Abstract
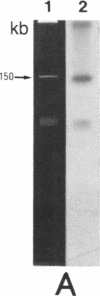
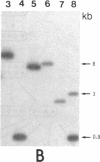
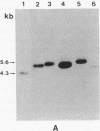
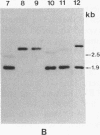
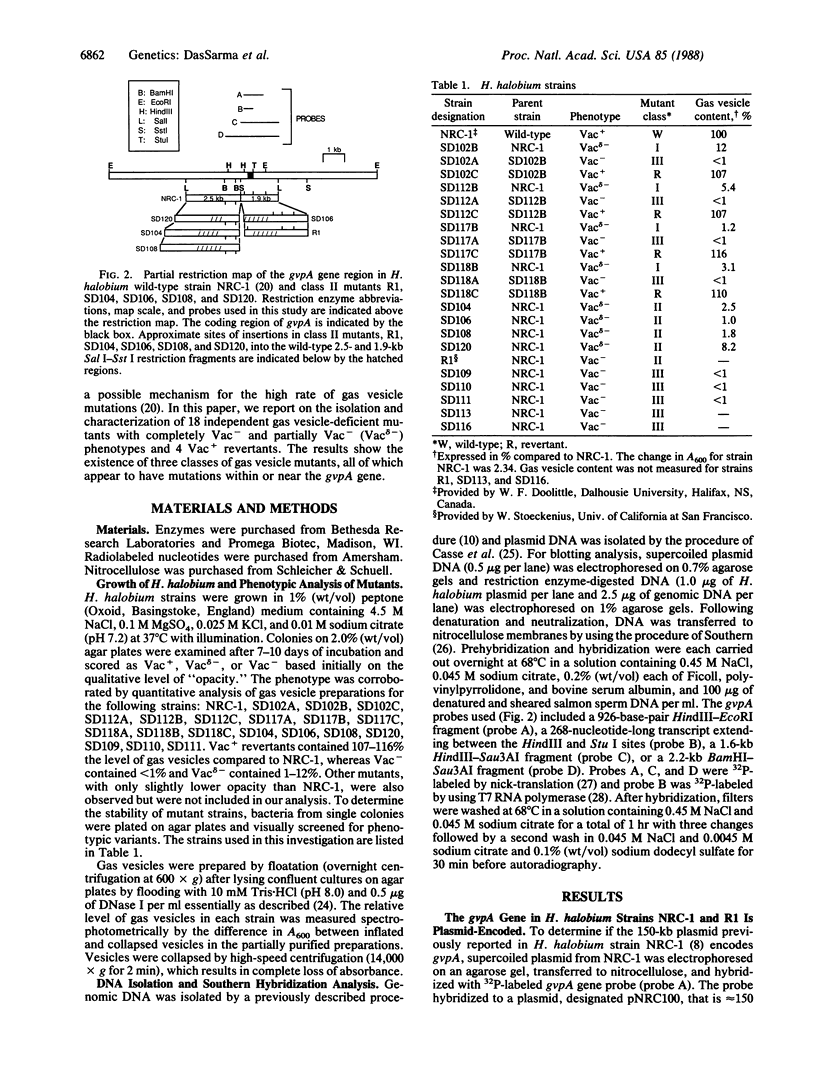
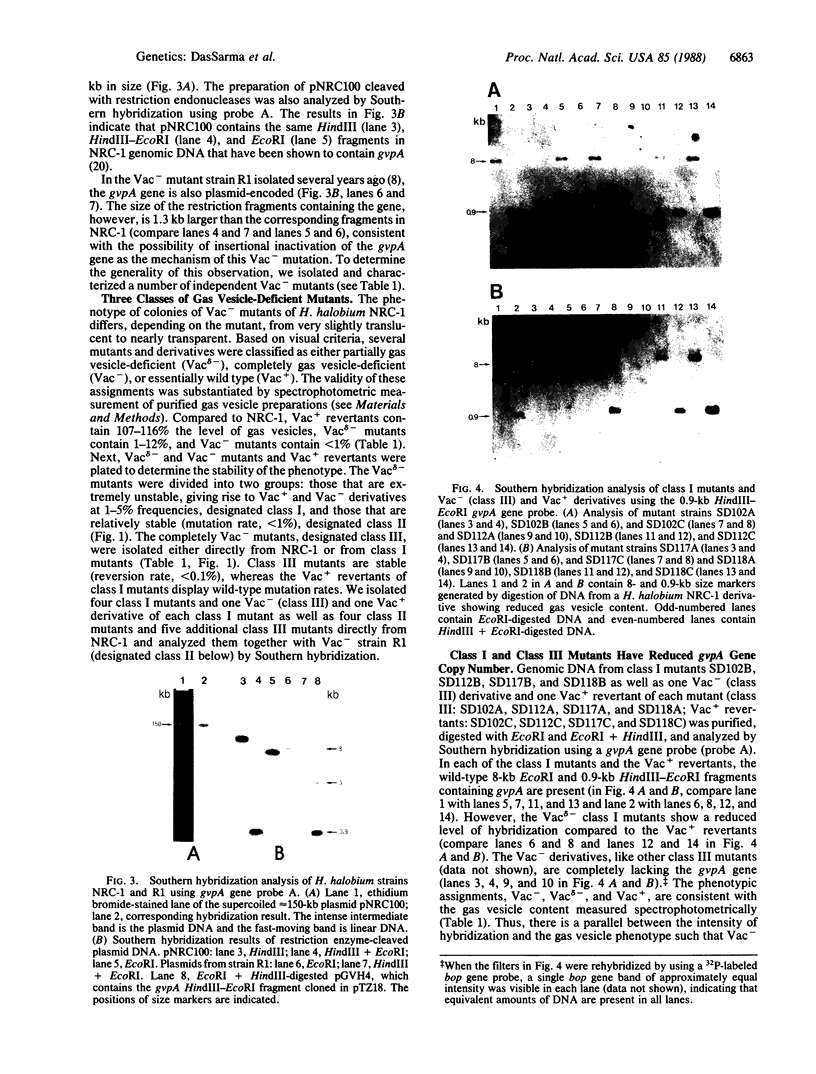
Gas vesicle-deficient mutants of Halobacterium halobium arise spontaneously at high frequency (about 1%). The mutants are readily detected, forming translucent colonies on agar plates in contrast to opaque wild-type colonies. To investigate the mechanism of this mutation, we recently cloned a plasmid-encoded gas vesicle protein gene, gvpA, from H. halobium. In the wild-type NRC-1 strain the gvpA gene is encoded by a multicopy plasmid of ≈150 kilobase pairs (kb). We have now characterized 18 gas vesicle-deficient mutants and 4 revertants by phenotypic and Southern hybridization analyses. Our results indicate that the mutants fall into three major classes. Class I mutants are partially gas vesicle-deficient (Vacδ-) and unstable, giving rise to completely gas vesicle-deficient (Vac-) derivatives and Vac+ revertants at frequencies of 1-5%. The restriction map of the gvpA gene region in class I mutants is unchanged but the gene copy number is reduced compared to the Vac+ strains. Class II mutants can be either Vacδ- or completely Vac- but are relatively stable. They contain insertion sequences within or upstream of the gvpA gene. A Vac- class II mutant, R1, contains the 1.3-kb insertion sequence, ISH3, within the gvpA gene, whereas four Vacδ- class II mutants contain other insertion sequences upstream of the gene. Class III mutants are stable Vac- derivatives of either the wild-type or class I mutants and have no detectable copies of the gvpA gene. Based on these results, we discuss the mechanisms of gas vesicle mutations in H. halobium.
Keywords: gvp gene, transposable elements, insertion sequence from Halobacterium, bacterio-opsin gene, plasmid instability
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayley S. T., Morton R. A. Recent developments in the molecular biology of extremely halophilic bacteria. CRC Crit Rev Microbiol. 1978;6(2):151–205. doi: 10.3109/10408417809090622. [DOI] [PubMed] [Google Scholar]
- Betlach M., Friedman J., Boyer H. W., Pfeifer F. Characterization of a halobacterial gene affecting bacterio-opsin gene expression. Nucleic Acids Res. 1984 Oct 25;12(20):7949–7959. doi: 10.1093/nar/12.20.7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betlach M., Pfeifer F., Friedman J., Boyer H. W. Bacterio-opsin mutants of Halobacterium halobium. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1416–1420. doi: 10.1073/pnas.80.5.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasSarma S., Damerval T., Jones J. G., Tandeau de Marsac N. A plasmid-encoded gas vesicle protein gene in a halophilic archaebacterium. Mol Microbiol. 1987 Nov;1(3):365–370. doi: 10.1111/j.1365-2958.1987.tb01943.x. [DOI] [PubMed] [Google Scholar]
- DasSarma S., RajBhandary U. L., Khorana H. G. High-frequency spontaneous mutation in the bacterio-opsin gene in Halobacterium halobium is mediated by transposable elements. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2201–2205. doi: 10.1073/pnas.80.8.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassarma S., Rajbhandary U. L., Khorana H. G. Bacterio-opsin mRNA in wild-type and bacterio-opsin-deficient Halobacterium halobium strains. Proc Natl Acad Sci U S A. 1984 Jan;81(1):125–129. doi: 10.1073/pnas.81.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn R., McCoy J., Simsek M., Majumdar A., Chang S. H., Rajbhandary U. L., Khorana H. G. The bacteriorhodopsin gene. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6744–6748. doi: 10.1073/pnas.78.11.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney T. D., Lessie T. G. Insertion-sequence-dependent rearrangements of Pseudomonas cepacia plasmid pTGL1. J Bacteriol. 1987 Jan;169(1):224–230. doi: 10.1128/jb.169.1.224-230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman J. D., Schalkwyk L. C., Doolittle W. F. ISH51: a large, degenerate family of insertion sequence-like elements in the genome of the archaebacterium, Halobacterium volcanii. Nucleic Acids Res. 1986 Sep 11;14(17):6983–7000. doi: 10.1093/nar/14.17.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz M. J., Ballou C. E. Analysis of Halobacterium halobium gas vesicles. J Bacteriol. 1973 Jun;114(3):1058–1067. doi: 10.1128/jb.114.3.1058-1067.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen H., Omang S., Steensland H. On the gas vacuoles of the halobacteria. Arch Mikrobiol. 1967;59(1):197–203. doi: 10.1007/BF00406332. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Plasmid incompatibility. Microbiol Rev. 1987 Dec;51(4):381–395. doi: 10.1128/mr.51.4.381-395.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B. C., Rownd R. H. Recombination sites in plasmid drug resistance gene amplification. J Bacteriol. 1985 Dec;164(3):1359–1361. doi: 10.1128/jb.164.3.1359-1361.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer F., Betlach M. Genome organization in Halobacterium halobium: a 70 kb island of more (AT) rich DNA in the chromosome. Mol Gen Genet. 1985;198(3):449–455. doi: 10.1007/BF00332938. [DOI] [PubMed] [Google Scholar]
- Pfeifer F., Weidinger G., Goebel W. Genetic variability in Halobacterium halobium. J Bacteriol. 1981 Jan;145(1):375–381. doi: 10.1128/jb.145.1.375-381.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sapienza C., Doolittle W. F. Unusual physical organization of the Halobacterium genome. Nature. 1982 Feb 4;295(5848):384–389. doi: 10.1038/295384a0. [DOI] [PubMed] [Google Scholar]
- Simon R. D. Halobacterium strain 5 contains a plasmid which is correlated with the presence of gas vacuoles. Nature. 1978 May 25;273(5660):314–317. doi: 10.1038/273314a0. [DOI] [PubMed] [Google Scholar]
- Simsek M., DasSarma S., RajBhandary U. L., Khorana H. G. A transposable element from Halobacterium halobium which inactivates the bacteriorhodopsin gene. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7268–7272. doi: 10.1073/pnas.79.23.7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Tandeau de Marsac N., Mazel D., Bryant D. A., Houmard J. Molecular cloning and nucleotide sequence of a developmentally regulated gene from the cyanobacterium Calothrix PCC 7601: a gas vesicle protein gene. Nucleic Acids Res. 1985 Oct 25;13(20):7223–7236. doi: 10.1093/nar/13.20.7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toeckenius W., Kunau W. H. Further characterization of particulate fractions from lysed cell envelopes of Halobacterium halobium and isolation of gas vacuole membranes. J Cell Biol. 1968 Aug;38(2):337–357. doi: 10.1083/jcb.38.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidinger G., Klotz G., Goebel W. A large plasmid from Halobacterium halobium carrying genetic information for gas vacuole formation. Plasmid. 1979 Jul;2(3):377–386. doi: 10.1016/0147-619x(79)90021-0. [DOI] [PubMed] [Google Scholar]
- Xu W. L., Doolittle W. F. Structure of the archaebacterial transposable element ISH50. Nucleic Acids Res. 1983 Jun 25;11(12):4195–4199. doi: 10.1093/nar/11.12.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]