the cytokines leads to a respiratory burst, an effect that is abrogated by apocynin, an NADPH oxidase inhibitor. Treatment with apocynin also decreased CXCL10 expression in Tat, IFN-γ, and TNF-α stimulated astrocytes. Western blot analysis of U-87 astrocytes treated with apocynin and the Tat/cytokine mixture demonstrated decreased activation of the signaling molecules Erk1/2, Jnk, and Akt, and decreased activation and nuclear translocation of NF-κB. Understanding the mechanisms involved in reducing both oxidative stress and the release of pro-inflammatory agents could aid in the development of therapies targeted at reduction of overall neuro-inflammation in patients affected by HAD.
Materials and Methods
Astrocyte cell culture and treatments
Human astrocytic cell line, U-87 (ATCC; American Type Culture Collection, Manassas, VA), was grown as described previously (Davis et al. 2002). The cells were treated for 24 hours with: 1) HIV-1 Tat at 200ng/ml (1–72) (supplied by Philip Ray, University of Kentucky), 2) a combination of the cytokines IFN-γ (50ng/ml) and TNF-α (5ng/ml) or, 3) HIV-1 Tat and the cytokines. In the instances where the apocynin (Sigma, St. Louis, MO) was utilized cells were pretreated for one hour with the inhibitor (50μM-1mM) before stimulation with Tat and cytokines.
Measurement of oxidative stress
U-87 astrocytes were untreated, treated with the Tat/IFN-γ/TNF-α mixture, or pre-treated with apocynin (250μM) for one hour followed by treatment with the Tat/IFN-γ/TNF-α mixture. Cells were then trypsinized, centrifuged and the resulting cell pellet was stained for 30min with 15μM of 5-(and −6)-carboxy-2',7'-dichlorodihydroflourescein diacetate (carboxy-H2-DCF-DA) (Molecular Probes, Inc, Eugene, OR), to assess cytoplasmic reactive oxygen species (ROS) (Ghosh et al. 2007). During the last five minutes of incubation Hoechst (1μM) was added to the cell suspension. After the 30min incubation period the cells were washed and resuspended in PBS containing 20mM glucose before being analyzed by a Tecan fluorescence plate reader. In the first setting the plate was read at an excitation of 485nm with an emission of 530nm for DCF, the second setting measured the Hoechst at an excitation of 355nm and emission of 460nm. The DCF fluorescent values were divided by their corresponding Hoechst fluorescent values for normalization. The data represents the mean ± SD from three independent experiments.
CXCL10 protein analysis by ELISA
Supernatants collected from treated astrocytes that were either untreated or treated with HIV-1 Tat and/or cytokines were examined for secreted CXCL10 protein levels using a commercially available CXCL10 ELISA kit (R&D Systems, Minneapolis, MN). The data represents the mean ± SD from three independent experiments.
Gp91phox knock down
U-87 astrocytes were incubated with the siRNA transfection reagent, Ribojuice (Novagen, Gibbstown, NJ), and 400nM of Accell Green Non-targeting siRNA, or a Human CYBB (gp91phox) siGENOME SMARTpool siRNA consisting of four different siRNA sequences (Dharmacon Inc, Chicago, IL). The sequences of the siRNA's are as follows 5'-GAAGACAACUGGACAGGAA-3', 5'-GGAACUGGGCUGUGAAUGA-3', 5'-GUGAAUGCCCGAGUCAAUA-3', and 5'-GAAACUACCUAAGAUAGCG-3'. Forty eight hours after transfection the cells were lysed using TriZol reagent for RNA extraction and assessed for gp91phox knock down using RT-PCR analysis. Primers for gp91phox were as follows: forward primer 5'-CAAGATGCGTGGAAACTACCTAAGAT-3' and reverse 5'-TCCCTGCTCCCACTAACATCA-3'. The RT-PCR set up consisted of 1 cycle of 50°C for 30 min, a 1 cycle 95°C, 15 min hot start, and 40 cycles of 94°C for 1min, 55°C for 1min, and 72 °C for 1min followed by one extension cycle of 72°C for 10min. The data represents the mean ± SD from three independent experiments.
Western Blot Analysis
Treated U-87 cells were lysed using the NE-PER Nuclear and Cytoplasmic Extraction kits (Pierce, Rockford, IL). Equal amounts of the corresponding proteins were electrophoresed in a sodium dodecyl sulfate-polyacrylamide gel (12%) in reducing conditions followed by transfer to PVDF membranes. The blots were blocked with 5% non fat dry milk in phosphate buffered saline. Western blots were then probed with antibodies recognizing the phosphorylated forms of Erk1/2, Jnk, Akt, p38, (Cell Signaling, Danvers, MA 1:200), Stat1-α (Cell Signaling, 1:500), NF-κB p65 (Cell Signaling, 1:1000), and β-actin (Sigma, St. Louis, MO,1:4000) The secondary antibodies were alkaline phosphatase conjugated to goat anti mouse/rabbit IgG (1:5000). Signals were detected by chemiluminescence (CDP-star; Tropix, Bedford, MA). All of Western blots were repeated at least three times.
MTT Assay
Astrocytes were untreated, treated with Tat/IFN-γ/TNF-α, or pre-treated for one hour with apocynin (250μM) then treated with Tat/IFN-γ/TNF-α in serum free neuronal media. After 48 hours the supernatants were placed on primary rat cortical neuronal cultures prepared as previously described (Yao et al. 2009). Half of the supernatants from the Tat/IFN-γ/TNF-α treated astrocytes were incubated for one hour at room temperature with a CXCL10 neutralizing antibody as described earlier (Dhillon et al. 2008). This was followed by an additional one hour incubation period with protein-G sepharose beads (Sigma, St. Louis, MO) to pull down the antibody/antigen complex by centrifugation. The resulting supernatant was added to the neurons. After 24 hours cell viability was measured by mitochondrial dehydrogenases [3(4,5-dimethylthiazol-2-yl)-2.5 diphenyltetrazolium bromide] (MTT) (Sigma, St. Louis, MO) assay as described earlier (Yao et al. 2005). The data represents the mean ± SD from three independent experiments.
Statistical Analysis
Data were expressed as mean ± SD. Significance of differences between control and samples treated with various drugs was determined by a one-tail, independent, Student's t-test. Values of p < 0.05 were taken as statistically significant.
Results
Tat/IFN-γ/TNF-α induce oxidative stress in human astrocytes
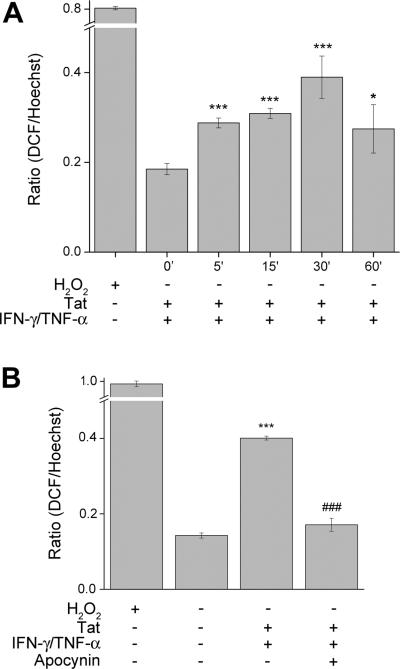
Since oxidative stress has been demonstrated to impact signaling pathways and cytokine/chemokine production, we sought to explore whether treatment of human astrocytes with Tat/IFN-γ/TNF-α could cause an oxidative burst. U-87 astrocytes were treated with Tat/IFN-γ/TNF-α over a period of 60min and assessed for the presence of reactive oxygen species by fluorometer. Our findings demonstrated that in stimulated cells there was a time dependent increase in the formation of ROS with a peak at 30min post treatment, indicative of an oxidative burst (Fig. 1A).
Figure 1.
Measurement of oxidative stress. (A) U-87 astrocytes were either untreated or treated with the Tat/IFN-γ/TNF-α mixture for 0–60min, prior to incubation with carboxy-H2-DCF-DA and assessed for oxidative stress. Values are displayed as a ratio of the DCF/Hoechst fluorescent value. A respiratory burst culminates after 30 min of stimulation. (B) U-87 astrocytes were untreated, treated with the Tat/IFN-γ/TNF-α mixture, or pre-treated with apocynin (250μM) followed by stimulation for 30min. Apocynin pre-treatment was able to abrogate the respiratory burst observed in stimulated astrocytes. The data represents the mean ± SD from three independent experiments. *p<0.05; ***p<0.001 vs control group;, ###p<0.001 vs Tat/IFN-γ/TNF-α group.
Recent reports have shown both Tat exposure and TNF-α receptor engagement can activate NADPH oxidase, a membrane protein capable of producing oxidative burst (Basuroy et al. 2009; Turchan-Cholewo et al. 2009). To examine whether NADPH oxidase was involved in the oxidative burst of Tat and cytokine stimulated of astrocytes, cells were pretreated for one hour with the apocynin (250μM) followed by stimulation and subsequent staining with H2DCF-DA. The data in Figure 1B demonstrated the ability of apocynin to abrogate the Tat and cytokine mix-mediated oxidative burst in U87 astrocytes. Taken together these data suggest that Tat/IFN-γ/TNF-α have the ability to activate NADPH oxidase, resulting in an oxidative burst.
Inhibition of NADPH oxidase resulted in decreased CXCL10 expression
Based on our findings that treatment of astrocytes with Tat/IFN-γ/TNF-α generated ROS through NADPH oxidase, it was of interest to examine first whether ROS played a role in the induction of CXCL10. We thus pre-treated U-87 astrocytes with various concentrations of apocynin (50μM-1mM) prior to stimulation with the Tat and cytokine mixture. Supernatants were collected after 24 hours and assessed for CXCL10 content by ELISA. As seen in Figure 2A, apocynin was able to dose-dependently decrease CXCL10 levels, with the optimal inhibition occurring at a concentration of 250μM apocynin. All further experiments were thus conducted with apocynin at 250μM.
Figure 2.
Apocynin decreases CXCL10 expression. (A) U-87 astrocytes were pre-treated with various doses of apocynin (50μM-1mM) for one hour prior to stimulation with the Tat/IFN-γ/TNF-α mixture. After 24hours of incubation supernatants were assessed for CXCL10 by ELISA. (B) U-87 astrocytes were pre-treated with 250μM apocynin followed by stimulation. mRNA level of CXCL10 was assessed by real time RT-PCR. Both U-87 (C) and primary human astrocytes (D) were treated either with the cytokines alone, the Tat/cytokine mixture, or pretreated with apocynin followed by stimulation with either the cytokines or the Tat/cytokine mixture. The data represents the mean ± SD from three independent experiments. **p<0.01; ***p<0.001 vs control group;, ###p<0.001 vs Tat/IFN-γ/TNF-α group.
The next step was to identify whether the apocynin-mediated reduction of CXCL10 in astrocytes was attributable to treatment with Tat, the cytokine mix, or both. Therefore both the U-87 and primary human astrocytes were pre-treated with apocynin prior to stimulation followed by assessment of cellular RNA and culture supernatant fluids 24hrs later for CXCL10 RNA by real-time RT-PCR and the protein by ELISA. As shown in Figure 2, in the presence of TNF-α and IFN-γ, Tat mediated up-regulation of both CXCL10 RNA (Fig 2B) as well as protein (Fig. 2C) in both U87 cells and in human primary astrocytes (Fig. 2D). Furthermore, apocynin treatment significantly decreased both the RNA and protein expression of CXCL10 induced by Tat/cytokine mixture. While apocynin was able to significantly decrease CXCL10 expression in both the Tat/cytokine and cytokine alone groups, it was unable to completely inhibit CXCL10 expression. Therefore, it is likely that while the ROS generated from NADPH oxidase activation is involved in the induction of CXCL10, there could be other pathways contributing to CXCL10 induction.
siRNA knockdown of gp91phox resulted in decreased CXCL10 expression
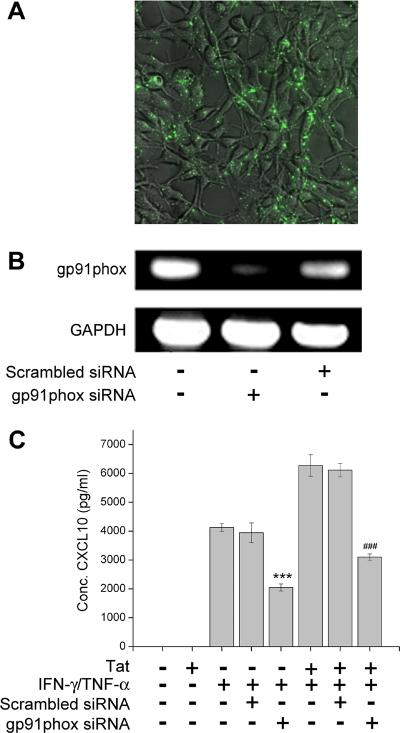
In order to confirm the role of NADPH oxidase in the induction of CXCL10, we used the approach of a siRNA targeted knock down of gp91phox, the critical subunit of NADPH oxidase. In the absence of gp91phox the activated cytosolic subunits of NADPH oxidase are unable to dock with the membrane components (gp91phox and gp22phox), resulting in the failure of enzyme activation. It has also been demonstrated that gp91phox is up-regulated in activated astrocytes (Abramov and Duchen 2005; Abramov et al. 2005), possibly through a positive feedback loop with the transcription factor NF-κB (Anrather et al. 2006).
We thus sought to examine whether blocking gp91phox expression in U-87 astrocytes by siRNA transfection could result in the inhibition of CXCL10 expression. U-87 astrocytes were transfected with either the siRNA pool against gp91phox or with the scrambled siRNA conjugated with GFP. Effective transfection was confirmed at 48 hrs in the scrambled-GFP transfected cells as shown in Fig 3A. Knockdown of gp91phox was subsequently confirmed by RT-PCR analysis (Fig. 3B). Having achieved the siRNA-mediated knockdown, we next stimulated U-87 astrocytes either with the Tat and cytokine mix or with the cytokine mix alone for 24 hours prior to collection of supernatants for assessment of CXCL10 content by ELISA. As shown in Figure 3C, there was a significant reduction of CXCL10 expression in cells transfected with gp91phox siRNA compared with cells transfected with scrambled siRNAs. These findings underscore the role of NADPH oxidase in Tat and cytokine-mediated induction of CXCL10.
Figure 3.
Knocking down NADPH oxidase subunit gp91phox decreased CXCL10 expression. (A) U-87 cells demonstrating transfection of the scrambled siRNA-GFP 24hours after transfection. (B) RT-PCR for gp91phox 48hours after transfection with gp91phox siRNA or scrambled siRNA. (C) CXCL10 ELISA demonstrating decreased CXCL10 in U-87 astrocytes transfected with gp91phox siRNA as compared to mock transfected or scrambled siRNA transfected cells. The data represents the mean ± SD from three independent experiments. ***p<0.001 vs control group;, ###p<0.001 vs Tat/IFN-γ/TNF-α group.
NADPH oxidase impacts the activation of the Erk1/2, Jnk, and Akt signaling pathways
NADPH oxidase activation can affect cell signaling by several different mechanisms. Its activation relies on the phosphorylation of Rac1 or Rac2, both of which, when activated can affect MAPK pathways (Ahn and Lee 2008; Babior 1999; Li et al. 2006). Another mechanism of action for NADPH oxidase is the production of superoxide, which is then dismutated to hydrogen peroxide, a critical redox signaling intermediate (Chanock et al. 1994; Li et al. 2006; Raad et al. 2008; Sundaresan et al. 1995). Increased levels of hydrogen peroxide can activate Ras, which in turn can activate MAPK pathways or the PI3-K-Akt pathway (Adachi et al. 2004; Ahn and Lee 2008; Kuan et al. 2006).
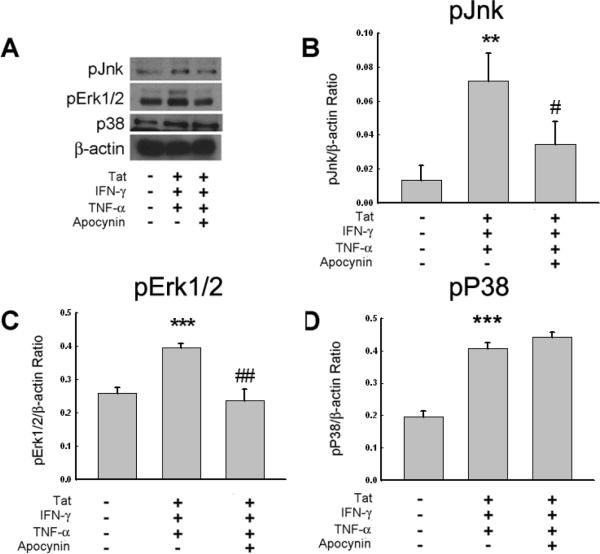
We next wanted to examine the downstream mediators of NADPH oxidase activation in stimulated astrocytes. Stimulated U-87 astrocytes in the presence or absence of apocynin (250μM) were lysed after 60 min (time based on our previous findings – unpublished results) and analyzed for MAPK activation by Western Blot analysis. As shown in Figure 4 Tat and cytokine mix-mediated activation of MAPK proteins, Jnk and Erk1/2, was inhibited in the presence of apocynin. Interestingly, the Tat and cytokine mix-mediated activation of p38 was not affected by apocynin treatment, thus leading to the speculation that p38 activation was independent of NADPH oxidase activation.
Figure 4.
Jnk and Erk1/2 signaling pathways, but not p38, are affected by apocynin treatment in stimulated U-87 astrocytes. (A) Western Blot analysis of cytosolic lysates collected from cells untreated, treated with Tat/IFN-γ/TNF-α, or pre-treated with apocynin prior to stimulation with Tat/IFN-γ/TNF-α for 60 min. The blots were probed with antibodies against phospho-p38, phospho-Jnk, and phospho-Akt. (B, C and D) Densitometric scans illustrating the ratio of phospho-Jnk, Erk1/2 and p38 normalized to β-actin levels. Data are representative of one of three replicate experiments. **p<0.01; ***p<0.001 vs control group; #p<0.05, ##p<0.01 vs Tat/IFN-γ/TNF-α group.
Since Akt signaling has been shown to be critical in the induction of CXCL10 in stimulated astrocytes (Williams et al. 2008), we next wanted to examine the role of NADPH oxidase in this signaling pathway. Stimulated U-87 astrocytes in the presence or absence of apocynin (250μM) were lysed and analyzed for Akt activation by Western Blot analysis. As shown in Figure 5 the Tat and cytokine mix-mediated activation of Akt was inhibited in the presence of apocynin. Taken together these findings underpin the role of NADPH oxidase-mediated activation of Jnk, Erk1/2, and Akt in CXCL10 induction in astrocytes.
Figure 5.
Akt signaling pathway is affected by apocynin treatment in stimulated U-87 astrocytes. (A) Western Blot analysis of cytosolic lysates collected from cells untreated, treated with Tat/IFN-γ/TNF-α, or pre-treated with apocynin prior to stimulation with Tat/IFN-γ/TNF-α for 60 min. The blots were probed with antibodies against phosphop38, phospho-Jnk, and phospho-Akt. (B) Densitometric scans illustrating the ratio of phospho-Akt to β-actin levels. Data are representative of one of three replicate experiments. ***p<0.001 vs control group; ###p<0.001 vs Tat/IFN-γ/TNF-α group.
Inhibition of NADPH oxidase decreased NF-κB activation and translocation
The Jnk, Erk1/2, and Akt pathways are capable of converging on a common transcription factor, NF-κB. NF-κB is one of several transcription factors sensitive to redox related signaling, and has been shown to affected by the generation of ROS (Park et al. 2004; Song et al. 2007). It has been previously reported that NADPH oxidase activation can be directly linked to NF-κB phosphorylation and nuclear translocation (Anrather et al. 2006; Kaul and Forman 1996; Li et al. 2006). Taking into account the decreased activation of the Jnk, Erk1/2, and Akt pathways in response to apocynin and the fact that NF-κB has been previously linking to NADPH oxidase, the effect of apocynin on NF-κB activation and translocation in Tat/IFN-γ/TNF-α stimulated U-87 cells was assessed by Western Blot analysis. As shown in Figure 6, treatment with apocynin was able to decrease the phosphorylation of IκB-α, which sequesters NF-κB in the cytosol until phosphorylation leads to its degradation, resulting in the activation and nuclear translocation of NF-κB. Since NF-κB is one of the critical transcription factors responsible for CXCL10 induction, its decreased activation could therefore negatively impact CXCL10 expression.
Figure 6.
NF-κB activation and nuclear translocation was affected by apocynin pre-treatment of stimulated astrocytes. (A) Western Blot analysis of the cytosolic (pIκBα) and nuclear (NF-κB p65) lysates collected from cells untreated, treated with Tat/IFN-γ/TNF-α, or pre-treated with apocynin prior to stimulation with Tat/IFN-γ/TNF-α for 60 min. The blots were probed with antibodies against phospho- IκBα and phospho-NF-κB p65. (B and C) Densitometric scans illustrating the ratio of phospho- IκBα and phospho-NF-κB p65. Data are representative of one of three replicate experiments. **p<0.01 vs control group; #p<0.05, #p<0.05vs Tat/IFN-γ/TNF-α group.
Treatment with apocynin decreased the overall toxicity to neurons
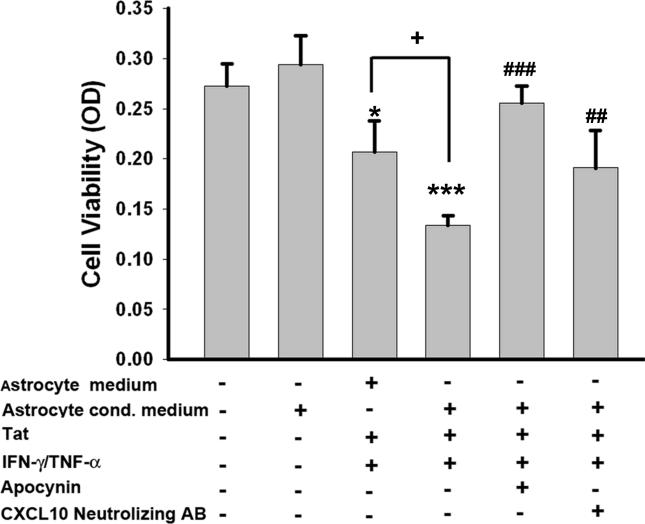
Since CXCL10 has been demonstrated to be neurotoxic, it was of interest to examine whether blocking CXCL10 expression in astrocytes via the NADPH oxidase inhibitor could result in decreased neuronal toxicity in conditioned-media experiments. In these studies, primary rat cortical neurons were cultured in the presence of conditioned media collected from stimulated astrocytes treated or untreated with apocynin. Additionally, conditioned media from stimulated astrocytes was also incubated with a CXCL10 neutralizing antibody prior to exposure to neurons. Neuronal survival was assessed after 24 hrs using the MTT survival assay. As shown in Figure 7 and as expected, astrocyte conditioned media from untreated astrocytes had no effect on neuronal survival, similar to the effect observed with neuronal media. However, the astrocyte conditioned media from stimulated astrocytes (Tat and cytokine treated) significantly decreased neuronal survival, and this effect was reversed in the presence of apocynin. We next wanted to confirm that increased neurotoxicity observed in astrocyte conditioned media from stimulated cells was not entirely due to the Tat and cytokine present in the supernatant fluids, but rather, was attributable to some other factor released from the stimulated cells. To answer this, astrocyte medium without the astrocytes was first treated with Tat and cyokines (same concentrations as used in the presence of astrocytes). The neurons were then exposed to either the astrocyte-conditioned media or the media with just the HIV protein/cytokine mix (no astrocytes) and monitored for neurotoxicity using the MTT assay. As expected, medium containing the factors without pre-exposure to the astrocytes was indeed toxic, however, there was enhanced toxicity of neurons in the presence of astrocyte-conditioned media.
Figure 7.
MTT cell survival assay utilizing primary rat cortical neurons treated with U-87 astrocytes conditioned media. Cells were untreated or treated as described earlier with and without apocynin. The conditioned media collected from treated cells was then added onto primary rat cortical neurons for 24 hours prior to the MTT assay. Portion of the Tat/IFN-γ/TNF-α treated conditioned media from the astrocytes was also incubated with a CXCL10 neutralizing antibody prior to addition to the neurons. The data represents the mean ± SD from three independent experiments. **p<0.01; ***p<0.001 vs astrocyte conditioned medium group; +p<0.05 vs astrocyte medium containing Tat/IFN-γ/TNF-α group; ##p< 0.01;###p< 0.001 vs Tat/IFN-γ/TNF-α group.
Since astrocytes are capable of releasing a plethora of cytokines and chemokines in response to the Tat/cytokine mixture, the CXCL10 neutralizing antibody was utilized to confirm the neurotoxic potential of CXCL10. Neurons treated with conditioned media exposed to the CXCL10 neutralizing antibody demonstrated significantly increased survival compared with the neurons treated with conditioned media from stimulated cells (Fig. 7). However, CXCL10 neutralizing antibody conditioned media did not exert complete reversal of cell toxicity, thus implying, while CXCL10 is partially responsible for the neurotoxicity, the release of other neurotoxic factors from stimulated astrocytes can contribute to neuronal damage. However, treatment with apocynin reversed the overall damage to the neurons, indicating its effectiveness in decreasing not only the concentration of CXCL10, but of other neurotoxic factors as well.
Discussion
Oxidative stress is a common denominator in several neurodegenerative diseases, including HAD, the most severe form of HAND. However, the exact cause and mechanism for its generation and the impact it has on disease pathogenesis is poorly understood. In this study we explored the mechanism by which HIV-1 Tat and the cytokines, IFN-γ and TNF-α, induce oxidative stress in astrocytes and the implications of NADPH oxidase in inducing the respiratory burst involved in generation of CXCL10. Furthermore, we have also demonstrated the ability of the NADPH oxidase inhibitor, apocynin, to diminish this response, ultimately sustaining neuronal health.
CXCL10 was of interest in these studies because levels of this neurotoxic chemokine are positively correlated with HAD progression (Kolb et al. 1999). Moreover, in macaque brains with SHIV-E, CXCL10 is significantly up-regulated and is apoptotic to neurons (Sui et al. 2004). Additionally, brain tissue derived from patients with HAD revealed increased astrocytic expression of CXCL10 mRNA (Sui et al. 2004; Sui et al. 2006; van Marle et al. 2004) (Kolson and Pomerantz 1996; McArthur et al. 1993; Sanders et al. 1998).
CXCL10 also has the capacity to be both directly and indirectly neurotoxic (Davis and Syapin 2004; Sui et al. 2004; Sui et al. 2006). CXCL10 exerts direct toxicity by initiating the activation of a calcium-dependent apoptotic pathway in neurons (Sui et al. 2004; Sui et al. 2006). Indirectly, CXCL10 has the ability to create a chemotactic gradient, allowing cells from the periphery to infiltrate the brain, thus increasing local neuro-inflammation (Davis and Syapin 2004).
We have previously demonstrated that HIV-1 Tat in combination with the cytokines, IFN-γ and TNF-α, is able to potentiate the expression of CXCL10 (Williams R 2009). The mechanism of Tat-mediated induction of CXCL10 involved enhanced activation of the Jnk, p38, and Akt pathways. This potentiation lead to the activation of NF-κB and STAT-1α, critical transcription factors involved in the synergistic induction of CXCL10 by the cytokines (Williams R 2009).
In the current studies we sought to explore additional pathways by which HIV Tat mediated CXCL10 potentiation in the presence of cytokines. The membrane associated enzyme, NADPH oxidase, has been garnering increased attention for its ability to participate in signal transduction, ultimately impacting cytokine and chemokine production (Abramov and Duchen 2005; Anrather et al. 2006; Li et al. 2006; Turchan-Cholewo et al. 2009). A recent study by Turchan-Cholewo et. al. demonstrated the ability of Tat to activate NADPH oxidase, inducing the production of ROS, ultimately leading to the increased expression of TNF-α, IL-6, and MCP-1 in microglia and macrophages (Turchan-Cholewo et al. 2009). Furthermore, MCP-1 has been shown in astrocytes to be regulated by the transcription factor, NF-κB, in response to ROS (Park et al. 2004). Based on these findings we hypothesized that CXCL10 expression, which is also a target of NF-κB, is modulated by ROS and possibly NADPH oxidase.
We thus decided to investigate the role of NADPH oxidase in the regulation of CXCL10 in astrocytes treated with Tat, IFN-γ, and TNF-α. First we confirmed that there was indeed the production of ROS in U-87 astrocytes treated with the Tat/cytokine mixture by DCF staining, which non-discriminatorily visualizes ROS in the cytoplasm. There was a time-dependent increase in DCF fluorescence with a peak at 30min following stimulation of astrocytes (Fig. 1A). To determine whether NADPH oxidase may be influencing the release of ROS in the stimulated cells, we pretreated the cells with the apocynin prior to stimulation of cells. The rationale for choosing apocynin was based on its specificity to block activation of NADPH oxidase, the proposed mechanism of action being its interference with the ability of p47phox subunit to associate with the membrane bound subunits (Stefanska and Pawliczak 2008). In the presence of apocynin there was abrogation of the Tat and cytokine-mediated respiratory burst observed at 30 min of cell stimulation (Fig. 1B), thus underscoring the role of NADPH oxidase activity in generation of ROS.
Since NADPH oxidase activity has been reported to impact the expression of several different immunomodulatory proteins (Abramov and Duchen 2005; Ahn and Lee 2008; Li et al. 2006; Turchan-Cholewo et al. 2009), we next sought to examine whether it also played a role in Tat and cytokine-mediated induction of CXCL10 in astrocytes. U-87 cells were pre-treated with apocynin, followed by stimulation with Tat and the cytokines to determine if inhibiting NADPH oxidase activity could decrease the expression of CXCL10. Our findings demonstrated a dose-dependent decrease in CXCL10 expression in the presence of apocynin in stimulated astrocytes (Fig. 2A), thereby confirming the role of NADPH oxidase in the induction of CXCL10.
In order to further confirm the role of NADPH oxidase in the induction of CXCL10 expression U-87 cells were transfected with either siRNAs against gp91phox conjugated to GFP. This critical membrane bound subunit was chosen because in the absence of gp91phox the activated cytosolic subunits of NADPH oxidase are unable to dock with the membrane components (gp91phox and gp22phox), consequently leading to lack of enzymatic activity. It has also been demonstrated that gp91phox is up-regulated in activated astrocytes (Abramov and Duchen 2005; Abramov et al. 2005), possibly through a positive feedback loop with NF-κB (Anrather et al. 2006). Additional support for selection of gp91phox as a target comes from the use of gp91phox knock out mice. The neuro-inflammation, and thus the neuronal toxicity, caused by cells in these mice is greatly reduced, indicating that therapies targeting NADPH oxidase could be beneficial (Abramov and Duchen 2005; Turchan-Cholewo et al. 2009).
To assess the role of NADPH oxidase in CXCL10 induction, 48 hours following transfection with siRNA against gp91phox U-87 cells were stimulated for 24 hours before the supernatants were collected and analyzed for CXCL10 content. Similar to findings with apocynin pretreatment, knock down of the gp91phox subunit also resulted in a concomitant reduction of CXCL10 expression, thus underlining the role of NADPH oxidase in this process.
Given that NADPH oxidase can participate in signal transduction in multiple ways (Adachi et al. 2004; Ahn and Lee 2008; Kuan et al. 2006; Li et al. 2006), ultimately impacting transcriptional regulation, several signaling pathways were assessed for changes in the presence of apocynin. Since the Rac1/2 subunit of NADPH oxidase can effect MAPK signal pathways (Li et al. 2006), and the dismutation of superoxide to hydrogen peroxide affects Ras activation leading to MAPK and Akt phosphorylation (Adachi et al. 2004; Ahn and Lee 2008; Kuan et al. 2006), these pathways were selected for further examination. Previously it has been shown that Tat in combination with the cytokines, was able to increase the phosphorylation of p38, Jnk, and Akt. Interestingly, in our previous findings we have demonstrated that Tat-mediated potentiation of CXCL10 did not involve Erk1/2 phosphorylation. In our current findings we demonstrated that pre-treatment of astrocytes with apocynin followed by stimulation with the Tat/cytokine mix resulted in decreased phosphorylation of MAPK proteins Jnk and Erk1/2, but not that of p38 (Fig. 4A). This data thus implicates that NADPH oxidase is involved, at least, in part, in the activation of Jnk and Erk1/2, but plays no role in Tat-mediated activation of p38.
In addition to diminished activation of MAPK, apocynin was also able to decrease the activation of the Akt survival pathway (Fig. 5A). One possible explanation for this could be the ability of superoxide (generated via NADPH oxidase) to impact Ras through its conversion to hydrogen peroxide, and Ras in turn, can result in activation of PI3K-Akt pathway (Adachi et al. 2004; Ahn and Lee 2008; Kuan et al. 2006; Li et al. 2006). These findings this suggest the pivotal role of NADPH oxidase in regulation of various signal transduction pathways at multiple levels.
It has been well documented that the Jnk, Erk1/2, and Akt pathways are capable of converging on a common transcription factor, NF-κB. NF-κB is sensitive to redox related signaling, and has been show to affected by the generation of ROS (Park et al. 2004; Song et al. 2007). It has been previously reported that NADPH oxidase activation can be directly linked to NF-κB phosphorylation and nuclear translocation (Anrather et al. 2006; Kaul and Forman 1996; Li et al. 2006). Taking into account apocynin-mediated reduction of Jnk, Erk1/2, and Akt activation, and the fact that NF-κB has been previously linked to NADPH oxidase, we next examined the effect of apocynin on NF-κB activation and translocation in U-87 cells stimulated with the Tat/cytokine mix Astrocytes pretreated with apocynin followed by stimulation with the Tat/cytokine mix resulted in the decreased phosphorylation of cytosolic IκBα (Fig. 6A). In addition to decreased levels of phosphorylated IκBα in the cytosol, apocynin treated astrocytes also displayed a corresponding reduction of activated NF-κBp65 subunit in the nuclear extracts of treated cells. Decreased expression of CXCL10 in apocynin pretreated, stimulated astrocytes could therefore be explained by the inhibition of NF-κB activation, which has been implicated as a critical regulatory factor in the transcription of CXCL10.
Having determined the mechanism by which apocynin pre-treatment of stimulated astrocytes resulted in decreased CXCL10 expression, it was of interest to examine the functional implications of this process in CXCL10-mediated neurotoxicity. Neuronal survival was monitored in the presence of astrocyte conditioned media collected from stimulated astrocytes in the presence or absence of apocynin pre-treatment.. The astrocyte conditioned media from the control astrocytes did not impact neuronal survival. However, as expected, astrocyte conditioned media from the Tat/cytokine mix stimulated cells was significantly toxic to the neurons. In contrast, apocynin pre-treated conditioned media from stimulated astrocytes was able to rescue neuronal toxicity. The role of CXCL10 as a player in astrocytes conditioned media was validated by blocking the CXCL10 effect using a CXCL10 neutralizing antibody. It was interest, however, that blocking CXCL10 activity by neutralizing antibody did not reverse the neuronal toxicity to control levels. This lead us to speculate that there must be other neurotoxic factors, such as MCP-1 and/or IL-6, that could be toxic to the neurons and are released by stimulated astrocytes. Both MCP-1 and IL-6 expression are regulated NADPH oxidase activation, and apocynin treatment has been shown to decrease their expression.
Taken together these data suggest the mechanism(s) by which Tat, IFN-γ, and TNF-α can activate NADPH oxidase to augment CXCL10 induction in astrocytes. Using pharmacological and gene knock down approaches, we demonstrate that Tat and cytokine-mediated activation of astrocytes and induction of CXCL10 involves generation of ROS, activation of Jnk, Erk1/2, and Akt pathways and subsequent activation of NF-κB leading to CXCL10 gene transcription. Released CXCL10 could in turn, be toxic for neurons, thereby enhancing neuropathogenesis. These findings have implications for patients affected by HAD in that it represents a mechanism whereby therapeutic reduction of both oxidative stress and the release of pro-inflammatory agents, can have beneficial effects for HIV-infected patients.
Acknowledgments
We acknowledge the technical assistance of Shannon Callen in the preparation of this manuscript.
This work was supported by grants MH-068212, DA020392, DA023397 and DA024442 from the National Institutes of Health (SB), and F31NS062665 (RW).
Works Cited
- Abramov AY, Duchen MR. The role of an astrocytic NADPH oxidase in the neurotoxicity of amyloid beta peptides. Philos Trans R Soc Lond B Biol Sci. 2005;360(1464):2309–14. doi: 10.1098/rstb.2005.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramov AY, Jacobson J, Wientjes F, Hothersall J, Canevari L, Duchen MR. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci. 2005;25(40):9176–84. doi: 10.1523/JNEUROSCI.1632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi T, Pimentel DR, Heibeck T, Hou X, Lee YJ, Jiang B, Ido Y, Cohen RA. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J Biol Chem. 2004;279(28):29857–62. doi: 10.1074/jbc.M313320200. [DOI] [PubMed] [Google Scholar]
- Adler V, Yin Z, Tew KD, Ronai Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999;18(45):6104–11. doi: 10.1038/sj.onc.1203128. [DOI] [PubMed] [Google Scholar]
- Ahn JH, Lee M. Tyrosine phosphorylation and Ras activation is required for hydrogen peroxide-mediated Raf-1 kinase activation. Mol Cell Biochem. 2008;317(1–2):121–9. doi: 10.1007/s11010-008-9839-9. [DOI] [PubMed] [Google Scholar]
- Albright AV, Soldan SS, Gonzalez-Scarano F. Pathogenesis of human immunodeficiency virus-induced neurological disease. J Neurovirol. 2003;9(2):222–7. doi: 10.1080/13550280390194073. [DOI] [PubMed] [Google Scholar]
- Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006;281(9):5657–67. doi: 10.1074/jbc.M506172200. [DOI] [PubMed] [Google Scholar]
- Asensio VC, Maier J, Milner R, Boztug K, Kincaid C, Moulard M, Phillipson C, Lindsley K, Krucker T, Fox HS, et al. Interferon-independent, human immunodeficiency virus type 1 gp120-mediated induction of CXCL10/IP-10 gene expression by astrocytes in vivo and in vitro. J Virol. 2001;75(15):7067–77. doi: 10.1128/JVI.75.15.7067-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior BM. NADPH oxidase: an update. Blood. 1999;93(5):1464–76. [PubMed] [Google Scholar]
- Basuroy S, Bhattacharya S, Leffler CW, Parfenova H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-{alpha} in cerebral vascular endothelial cells. Am J Physiol Cell Physiol. 2009;296(3):C422–32. doi: 10.1152/ajpcell.00381.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot (Lond) 2003;91:179–94. doi: 10.1093/aob/mcf118. Spec No. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanock SJ, el Benna J, Smith RM, Babior BM. The respiratory burst oxidase. J Biol Chem. 1994;269(40):24519–22. [PubMed] [Google Scholar]
- Davis RL, Dertien J, Syapin PJ. Ethanol-induced modulation of inducible nitric-oxide synthase activity in human A172 astrocytoma cells. Alcohol Clin Exp Res. 2002;26(9):1404–11. doi: 10.1097/01.ALC.0000030841.92766.80. [DOI] [PubMed] [Google Scholar]
- Davis RL, Syapin PJ. Chronic ethanol inhibits CXC chemokine ligand 10 production in human A172 astroglia and astroglial-mediated leukocyte chemotaxis. Neurosci Lett. 2004;362(3):220–5. doi: 10.1016/j.neulet.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Dhillon N, Zhu X, Peng F, Yao H, Williams R, Callen S, Ladner AO, Buch S, Qiu J. Molecular mechanism(s) involved in the synergistic induction of CXCL10 by human immunodeficiency virus type 1 Tat and interferon-gamma in macrophages. J Neurovirol. 2008;14(3):196–204. doi: 10.1080/13550280801993648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36(2):180–90. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Dou H, Morehead J, Bradley J, Gorantla S, Ellison B, Kingsley J, Smith LM, Chao W, Bentsman G, Volsky DJ, et al. Neuropathologic and neuroinflammatory activities of HIV-1-infected human astrocytes in murine brain. Glia. 2006;54(2):81–93. doi: 10.1002/glia.20358. [DOI] [PubMed] [Google Scholar]
- El-Benna J, Dang PM, Gougerot-Pocidalo MA, Elbim C. Phagocyte NADPH oxidase: a multicomponent enzyme essential for host defenses. Arch Immunol Ther Exp (Warsz) 2005;53(3):199–206. [PubMed] [Google Scholar]
- Firth CA, Yang YT, Gieseg SP. Lipid oxidation predominates over protein hydroperoxide formation in human monocyte-derived macrophages exposed to aqueous peroxyl radicals. Free Radic Res. 2007;41(7):839–48. doi: 10.1080/10715760701416442. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Patel N, Rahn D, McAllister J, Sadeghi S, Horwitz G, Berry D, Wang KX, Swerdlow RH. The thiazolidinedione pioglitazone alters mitochondrial function in human neuron-like cells. Mol Pharmacol. 2007;71(6):1695–702. doi: 10.1124/mol.106.033845. [DOI] [PubMed] [Google Scholar]
- Giunta B, Obregon D, Hou H, Zeng J, Sun N, Nikolic V, Ehrhart J, Shytle D, Fernandez F, Tan J. EGCG mitigates neurotoxicity mediated by HIV-1 proteins gp120 and Tat in the presence of IFN-gamma: role of JAK/STAT1 signaling and implications for HIV-associated dementia. Brain Res. 2006;1123(1):216–25. doi: 10.1016/j.brainres.2006.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5(1):69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, Vesselingh SL, Purcell DF. Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr HIV Res. 2003;1(4):463–73. doi: 10.2174/1570162033485122. [DOI] [PubMed] [Google Scholar]
- Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386(Pt 3):401–16. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res. 2006;4(3):307–18. doi: 10.2174/157016206777709384. [DOI] [PubMed] [Google Scholar]
- Kaul N, Forman HJ. Activation of NF kappa B by the respiratory burst of macrophages. Free Radic Biol Med. 1996;21(3):401–5. doi: 10.1016/0891-5849(96)00178-5. [DOI] [PubMed] [Google Scholar]
- Kolb SA, Sporer B, Lahrtz F, Koedel U, Pfister HW, Fontana A. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-gamma inducible protein 10. J Neuroimmunol. 1999;93(1–2):172–81. doi: 10.1016/s0165-5728(98)00223-9. [DOI] [PubMed] [Google Scholar]
- Kolson DL, Pomerantz RJ. AIDS Dementia and HIV-1-Induced Neurotoxicity: Possible Pathogenic Associations and Mechanisms. J Biomed Sci. 1996;3(6):389–414. doi: 10.1007/BF02258044. [DOI] [PubMed] [Google Scholar]
- Kuan YH, Lin RH, Chen YL, Tsao LT, Tzeng CC, Wang JP. Effective attenuation of acute lung injury in vivo and the formyl peptide-induced neutrophil activation in vitro by CYL-26z through the phosphoinositide 3-kinase gamma pathway. Biochem Pharmacol. 2006;72(6):749–60. doi: 10.1016/j.bcp.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Kutsch O, Oh J, Nath A, Benveniste EN. Induction of the chemokines interleukin-8 and IP-10 by human immunodeficiency virus type 1 tat in astrocytes. J Virol. 2000;74(19):9214–21. doi: 10.1128/jvi.74.19.9214-9221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Harraz MM, Zhou W, Zhang LN, Ding W, Zhang Y, Eggleston T, Yeaman C, Banfi B, Engelhardt JF. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol. 2006;26(1):140–54. doi: 10.1128/MCB.26.1.140-154.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, Graham NM, McArthur JH, Selnes OA, Jacobson LP, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43(11):2245–52. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002;202(1–2):13–23. doi: 10.1016/s0022-510x(02)00207-1. [DOI] [PubMed] [Google Scholar]
- Muscoli C, Salvemini D, Paolino D, Iannone M, Palma E, Cufari A, Rotiroti D, Perno CF, Aquaro S, Mollace V. Peroxynitrite decomposition catalyst prevents apoptotic cell death in a human astrocytoma cell line incubated with supernatants of HIV-infected macrophages. BMC Neurosci. 2002;3:13. doi: 10.1186/1471-2202-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(Suppl 2):S193–8. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986;19(6):525–35. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Ozdener H. Molecular mechanisms of HIV-1 associated neurodegeneration. J Biosci. 2005;30(3):391–405. doi: 10.1007/BF02703676. [DOI] [PubMed] [Google Scholar]
- Park J, Choi K, Jeong E, Kwon D, Benveniste EN, Choi C. Reactive oxygen species mediate chloroquine-induced expression of chemokines by human astroglial cells. Glia. 2004;47(1):9–20. doi: 10.1002/glia.20017. [DOI] [PubMed] [Google Scholar]
- Pocernich CB, Poon HF, Boyd-Kimball D, Lynn BC, Nath A, Klein JB, Butterfield DA. Proteomic analysis of oxidatively modified proteins induced by the mitochondrial toxin 3-nitropropionic acid in human astrocytes expressing the HIV protein tat. Brain Res Mol Brain Res. 2005a;133(2):299–306. doi: 10.1016/j.molbrainres.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Pocernich CB, Sultana R, Mohmmad-Abdul H, Nath A, Butterfield DA. HIV-dementia, Tat-induced oxidative stress, and antioxidant therapeutic considerations. Brain Res Brain Res Rev. 2005b;50(1):14–26. doi: 10.1016/j.brainresrev.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Quinn MT, Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leukoc Biol. 2004;76(4):760–81. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- Raad H, Paclet MH, Boussetta T, Kroviarski Y, Morel F, Quinn MT, Gougerot-Pocidalo MA, Dang PM, El-Benna J. Regulation of the phagocyte NADPH oxidase activity: phosphorylation of gp91phox/NOX2 by protein kinase C enhances its diaphorase activity and binding to Rac2, p67phox, and p47phox. Faseb J. 2008 doi: 10.1096/fj.08-114553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha RN, Pahan K. Differential regulation of Mn-superoxide dismutase in neurons and astroglia by HIV-1 gp120: Implications for HIV-associated dementia. Free Radic Biol Med. 2007;42(12):1866–78. doi: 10.1016/j.freeradbiomed.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders VJ, Pittman CA, White MG, Wang G, Wiley CA, Achim CL. Chemokines and receptors in HIV encephalitis. Aids. 1998;12(9):1021–6. [PubMed] [Google Scholar]
- Song HY, Ryu J, Ju SM, Park LJ, Lee JA, Choi SY, Park J. Extracellular HIV-1 Tat enhances monocyte adhesion by up-regulation of ICAM-1 and VCAM-1 gene expression via ROS-dependent NF-kappaB activation in astrocytes. Exp Mol Med. 2007;39(1):27–37. doi: 10.1038/emm.2007.4. [DOI] [PubMed] [Google Scholar]
- Stefanska J, Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm. 2008;2008:106507. doi: 10.1155/2008/106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Y, Potula R, Dhillon N, Pinson D, Li S, Nath A, Anderson C, Turchan J, Kolson D, Narayan O, et al. Neuronal apoptosis is mediated by CXCL10 overexpression in simian human immunodeficiency virus encephalitis. Am J Pathol. 2004;164(5):1557–66. doi: 10.1016/S0002-9440(10)63714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Y, Stehno-Bittel L, Li S, Loganathan R, Dhillon NK, Pinson D, Nath A, Kolson D, Narayan O, Buch S. CXCL10-induced cell death in neurons: role of calcium dysregulation. Eur J Neurosci. 2006;23(4):957–64. doi: 10.1111/j.1460-9568.2006.04631.x. [DOI] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270(5234):296–9. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- Thompson KA, McArthur JC, Wesselingh SL. Correlation between neurological progression and astrocyte apoptosis in HIV-associated dementia. Ann Neurol. 2001;49(6):745–52. doi: 10.1002/ana.1011. [DOI] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Dimayuga VM, Gupta S, Gorospe RM, Keller JN, Bruce-Keller AJ. NADPH oxidase drives cytokine and neurotoxin release from microglia and macrophages in response to HIV-Tat. Antioxid Redox Signal. 2009;11(2):193–204. doi: 10.1089/ars.2008.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marle G, Henry S, Todoruk T, Sullivan A, Silva C, Rourke SB, Holden J, McArthur JC, Gill MJ, Power C. Human immunodeficiency virus type 1 Nef protein mediates neural cell death: a neurotoxic role for IP-10. Virology. 2004;329(2):302–18. doi: 10.1016/j.virol.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci. 2002;59(9):1428–59. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani B, Corsini E, Binaglia M, Galli CL, Marinovich M. Reactive oxygen species generated by glia are responsible for neuron death induced by human immunodeficiency virus-glycoprotein 120 in vitro. Neuroscience. 2001;107(1):51–8. doi: 10.1016/s0306-4522(01)00332-3. [DOI] [PubMed] [Google Scholar]
- Wang X, Martindale JL, Liu Y, Holbrook NJ. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J. 1998;333(Pt 2):291–300. doi: 10.1042/bj3330291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Trillo-Pazos G, Kim SY, Canki M, Morgello S, Sharer LR, Gelbard HA, Su ZZ, Kang DC, Brooks AI, et al. Effects of human immunodeficiency virus type 1 on astrocyte gene expression and function: potential role in neuropathogenesis. J Neurovirol. 2004;10(Suppl 1):25–32. doi: 10.1080/753312749. [DOI] [PubMed] [Google Scholar]
- Williams R, Dhillon NK, Hegde ST, Yao H, Peng F, Callen S, Chebloune Y, Davis RL, Buch SJ. Proinflammatory cytokines and HIV-1 synergistically enhance CXCL10 expression in human astrocytes. Glia. 2008 doi: 10.1002/glia.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R, Yao H, Dhillon NK, Buch SJ. HIV-1 Tat co-operates with IFN-γ and TNF-α to increase CXCL10 in human astrocytes. PLoS One. 2009 doi: 10.1371/journal.pone.0005709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Peng F, Dhillon N, Callen S, Bokhari S, Stehno-Bittel L, Ahmad SO, Wang JQ, Buch S. Involvement of TRPC channels in CCL2-mediated neuroprotection against tat toxicity. J Neurosci. 2009;29(6):1657–69. doi: 10.1523/JNEUROSCI.2781-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HH, Ding JH, Zhou F, Wang F, Hu LF, Sun T, Hu G. Enhancement of glutamate uptake mediates the neuroprotection exerted by activating group II or III metabotropic glutamate receptors on astrocytes. J Neurochem. 2005;92(4):948–61. doi: 10.1111/j.1471-4159.2004.02937.x. [DOI] [PubMed] [Google Scholar]