Abstract
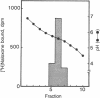
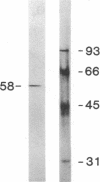
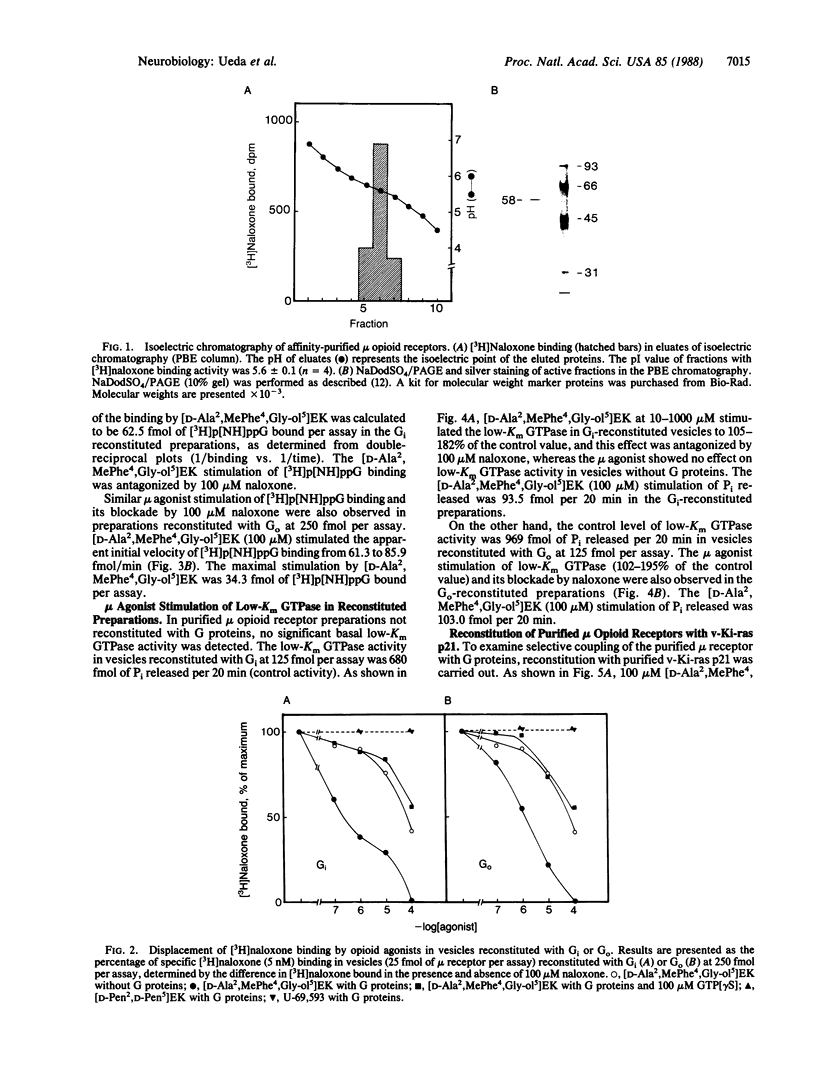
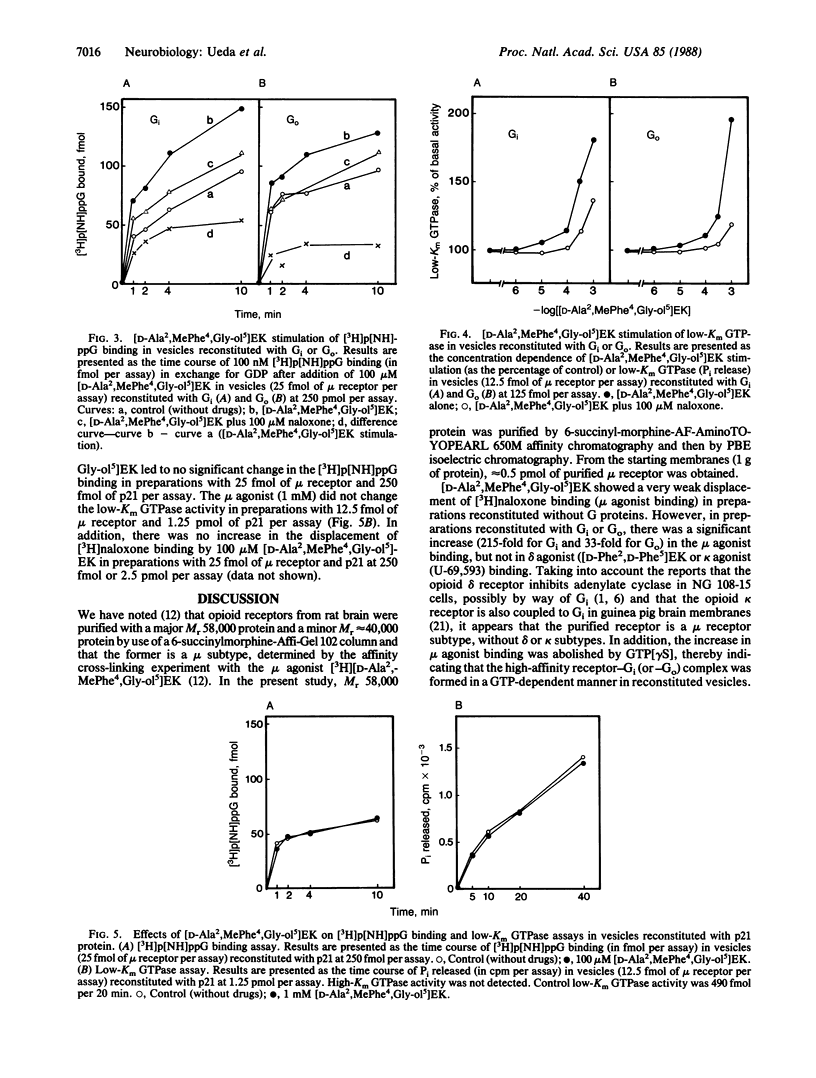
Reconstitution of purified mu opioid receptors with purified guanine nucleotide-binding regulatory proteins (G proteins) was investigated. mu opioid receptors were purified by 6-succinylmorphine AF-AminoTOYOPEARL 650M affinity chromatography and by PBE isoelectric chromatography. The purified mu opioid receptor (pI 5.6) migrated as a single Mr 58,000 polypeptide by NaDodSO4/PAGE, a value identical to that obtained by affinity cross-linking purified mu receptors. When purified mu receptors were reconstituted with purified Gi, the G protein that mediates the inhibition of adenylate cyclase, the displacement of [3H]naloxone (a mu opioid antagonist) binding by [D-Ala2,MePhe4,Gly-ol5]enkephalin (a mu opioid agonist) was increased 215-fold; this increase was abolished by adding 100 microM (guanosine 5'-[gamma-thio]triphosphate. Similar increases in agonist displacement of [3H]naloxone binding (33-fold) and its abolition by guanosine 5'-[gamma-thio]triphosphate were observed with Go, the G protein of unknown function, but not with the v-Ki-ras protein p21. In reconstituted preparations with Gi or Go, neither [D-Pen2,D-Pen5]enkephalin (a delta opioid agonist; where Pen is penicillamine) nor U-69,593 (a kappa opioid agonist) showed displacement of the [3H]naloxone binding. In addition, the mu agonist stimulated both [3H]guanosine 5'-[beta,gamma-imido]triphosphate binding (in exchange for GDP) and the low-Km GTPase in such reconstituted preparations, with Gi and Go but not with the v-Ki-ras protein p21, in a naloxone-reversible manner. The stoichiometry was such that the stimulation of 1 mol of mu receptor led to the binding of [3H]guanosine 5'-[beta,gamma-imido]triphosphate to 2.5 mol of Gi or to 1.37 mol of Go. These results suggest that the purified mu opioid receptor is functionally coupled to Gi and Go in the reconstituted phospholipid vesicles.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abood M. E., Law P. Y., Loh H. H. Pertussis toxin treatment modifies opiate action in the rat brain striatum. Biochem Biophys Res Commun. 1985 Mar 15;127(2):477–483. doi: 10.1016/s0006-291x(85)80185-6. [DOI] [PubMed] [Google Scholar]
- Beals C. R., Wilson C. B., Perlmutter R. M. A small multigene family encodes Gi signal-transduction proteins. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7886–7890. doi: 10.1073/pnas.84.22.7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Gross R. A., Macdonald R. L. Dynorphin A selectively reduces a large transient (N-type) calcium current of mouse dorsal root ganglion neurons in cell culture. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5469–5473. doi: 10.1073/pnas.84.15.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga K., Haga T. Purification of the muscarinic acetylcholine receptor from porcine brain. J Biol Chem. 1985 Jul 5;260(13):7927–7935. [PubMed] [Google Scholar]
- Hara M., Tamaoki T., Nakano H. Guanine nucleotide binding properties of purified v-Ki-ras p21 protein produced in Escherichia coli. Oncogene Res. 1988 May;2(4):325–333. [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Trautwein W., Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. 1987 Jan 29-Feb 4Nature. 325(6103):445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Itoh H., Kozasa T., Nagata S., Nakamura S., Katada T., Ui M., Iwai S., Ohtsuka E., Kawasaki H., Suzuki K. Molecular cloning and sequence determination of cDNAs for alpha subunits of the guanine nucleotide-binding proteins Gs, Gi, and Go from rat brain. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3776–3780. doi: 10.1073/pnas.83.11.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katada T., Oinuma M., Kusakabe K., Ui M. A new GTP-binding protein in brain tissues serving as the specific substrate of islet-activating protein, pertussis toxin. FEBS Lett. 1987 Mar 23;213(2):353–358. doi: 10.1016/0014-5793(87)81521-1. [DOI] [PubMed] [Google Scholar]
- Katada T., Oinuma M., Ui M. Two guanine nucleotide-binding proteins in rat brain serving as the specific substrate of islet-activating protein, pertussis toxin. Interaction of the alpha-subunits with beta gamma-subunits in development of their biological activities. J Biol Chem. 1986 Jun 25;261(18):8182–8191. [PubMed] [Google Scholar]
- Kim M. H., Neubig R. R. Membrane reconstitution of high-affinity alpha 2 adrenergic agonist binding with guanine nucleotide regulatory proteins. Biochemistry. 1987 Jun 16;26(12):3664–3672. doi: 10.1021/bi00386a061. [DOI] [PubMed] [Google Scholar]
- Kurose H., Katada T., Amano T., Ui M. Specific uncoupling by islet-activating protein, pertussis toxin, of negative signal transduction via alpha-adrenergic, cholinergic, and opiate receptors in neuroblastoma x glioma hybrid cells. J Biol Chem. 1983 Apr 25;258(8):4870–4875. [PubMed] [Google Scholar]
- Kurose H., Katada T., Haga T., Haga K., Ichiyama A., Ui M. Functional interaction of purified muscarinic receptors with purified inhibitory guanine nucleotide regulatory proteins reconstituted in phospholipid vesicles. J Biol Chem. 1986 May 15;261(14):6423–6428. [PubMed] [Google Scholar]
- Kurose H., Ui M. Dual pathways of receptor-mediated cyclic GMP generation in NG108-15 cells as differentiated by susceptibility to islet-activating protein, pertussis toxin. Arch Biochem Biophys. 1985 May 1;238(2):424–434. doi: 10.1016/0003-9861(85)90183-3. [DOI] [PubMed] [Google Scholar]
- Lahti R. A., Mickelson M. M., McCall J. M., Von Voigtlander P. F. [3H]U-69593 a highly selective ligand for the opioid kappa receptor. Eur J Pharmacol. 1985 Feb 26;109(2):281–284. doi: 10.1016/0014-2999(85)90431-5. [DOI] [PubMed] [Google Scholar]
- North R. A., Williams J. T., Surprenant A., Christie M. J. Mu and delta receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5487–5491. doi: 10.1073/pnas.84.15.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper C. M., Henderson G. Opiates and opioid peptides hyperpolarize locus coeruleus neurons in vitro. Science. 1980 Jul 18;209(4454):394–395. doi: 10.1126/science.7384811. [DOI] [PubMed] [Google Scholar]
- Sharma S. K., Nirenberg M., Klee W. A. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci U S A. 1975 Feb;72(2):590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E. J., Dole W. P., Hiller J. M. Coupling of a new, active morphine derivative to sepharose for affinity chromatography. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1835–1837. doi: 10.1073/pnas.69.7.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H., Harada H., Misawa H., Nozaki M., Takagi H. Purified opioid mu-receptor is of a different molecular size than delta- and kappa-receptors. Neurosci Lett. 1987 Apr 10;75(3):339–344. doi: 10.1016/0304-3940(87)90546-5. [DOI] [PubMed] [Google Scholar]
- Ueda H., Misawa H., Fukushima N., Takagi H. The specific opioid kappa-agonist U-50,488H inhibits low Km GTPase. Eur J Pharmacol. 1987 Jun 12;138(1):129–132. doi: 10.1016/0014-2999(87)90348-7. [DOI] [PubMed] [Google Scholar]