Abstract
The nuclear factor-κB (NF-κB) family of transcription factors plays a key role in inflammation and augments the initiation, promotion, and progression of cancer.NF-κB activation generally leads to transcriptional enhancement of genes important in cell survival and cell growth, which is exploited in cancer cells. In this study, we identify an additional oncogene, PPM1D, which encodes for Wip1, as a transcriptional target of NF-κB in breast cancer cells. Inhibition of NF-κB or activation of NF-κB resulted in decreased or increased Wip1 expression, respectively, at both the mRNA and protein levels. PPM1D promoter activity was positively regulated by NF-κB, and this regulation was dependent on the presence of the conserved κB site in the PPM1D promoter region. Chromatin immunoprecipitation analysis showed basal binding of the p65 NF-κB subunit to the PPM1D promoter region encompassing the κB site, which is enhanced after NF-κB activation by tumor necrosis factor-α. Finally, we show that Wip1 expression is induced in lipopolysaccharide-stimulated mouse splenic B-cells and is required for maximum proliferation. Taken together, these data suggest an additional mechanism by which NF-κB may promote tumorigenesis, support the selective use of NF-κB inhibitors as chemotherapeutic agents for the treatment of human cancers, and further define a function for Wip1 in inflammation.
Keywords: NF-κB, PPM1D, Wip1, Cancer, Inflammation, p65
Introduction
The family of transcription factors named nuclear factor-κB (NF-κB)2 has been extensively studied and plays an important role in inflammation, in cancer, and in the cellular response to a variety of stresses such as genotoxic stress (1). The mechanisms of NF-κB activation have been extensively reviewed and are dependent on the cell type and stimulus (1, 2). Briefly, the NF-κB subunits are held in the cytoplasm by binding to the inhibitor protein, IκB (inhibitor of NF-κB). Upon stimulation, IκB is phosphorylated by the inhibitor of NF-κB kinase (IKK) complex and then ubiquitinated and targeted for proteasome-mediated degradation. Once IκB is degraded, the NF-κB subunits translocate into the nucleus, are further regulated by post-translational modifications, and are able to either promote or repress transcription by directly binding to κB sites in the promoter regions of target genes (1).
NF-κB signaling has gained significant attention in cancer research, especially because there is increasingly more evidence linking inflammation to the initiation, promotion, and progression of cancer through NF-κB activation (3–5). NF-κB can be activated in normal tissue by carcinogens, DNA-damaging agents, or pro-inflammatory cytokines secreted by stimulated leukocytes during chronic inflammation such as that associated with bacterial or viral infection. Additionally, NF-κB activation occurs in tumor cells either by leukocytes surrounding the tumor microenvironment or acquired constitutive NF-κB activation. Once activated, the NF-κB transcription factors increase the expression of genes important for cell growth, inhibition of apoptosis, metastasis, and angiogenesis, all of which promote cancer progression at various levels (4). There are many examples of the pro-survival features of NF-κB, e.g. an activator for this pathway substantially reduced radiation-induced killing in mice at doses that are normally lethal (6).
Wip1 (wild type p53-induced phosphatase 1) is a nuclear type 2C protein phosphatase (PP2C) that is overexpressed and amplified in many types of cancers such as breast cancer, gastric carcinomas, ovarian clear cell adenocarcinoma, and pancreatic adenocarcinoma (7). Further studies have shown Wip1 to act as an oncogene by complementing other oncogenes such as c-myc, H-Ras, and ErbB2 (shown through the use of mouse models) and inhibiting tumor suppressors such as p16/p19, p53, and ATM (8, 9). Another possible oncogenic property of Wip1 involves the ability to reverse signaling induced by stresses, such as genotoxic stress (7, 10). Wip1 overexpression, such as that seen in cancer cells, may lead to premature inhibition of stress signaling, a situation that may enhance genomic instability and facilitate tumorigenesis. Additionally, chemical inhibition of Wip1 suppresses tumor growth in vitro and in vivo (11), and inhibition of Wip1 expression by siRNA in breast cancer cells leads to enhanced apoptosis and decreased cell growth (12, 13). These studies clearly indicate a function(s) for Wip1 in human cancer cell growth. Therefore, Wip1 is an attractive drug target for the treatment of cancers, especially those in which Wip1 is amplified and overexpressed.
Due to the oncogenic properties of Wip1, understanding the regulation of Wip1 activity is crucial. Although Wip1 activity may possibly be modulated by post-translational modifications, Wip1 activity is currently only known to be regulated at the level of expression. This is evident by the dramatic increases in both transcript and protein levels of Wip1 induced by a variety of stresses, some of which are p53-independent (14–17). Thus far, p53, cAMP-response element-binding protein, and E2F have been identified as transcription factors that regulate transcription of PPM1D, the gene encoding Wip1 (16, 17). Given that Wip1 expression is increased by several types of stress, there are likely other transcription factors involved in PPM1D transcriptional regulation that may be important in the context of a cancer cell, and this remains largely unexplored.
In this study, we identify the oncogenic Wip1 phosphatase as a positively regulated target gene of NF-κB. Wip1 expression, at both mRNA and protein levels, is enhanced or repressed in cells that have stimulated or inhibited NF-κB activity, respectively. Furthermore, NF-κB regulates PPM1D transcription by directly binding to the PPM1D promoter region. Finally, we show that Wip1 expression induced in an NF-κB-dependent manner in LPS-stimulated mouse splenic B-cells facilitates proliferation. These data have important implications for Wip1 oncogene regulation by NF-κB in human cancers.
EXPERIMENTAL PROCEDURES
Materials
IMD-0354 (Sigma, catalog no. I3159) was used at a concentration of 2 μm. Recombinant mouse tumor necrosis factor-α (TNF-α) was used at a concentration of 1 ng/ml (Sigma, catalog no. T7539). Lipopolysaccharide (Sigma, catalog no. L4130) was used at a concentration of 10 μg/ml. SB203580 (Sigma, catalog no. S8307) was used at a concentration of 10 μm.
Cell Culture
MCF-7 and HCT-116 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 2 mm glutamine at 37 °C in 5% CO2. Primary splenic lymphocytes were cultured following a protocol described previously (18).
Immunoblotting
At the time of harvest, cells were washed with cold phosphate-buffered saline, scraped, and centrifuged (2000 rpm for 2 min) to remove phosphate-buffered saline. Nuclear extracts were prepared as described previously (19). For whole cell extract isolation, cell pellets were resuspended in tissue lysis buffer (20 mm Tris, pH 7.4, 137 mm NaCl, 2 mm EDTA, 1% Triton X-100, 25 mm β-glycerophosphate, 2 mm sodium pyrophosphate, 10% glycerol, and protease inhibitor mixture) and clarified by centrifugation at 13,000 rpm for 10 min. The protein concentration of each sample was measured using a Bradford assay (Bio-Rad, catalog no. 500-0205) with bovine serum albumin standards. Approximately 50 μg of total protein from each sample was separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane, and individual proteins were then detected as described previously (19). Specific primary antibodies used include p65 (catalog no. sc-372), β-actin (catalog no. sc-47778), and p38 (catalog no. sc-7972) from Santa Cruz Biotechnology (Santa Cruz, CA). Wip1 (Bethyl Laboratories, Montgomery, TX; catalog no. A300-664A) and phospho-p38 (Thr-180/Tyr-182) (Cell Signaling Technology, Danvers, MA; catalog no. 9216) were also used.
siRNA and Plasmid Transfections
RELA (p65) and green fluorescent protein-targeted siRNA were purchased from Dharmacon, Inc. (Chicago, catalog no. J-003533-06 and P-002048-01-20, respectively). Cells were transfected using Lipofectamine 2000 (Invitrogen, catalog no. 11668027) following the manufacturer's protocol with 100 pmol of siRNA (and 2 μg of plasmid DNA for cotransfections) per 60-mm tissue culture plate.
Quantitative Real Time PCR
At the time of harvest, cells were homogenized in TRIzol (Invitrogen, catalog no. 15596018), and RNA was isolated following the manufacturer's protocol. RNA cleanup and concentration were performed using the RNeasy MinElute Cleanup kit (Qiagen, Valencia CA; catalog no. 74204). 500 ng of RNA from each sample was used in a single qRT-PCR. Brilliant®II qRT-PCR Master Mix, 1-step (Stratagene, La Jolla, CA; catalog no. 600809), was used with the specified TaqMan probe/primer mix from Applied Biosystems, Inc. (Foster City, CA), including human PPM1D (catalog no. NM_003620.2), mouse Ppm1d (catalog no. NM_016910.2), human GAPDH (catalog no. 4326317E), and mouse GAPDH (catalog no. 4352339E). qRT-PCRs were run with the iCycler (Bio-Rad) and the following parameters: 1) 95 °C for 3 min (1 time); 2) 95 °C for 15 s followed by 60 °C for 1 min (50 times); 3) 95 °C for 1 min (1 time); and 4) 60 °C for 20 s (50 times). Data analysis was performed using the iCycler IQ version 3.1 software (Bio-Rad).
Plasmid Construction and Luciferase Assays
For the construction of the two PPM1D reporter plasmids used, “pWip1-luc” and “pWip1_ΔκB-luc,” the appropriate sequences were cloned into the RapidReporter®pRR-High vector (Active Motif, Carlsbad, CA; catalog no. 33003). Cloning of the 855-bp PPM1D promoter sequence was based on cloning of this region as described previously (17). Briefly, a section of the PPM1D promoter region, including the 855-bp sequence between AvrII and BamHI restriction sites, was amplified from genomic DNA isolated from MCF-7 cells using primers FP1 and RP1. The κB site was deleted by using two different sets of primers that, after amplification, result in two overlapping fragments that have the κB site deleted (FP1 and RP2, FP2 and RP1). The primer names and sequences used were as follows: FP1, ACATTTTCTTGAGCTGATTTTGCTT; FP2, ACCGAGACTGTGCAGCTGACGGTTAAGTGCTT; RP1, TCGGAGAAGACGCTCACTCC; and RP2, GTTTAAAAAGCACTTAACCGTCAGCT. Primers were obtained from Eurofins MWG Operon (Huntsville, AL). The two overlapping (κB site deleted) fragments were then annealed by overlap extension in a PCR. The wild type and the κB site-deleted amplicons were digested with AvrII and BamHI enzymes and cloned into the SpeI and BglII sites of the RapidReporter®pRR-High vector to generate pWip1-luc and pWip1_ΔκB-luc, respectively. Correct plasmid construction was confirmed by sequencing.
Luciferase activity of cells transfected with the luciferase reporter vectors was measured using the RapidReporter®Gaussia luciferase assay (Active Motif, catalog no. 33001) according to the manufacturer's protocol.
Chromatin Immunoprecipitation (ChIP) Assays
ChIP analysis was run on three 100-cm plates per treatment of MCF-7 cells using the ChIP-ITTM Express enzymatic kit (Active Motif, catalog no. 53009) according to the manufacturer's protocol. A ChIP grade NF-κB p65 antibody was used and purchased from Abcam, Inc. (Cambridge, MA, catalog no. ab7970). The IgG negative control antibody and the forward and reverse negative control primers used were from the ChIP-ITTM Control Kit-Human (Active Motif, catalog no. 53010). The sequences of the NFKBIA (20) and PPM1D primers are as follows: PPM1D-FP1, TCTGCCTAGGAATTCGT; PPM1D-RP1, TGGCCGGAACAAGTGCT; NFKBIA-FP1, GACGACCCCAATTCAAATCG; and NFKBIA-RP1, TCAGGCTCGGGGAATTTCC. Primers were obtained from Eurofins MWG Operon.
RESULTS
PPM1D Promoter Region Includes an Evolutionarily Conserved κB Site
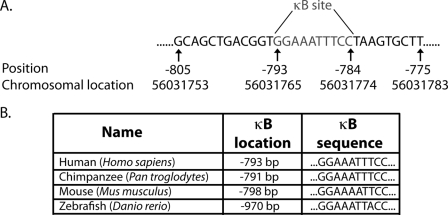
In a search for transcription factors that regulate PPM1D expression, analysis of the 1000 bp immediately upstream of the PPM1D translational start site revealed a κB site, which is a conserved binding sequence for the NF-κB transcription factors (Fig. 1A). Further analysis of the promoter region in several organisms among the evolutionary hierarchy ranging from humans to zebrafish showed conservation of this κB site (Fig. 1B). Both the sequence and the position (relative to the translational start site) of the κB site are highly homologous among the different species, implicating evolutionary importance of the κB site in the PPM1D promoter region and, most likely, PPM1D transcriptional regulation.
FIGURE 1.
PPM1D promoter region includes an evolutionarily conserved κB site. A, conserved κB site was found at position −793 bp in the PPM1D promoter region using the Alibaba 2.1 software. B, κB site is highly homologous in sequence and in position with respect to the translational start site among the various organisms indicated.
Inhibition of NF-κB Decreases Wip1 Expression
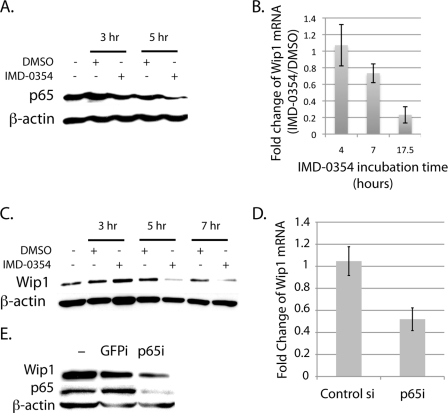
The presence of a κB site implies that the NF-κB transcription factors regulate PPM1D transcription; therefore, we next sought to determine whether alteration of NF-κB activity affected Wip1 expression. To inhibit NF-κB activity, we used the chemical inhibitor of IKKβ, IMD-0354 (21, 22), which ultimately inhibits IκBα degradation and translocation of the NF-κB subunits into the nucleus. After incubation of IMD-0354 for various time periods with MCF-7 breast cancer cells, which have about a 3-fold increase in Wip1 expression (23), nuclear extracts were immunoblotted for the NF-κB subunit p65 to confirm that IMD-0354 effectively inhibits NF-κB in these cells. As shown in Fig. 2A, nuclear levels of p65 decreased in cells treated with IMD-0354 compared with the Me2SO controls in a time-dependent manner, which indicates a reduction in p65 activity. qRT-PCR analysis of similarly treated cells revealed that Wip1 mRNA levels decreased in IMD-0354-treated cells with time compared with the controls (Fig. 2B). The effect of IMD-0354 on Wip1 expression is not solely at the mRNA level, because immunoblotting of whole cell lysates from these cells for Wip1 revealed that IMD-0354 treatment also decreased total Wip1 protein (Fig. 2C).
FIGURE 2.
Inhibition of NF-κB decreases Wip1 expression. A, nuclear extracts were immunoblotted for p65 and β-actin (loading control). Wip1 mRNA levels were quantified (B), or whole cell lysates were immunoblotted for Wip1 and β-actin (loading control) from MCF-7 cells incubated with IMD-0354 for the time periods indicated (C). D and E, MCF-7 cells were transfected with either control siRNA or p65 siRNA for 24 h. Wip1 mRNA (D) and total Wip1 protein from these cells were analyzed (E). Wip1 mRNA levels were quantified by qRT-PCR and normalized to GAPDH. E, whole cell lysates were immunoblotted for Wip1, p65, and β-actin (loading control).
As an alternative method of NF-κB inhibition, MCF-7 cells were transfected with siRNA targeted to p65. As with inhibition of NF-κB by IMD-0354, qRT-PCR and immunoblotting analysis demonstrated that knockdown of p65 expression also resulted in a reduction of Wip1 mRNA levels (Fig. 2D) and total protein (Fig. 2E), respectively, by about 50%. We would expect there to be more dramatic changes in Wip1 expression if the transfection efficiency of the siRNA in these cells was higher. Taken together, these findings show that inhibition of p65 activity in MCF-7 cells inhibits the expression of Wip1 at both the protein and mRNA levels, showing that p65 is a positive regulator of Wip1 expression.
Activation of NF-κB Increases Wip1 Expression
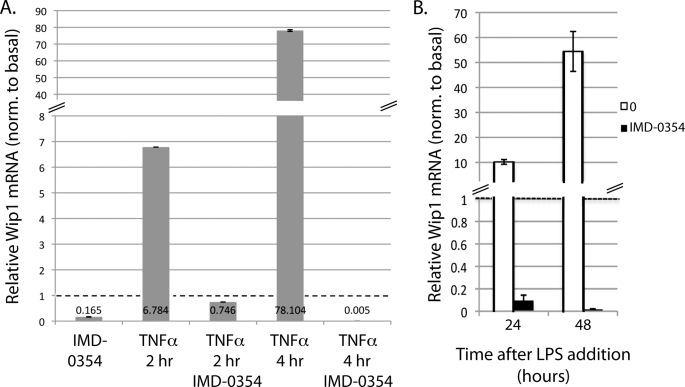
Because reducing NF-κB activity decreased Wip1 expression, enhancing NF-κB activity should increase Wip1 expression. To test this, TNFα was incubated with MCF-7 cells because it is a well characterized activator of NF-κB (24, 25). Two and four hours after incubation of TNFα, qRT-PCR analysis shows that Wip1 mRNA levels dramatically increase (Fig. 3A). Furthermore, preincubation with IMD-0354 severely inhibits this increase (Fig. 3A), indicating that TNFα-stimulated Wip1 induction is NF-κB-dependent.
FIGURE 3.
Activation of NF-κB increases Wip1 expression. Wip1 mRNA levels were quantified by qRT-PCR and normalized to GAPDH after NF-κB activation. A, MCF-7 cells were incubated with either IMD-0354 alone, TNFα alone, or TNFα with pretreatment of IMD-0354 for the indicated time periods. B, primary splenic lymphocytes from wild type mice were incubated with LPS with or without pretreatment of IMD-0354 for the indicated time periods. Dotted lines represent the value of the untreated control samples, which are set to 1.
To supplement the above results, we also measured Wip1 mRNA levels in LPS-stimulated primary splenic lymphocytes harvested from wild type mice. LPS-stimulated B-cell proliferation is a process in which NF-κB activation is required (26–28). Additionally, Wip1 has been shown to be important in LPS-stimulated B-cell proliferation, particularly at 24 and 48 h after LPS addition (18), suggesting that Wip1 expression might be modulated in this system. As expected, Wip1 mRNA levels greatly increase at 24 and 48 h after LPS stimulation (Fig. 3B). Similar to the TNFα results above, preincubation of IMD-0354 with the lymphocytes inhibited the LPS-stimulated increase in Wip1 mRNA (Fig. 3B), indicating that the regulation of Wip1 expression is dependent on NF-κB activation. Taken together, these data show that Wip1 expression increases in response to LPS and TNFα stimulation through NF-κB activation in splenic B-cells and MCF-7 cells, respectively.
NF-κB Regulates PPM1D Promoter Activity and Binds to the PPM1D Promoter Region
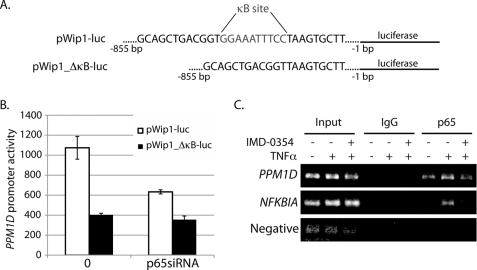
Thus far, we have shown that NF-κB regulates Wip1 expression. Because the mechanism of action of the NF-κB family members is through transcriptional modulation of target genes (1), we wanted to test whether NF-κB directly regulates PPM1D transcription. First, PPM1D promoter activity was measured with luciferase reporter constructs in cells with and without altered NF-κB activity. Fig. 4A highlights the design of the two constructs; they are identical with the exception of the deletion of the κB site in one of the vectors. As shown in Fig. 4B, the activity of the wild type promoter (pWip1-luc) decreases by more than 60% in cells with depleted p65 expression by siRNA treatment. On the other hand, the basal activity of the κB site-deleted PPM1D promoter (pWip1_ΔκB-luc) is substantially reduced compared with the wild type promoter (Fig. 4B), and depletion of p65 by siRNA does not significantly alter the activity of the mutated PPM1D promoter. Similar results were seen after ionizing radiation exposure, which is also an NF-κB activator, and TNFα stimulation (data not shown). These data indicate the following: (i) full basal PPM1D promoter activity requires the conserved κB site, and (ii) the NF-κB subunit p65 regulates PPM1D transcription in MCF-7 cells.
FIGURE 4.
p65 activity regulates PPM1D promoter activity and directly binds to the PPM1D promoter region. A, schematic of the construction of the luciferase reporter vectors used to measure PPM1D promoter activity. pWip1-luc includes the complete 855-bp sequence directly upstream from the PPM1D translational start site, and pWip1_ΔκB-luc includes the same sequence with the exception of the putative κB site. B, PPM1D promoter activity assay. MCF-7 cells were transfected with either pWip1-luc or pWip1_ΔκB-luc alone or cotransfected with p65 siRNA. Luciferase activity was measured 48 h later. C, ChIP analysis of NF-κB promoter binding. MCF-7 cells were either untreated, treated with TNFα alone, or treated with TNFα after a 5-h pretreatment with IMD-0354. The cells were harvested for ChIP assays using either IgG (negative control) or a p65-specific antibody. The primers used were specific for either the region of the PPM1D promoter, including the κB site, the promoter region of the NFKBIA gene (positive control), or the region between the GAPDH and CNAP1 genes (Negative, negative control).
To test the possibility that the NF-κB subunits directly regulate PPM1D transcription by binding to the promoter region, ChIP assays were performed. The p65 subunit constitutively binds to the PPM1D promoter region in MCF-7 cells, indicated by the amplification of the promoter region that encompasses the κB site from chromatin samples immunoprecipitated with a p65 antibody but not IgG serum (Fig. 4C). After a 3-h treatment with TNFα, the amount of p65 bound to the PPM1D promoter region increases compared with basal levels (Fig. 4C), which is similar to p65 binding to the promoter of NFKBIA, the gene encoding IκBα (positive control). The reason why there appears to be a larger effect of TNFα-induced p65 binding to the NFKBIA than to the PPM1D promoter is unclear, but this may be due to the higher amount of basal p65 binding to the PPM1D and not the NFKBIA promoter region. Furthermore, inhibition of TNFα-induced NF-κB activation by pretreatment with IMD-0354 reduces the amount of p65 subunits bound to the PPM1D promoter region to basal levels (Fig. 4C). These results demonstrate that the p65 NF-κB subunit directly regulates PPM1D transcription basally and after TNFα stimulation in MCF-7 breast cancer cells.
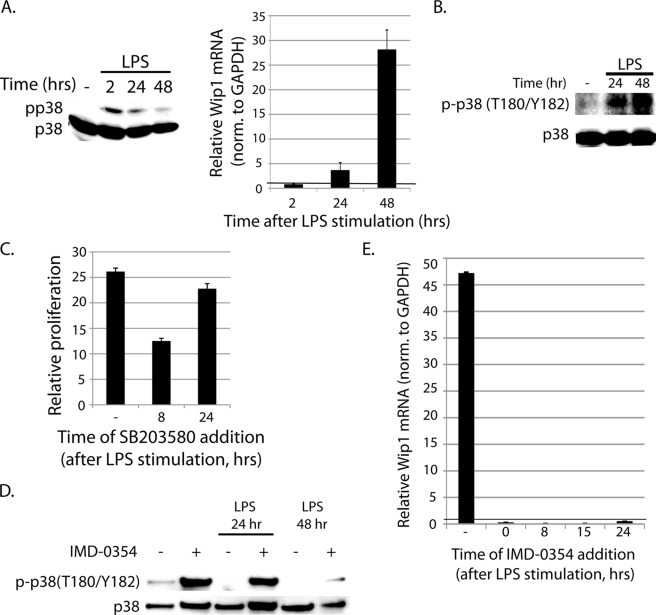
Wip1 Expression in LPS-stimulated Splenic B-cells Is Inversely Correlated with p38 Activation and Promotes Proliferation
We next sought to determine the function of NF-κB-dependent Wip1 expression in LPS-stimulated B-cells, because Wip1−/− LPS-stimulated B-cells show reduced proliferation (18). In doing so, we focused on determining whether p38 may play a role in the proliferation deficiency seen in Wip1−/− B-cells because p38 is a known Wip1 target (8, 15, 16, 29–31) and plays a major role in cytokine production and B-cell proliferation (32, 33). p38 activation at early time points after LPS stimulation is known to positively regulate B-cell proliferation (32, 33), yet the effects of p38 activity at later time points when proliferation is high and Wip1 expression is increased (24 and 48 h) have not been extensively studied. Therefore, we measured the levels of active p38 at various times after LPS stimulation. As seen in Fig. 5A, phospho-p38 (Thr-180/Tyr-182, active p38) increases 2 h after LPS stimulation, as expected, and then decreases at the 24- and 48-h time points. Wip1 expression, on the other hand, is only increased at 24 and 48 h after LPS addition and not at 2 h (Fig. 5A). Furthermore, lymphocytes isolated from Wip1−/− mice show heightened phospho-p38 levels 24 and 48 h after LPS stimulation (Fig. 5B). These results demonstrate an inverse correlation of p38 activation and Wip1 expression, suggesting that a possible role for Wip1 is to inactivate p38 at later time points of LPS stimulation in B-cells.
FIGURE 5.
Wip1 expression in LPS-stimulated splenic B-cells inversely correlates with p38 activity. A, primary splenic lymphocytes from wild type mice were incubated with LPS for the indicated time periods. Whole cell lysates were subject to immunoblot analysis, and the fold change in Wip1 mRNA levels (normalized to GAPDH) was measured by qRT-PCR. B, primary splenic lymphocytes from Wip1−/− mice were stimulated with LPS (or not, −), and whole cell lysates were subject to immunoblot analysis. C, proliferation was measured in LPS-stimulated wild type lymphocytes at 48 h without SB203580 (−) or with SB203580 added 8 or 24 h after LPS addition. D, primary splenic lymphocytes from wild type mice were incubated with LPS (or not), with or without IMD-0354 pretreatment, for the indicated time periods. Whole cell lysates were subject to immunoblot analysis. E, fold change in Wip1 mRNA levels was measured at 48 h after LPS stimulation without IMD-0354 (−) or with IMD-0354 added at the indicated time points after LPS stimulation. A black horizontal line indicates the value of “1” on the x axis and highlights that all IMD-0354-treated cells show no proliferation in response to LPS (specific values: 0, 0.3 ± 0.0015; 8, 0.08 ± 0.0005; 15, 0.1 ± 0.001; 24, 0.522 ± 0.007). All immunoblotting was analyzed using specific antibodies for phospho-p38 (Thr-180/Tyr-182) and p38. For the analysis of Wip1 mRNA fold changes, values were normalized to those of nonstimulated controls, which are set to 1 (indicated by a black horizontal line).
The fact that p38 activity decreases 24 and 48 h after LPS treatment suggests that p38 needs to be inactivated at these late time points. Given that p38 plays a major role in cytokine production, which is crucial for proliferation, a reduction of LPS-stimulated B-cell proliferation by inhibition of p38 would be expected (32, 33). However, whether inhibition of p38 at later times after LPS stimulation affects B-cell proliferation is unknown. Proliferation (measured at 48 h after LPS stimulation) is decreased when the p38 inhibitor SB203580 is added at 8 h after LPS stimulation, but it is not significantly altered when p38 is inhibited at 24 h post-LPS stimulation (Fig. 5C). These results indicate that p38 activity is low and plays a limited role in proliferation at late time points after LPS stimulation.
If NF-κB induces Wip1 expression, and Wip1 inhibits p38, then inhibition of NF-κB should result in increased levels of active p38. To determine whether p38 activity (like Wip1 expression) is affected by inhibition of NF-κB, LPS-stimulated lymphocytes were either untreated or pretreated with IMD-0354. Pretreatment of IMD-0354 enhances p38 activation levels at 24 and 48 h (Fig. 5D). These data indicate that NF-κB decreases p38 activity at later times after LPS addition, presumably through Wip1.
To eliminate the possibility that IMD-0354 pretreatment indirectly decreases Wip1 expression by affecting signaling at early time points, Wip1 expression was measured when IMD-0354 was added at various times after LPS stimulation. As seen in Fig. 5E, Wip1 mRNA levels still decrease when IMD-0354 is added 8, 15, and 24 h after LPS stimulation, which indicates that the effect of IMD-0354 on LPS-induced Wip1 expression is not due to inhibition of signaling at early time points.
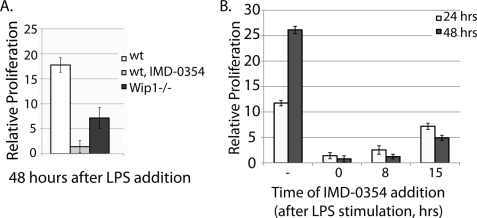
A functional role of NF-κB-induced Wip1 expression is to facilitate LPS-stimulated B-cell proliferation, because Wip1−/− B-cells show reduced proliferation compared with wild type controls (Fig. 6A) (18). Because NF-κB induces Wip1 expression, which then regulates LPS-stimulated proliferation, the inhibition of NF-κB by IMD-0354 should also reduce proliferation. Preincubation of IMD-0354 with the wild type splenic lymphocytes showed a significant deficiency in LPS-stimulated proliferation at 48 h (Fig. 6A). Again, to rule out the possibility that IMD-0354 preincubation affects proliferation by regulating early signaling and not later Wip1 induction, incubation of the B-cells with IMD-0354 at various times after LPS addition also dramatically reduced proliferation at 24 and 48 h compared with the untreated controls (Fig. 6B). In fact, addition of IMD-0354 15 h after LPS addition (Fig. 6B) results in a reduced proliferation level at 48 h similar to that of the Wip1−/− lymphocytes (Fig. 6A). However, the fact that there is a more dramatic decrease in proliferation when IMD-0354 is preincubated in wild type lymphocytes indicates that there are probably other NF-κB targets that play a role in this process. Therefore, although Wip1 may not be sufficient for LPS-stimulated lymphocyte proliferation, it is necessary for full proliferation. Taken together, these data indicate that NF-κB-dependent Wip1 expression facilitates LPS-stimulated proliferation in lymphocytes possibly (at least in part) through a p38-dependent mechanism.
FIGURE 6.
NF-κB-induced Wip1 promotes LPS-stimulated B-cell proliferation. A, relative proliferation was calculated for primary splenic lymphocytes at the indicated times of LPS incubation (wt, lymphocytes harvested from wild type mice; wt, IMD-0354, lymphocytes harvested from wild type mice treated with IMD-0354; Wip1−/−, lymphocytes harvested from Wip1−/− mice). B, proliferation was measured at 24 and 48 h after LPS stimulation in wild type splenic lymphocytes without IMD-0354 (−) or with IMD-0354 added at the indicated time periods after LPS addition.
DISCUSSION
Our results demonstrate that NF-κB regulates Wip1 expression by directly binding to the PPM1D promoter region to enhance transcription and that NF-κB-dependent Wip1 expression facilitates proliferation in stimulated B-cells. Our results also indicate that NF-κB-driven Wip1 expression in LPS-stimulated B-cells promotes proliferation by dampening p38 activity at late time points after stimulation. However, the entire scope of NF-κB and Wip1 involvement in B-cell proliferation most likely exceeds that of the single mechanism presented in this study. Nevertheless, these findings have multiple implications in the role of Wip1 in the progression and treatment of cancer as well as inflammation.
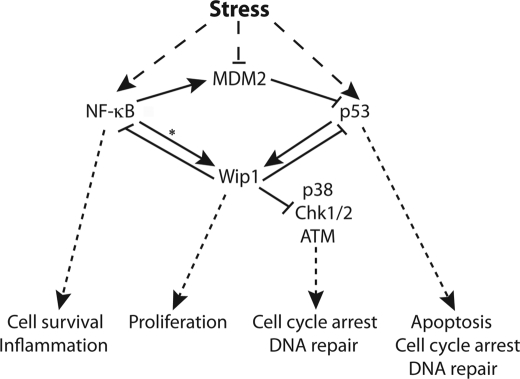
NF-κB regulation of Wip1 expression adds to the complex network of stress-signaling pathways, including several feedback loops (Fig. 7), which if improperly regulated can contribute to tumorigenesis. The function of both NF-κB and Wip1 proteins is to negate stress signaling and facilitate returning the cell to homeostasis. NF-κB inhibits the pro-apoptotic signaling of p53 (34, 35) and increases anti-apoptotic gene expression (1), which result in cell survival. Wip1 signaling has similar effects, because Wip1 inhibits apoptosis and cell cycle arrest by inactivating several key stress-signaling proteins (7). Another common theme of NF-κB and Wip1 signaling is that both enhance MDM2 expression; NF-κB enhances MDM2 mRNA levels, and Wip1 dephosphorylates and stabilizes MDM2 protein. By stabilizing MDM2, NF-κB and Wip1 negatively regulate p53-dependent apoptotic signaling (7, 34). Additionally, Chew et al. (36) recently showed that Wip1 can negatively regulate NF-κB signaling by directly dephosphorylating the p65 subunit on Ser-536. As outlined in Fig. 7, there are several feedback loops that exist in this complex stress-signaling network. p53 directly up-regulates Wip1, which then inhibits p53. NF-κB directly up-regulates Wip1 (Fig. 7, * means as described in this study), which then inhibits NF-κB signaling, at least in the context of inflammation. p53 enhances MDM2 expression, which then inhibits p53 as the stress response subsides (37). Thus, our results provide a link between multiple stress-signaling pathways that return the cell to homeostasis, which may promote tumorigenesis when these pathways are not properly regulated.
FIGURE 7.
Diagram of Wip1 cross-talk with stress signaling. Asterisk designates finding from our studies.
Enhancing the expression of Wip1 may also be an additional way in which NF-κB activity contributes to the growth of cancer cells. The fact that Wip1 deletion stunted LPS-stimulated proliferation demonstrates a proliferative function for NF-κB-dependent Wip1 induction in lymphocytes, but a similar mechanism may also exist in cancer cells. Evidence for this lies in the fact that we show NF-κB as a direct enhancer of Wip1 expression in human breast cancer cells, and others (10, 12) have shown that Wip1 enhances proliferation and inhibits apoptosis in these cells. Inhibitors of NF-κB have been suggested as chemotherapeutic agents for cancer patients (4, 5, 38, 39). Inhibition of Wip1 expression could be an added benefit of inhibiting NF-κB in this setting, which may be especially important in patients whose tumors have PPM1D amplified and Wip1 overexpressed.
It should be noted, however, that the negative feedback loop between Wip1 and NF-κB may not occur in some cancer cells because Wip1 regulation of NF-κB may be context-dependent. NF-κB activity regulation, including p65 phosphorylation, is extremely complex and has been shown to be dependent on the type of stimulus and tissue (1, 2, 40). Additionally, the biological consequences of NF-κB activation depend on tissue and stimulus type. For example, constitutive NF-κB activity in the background of inflammation promotes tumorigenesis in some tissues (4), whereas NF-κB activity inhibits tumorigenesis in some types of tissues, such as the skin (41). Therefore, there are probably several effects of NF-κB-induced Wip1 on tumorigenesis depending on the context. As shown previously, Wip1 may inhibit tumor suppressors such as p53 or p16/p19 (7, 8, 42). Wip1 may inhibit NF-κB in some tissues, or Wip1 may not inhibit constitutive NF-κB activity in certain types of cancer cells to promote tumorigenesis.
Not only could inhibition of NF-κB activity, and therefore Wip1 expression, be used for the killing of tumor cells, but it may possibly be used to sensitize tumor cells to radiotherapy. NF-κB activation is a means of adaptive radioresistance in cancer cells (43–45), and inhibition of NF-κB has been shown to radiosensitize a number of human cancers (46–50). Enhancing Wip1 expression in tumor cells (either basally or after radiation exposure) may be one way in which NF-κB facilitates tumor cell survival after radiotherapy, because Wip1 is a known inhibitor of many pathways of the DNA damage response that lead to apoptosis, cell cycle arrest, and DNA repair (7).
Our data also provide additional evidence for a role for Wip1 in inflammation. The first evidence of this came from the analysis of the phenotype of the Wip1−/− mouse, which revealed that these mice have compromised immunocompetency due to the presence of abnormal lymphoid structure, increased susceptibility to ulcerated skin lesions and pathogens, and defective T- and B-cell responses as we also demonstrate here (7). NF-κB is highly activated by inflammatory signals and has a crucial role in inflammatory processes (1, 28); as mentioned above, Chew et al. (36) recently showed that Wip1 can dampen the inflammatory process by negatively regulating NF-κB signaling by directly dephosphorylating p65. These data, together with the data presented here, provide a mechanism for the role for Wip1 in inflammation; NF-κB is activated by inflammatory signals and enhances the expression of Wip1, which then inhibits NF-κB signaling through a negative feedback loop to presumably turn off the inflammatory process and return the cell to homeostasis.
In conclusion, we have demonstrated NF-κB as a direct transcriptional enhancer of Wip1 expression. These data show an additional mechanism by which NF-κB may promote tumorigenesis and tumor cell growth, support the use of NF-κB inhibitors for chemotherapeutic and radiosensitizing purposes in the treatment of various human cancers, and further define Wip1 function in inflammation. Although we clearly show that NF-κB constitutively activates Wip1 transcription in breast cancer cells, more studies need to be done to further develop the downstream mechanisms of NF-κB-induced Wip1 expression in the context of cancer cells.
This work was partially supported by Grant R31-10069 (WCU program) through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology.
- NF-κB
- nuclear factor-κB
- LPS
- lipopolysaccharide
- ChIP
- chromatin immunoprecipitation
- TNF-α
- tumor necrosis factor-α
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- qRT
- quantitative reverse transcription
- siRNA
- small interfering RNA.
REFERENCES
- 1.Hayden M. S., Ghosh S. (2008) Cell 132, 344–362 [DOI] [PubMed] [Google Scholar]
- 2.Hayden M. S., Ghosh S. (2004) Genes. Dev. 18, 2195–2224 [DOI] [PubMed] [Google Scholar]
- 3.Karin M., Greten F. R. (2005) Nat. Rev. Immunol. 5, 749–759 [DOI] [PubMed] [Google Scholar]
- 4.Naugler W. E., Karin M. (2008) Curr. Opin. Genet. Dev. 18, 19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escarcega R. O., Fuentes-Alexandro S., Garcia-Carrasco M., Gatica A., Zamora A. (2007) Clin. Oncol. 19, 154–161 [DOI] [PubMed] [Google Scholar]
- 6.Burdelya L. G., Krivokrysenko V. I., Tallant T. C., Strom E., Gleiberman A. S., Gupta D., Kurnasov O. V., Fort F. L., Osterman A. L., Didonato J. A., Feinstein E., Gudkov A. V. (2008) Science 320, 226–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu X., Nguyen T. A., Moon S. H., Darlington Y., Sommer M., Donehower L. A. (2008) Cancer Metastasis Rev. 27, 123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulavin D. V., Phillips C., Nannenga B., Timofeev O., Donehower L. A., Anderson C. W., Appella E., Fornace A. J., Jr. (2004) Nat. Genet. 36, 343–350 [DOI] [PubMed] [Google Scholar]
- 9.Shreeram S., Demidov O. N., Hee W. K., Yamaguchi H., Onishi N., Kek C., Timofeev O. N., Dudgeon C., Fornace A. J., Anderson C. W., Minami Y., Appella E., Bulavin D. V. (2006) Mol. Cell 23, 757–764 [DOI] [PubMed] [Google Scholar]
- 10.Xia Y., Ongusaha P., Lee S. W., Liou Y. C. (2009) J. Biol. Chem. 284, 17428–17437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belova G. I., Demidov O. N., Fornace A. J., Jr., Bulavin D. V. (2005) Cancer Biol. Ther. 4, 1154–1158 [DOI] [PubMed] [Google Scholar]
- 12.Kong W., Jiang X., Mercer W. E. (2009) Cancer Biol. Ther. 8, 555–563 [DOI] [PubMed] [Google Scholar]
- 13.Pärssinen J., Alarmo E. L., Karhu R., Kallioniemi A. (2008) Cancer Genet. Cytogenet. 182, 33–39 [DOI] [PubMed] [Google Scholar]
- 14.Fiscella M., Zhang H., Fan S., Sakaguchi K., Shen S., Mercer W. E., Vande Woude G. F., O'Connor P. M., Appella E. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 6048–6053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takekawa M., Adachi M., Nakahata A., Nakayama I., Itoh F., Tsukuda H., Taya Y., Imai K. (2000) EMBO J. 19, 6517–6526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hershko T., Korotayev K., Polager S., Ginsberg D. (2006) J. Biol. Chem. 281, 31309–31316 [DOI] [PubMed] [Google Scholar]
- 17.Rossi M., Demidov O. N., Anderson C. W., Appella E., Mazur S. J. (2008) Nucleic Acids Res. 36, 7168–7180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi J., Nannenga B., Demidov O. N., Bulavin D. V., Cooney A., Brayton C., Zhang Y., Mbawuike I. N., Bradley A., Appella E., Donehower L. A. (2002) Mol. Cell. Biol. 22, 1094–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cha H., Wang X., Li H., Fornace A. J., Jr. (2007) J. Biol. Chem. 282, 22984–22992 [DOI] [PubMed] [Google Scholar]
- 20.Tian B., Nowak D. E., Jamaluddin M., Wang S., Brasier A. R. (2005) J. Biol. Chem. 280, 17435–17448 [DOI] [PubMed] [Google Scholar]
- 21.Tanaka A., Konno M., Muto S., Kambe N., Morii E., Nakahata T., Itai A., Matsuda H. (2005) Blood 105, 2324–2331 [DOI] [PubMed] [Google Scholar]
- 22.Tanaka A., Muto S., Konno M., Itai A., Matsuda H. (2006) Cancer Res. 66, 419–426 [DOI] [PubMed] [Google Scholar]
- 23.Bulavin D. V., Demidov O. N., Saito S., Kauraniemi P., Phillips C., Amundson S. A., Ambrosino C., Sauter G., Nebreda A. R., Anderson C. W., Kallioniemi A., Fornace A. J., Jr., Appella E. (2002) Nat. Genet. 31, 210–215 [DOI] [PubMed] [Google Scholar]
- 24.Pahl H. L. (1999) Oncogene 18, 6853–6866 [DOI] [PubMed] [Google Scholar]
- 25.Doman R. K., Perez M., Donato N. J. (1999) J. Interferon Cytokine Res. 19, 261–269 [DOI] [PubMed] [Google Scholar]
- 26.Doi T. S., Takahashi T., Taguchi O., Azuma T., Obata Y. (1997) J. Exp. Med. 185, 953–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janeway C. A., Jr., Medzhitov R. (2002) Annu. Rev. Immunol. 20, 197–216 [DOI] [PubMed] [Google Scholar]
- 28.Hayden M. S., West A. P., Ghosh S. (2006) Oncogene 25, 6758–6780 [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi H., Minopoli G., Demidov O. N., Chatterjee D. K., Anderson C. W., Durell S. R., Appella E. (2005) Biochemistry 44, 5285–5294 [DOI] [PubMed] [Google Scholar]
- 30.Yu E., Ahn Y. S., Jang S. J., Kim M. J., Yoon H. S., Gong G., Choi J. (2007) Breast Cancer Res. Treat. 101, 269–278 [DOI] [PubMed] [Google Scholar]
- 31.Demidov O. N., Kek C., Shreeram S., Timofeev O., Fornace A. J., Appella E., Bulavin D. V. (2007) Oncogene 26, 2502–2506 [DOI] [PubMed] [Google Scholar]
- 32.Saklatvala J. (2004) Curr. Opin. Pharmacol. 4, 372–377 [DOI] [PubMed] [Google Scholar]
- 33.Schieven G. L. (2005) Curr. Top. Med. Chem. 5, 921–928 [DOI] [PubMed] [Google Scholar]
- 34.Perkins N. D. (2007) Nat. Rev. Mol. Cell Biol. 8, 49–62 [DOI] [PubMed] [Google Scholar]
- 35.Fujioka S., Schmidt C., Sclabas G. M., Li Z., Pelicano H., Peng B., Yao A., Niu J., Zhang W., Evans D. B., Abbruzzese J. L., Huang P., Chiao P. J. (2004) J. Biol. Chem. 279, 27549–27559 [DOI] [PubMed] [Google Scholar]
- 36.Chew J., Biswas S., Shreeram S., Humaidi M., Wong E. T., Dhillion M. K., Teo H., Hazra A., Fang C. C., López-Collazo E., Bulavin D. V., Tergaonkar V. (2009) Nat. Cell Biol. 11, 659–666 [DOI] [PubMed] [Google Scholar]
- 37.Wu X., Bayle J. H., Olson D., Levine A. J. (1993) Genes Dev. 7, 1126–1132 [DOI] [PubMed] [Google Scholar]
- 38.Van Waes C. (2007) Clin. Cancer Res. 13, 1076–1082 [DOI] [PubMed] [Google Scholar]
- 39.Baud V., Karin M. (2009) Nat. Rev. Drug Discov. 8, 33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viatour P., Merville M. P., Bours V., Chariot A. (2005) Trends Biochem. Sci. 30, 43–52 [DOI] [PubMed] [Google Scholar]
- 41.Dajee M., Lazarov M., Zhang J. Y., Cai T., Green C. L., Russell A. J., Marinkovich M. P., Tao S., Lin Q., Kubo Y., Khavari P. A. (2003) Nature 421, 639–643 [DOI] [PubMed] [Google Scholar]
- 42.Demidov O. N., Timofeev O., Lwin H. N., Kek C., Appella E., Bulavin D. V. (2007) Cell Stem Cell 1, 180–190 [DOI] [PubMed] [Google Scholar]
- 43.Fan M., Ahmed K. M., Coleman M. C., Spitz D. R., Li J. J. (2007) Cancer Res. 67, 3220–3228 [DOI] [PubMed] [Google Scholar]
- 44.Chen X., Shen B., Xia L., Khaletzkiy A., Chu D., Wong J. Y., Li J. J. (2002) Cancer Res. 62, 1213–1221 [PubMed] [Google Scholar]
- 45.Ahmed K. M., Li J. J. (2008) Free Radic. Biol. Med. 44, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim B. Y., Kim K. A., Kwon O., Kim S. O., Kim M. S., Kim B. S., Oh W. K., Kim G. D., Jung M., Ahn J. S. (2005) Carcinogenesis 26, 1395–1403 [DOI] [PubMed] [Google Scholar]
- 47.Eliseev R. A., Zuscik M. J., Schwarz E. M., O'Keefe R. J., Drissi H., Rosier R. N. (2005) J. Cell. Biochem. 96, 1262–1273 [DOI] [PubMed] [Google Scholar]
- 48.Munshi A., Kurland J. F., Nishikawa T., Chiao P. J., Andreeff M., Meyn R. E. (2004) Mol. Cancer Ther. 3, 985–992 [PubMed] [Google Scholar]
- 49.Aravindan N., Madhusoodhanan R., Ahmad S., Johnson D., Herman T. S. (2008) Cancer Biol. Ther. 7, 569–576 [DOI] [PubMed] [Google Scholar]
- 50.Tsuboi Y., Kurimoto M., Nagai S., Hayakawa Y., Kamiyama H., Hayashi N., Kitajima I., Endo S. (2009) J. Neurosurg. 110, 594–604 [DOI] [PubMed] [Google Scholar]