Abstract
Thought disorder as well as language and communication disturbances are associated with schizophrenia and are over-represented in clinically unaffected relatives of schizophrenics. All three kinds of dysfunction involve some element of deviant verbalizations, most notably, semantic anomalies. Of particular importance, thought disorder characterized primarily by deviant verbalizations has a higher recurrence in relatives of schizophrenic patients than schizophrenia itself. These findings suggest that deviant verbalizations may be more penetrant expressions of schizophrenia susceptibility genes than schizophrenia. This paper reviews the evidence documenting the presence of thought, language and communication disorders in schizophrenic patients and in their first-degree relatives. This familial aggregation potentially implicates genetic factors in the etiology of thought disorder, language anomalies, and communication disturbances in schizophrenia families. We also present two examples of ways in which thought, language and communication disorders can enrich genetic studies, including those involving schizophrenia.
Keywords: Schizophrenia, Thought disorder, Language disorders, Communication disorders, Semantic anomalies, Genetics, Family studies, Linkage analyses, Endophenotypes
Thought Disorder and Schizophrenia
Due largely to the influence of Eugen Bleuler (Bleuler, 1911/1950), thought disorder was considered virtually pathognomonic of the diagnosis of schizophrenia for many years. Kraepelin (Kraepelin, 1896/1919) is not credited with having had as much influence as Bleuler in this respect, but he too was impressed with the centrality of disturbed thinking in dementia praecox. Kraepelin considered “derailments in the train of thought,” which he named “akataphasia,” to be a fundamental characteristic of the dementia of dementia praecox (Kraepelin, 1896/1919) (p. 72). Manifestations of derailment included loosening of associations (“the most different ideas follow one another with most bewildering want of connection...” p. 56) and incoherence (“bewilderingly nonsensical utterances ... apparently represent a senseless jiggling of words” p. 71).1 Bleuler (Bleuler, 1911/1950) considered “disorders of association” to be one of the four fundamental characteristics of the schizophrenias. He relegated the more conspicuous psychotic indicators, delusions and hallucinations, to secondary status as “accessory” symptoms, or derivatives of the primary ones. Bleuler described the disrupted associative process as follows: “... associations lose their continuity ... thinking becomes illogical and often bizarre ... two or more ideas are condensed into a single one.” (p. 14). Further, “... associations do not become entirely senseless, but they still appear odd, bizarre, distorted ...” (p. 19), and “in some cases, all the threads between thoughts are torn.” (p.20) Such disorders of association could, according to both Bleuler and Kraepelin, present in a variety of ways - as incoherence, bizarre associations, clangs, condensations, stereotypy, illogicality, etc. The persisting primacy of thought disorder in the diagnostic schema is also evident in Meehl’s much later answer to the question, “What kind of behavioral fact about the patient leads us to diagnose schizophrenia?” Despite the presence of a variety of other symptoms of schizophrenia, the statement, “‘Naturally, I am growing my father’s hair’,” was, for Meehl, the “diagnostic bell-ringer.” (Meehl, 1977) (p. 138)
Many efforts have been made to capture the essence of schizophrenic thought disorder since the pioneering descriptions of Kraepelin and Bleuler. Some formulations focused on specific attributes of thinking like overinclusive (Cameron, 1938) or bizarre-idiosyncratic qualities (M. Harrow & Quinlan, 1985). Others emphasized dichotomies like concrete vs abstract thinking (Goldstein, 1944) and primary vs secondary process thought (Fenichel, 1945). Chapman and Chapman (Chapman & Chapman, 1973) identified a number of key domains of disordered thought in schizophrenia (e.g., reasoning, concept formation, reality testing). A variety of scales were developed to assess the varied manifestations of thought disorder, usually in the context of an interview or standardized tests (N. Andreasen, 1979a, 1979b; N. Andreasen & Grove, 1986; Cancro, 1968; Caplan, 1994; M. Harrow, Harkavy, Bromet, & Tucker, 1973; M. Harrow & Quinlan, 1977; M. Harrow, Tucker, Himmelhoch, & Putnam, 1972; Johnston & Holzman, 1979; Liddle et al., 2002; Perry, Geyer, & Braff, 1999; Perry, Minassian, Cadenhead, Sprock, & Braff, 2003; Solovay et al., 1986). Many of the categories for classifying thought disorder overlap across scales; moreover, what appears to be the same quality of disturbed thinking is named differently in the various scales.
There is now general agreement that thought disorder is multidimensional, that it occurs both in schizophrenic and nonschizophrenic psychotic conditions, and that its manifestations cover a spectrum of severity (N. Andreasen, 1979b; N. Andreasen & Grove, 1986; M. Harrow, Grossman, & Silverstein, 1986; M. Harrow et al., 1973; M. Harrow & Quinlan, 1977; P. Harvey, Earle-Boyer, & Wielgus, 1984; Holzman, Shenton, & Solovay, 1986; Johnston & Holzman, 1979; Kufferle, Lenz, & Schanda, 1985; Oltmanns, Murphy, Berenbaum, & Dunlop, 1985; Shenton, Solovay, & Holzman, 1987; Solovay, Shenton, & Holzman, 1987; Spohn et al., 1986; Taylor, Reed, & Berenbaum, 1994). Andreasen and colleagues (N. Andreasen, 1979b; N. Andreasen & Grove, 1986), for example, noted that both manic and schizophrenic patients had elevated scores on the Scale for the Assessment of Thought, Language and Communication (TLC). Similarly, Holzman and colleagues showed that psychotic patients, whether schizophrenic, schizo-affective or manic, had elevated total Thought Disorder Index (TDI) scores compared with nonpsychotic patients and controls (Holzman et al., 1986; Johnston & Holzman, 1979; Shenton et al., 1987; Solovay et al., 1987; Spohn et al., 1986).
Despite the non-specificity of thought disorder per se, certain qualities of disturbed thought tend to be distinctive of one psychotic disorder but not of others, whereas other forms of thought disorder are nonspecific concomitants of psychotic state. Harrow and colleagues (M. Harrow et al., 1973; M Harrow & Marengo, 1986; M. Harrow et al., 1972) showed that overinclusiveness was a non-specific feature of acute psychosis; in contrast, idiosyncratic thinking was present in acutely psychotic schizophrenic patients, tended to persist during partial remission and was unusual in nonschizophrenics. Andreasen (N. Andreasen, 1979b) found that poverty of speech and poverty of content were more characteristic of schizophrenia than of mania. Pressured speech, clanging, distractible speech, and circumstantiality were more strongly associated with mania. The two patient groups did not differ in the frequency of other indicators of thought disorder (derailment, tangentiality, illogicality, incoherence, and loss of goal). During the initial stage of an acute episode, the distinguishing features of thought disorder in manic and schizophrenic patients remain stable (P. Harvey et al., 1984). Holzman and colleagues (Holzman et al., 1986; Shenton et al., 1987; Solovay et al., 1987) noted that vagueness and inappropriate distance were nonspecific expressions of thought disorder in psychotic patients. Manic thought disorder was characterized primarily by extravagantly (and often playfully) combined ideas and irrelevant intrusions. The thought disorder of schizophrenics, on the other hand, was characterized by disorganization and fluidity, and included many idiosyncratic words or phrases. Adolescent-onset psychotic disorders show the same pattern of shared and distinctive components of thought disorder observed in adult-onset cohorts (Makowski et al., 1997). The thought disorder of schizo-affective patients tends to resemble primarily that of schizophrenic patients (N. Andreasen & Grove, 1986; Holzman et al., 1986; Shenton et al., 1987; Solovay et al., 1987; Spohn et al., 1986). Regardless of the particular method of measuring it, all forms of thought disorder are not present in any single patient. Further, individual patients vary in the severity and specific expressions of thought disorder that they show at any given time. Thus, substantial evidence supports the idea that some thought disorder profiles are strongly associated with individual psychotic disorders and others are non-specific indicators of psychotic state.
The persistence of some level of thought disorder, even during periods of remission and drug treatment, is especially notable in schizophrenic patients. Severity of thought disorder is, in part, state- and medication-related (N. M. Docherty, Schnur, & Harvey, 1988; M. Harrow, Grossman, Silverstein, & Meltzer, 1982; M Harrow & Marengo, 1986; Hurt, Holzman, & Davis, 1983; Spohn et al., 1986). Antipsychotic medication reduces thought disorder, as measured with the TDI (Hurt et al., 1983; Spohn et al., 1986). Drug treatment tends to target the more severe forms of thought disorder, but mild forms of thought disorder remain detectable, acutely, chronically and episodically (M Harrow & Marengo, 1986; Hurt et al., 1983; Marengo & Harrow, 1987; Spohn et al., 1986). These residual forms of thought disorder, which are present during both acute episodes and remission, appear to be more trait-related and less state-dependent than the more severe forms of thought disorder; they include peculiar use of language, disorganization, disconnected speech, and verbal underproductivity (N. Andreasen & Grove, 1986; P. Harvey et al., 1984; P. D. Harvey, Docherty, Serper, & Rasmussen, 1990; Marengo & Harrow, 1987; Spohn et al., 1986). Thought disorder in manic patients is much more state-dependent, including a frequent return to normal levels during clinical remission (N. Andreasen & Grove, 1986; M Harrow & Marengo, 1986; Spohn et al., 1986). More severe thought disorder, especially in schizophrenic patients, is strongly predictive of worse functional and social outcomes (Bowie & Harvey, 2008; M Harrow & Marengo, 1986; M Harrow, Silverstein, & Marengo, 1983; Keefe et al., 1987; Marengo & Harrow, 1987; Racenstein, Penn, Harrow, & Schleser, 1999).
Thought Disorder, Language Disorder, Speech Disorder, and Communication Disorder
The relative contributions of thought, language, speech, and communication to what is called “thought disorder” are a topic of active debate and vigorous investigation. Although Kraepelin (Kraepelin, 1896/1919) and Bleuler (Bleuler, 1911/1950) considered a disorder of thought to have primacy over a disorder of speech or language, they also recognized that thought, language, and speech were inextricably linked. They viewed verbal communication as the primary vehicle through which disordered thoughts were conveyed. Indeed, Bleuler explicitly bemoaned the fact that “we are forced to deduce most of the anomalies from the oral and written productions of the patients.” (p. 39) Kraepelin referred not only to “derailments in the train of thought” (p. 72), but also to “derailments in the expression of thought in speech” (p. 70) and to “disorders in connected speech” (p. 67) as well as to “confused syntax” (p. 71). “Derailments in linguistic expression,” he wrote, “form a specially important domain in the speech disorders of dementia praecox” (p. 65) and “influence in a morbid way the form of speech.” (p. 70)
Later investigators also grappled with the unclear boundaries between thought, language and speech. The distinctions among these three domains remain incompletely resolved (see (McKenna & Oh, 2005) for an extensive discussion of this topic). In particular, the validity of the assumption that language or speech can be used as proxies for thought has provoked considerable commentary (Brown, 1973; E. Chaika, 1974, 1990; P. D. Harvey & Neale, 1983; Holzman et al., 1986; M.T. Singer & Wynne, 1963). Rochester and Martin (Rochester & Martin, 1979) explicitly noted the circular reasoning that surrounds efforts to distinguish between disordered thought and disordered speech or language. They wrote, “... thought disorder is when talk is incoherent. And talk is incoherent when the thought is disordered.” (p. 6). Andreasen (N. Andreasen, 1979a) also acknowledged this close but unclear connection, observing that “disorganized speech is a more accurate term for the behavior” that is being assessed than thought disorder (p. 1316). She described the 18 components of the TLC as “language behaviors” that could be considered “subtypes of thought disorder” (p. 1316). McKenna and Oh (McKenna & Oh, 2005) refer to “thought-disordered schizophrenic speech” (p. 183). Even the names of the scales, for example, the Scale for the Assessment of Thought, Language and Communication (N. Andreasen, 1979a) and the Thought and Language Index (TLI) (Liddle et al., 2002) attest to the difficulty separating thought, language, and speech in evaluating behavior that is largely dependent upon verbal productions. Indeed, the somewhat convoluted language evident in the quotes above reflects the persisting ambiguities about the boundaries between these domains. In recognition of how interwoven thought, language, and speech are, some investigators instead emphasize failures of communication: “communication deviance” (M. Singer & Wynne, 1966a; M.T. Singer & Wynne, 1963; M.T. Singer & Wynne, 1966b; Wynne, 1967; Wynne & Singer, 1965; Wynne, Singer, Bartko, & Toohey, 1977), “communication disturbance” (N. M. Docherty, DeRosa, & Andreasen, 1996), “discourse failures” (Rochester & Martin, 1979), “communicative competence” (McKenna & Oh, 2005), and “communication abnormalities” (Bowie & Harvey, 2008). Whether this is a semantic “solution” or a substantive one is yet to be determined. It is, however, far from surprising that certain categories of communication disturbance, like certain categories of thought and language disorder, are non-specific concomitants of psychosis (e.g., confused references, structural ambiguities). Others are over-represented in schizophrenic patients (vague references, missing information references) or in manic patients (ambiguous word meanings). Some are stable features of the illness that appear to be independent of symptom (or thought disorder) severity (N. M. Docherty, Cohen, Nienow, Dinzeo, & Dangelmaier, 2003; N. M. Docherty et al., 1996; N. M. Docherty et al., 1988).
Despite these persisting ambiguities, there is growing recognition that aspects of language and speech production in schizophrenia are compromised at various linguistic processing levels (Covington et al., 2005; DeLisi, 2001; Marini et al., 2008). Crow (Crow, 1997, 1998, 2008) has linked language deviance and its associated reduced cerebral asymmetry to the genetic etiology of schizophrenia (cf also (Manoach, 1994) and Crow, this issue). Various kinds of linguistic abnormalities have been reported in schizophrenic patients. Starting with the seminal studies by the Chapmans (Chapman & Chapman, 1973; Chapman, Chapman, & Miller, 1964), numerous investigators have examined two types of semantic deficits in schizophrenia: abnormal semantic activation (both hyper-priming and hypo-priming), and impaired use of context. Although a review of these vast literatures is beyond the scope of this paper, it is important to note that a general consensus is emerging regarding the relation between semantic processing deficits and thought disorder.
With respect to abnormal semantic activation in schizophrenia, a recent meta-analysis (Pomarol-Clotet, Oh, Laws, & McKenna, 2008) identified 36 semantic priming studies involving schizophrenic and control participants. They examined whether group differences in priming differed as a function of variables known to be important for the interpretation of semantic priming data (e.g., stimulus onset asynchrony, type of semantic relationship, patient characteristics). Group comparisons of schizophrenic patients and control subjects showed little evidence of hyper- or hypo-priming in schizophrenia. However, when schizophrenics were stratified on the basis of high and low levels of thought disorder, high levels of thought disorder were associated with greater priming. Thus, excessive semantic activation during the initial stages of word processing may be fundamentally related to schizophrenic thought disorder, a conclusion that is also consistent with recent work (Kreher, Goff, & Kuperberg, 2009; Kuperberg, Deckersbach, Holt, Goff, & West, 2007).
Other studies have shown that schizophrenic patients have difficulty making optimal use of context. People with schizophrenia have difficulty noticing semantic violations in sentences [e.g., (Kuperberg, Kreher, Goff, McGuire, & David, 2006)], using context to resolve lexical ambiguity [e.g., (Salisbury, 2008; Sitnikova, Salisbury, Kuperberg, & Holcomb, 2002; Titone, Levy, & Holzman, 2000)], comprehending figurative language such as idioms and metaphors [e.g., (Titone, Holzman, & Levy, 2002; Titone, Libben, Niman, Ranbom, & Levy, 2007)], and quickly generating coherent inferences during discourse processing [e.g., (Ditman & Kuperberg, 2007)]. The causes of these “context processing” deficits remain unclear, however. One possibility is that schizophrenic individuals experience impaired maintenance of context information in working memory (Cohen, Barch, Carter, & Servan-Schreiber, 1999). Accordingly, a failure to make use of context would arise because relevant contextual information is not available. A second possibility is that schizophrenic individuals successfully encode and maintain context in working memory but nevertheless have difficulty inhibiting or suppressing irrelevant meanings on the basis of this information (Titone et al., 2002; Titone & Levy, 2004; Titone et al., 2000; Titone et al., 2007). Such deficient inhibition might be especially profound under conditions in which there is excessive semantic activation of contextually irrelevant meanings, as described previously. Moreover, some combination of both mechanisms may simultaneously contribute to context processing deficits in schizophrenia [e.g., (Salisbury, 2008)].
Abnormal syntax has also been reported in schizophrenia by some investigators (Fraser, King, Thomas, & Kendell, 1986; Morice & Ingram, 1982; Tavano et al., 2008), but not by others (E. O. Chaika & Alexander, 1986; Maher, 1972, 1983; Rodriguez-Ferrera, McCarthy, & McKenna, 2001). One possible explanation for conflicting findings was offered by Oh et al. (Oh, McCarthy, & McKenna, 2002), who showed that syntactic errors occurred independent of thought disorder in schizophrenics, whereas semantic anomalies were strongly associated with the presence of thought disorder. Thus, stratifying schizophrenics on the basis of the presence or absence of thought disorder may be essential for distinguishing linguistic correlates of schizophrenia from linguistic correlates of schizophrenic thought disorder.
The strongest support for language deviations in schizophrenia comes from the study of semantic anomalies. An excess of semantic errors has been reported in many studies of schizophrenic patients (Cameron, 1938; Covington et al., 2005; Fraser et al., 1986; Morice & Ingram, 1982; Rochester & Martin, 1979; Rodriguez-Ferrera et al., 2001). Semantic anomalies and deficits in semantic processing are also associated with thought disorder in schizophrenic patients (Goldberg et al., 1998; J.G. Kerns & Berenbaum, 2002; John G. Kerns, Berenbaum, Barch, Banich, & Stolar, 1999; Oh et al., 2002; Stirling, Hellewell, Blakey, & Deakin, 2006). McKenna & Oh (McKenna & Oh, 2005) recently speculated that, if “language abnormality does make a contribution to the phenomenon of thought disorder in schizophrenia,” it would be “characterized chiefly by semantic errors in expressed speech.” (p. 99)
The Key Feature of Deviant Verbalizations is Anomalous Semantics
“‘When I use a word,’ Humpty Dumpty said in a rather scornful tone, ‘it means just what I choose it to mean - neither more nor less.’” (Carroll, 1872) (p. 124)
“‘When I make a word do a lot of work like that,’ said Humpty Dumpty, ‘I always pay it extra.’
‘Oh!’ said Alice. She was too much puzzled to make any other remark.” (Carroll, 1872) (p. 125).
Idiosyncratic use of language involves a real word or phrase that is used in an awkward, peculiar, or strange way. A word that means one thing is used to mean something else, or individual words are combined in a way that does not make sense given their individual meanings.2 As a result of these semantic violations, the intended meaning of the utterance is unknown to the listener. When words acquire such private meanings, the ideas being conveyed leave the listener confused. Indeed, the very concept of assigning personal definitions to words is inherently absurd, because it undermines the dependence of social interaction on using words in ways that reflect their shared meanings. Witness Alice’s consternation at Humpty Dumpty’s readiness to violate this implicit principle of social discourse. Privatizing the meaning of real words, as Humpty Dumpty proposed to do, derails not only “linguistic expression” but also human engagement in a fundamental way.
Word usage involving such semantic anomalies is a prominent feature of most attempts to systematize the thought, language and communication disorders associated with schizophrenia. Table 1 contains a number of examples (the awkwardness of translation aside). These semantic irregularities are a core feature of schizophrenic thought pathology. Deviant word usage has been incorporated into scales designed to assess formal thought disorder (M Harrow & Prosen, 1979; M. Harrow & Quinlan, 1985; Johnston & Holzman, 1979; Solovay et al., 1986) as well as those that examine language and thought (N. Andreasen, 1979a; Liddle et al., 2002) and language and speech (Chen et al., 1996). Some of the examples of poverty of content of speech, word approximations, derailment, and incoherence in the TLC ((N. Andreasen, 1979a, 1979b) would be scored as peculiar word usage in other systems, such as the TDI and TLI. Semantic anomalies have also been included in scales designed to characterize interference with effective communication (N. M. Docherty, 1993; N. M. Docherty et al., 1996; N.M. Docherty, Miller, & Lewis, 1997; N. M. Docherty, Rhinewine, Labhart, & Gordiner, 1998a; McKenna & Oh, 2005; Rochester & Martin, 1979; Wynne & Singer, 1965). Although Singer & Wynne’s work (M.T. Singer, 1967; M. T. Singer & Wynne, 1965a; M.T. Singer & Wynne, 1966b; M. T. a. W. Singer, L. C., 1965b; Wynne, 1967; Wynne & Singer, 1965) was directed at transactional/communication patterns within families, some of their categories of communication deviance (“underspecific syncretic terms,” “intrusions of primary process,” “crypticness,” and “offbeat responses conveyed in an arbitrary, unexplained fashion”) would be classified in scoring schemes like the TDI and the TLI as peculiar word usage (M. T. Singer & Wynne, 1965a) (pp. 193-195). The “approximation metonyms” (word approximations) described by Cameron (Cameron, 1938) (p. 20) and included in the TLC also bear striking similarity to verbalizations that would be classified as peculiar word usage in other scales. “Peculiar language” and “unintelligible remarks” are two of the categories in Docherty’s scale of language deviance (N. M. Docherty, 1993) (p. 753). Wrong word references and ambiguous word meanings are key components of Docherty’s Communication Deviance Index (CDI) (N. M. Docherty et al., 2003; N. M. Docherty et al., 1996; N.M. Docherty et al., 1997; N. M. Docherty et al., 1998a), which was developed explicitly “to assess ... clarity or unclarity of meanings in speech” (N. M. Docherty et al., 1996) (p. 361). Thus, deviant verbalizations involving semantic violations have been recognized as a core characteristic of thought disorder, anomalous language, and communication disturbances. Although the various scales use different names to describe deviant verbalizations, we would suggest that all of these terms refer to the same phenomenon.
Table 1. Examples of Deviant Use of Language, or Anomalous Semantics.
| Kraepelin (Kraepelin, 1896/1919) |
| “The brain-navel of the merchants’ association” a |
| “One cannot take the direction from the reflection” a |
| “Suffering hunger is stronger than in all deaf-mutes.” a |
| “I have gone through much for the German language.” a |
| “I have voluntary disease of the eyes.” b |
| “I am national-liberal chased away.” b |
| “I have a suspended appetite.” c |
| “Life is a dessert-spoon.” d |
| “We are already standing in the spiral under a hammer.” d |
| “Death will be awakened by the golden dagger.” d |
| “The consecrated discourse cannot be over split in any movement.” d |
| “I don’t know what I am to do here, it must be the aim, that means to steal with the gentleman.” e |
| The doctor has collected my four senses.” f |
| Bleuler (Bleuler, 1911/1950) |
| “The mountains which are outlined in the swellings of the oxygen are beautiful.” g |
| “The rosary ‘was a prayer multiplier and this in turn is a prayer for multiplying and as such is nothing else but a prayer-mill, and is therefore a mill-prayer machine which is again a prayer-machine mill.’” g |
| “I have never been in Hamburg, Lubeck or Berne; I have never seen Professor Hilty; I have never been to the University of Basel; I have never seen Luther, not ever had the lütter (a vulgar expression for diarrhea). But I have already seen all the members of the legislature...” I |
| Wynne & Singer (Wynne & Singer, 1965) (p. 194) |
| “some sort of species for a special occasion” |
| “a blended color species” |
| “Imagination is the worst nation in the world.” |
| Scale for the Assessment of Thought, Language, and Communication (N. Andreasen, 1979a) (p. 1320) |
| “handshoes” [referring to gloves] |
| “time vessel” [referring to a watch] |
| Thought Disorder Index (Johnston & Holzman, 1979; Solovay et al., 1986) (includes only peculiar verbalizations, identified by italic font) |
| “They are an equal species.” [referring to a mirror image] |
| “It had the fishing smell.” |
| “Rectangularly speaking” |
| “It has a nasal look to it.” |
| “The mineral of its substance” |
| “The capitalization of native Americans” |
| “It was up to place with the surroundings.” |
| “It looks like an x axis in origin.” |
| “It is darker here and interacts the eye.” |
| “A heartwhelmed blessing” |
| “This is a duplicate side leaf. It’s geometrical.” |
| “Because of the dimensions of vision, it looks like it is ...” |
p. 72
p. 71
p. 70-71
p.56
pp. 56-57
p. 67
p. 19
p. 28
p. 30
The Co-familiality of Thought, Language and Communication Disorders
The presence of subtle cognitive slippage in nonschizophrenic relatives of schizophrenic patients and in individuals who presented with symptoms that used to be called latent or pseudoneurotic schizophrenia has been noted for many years (Bleuler, 1911/1950) [cf (K. S. Kendler, 1985)]. Meehl (Meehl, 1977) purposefully included such mild thought disorder, which he subsumed under the concept of “cognitive slippage,” as part of the “schizotypic tetrad” (p. 145), or the four core “schizotypal source traits “ (p. 143): cognitive slippage, interpersonal aversiveness, anhedonia and ambivalence. Thus, from early on it was recognized that qualitatively less severe cognitive disturbances could be present in nonschizophrenic individuals who either had attenuated forms of schizophrenia or who were biologically related to individuals with the disorder. Early empirical studies emphasized that the cognitive slippage observed in family members was “subclinical,” in order to distinguish it from the more florid thought disorder observed in patients.
The first modern empirical studies of thought disorder in clinically unaffected relatives were carried out by McConaghy (McConaghy, 1959) and Rosenthal (Rosenthal, 1962). In their combined samples, they observed that at least one parent of each of 15 schizophrenic patients showed indications of subtle thought slippage. Since then, an impressive literature has consistently documented that adult nonschizophrenic first-degree relatives of schizophrenics have increased levels of thought disorder compared with relatives of nonpsychotic patients and nonpsychiatric controls (Arboleda & Holzman, 1985; Hain, Maier, Hoechst-Janneck, & Francke, 1995; Johnston & Holzman, 1979; Perry et al., 2003; Shenton, Solovay, Holzman, Coleman, & Gale, 1989a; M.T. Singer & Wynne, 1963; Solovay et al., 1987). Mild thought disorder was one of several nonpsychotic signs and behaviors that Kendler et al (K.S. Kendler, McGue, Gruenberg, & Walsh, 1995) found to be over-represented in relatives of schizophrenics. Children of schizophrenic mothers and children who later developed schizophrenia have also been shown to have increased attentional disturbances as well as cognitive dysfunctions, including mild thought disorder (Arboleda & Holzman, 1985; Parnas, Schulsinger, Schulsinger, Mednick, & Teasdale, 1982; Watt & Anthony, 1984). Both clinical and psychometric schizotypes show increased amounts of thought disorder (Coleman, Levy, Lenzenweger, & Holzman, 1996; Holzman et al., 1995; Perry et al., 2003). Of particular importance, studies that used the TDI showed that the thought disorder found in well relatives, though usually milder, was qualitatively similar to that found in schizophrenic patients. Idiosyncratic verbalizations were the distinctive feature of both the patients and their relatives(Johnston & Holzman, 1979; Shenton et al., 1989a). Consistent with these findings, Baskak et al. (Baskak, Ozel, Atbasoglu, & Baskak, 2008) reported increased peculiar word usage in nonpsychotic siblings of schizophrenics. Studies using the TDI have also shown that manic patients and their clinically unaffected relatives have similar thought disorder profiles. The distinguishing quality of the thought disorder in these latter two groups was the over-representation of extravagantly combinatory thinking, but not idiosyncratic verbalizations (Johnston & Holzman, 1979; Shenton et al., 1989a).
Communication deviance and communication disturbances also show a propensity to aggregate significantly among biological relatives of schizophrenics. Several independent studies have shown that families of adult schizophrenic patients could be differentiated from families of nonschizophrenics on the basis of communication deviance (Hirsch & Leff, 1975; M. T. Singer & Wynne, 1965a; M. T. a. W. Singer, L. C., 1965b; Wynne & Singer, 1965; Wynne et al., 1977). Similarly, elevated language deviance and CDI scores have been reported in nonschizophrenic parents of schizophrenic patients. Moreover, higher CDI scores in parents were associated with greater severity of illness in probands (N. M. Docherty, 1993; N. M. Docherty, Gordinier, Hall, & Cutting, 1999; N.M. Docherty et al., 1997; N. M. Docherty et al., 1998a). Children of schizophrenics show a higher frequency of unclear references than children of patients with affective disorders or controls (P. Harvey, Weintraub, & Neale, 1982). Notably, a key factor that distinguished parents of young adult schizophrenics from parents of children with other psychiatric conditions was thought disorder with “peculiar content” (M.T. Singer & Wynne, 1963) (p.240). In Docherty’s studies of communication disturbance (N. M. Docherty et al., 1999; N.M. Docherty et al., 1997), the categories that were over-represented in parents were what could be considered variations of idiosyncratic verbalizations (use of vague, overinclusive words, use of words with ambiguous meanings or vague referents).
There is a remarkable parallelism between studies of relatives and those of patients. Whether described as language, communication or thought disorders, mild cognitive slippage has consistently been reported in clinically unaffected relatives of schizophrenics, a finding that suggests that it can occur independent of the disease or its treatment.
Whether the origin of the co-familiality is genetic, environmental, or some interaction between them cannot be determined conclusively from these studies. However, twin and adoption studies are relevant to this issue. Data from twin studies are inconclusive, but provide some intriguing clues. Although one study of normal twins (Gambini, Campana, Macciardi, & Scarone, 1997) found significantly higher intra-class coefficients for total TDI scores in MZ twins (0.87) compared with DZ twins (0.39), the sample was too small to permit a definitive conclusion. They were able to reject a model of no additive genetic variance with heritability between 80-90%, but could not distinguish a model with additive genetic variance from one in which shared family environment accounted for within-pair similarity. Using an abbreviated version of the TLC, Berenbaum and colleagues (Berenbaum, Oltmanns, & Gottesman, 1985) found no difference between the intra-class correlations of MZ and DZ twin pairs in which at least one member of a pair was schizophrenic. They concluded that there were familial resemblances, but that thought disorder was not under genetic control. Such a conclusion is premature in part because the categories of the TLC that pertain to the kinds of mild word peculiarities that are characteristic of schizophrenia in remission and that are over-represented in nonpsychotic relatives were excluded from consideration. For a discussion of additional issues that limit the conclusiveness of this study see Matthysse & Holzman (S. Matthysse & Holzman, 1988). Finally, Docherty and Gottesman (N. M. Docherty & Gottesman, 2000) examined three types of referential communication disturbances measured by the CDI (missing information, ambiguous word meanings, structural unclarities) in the same Maudsley twin pairs from the Gottesman & Shields (Gottesman & Shields, 1972) sample that had been studied by the Berenbaum group (Berenbaum et al., 1985). They found a significantly higher frequency of missing references in the non-schizophrenic MZ co-twins than in their DZ counterparts. This difference was even larger when the diagnoses were based on those of the diagnostician with the highest MZ:DZ ratio. Interestingly, a similar finding emerged in the Berenbaum study (Berenbaum et al., 1985): thought disorder was more strongly related to the diagnoses of the diagnostician with the highest MZ:DZ ratio, who considered mild instances of idiosyncratic verbalizations as one of the criteria for a diagnosis of schizophrenia.
Although the co-familiality of thought and language disorders could suggest that these traits may be potentially helpful in identifying non-penetrant gene carriers, the possible contribution of environmental factors cannot be ruled out. Adoption studies allow the influence of biological and environmental contributions to be distinguished. Two such studies have been carried out. Kinney and colleagues (Kinney et al., 1997) found that the biological relatives of schizophrenic adoptees, who shared genetic risk but not environmental risk with the probands, had significantly higher TDI scores than the adoptive relatives of schizophrenics, who shared environmental risk but not genetic risk with the probands. Similar findings were reported by Wahlberg et al. (Wahlberg et al., 2000). They compared the TDI scores of the offspring of schizophrenic and control mothers who had been adopted soon after birth. No one in either group of adoptees was diagnosed with schizophrenia or with a schizophrenia spectrum disorder. The total TDI scores of the two groups of adoptees did not differ significantly, but adopted biological offspring of schizophrenic mothers had a significantly larger proportion of idiosyncratic verbalizations than the adopted offspring of control mothers. That the total TDI score would be a less specific discriminator than deviant verbalizations is not surprising since the total score is comprised of many categories that are not specifically associated with schizophrenia. Consistent with both adoption studies, Zahn (Zahn, 1968) found that the biological parents of schizophrenics had significantly more deviant associations than their adoptive parents.
Taken together, thought, language and communication disorders seem to be both symptoms of schizophrenia as well as possible indicators of a genetic predisposition that becomes exacerbated with the onset of the disease. They are over-represented in relatives of schizophrenics compared with controls. Whether there is a genetic basis for this familial aggregation remains unclear because the evidence for the heritability of this form of cognitive dysfunction is still inconclusive.
Incorporating Thought, Language and Communication Disorders into Genetic Studies of Schizophrenia
The strong association of thought, language and communication disorders with schizophrenia and their over-representation in clinically unaffected first-degree relatives of schizophrenics suggest that one or more aspects of these behaviors may productively inform genetic studies of schizophrenia. Semantic anomalies, in particular, seem to be a consistently identified linguistic component of the disease and possibly of genetic liability for it. In the discussion below we present two examples, each illustrating a different stage of genetic exploration, of ways in which disturbances in thought, language and communication can enrich genetic studies, including those on schizophrenia.
Example 1
We begin with a case study of a language disorder with a known genetic cause and describe how the discovery of that gene and knowledge about its molecular biology and inheritance informed genetic studies of diseases in which language anomalies are a prominent component. Although the interaction of many genes is undoubtedly involved in a trait as complex as language (Marcus & Fisher, 2003), identifying any one of these genes and its functions can help to clarify the molecular genetic foundations of language and of the ways in which its normal expressions can be disrupted. In this particular example, interactions between this gene and other genes turned out to be relevant to the genetic dissection of both schizophrenia and autism.
This case study begins with the discovery of family KE, a multigenerational pedigree in which a developmental speech and language disorder segregated as an autosomal dominant trait (Hurst, Baraitser, Auger, Graham, & Norell, 1990). The clinical phenotype includes difficulties with speech articulation, impaired use of grammar, and abnormalities in language production and comprehension as well as nonverbal learning disabilities (Vargha-Khadem, Watkins, Alcock, Fletcher, & Passingham, 1995). The relative contributions of language deficits and motor control to the clinical presentation has been controversial (Marcus & Fisher, 2003; Stromswold, 2008). A mutation involving a single nucleotide sequence change in the FOXP2 gene has been shown to segregate with this clinical disorder (Lai, Fisher, Hurst, Vargha-Khadem, & Monaco, 2001). Moreover, disruptions in FOXP2 are associated more generally with anomalous language and speech milestones (MacDermot et al., 2005; Shriberg et al., 2006; Zeesman et al., 2006).
The FOXP2 gene is just one of a number of genes that encode forkhead box (FOX) transcription factors (see (Hannenhalli & Kaestner, 2009) for an overview). Among other things, transcription factors are proteins that regulate expression of downstream target genes, thereby influencing the rate and amount of protein transcribed by the targets (Marcus & Fisher, 2003). FOXP2 has at least 285 targets in the fetal human brain, including the inferior frontal cortex (Spiteri et al., 2007; S.C. Vernes et al., 2007), a region that is involved in linguistic processing (Buckner, Raichle, & Petersen, 1995; Demonet et al., 1995; Fiez, 1997; Gough, Nobre, & Devlin, 2005; Petersen, Fox, Posner, Mintun, & Raichle, 1989). These targets play a role in regulating synaptic plasticity, axon guidance, and cortical patterning (Fukuchi-Shimogori & Grove, 2001; Terzic & Saraga-Babic, 1999), among others. Thus, a disruption in the FOXP2 gene is likely to have far-reaching consequences. Indeed, the complex phenotype associated with the FOXP2 mutation in family KE is likely to result from the effects of the disrupted FOXP2 protein on multiple targets.
Genes that are regulated by FOXP2 are plausible candidates for having a role in speech and language. Indeed, Crespi (Crespi, 2007) has hypothesized that FOXP2 may play a critical role in the evolution of human language. One of the target genes regulated by FOXP2 is CNTNAP2; expression of CNTAP2 is decreased when expression of FOXP2 is increased (S.C. Vernes et al., 2008). Expression of CNTNAP2 is preferentially enriched in anterior cortical regions of the human brain that are involved in higher cognitive functions, including language (Abrahams et al., 2007). Variants in CNTNAP2 have been associated with a number of clinical disorders in which disturbances in language and/or speech are a prominent feature. Both common (Alarcon et al., 2008; Arking et al., 2008) and rare (Bakkaloglu et al., 2008; Strauss et al., 2006) variants of CNTNAP2 are associated with autism spectrum disorders as well as with language delay (age at first word) in children from multiplex autism families (Alarcon et al., 2008). A mutation in CNTNAP2 also segregates with a recessive seizure disorder associated with a complex phenotype that includes language regression (Stromswold, 2008). Vernes and colleagues recently reported an association between a quantitative trait, nonsense-word (pronounceable non-real words, e.g., “brufid”) repetitions, and variants in CNTNAP2 in individuals with specific language impairment (S.C. Vernes et al., 2008). The sample had been screened specifically to exclude other neurodevelopmental disorders (such as autism spectrum disorders) that are commonly accompanied by language-related deficits (S.C. Vernes et al., 2008), including deficits in nonsense-word repetition (Kjelgaard & Tager-Flusberg, 2001; A.J. Whitehouse, Barry, & Bishop, 2007; A.J. Whitehouse, Barry, & Bishop, 2008). Rare variants in CNTNAP2 are one of a number of genomic rearrangement “hotspots” that are also associated with schizophrenia (Friedman et al., 2008; Stone, O’Donovan, Gurling, al, & Consortium, 2008), another disorder in which misuse of language is associated with the illness (and over-represented in unaffected relatives). Even more intriguing, CNTNAP2 is a member of the Neurexin gene family and an excess of rare variants in neurexin1 has been associated with both schizophrenia ((Kirov et al., 2008; Rujescu et al., 2008; Walsh et al., 2008) and autism (Kim et al., 2008; Marshall et al., 2008; Szatmari et al., 2007).
These findings suggest that alterations in CNTNAP2 (and possibly other related genes) may disrupt a neurodevelopmental process that plays a critical role in the timing and maintenance of various language functions. CNTNAP2 variants are implicated in specific language/speech disorders as well as in clinical syndromes in which language disturbance is one of several behavioral deficits. Thus, a shared genetic risk factor may contribute to certain language-related deficits in a variety of clinical disorders even though different genetic variants are risk factors for other behavioral features of complex phenotypes. The FOXP2-CNTNAP2 pathway is only one of many that warrants examination, but it provides a worked example of the ways in which a diversity of language-related phenotypes can inform the insights gleaned from genetic studies.
Example 2
In contrast to the case study above, specific causal genes are often not known. This is the case for schizophrenia. Power to detect risk genes is limited, in part by genetic heterogeneity, non-Mendelian modes of transmission, and relatively low recurrence of the disease in first-degree relatives. Traits associated with schizophrenia may, however, have higher heritability and result from simpler genetic mechanisms than schizophrenia. Thus, it may be easier to detect schizophrenia risk genes through their effects on such associated traits (i.e., endophenotypes) than through their effects on the disease phenotype. In this second example, we consider how one such trait, thought disorder, can be useful for augmenting the power of linkage analysis of schizophrenia susceptibility loci. Improving power to detect susceptibility loci common to both the disease and the endophenotype is the primary rationale for incorporating endophenotypes into linkage studies (Holzman & Matthysse, 1990; S. Matthysse & Parnas, 1992). Recurrence risk for schizophrenia in first-degree relatives of schizophrenic patients is only about 6.5% (K.S. Kendler et al., 1993a), much lower than for disorders with classic Mendelian transmission. This recurrence risk is too low to have adequate power to detect linkage in feasible sample sizes even when it is present, and results in an unacceptably high false negative rate (S. Matthysse & Parnas, 1992). Traits that are both strongly associated with schizophrenia and that occur in relatives at a higher rate than schizophrenia may be variable (or pleiotropic) manifestations of the same gene or genes that confer risk for the clinical disorder (S. Matthysse, Holzman, & Lange, 1986). Such “enriched” traits, or endophenotypes (Gottesman & Gould, 2003), may have a higher genetic “signal” than the clinical disorder alone and could serve as useful pointers to a gene(s) (Lander, 1988). Thus, the higher prevalence of these schizophrenia-related co-familial traits may increase power to detect a major locus that is associated with the genetic transmission of schizophrenia by improving the accurate identification of non-penetrant gene carriers (Botstein & Risch, 2003; Holzman & Matthysse, 1990; S. Matthysse & Parnas, 1992).
In the typical family study of endophenotypes, probands are ascertained through a diagnosis of schizophrenia and siblings are examined for recurrence on an endophenotype. This is an “asymmetrical” ascertainment design and it has different requirements for estimating power from the more typical symmetrical ascertainment design, whose power parameters have been delineated extensively in the elegant work of Risch (Risch, 1990c). We recently calculated power to detect linkage of an endophenotype when an asymmetrical ascertainment design is used. We noted that, for this design, power is a function of the difference between the endophenotype risk in siblings of individuals with a disease and the endophenotype risk in the general population when the penetrances of both the endophenotype and the disease are non-additive (Sung, Ji, Levy, Matthysse, & Mendell, 2009). Figure 1 graphs the power of model-free analysis vs. risk difference for 40 additive and non-additive genetic models and shows the close correspondence between power and the risk difference. In this model-free analysis, we compared the average number of alleles identical by descent with the proband in siblings with the abnormal endophenotype and in siblings with the normal endophenotype. The graph shows that there is a near linear relation between power and risk difference when the penetrances of both the endophenotype and the disease depart significantly from additivity if the pleiotropic allele frequency is not high (e.g., <0.06) (the two circled values). The model-based method of analysis results in power uniformly > 90% whenever the risk difference in siblings of schizophrenics and the general population is at least 0.20. Since the genetic model for schizophrenia is unknown, the data for the power function in the model-free case are particularly relevant: as relative risk differences approach ~ 0.25, power to detect linkage exceeds 80% and as the relative risk differences approach 0.30, power is close to 100%.
Figure 1.
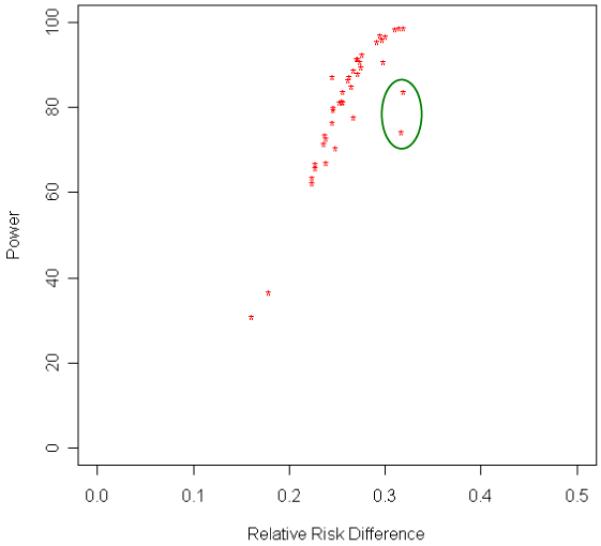
Power as a Function of the Difference in Risk for a Disease-related Endophenotype (thought disorder with “schizophrenic” features) in Siblings of Probands with a Disease (schizophrenia) and in the General Population Using Model-free Methods. The generating genetic parameters include both additive and non-additive penetrance values. The near linear relation between power and risk difference holds when the penetrances of both the endophenotype and the disease are not additive and the pleiotropic allele frequency is <0.06. Smaller departures from additivity do not impact power as much. When the allele frequency is high (>0.06), departures from this near linear relation between power and risk difference occur (two circled model-free analysis power values). This graph is based on a sample of 174 asymmetrically ascertained sib pairs. The recombination fraction between marker and disease/endophenotype locus equals 0.01.
The genetic parameter values used to obtain the results shown in figure 1 were those values that fit the observed data on five epidemiological variables: the frequency of thought disorder in schizophrenic patients, the frequency of thought disorder in siblings of schizophrenics, the frequency of thought disorder in the general population, the frequency of schizophrenia in siblings of schizophrenic patients, and the frequency of schizophrenia in the general population. The linkage parameter value, the recombination fraction, was set at 0.01. The parameter values were identified on the basis of an unbiased search of the range of parameter values for a major gene model with pleiotropic effects. The values reported are those parameter values that generated risk values that were consistent with the interval estimates provided by our data. Full details can be found in elsewhere (Levy et al., 2009; Sung et al., 2009).
The data on the observed frequency of thought disorder were based on data obtained using the TDI (Johnston & Holzman, 1979; Solovay et al., 1986). Verbatim transcripts of tape-recorded responses to 10 cards of the Rorschach were scored for various kinds of thought slippage by expert raters who were blind to group identity. The raters then assigned each protocol a “TDI diagnosis,” a qualitative designation that indicates whether or not the thought disorder that was present had “schizophrenic features,” which is defined primarily by excess deviant verbalizations. The sample consisted of 105 schizophrenic patients, 133 of their clinically unaffected full siblings and 86 nonpsychiatric controls. Individuals were considered unaffected if they did not meet criteria for a psychosis or bipolar disorder without psychosis (lifetime) or for schizotypal, schizoid or paranoid personality disorder. Full details about the clinical assessment and diagnostic procedures can be found elsewhere (Levy et al., 2009). The values for the five epidemiological values were as follows: risk of thought disorder with schizophrenic features - 72.4%, 39.85% and 11.6% in schizophrenic patients, siblings of schizophrenics and nonpsychiatric controls, respectively; risk of schizophrenia - 8.9% and 0.65% in siblings of schizophrenics and the general population, respectively (Slater, 1968).
We calculated the power of an analysis based on a sample of 174 sib pairs for each of the 40 sets of parameter values that fit the observed data. Power to detect linkage in a sample of this size was >70% in 33/40 of these parameter values.
These findings suggest that if schizophrenia and deviant verbalizations are pleiotropic effects of a major gene, power to detect such a locus is excellent in sample sizes of sibling pairs that can be realistically obtained when an asymmetric ascertainment scheme is used. Moreover, the power calculations described above take into account endophenotype-positive and endophenotype-negative siblings and thus are not restricted to affected sibling pairs. The results provide a more optimistic justification for incorporating endophenotypes into linkage studies of bivariate phenotypes than was previously thought using designs that did not make use of the asymmetric sampling technique discussed here (Faraone et al., 1995). This same approach could be applied to other indices of thought, language and communication disorders in schizophrenia and in other conditions in which impairments in these functions are a prominent component of the behavioral phenotype.
The two examples described above reinforce the potential value of using measures of thought, language and communication disturbances to enrich the potential yield of genetic studies of psychiatric and neurodevelopmental disorders.
In summary, we have reviewed research demonstrating that thought disorder as well as language and communication disturbances are associated with schizophrenia. Deviant verbalizations, manifested primarily in semantic anomalies, are a conspicuous feature of all three types of dysfunction. Thought disorder characterized by deviant verbalizations is also over-represented in the first-degree biological relatives of schizophrenic patients, occurring at a rate substantially higher than that of schizophrenia itself. This familial aggregation suggests that genetic factors may be involved in the etiology of thought disorder, language anomalies, and communication disturbances in schizophrenia families. Two examples, each illustrating a different stage of genetic exploration, highlight the potential usefulness of thought, language and communication disorders in enriching genetic studies of schizophrenia.
Acknowledgements
This study was supported in part by NIMH grants R01 MH071523 and MH31340, the Sidney R. Baer, Jr. Foundation, the Essel Foundation, and the National Association for Research on Schizophrenia and Depression.
Footnotes
“... we hear from our patients a great many quite incomprehensible and disconnected utterances, in which it can scarcely be only a question of disorders of linguistic expression, even though it is impossible in the individual case to discover the inner mechanism by which the utterances arose....” (p. 72)
“Here it is no longer the transference to expression in speech that is morbidly influenced, but the ideas aroused by the circumstances are themselves already in their origin pushed aside or suppressed by ideas related but lying remote or opposed to the original ones.” (p. 73)
Neologisms are a severe form of idiosyncratic verbalization, but they occur rarely. Other severe forms, such as the absurd category of the TDI, are also relatively rare in patients and are uncharacteristic of the milder thought slippage found in relatives.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams BS, Tentler D, Perederiy JV, Oldham MC, Coppola G, Geschwind DH. Genome-wide analyses of human perisylvian cerebral cortical patterning. Proc Nat’l Acad Sci. 2007;104:17849–17854. doi: 10.1073/pnas.0706128104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N. Thought, language and communication disorders. I. Clinical assessment, definition of terms, and evaluation of their reliability. Arch Gen Psychiatry. 1979a;36:1315–1321. doi: 10.1001/archpsyc.1979.01780120045006. [DOI] [PubMed] [Google Scholar]
- Andreasen N. Thought, language, and communication disorders: II. Diagnostic significance. Arch Gen Psychiatry. 1979b;36:1325–1330. doi: 10.1001/archpsyc.1979.01780120055007. [DOI] [PubMed] [Google Scholar]
- Andreasen N, Grove W. Thought, language and communication in schizophrenia: diagnosis and prognosis. Schiz Bull. 1986;12:348–359. doi: 10.1093/schbul/12.3.348. [DOI] [PubMed] [Google Scholar]
- Arboleda C, Holzman PS. Thought disorder in children at risk for psychosis. Arch Gen Psychiatry. 1985;42:1004–1013. doi: 10.1001/archpsyc.1985.01790330084010. [DOI] [PubMed] [Google Scholar]
- Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, et al. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 2008;82:160–164. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkaloglu B, O’Roak BJ, Louvi A, Gupta AR, Abelson JF, Morgan TM, et al. Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am J Hum Genet. 2008;82:165–173. doi: 10.1016/j.ajhg.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskak B, Ozel ET, Atbasoglu EC, Baskak SC. Peculiar word use as a possible trait marker in schizophrenia. Schiz Res. 2008;103:311–317. doi: 10.1016/j.schres.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, Oltmanns TF, Gottesman II. Formal thought disorder in schizophrenics and their twins. J Abnormal Psychol. 1985;94:3–16. doi: 10.1037//0021-843x.94.1.3. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Dementia Praecox or the Group of Schizophrenias. International Universities Press; New York: 19111950. [Google Scholar]
- Botstein D, Risch N. Discovering genotypes underlying human phenotypes: Past successes for Mendelian disease, future approaches for complex disease. Nat Genet. 2003;33(suppl):228–237. doi: 10.1038/ng1090. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Harvey PD. Communication abnormalities predict functional outcomes in chronic schizophrenia: Differential association with social and adaptive functions. Schiz Res. 2008;103:240–247. doi: 10.1016/j.schres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RW. Schizophrenia, language, and reality. Amer Psychologist. 1973;28:395–403. doi: 10.1037/h0034694. [DOI] [PubMed] [Google Scholar]
- Buckner RI, Raichle ME, Petersen SE. Dissociation of human prefrontal areas across different speech production tasks and gender groups. J Neurophysiol. 1995;74:2163–2173. doi: 10.1152/jn.1995.74.5.2163. [DOI] [PubMed] [Google Scholar]
- Cameron N. Reasoning, regression and communication in schizophrenics. Psychol Monogr (Whole No. 221) 1938 [Google Scholar]
- Cancro R. Thought disorder and schizophrenia. Diseases of the Nervous System. 1968;28:846–848. [PubMed] [Google Scholar]
- Caplan R. Communication deficits in childhood schizophrenia spectrum disorders. Schiz Bull. 1994;20:671–683. doi: 10.1093/schbul/20.4.671. [DOI] [PubMed] [Google Scholar]
- Carroll L. Through the Looking-glass and What Alice Found There. William Morrow & Co., Inc.; New York: 1872. [Google Scholar]
- Chaika E. A linguist looks at schizophrenic thought disorder. Brain & Language. 1974;1:257–276. [Google Scholar]
- Chaika E. Understanding Psychotic Speech: Beyond Freud and Chomsky. Thompsoon; Springfield, IL: 1990. [Google Scholar]
- Chaika EO, Alexander P. The ice cream stories: A study of normal and psychotic narrations. Discourse Processes. 1986;7:305–328. [Google Scholar]
- Chapman LJ, Chapman JP. Disordered Thought in Schizophrenia. Apple-Century-Crofts; New York: 1973. [Google Scholar]
- Chapman LJ, Chapman JP, Miller GA. A theory of verbal behavior in schizophrenia. In: Maher BA, editor. Progress in Experimental Personality Research. Vol. 1. Academic press; New York: 1964. pp. 49–77. [PubMed] [Google Scholar]
- Chen EYH, Lam LCW, Kan CS, Chan CKY, Kwok CL, Nguyen DGH, et al. Language disorganization in schizophrenia: Validation and assessment with a new clinical rating instrument. Hong Kong J Psychiatry. 1996;6:4–13. [Google Scholar]
- Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: Converging evidence from three theoretically motivated cognitive tasks. Journal of Abnormal Psychology. 1999;108:120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Levy DL, Lenzenweger MF, Holzman PS. Thought disorder, perceptual aberrations and schizotypy. J Abnorm Psychol. 1996;105:469–473. doi: 10.1037//0021-843x.105.3.469. [DOI] [PubMed] [Google Scholar]
- Covington MA, He C, Brown C, Naçi L, McClain JT, Fjordbak BS, et al. Schizophrenia and the structure of language: The linguist’s view. Schizophrenia Research. 2005;77(1):85–98. doi: 10.1016/j.schres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Crespi BJ. Sly FOXP2: genomic conflict in the evolution of language. Trends Ecol Evol. 2007;22:174–175. doi: 10.1016/j.tree.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Is schizophrenia the price homo sapiens pay for language? Schiz Res. 1997;28:127–141. doi: 10.1016/s0920-9964(97)00110-2. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Sexual selection, timing and the descent of man: A theory of the genetic origins of language. Current Pschology of Cognition. 1998;17:1079–1114. [Google Scholar]
- Crow TJ. The ‘big bang’ theory of the origin of psychosis and the faculty of language. Schiz Res. 2008;102:31–52. doi: 10.1016/j.schres.2008.03.010. [DOI] [PubMed] [Google Scholar]
- DeLisi LE. Speech disorder in schizophrenia: Review of the literature and exploration of its relation to the uniquely human capacity for language. Schizophr Bull. 2001;27(3):481–496. doi: 10.1093/oxfordjournals.schbul.a006889. [DOI] [PubMed] [Google Scholar]
- Demonet J-F, Chollet F, Ramsay S, Cardebat D, Nespoulous J-L, Wise R, et al. The anatomy of phonological and semantic processing in normal subjects. Brain. 1995;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- Ditman T, Kuperberg GR. The time course of building discourse coherence in schizophrenia: An ERP investigation. Psychophysiology. 2007;44:991–1001. doi: 10.1111/j.1469-8986.2007.00565.x. [DOI] [PubMed] [Google Scholar]
- Docherty NM. Communication deviance, attention and schizotypy in parents of schizophrenic patients. J Nerv Ment Dis. 1993;181:750–756. doi: 10.1097/00005053-199312000-00007. [DOI] [PubMed] [Google Scholar]
- Docherty NM, Cohen AS, Nienow TM, Dinzeo TJ, Dangelmaier RE. Stability of formal thought disorder and referential communication disturbances in schizophrenia. J Abnorm Psychol. 2003;112(3):469–475. doi: 10.1037/0021-843X.112.3.469. [DOI] [PubMed] [Google Scholar]
- Docherty NM, DeRosa M, Andreasen NC. Communication disturbances in schizophrenia and mania. Arch Gen Psychiatry. 1996;53:358–364. doi: 10.1001/archpsyc.1996.01830040094014. [DOI] [PubMed] [Google Scholar]
- Docherty NM, Gordinier SW, Hall MJ, Cutting LP. Communication disturbances in relatives beyond the age of risk for schizophrenia and their associations with symptoms in patients. Schiz Bull. 1999;25:851–862. doi: 10.1093/oxfordjournals.schbul.a033424. [DOI] [PubMed] [Google Scholar]
- Docherty NM, Gottesman I. A twin study of communication disturbances in schizophrenia. J Nerv Ment Dis. 2000;188(7):395–401. doi: 10.1097/00005053-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Docherty NM, Miller TN, Lewis MA. Communication disturbances in the natural speech of schizophrenic patients and their nonschizophrenic parents. Acta Psychiatr Scand. 1997;95:500–507. doi: 10.1111/j.1600-0447.1997.tb10138.x. [DOI] [PubMed] [Google Scholar]
- Docherty NM, Rhinewine JP, Labhart RP, Gordiner SW. Communication disturbances and family psychiatric history in parents of schizophrenic patients. J Nerv Ment Dis. 1998a;186:761–768. doi: 10.1097/00005053-199812000-00004. [DOI] [PubMed] [Google Scholar]
- Docherty NM, Schnur M, Harvey PD. Reference performance and positive and negative thought disorder: A follow-up study of manics and schizophrenics. J Abnormal Psychol. 1988;97:437–442. doi: 10.1037//0021-843x.97.4.437. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Kremen WS, Lyons MJ, Pepple JR, Seidman LJ, Tsuang MT. Diagnostic accuracy and linkage analysis: How useful are schizophrenia spectrum phenotypes? Am J Psychiatry. 1995;152:1286–1290. doi: 10.1176/ajp.152.9.1286. [DOI] [PubMed] [Google Scholar]
- Fenichel O. The Psychoanalytic Theory of Neurosis. Norton; New York: 1945. [Google Scholar]
- Fiez JA. Phonology, semantics and the role of the left inferior prefrontal cortex. Hum Brain Mapp. 1997;5:79–83. [PubMed] [Google Scholar]
- Fraser WI, King KM, Thomas P, Kendell RE. The diagnosis of schizophrenia by language analysis. Br J Psychiatry. 1986;148:275–278. doi: 10.1192/bjp.148.3.275. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Vrijenhoek T, Marks S, Janssen IM, van der Vliet WA, Faas BH, et al. CNTNAP2 gene dosage variation is associated with schizophrenia and epilepsy. Mol Psychiatry. 2008;13:261–266. doi: 10.1038/sj.mp.4002049. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- Gambini O, Campana A, Macciardi F, Scarone S. A preliminary report of a strong genetic component for thought disorder in normals. Neuropsychobiology. 1997;36:13–18. doi: 10.1159/000119353. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Aloia MS, Gourovitch ML, Missar D, Pickar D, Weinberger DR. Cognitive substrates of thought disorder. I: The semantic system. Am J Psychiatry. 1998;155(12):1671–1676. doi: 10.1176/ajp.155.12.1671. [DOI] [PubMed] [Google Scholar]
- Goldstein K. Methodical approach to the study of schizophrenic thought disorder. In: Kasanin JS, editor. Language and Thought in Schizophrenia. Norton; New York: 1944. pp. 17–40. [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry. 2003;130:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Shields J. Schizophrenia and Genetics. Academic Press; New York: 1972. [Google Scholar]
- Gough PM, Nobre AC, Devlin JT. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J Neurosci. 2005;25:8010–8016. doi: 10.1523/JNEUROSCI.2307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hain C, Maier W, Hoechst-Janneck S, Francke P. Subclinical thought disorder in first-degree relatives of schizophrenic patients. Results from a matched-pairs study of the Thought Disorder Index. Acta Psychiatr Scand. 1995;92:305–309. doi: 10.1111/j.1600-0447.1995.tb09587.x. [DOI] [PubMed] [Google Scholar]
- Hannenhalli S, Kaestner KH. The evolution of Fox genes and their role in development and disease. Nat Rev Genet. 2009;10:233–240. doi: 10.1038/nrg2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrow M, Grossman L, Silverstein ML. A longitudinal study of thought disorder in manic patients. Arch Gen Psychiatry. 1986;43:781–785. doi: 10.1001/archpsyc.1986.01800080067009. [DOI] [PubMed] [Google Scholar]
- Harrow M, Grossman LS, Silverstein ML, Meltzer HY. Thought pathology in manic and schizophrenic patients: Its occurrence at hospital admission and seven weeks later. Arch Gen Psychiatry. 1982;39:665–671. doi: 10.1001/archpsyc.1982.04290060027006. [DOI] [PubMed] [Google Scholar]
- Harrow M, Harkavy K, Bromet E, Tucker GJ. A longitudinal study of schizophrenic thinking. Arch Gen Psychiatry. 1973;28:179–182. doi: 10.1001/archpsyc.1973.01750320019003. [DOI] [PubMed] [Google Scholar]
- Harrow M, Marengo J. The early course of thought disorder at followup: Its persistence and prognostic significance. Schiz Bull. 1986;12:208–224. doi: 10.1093/schbul/12.3.373. [DOI] [PubMed] [Google Scholar]
- Harrow M, Prosen M. Schizophrenic thought disorders: bizarre associations and intermingling. Am J Psychiatry. 1979;136(3):293–296. doi: 10.1176/ajp.136.3.293. [DOI] [PubMed] [Google Scholar]
- Harrow M, Quinlan D. Is disordered thinking unique to schizophrenia? Arch Gen Psychiatry. 1977;34:15–21. doi: 10.1001/archpsyc.1977.01770130017001. [DOI] [PubMed] [Google Scholar]
- Harrow M, Quinlan D. Disordered Thinking and Schizophrenic Psychopathology. Gardner press; New York: 1985. [Google Scholar]
- Harrow M, Silverstein M, Marengo J. Disordered thinking. Arch Gen Psychiatry. 1983;40:765–771. doi: 10.1001/archpsyc.1983.01790060063008. [DOI] [PubMed] [Google Scholar]
- Harrow M, Tucker G, Himmelhoch J, Putnam NJ. Schizophrenic “thought disorders” after the acute phase. Am J Psychiatry. 1972;128(7):824–829. doi: 10.1176/ajp.128.7.824. [DOI] [PubMed] [Google Scholar]
- Harvey P, Earle-Boyer EA, Wielgus MS. The consistency of thought disorder in mania and schizophrenia. J Nerv Ment Dis. 1984;172:458–463. doi: 10.1097/00005053-198408000-00003. [DOI] [PubMed] [Google Scholar]
- Harvey P, Weintraub S, Neale J. Speech competence of children vulnerable to psychopathology. J Abnorm Child Psychol. 1982;10:373–388. doi: 10.1007/BF00912328. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Docherty NM, Serper MR, Rasmussen M. Cognitive deficits and thought disorder: II. An 8-month follow-up study. Schiz Bull. 1990;16:147–156. doi: 10.1093/schbul/16.1.147. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Neale JM. The specificity of thought disorder to schizophrenia: Research methods in their historical perspective. Prog Exp Pers Res. 1983;12:153–180. [PubMed] [Google Scholar]
- Hirsch SR, Leff JP. Abnormalities in Parents of Schizophrenics. Oxford University Press; London: 1975. [Google Scholar]
- Holzman PS, Coleman MJ, Lenzenweger MF, Levy DL, Matthysse S, O’Driscoll G, et al. Working memory deficits, antisaccades, and thought disorder in relation to perceptual aberration. In: Raine A, Lencz T, Mednick S, editors. Schizotypal Personality. Cambridge University Press; Cambridge: 1995. pp. 353–381. [Google Scholar]
- Holzman PS, Matthysse S. The genetics of schizophrenia: A review. Psychol Sci. 1990;1:279–286. [Google Scholar]
- Holzman PS, Shenton M, Solovay M. Quality of thought disorder in differential diagnosis. Schiz Bull. 1986;12:360–372. doi: 10.1093/schbul/12.3.360. [DOI] [PubMed] [Google Scholar]
- Hurst JA, Baraitser M, Auger E, Graham F, Norell S. An extended family with a dominantly inherited speech and language disorder. Dev Med Child Neurol. 1990;32:352–355. doi: 10.1111/j.1469-8749.1990.tb16948.x. [DOI] [PubMed] [Google Scholar]
- Hurt SW, Holzman PS, Davis JM. Thought disorder: The measurement of its changes. Arch Gen Psychiatry. 1983;40:1281–1285. doi: 10.1001/archpsyc.1983.01790110023005. [DOI] [PubMed] [Google Scholar]
- Johnston MH, Holzman PS. Assessing Schizophrenic Thinking. Jossey-Bass, Inc.; San Francisco: 1979. [Google Scholar]
- Keefe RSE, Mohs RC, Losonczy MF, Davidson M, Silverman JM, Kendler KS, et al. Characteristics of very poor outcome. Am J Psychiatry. 1987;144:889–895. doi: 10.1176/ajp.144.7.889. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Diagnostic approaches to schizotypal personality disorder: a historical perspective. Schiz Bull. 1985;11:538–553. doi: 10.1093/schbul/11.4.538. [DOI] [PubMed] [Google Scholar]
- Kendler KS, McGue M, Gruenberg AM, Walsh D. Schizotypal symptoms and signs in the Roscommon Family Study. Their factor structure and familial relationship with psychotic and affective disorders. Arch Gen Psychiatry. 1995;52:296–303. doi: 10.1001/archpsyc.1995.03950160046009. [DOI] [PubMed] [Google Scholar]
- Kendler KS, McGuire M, Gruenberg A, O’Hare A, Spellman M, Walsh D. The Roscommon family study. I. Methods, diagnosis of probands, and risk of schizophrenia in relatives. Arch Gen Psychiatry. 1993a;50:527–540. doi: 10.1001/archpsyc.1993.01820190029004. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Berenbaum H. Cognitive impairments associated with formal thought disorder in people with schizophrenia. J Abnormal Psychol. 2002;111:211–224. [PubMed] [Google Scholar]
- Kerns JG, Berenbaum H, Barch DM, Banich MT, Stolar N. Word production in schizophrenia and its relationship to positive symptoms. Psychiatry Research. 1999;87(1):29–37. doi: 10.1016/s0165-1781(99)00052-9. [DOI] [PubMed] [Google Scholar]
- Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, Shen Y, et al. Disruption of neurexin 1 associated with autism spectrum disorder. Am. J. Hum. Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney DK, Holzman PS, Jacobsen B, Jansson L, Faber B, Hildegrand W, et al. Thought disorder in schizophrenic and control adoptees and their relatives. Arch Gen Psychiatry. 1997;54:475–479. doi: 10.1001/archpsyc.1997.01830170101013. [DOI] [PubMed] [Google Scholar]
- Kirov G, Zaharieva I, Georgieva L, Moskvina V, Nikolov I, Cichon S, et al. A genome-wide association study in 574 schizophrenia trios using DNA pooling. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.33. e-pub. [DOI] [PubMed] [Google Scholar]
- Kjelgaard MM, Tager-Flusberg H. An investigation of language impairment in autism: Implications for genetic subgroups. Lang Cogn Process. 2001;16:287–308. doi: 10.1080/01690960042000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E. Dementia Praecox and Paraphrenia. Chicago Medical Book Company; Chicago: 18961919. [Google Scholar]
- Kreher DA, Goff D, Kuperberg GR. Why all the confusion? Experimental task explains discrepant semantic priming effects in schizophrenia under “automatic” conditions: Evidence from event-related potentials. Schiz Res. 2009;111:174–181. doi: 10.1016/j.schres.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufferle B, Lenz G, Schanda H. Clinical evaluation of language and thought disorders in patients with schizophrenia and affective psychoses. Psychopathology. 1985;18:126–132. doi: 10.1159/000284225. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Deckersbach T, Holt DJ, Goff D, West C. Increased temporal and prefrontal activity in response to semantic associations in schizophrenia. Arch Gen Psychiatry. 2007;64:138–151. doi: 10.1001/archpsyc.64.2.138. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Kreher DA, Goff D, McGuire PK, David AS. Building up linguistic context in schizophrenia: Evidence from self-paced reading. Neuropsychology. 2006;20:442–452. doi: 10.1037/0894-4105.20.4.442. [DOI] [PubMed] [Google Scholar]
- Lai CSL, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- Lander E. Splitting schizophrenia. Nature. 1988;336:105–106. doi: 10.1038/336105a0. [DOI] [PubMed] [Google Scholar]
- Levy DL, Ji F, Krastoshevsky O, Holzman PS, Mendell NR, Matthysse S. The power of asymmetric ascertainment in linkage analysis. In preparation. 2009 [Google Scholar]
- Liddle PF, Ngan ET, Caissie SL, Anderson CM, Bates AT, Quested DJ, et al. Thought and Language Index: An instrument for assessing thought and language in schizophrenia. Br J Psychiatry. 2002;181:326–330. doi: 10.1192/bjp.181.4.326. [DOI] [PubMed] [Google Scholar]
- MacDermot KD, Bonora E, Sykes N, Coupe A-M, Lai CSL, Vernes SC, et al. Identification of FOXP2 truncation as a novel cause of developmental speech and language deficits. Am J Hum Genet. 2005;76:1074–1080. doi: 10.1086/430841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher BA. The language of schizophrenia: A review and interpretation. Br J Psychiatry. 1972;120:3–17. doi: 10.1192/bjp.120.554.3. [DOI] [PubMed] [Google Scholar]
- Maher BA. A tentative theory of schizophrenic utterance. In: Maher BA, Maher WB, editors. Progress in Experimental Personality Research. Vol. 12. Academic press; New York: 1983. pp. 1–52. [PubMed] [Google Scholar]
- Makowski DG, Waternaux C, Lajonchere CM, Dicker R, Smoke N, Koplewicz H, et al. Thought disorder in adolescent-onset schizophrenia. Schiz Res. 1997;23:147–165. doi: 10.1016/s0920-9964(96)00097-7. [DOI] [PubMed] [Google Scholar]
- Manoach D. Handednes is related to formal thought disorder and language dysfunction in schizophrenia. J Clin Expt’l Neuropsychology. 1994;16:2–14. doi: 10.1080/01688639408402613. [DOI] [PubMed] [Google Scholar]
- Marcus GF, Fisher SE. FOXP2 in focus: What can genes tell us about speech and language? Trends Cognitive Sciences. 2003;7:257–262. doi: 10.1016/s1364-6613(03)00104-9. [DOI] [PubMed] [Google Scholar]
- Marengo J, Harrow M. Schizophrenic thought disorder at follow-up. A persistent or episodic course? Arch Gen Psychiatry. 1987;44:651–659. doi: 10.1001/archpsyc.1987.01800190071011. [DOI] [PubMed] [Google Scholar]
- Marini A, Spoletini I, Rubino IA, Ciuffa M, Bria P, Martinotti G, et al. The language of schizophrenia:An analysis of micro and macrolinguistic abilities and their neuropsychological correlates. Schiz Res. 2008;105:144–155. doi: 10.1016/j.schres.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, Skaug J, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse S, Holzman PS. Comment on Berenbaum, Oltmanns, and Gottesman (1985): “Formal thought disorder in schizophrenics and their twins”. J Abnormal Psychol. 1988;97:105–107. doi: 10.1037//0021-843x.97.1.105. [DOI] [PubMed] [Google Scholar]
- Matthysse S, Holzman PS, Lange K. The genetic transmission of schizophrenia: Application of Mendelian latent structure analysis to eye tracking dysfunctions in schizophrenia and affective disorders. J Psychiatr Res. 1986;20:57–76. doi: 10.1016/0022-3956(86)90023-3. [DOI] [PubMed] [Google Scholar]
- Matthysse S, Parnas J. Extending the phenotype of schizophrenia: Implications for linkage analysis. J Psychiatr Res. 1992;26:329–344. doi: 10.1016/0022-3956(92)90039-q. [DOI] [PubMed] [Google Scholar]
- McConaghy N. The use of an object sorting test in elucidating the hereditary factor in schizophrenia. J Neurology, Neurosurgery and Psychiatry. 1959;22:243–246. doi: 10.1136/jnnp.22.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna P, Oh T. Schizophrenic Speech. Making Sense of Bathroots and Ponds that Fall in Doorways. Cambridge University Press; Cambridge: 2005. [Google Scholar]
- Meehl PE. Psychodiagnosis. Selected Papers. W.W. Norton & Co.; New York: 1977. Schizotaxia, schizotypy, and schizophrenia; pp. 135–155. [Google Scholar]
- Morice R, Ingram JCL. Language analysis in schizophrenia: Diagnostic implications. Australian and New Zealand J Psychiatry. 1982;16:11–21. doi: 10.3109/00048678209161186. [DOI] [PubMed] [Google Scholar]
- Oh TM, McCarthy RA, McKenna PJ. Is there a schizophasia? A study applying the single case study approach to formal thought disorder in schizophrenia. Neurocase. 2002;8:233–244. doi: 10.1093/neucas/8.3.233. [DOI] [PubMed] [Google Scholar]
- Oltmanns TF, Murphy R, Berenbaum H, Dunlop SA. Rating verbal communication impairment in schizophrenia and affective disorders. Schiz Bull. 1985;11:292–299. doi: 10.1093/schbul/11.2.292. [DOI] [PubMed] [Google Scholar]
- Parnas J, Schulsinger F, Schulsinger H, Mednick S, Teasdale T. Behavioral precursors of the schizophrenia spectrum. Arch Gen Psychiatry. 1982;39:658–664. doi: 10.1001/archpsyc.1982.04290060020005. [DOI] [PubMed] [Google Scholar]
- Perry W, Geyer MA, Braff DL. Sensorimotor gating and thought disturbance measured in close temporal proximity in schizophrenic patients. Arch Gen Psychiatry. 1999;56:277–281. doi: 10.1001/archpsyc.56.3.277. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Cadenhead K, Sprock J, Braff D. The use of the Ego Impairment Index across the schizophrenia spectrum. J Personality Assessment. 2003;80:50–57. doi: 10.1207/S15327752JPA8001_13. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun MA, Raichle ME. Positron emission tomographic studies of the processing of single words. J Cogn Neurosci. 1989;1:153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Oh TMSS, Laws KR, McKenna PJ. Semantic priming in schizophrenia: Systematic review and meta-analysis. British Journal of Psychiatry. 2008;192:92–97. doi: 10.1192/bjp.bp.106.032102. [DOI] [PubMed] [Google Scholar]
- Racenstein JM, Penn D, Harrow M, Schleser R. Thought disorder and psychosocial functioning in schizophrenia: The concurrent and predictive relationships. J Nerv Ment Dis. 1999;187:281–289. doi: 10.1097/00005053-199905000-00003. [DOI] [PubMed] [Google Scholar]
- Risch N. Linkage strategies for genetically complex traits: II The power of affected relative pairs. Am J Hum Genet. 1990c;46:219–221. [PMC free article] [PubMed] [Google Scholar]
- Rochester SR, Martin JR. Crazy Talk: A Study of the Discourse of Schizophrenic Speakers. Plenum; New York: 1979. [Google Scholar]
- Rodriguez-Ferrera S, McCarthy RA, McKenna PJ. Language in schizophrenia and its relationship to formal thought disorder. Psychol Med. 2001;31:197–205. doi: 10.1017/s003329170100321x. [DOI] [PubMed] [Google Scholar]
- Rosenthal D. Problems of sampling and diagnosis in the major twin studies of schizphrenia. J Psychiatr Res. 1962;1:116–134. [Google Scholar]
- Rujescu D, Ingason A, Cichon S, Pietilainen OPH, Barnes MR, Toulopoulou T, et al. Disruption of the neurexin 1 gene is associated with schizophrenia. Hum. Mol. Genet. 2008;18:988–996. doi: 10.1093/hmg/ddn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF. Semantic activation and verbal working memory maintenance in schizophrenic thought disorder: Insights from electrophysiology and lexical amibiguity. Clin EEG Neurosci. 2008;39:103–107. doi: 10.1177/155005940803900217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Solovay MR, Holzman PS. Comparative studies of thought disorders. II. Schizoaffective disorder. Arch Gen Psychiatry. 1987;44:21–30. doi: 10.1001/archpsyc.1987.01800130023004. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Solovay MR, Holzman PS, Coleman MJ, Gale HJ. Thought disorder in the relatives of psychotic patients. Arch Gen Psychiatry. 1989a;46:897–901. doi: 10.1001/archpsyc.1989.01810100039007. [DOI] [PubMed] [Google Scholar]
- Shriberg LD, Ballard KJ, Tomblin JB, Duffy JR, Odell KH, Williams CA. Speech, prosody, and voice characteristics of a mother and daughter with a 7;13 translocation affecting FOXP2. J Speech Lang Hear Res. 2006;49:500–525. doi: 10.1044/1092-4388(2006/038). [DOI] [PubMed] [Google Scholar]
- Singer M, Wynne L. Communication styles in parents of normals, neurotics and schizophrenics. Psychiatric Research Reports. 1966a;20:25–38. [PubMed] [Google Scholar]
- Singer MT. Family transactions and schizophrenia. I: Recent research findings. In: Romano J, editor. The Origins of Schizophrenia; Excerpta Medica International Congress Series; Amsterdam. 1967.pp. 147–153. [Google Scholar]
- Singer MT, Wynne LC. Differentiating characteristics of parents of childhood schizophrenics, childhood autistics, and young adult schizophrenics. Am J Psychiatry. 1963;120:234–243. doi: 10.1176/ajp.120.3.234. [DOI] [PubMed] [Google Scholar]
- Singer MT, Wynne LC. Thought disorder and family relations of schizophrenics. III: Methodology using projective techniques. Arch Gen Psychiatry. 1965a;12:187–212. doi: 10.1001/archpsyc.1965.01720320075009. [DOI] [PubMed] [Google Scholar]
- Singer MT, Wynne LC. Principles for scoring communication defects and deviances in parents of schizophrenics: Rorschach and TAT scoring manuals. Psychiatry. 1966b;29:260–288. [PubMed] [Google Scholar]
- Singer MT, W LC. Thought disorder and family relations of schizophrenics. IV: results and implications. Arch Gen Psychiatry. 1965b;12:201–212. doi: 10.1001/archpsyc.1965.01720320089010. [DOI] [PubMed] [Google Scholar]
- Sitnikova T, Salisbury DF, Kuperberg G, Holcomb PJ. Electrophysiological insights into language processing in schizophrenia. Psychophysiol. 2002;39:851–860. doi: 10.1111/1469-8986.3960851. [DOI] [PubMed] [Google Scholar]
- Slater E. A review on earlier evidence of genetic factors in schizophrenia. In: Rosenthal D, Kety SS, editors. The Transmission of Schizophrenia. Pergamon; Oxford: 1968. pp. 15–26. [Google Scholar]
- Solovay M, Shenton M, Gasperetti C, Coleman M, Kestnbaum E, Carpenter J, et al. Scoring manual for the thought disorder index. Schiz Bull. 1986;12:483–496. doi: 10.1093/schbul/12.3.483. [DOI] [PubMed] [Google Scholar]
- Solovay M, Shenton M, Holzman P. Comparative studies of thought disorders. I. Mania and schizophrenia. Arch Gen Psychiatry. 1987;44:13–20. doi: 10.1001/archpsyc.1987.01800130015003. [DOI] [PubMed] [Google Scholar]
- Spiteri E, Konopka G, Coppola G, Bomar J, Oldham M, Ou J, et al. Identification of the transcriptional targets of FOXP2, a gene linked to speech and language, in developing human brain. Am J Hum Genet. 2007;81:1144–1157. doi: 10.1086/522237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spohn HE, Coyne L, Larson J, Mittleman F, Spray J, Hayes K. Episodic and residual thought pathology in chronic schizophrenia. Schiz Bull. 1986;12:394–407. doi: 10.1093/schbul/12.3.394. [DOI] [PubMed] [Google Scholar]
- Stirling J, Hellewell J, Blakey A, Deakin W. Thought disorder in schizophrenia is associated with both executive dysfunction and circumscribed impairments in semantic function. Pscyhol Med. 2006;36:475–484. doi: 10.1017/S0033291705006884. [DOI] [PubMed] [Google Scholar]
- Stone JL, O’Donovan MC, Gurling H, Consortium IS. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–241. doi: 10.1038/nature07239. al, e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss KA, Puffenberger EG, Huentelman MJ, Gottlieb S, Dobrin SE, Parod JM, et al. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N Engl J Med. 2006;354:1370–1377. doi: 10.1056/NEJMoa052773. [DOI] [PubMed] [Google Scholar]
- Stromswold K. The genetics of speech and language impairments. N Engl J Med. 2008;359:2381–2383. doi: 10.1056/NEJMe0807813. [DOI] [PubMed] [Google Scholar]
- Sung H, Ji F, Levy DL, Matthysse S, Mendell NR. The power of linkage analysis of a disease-related endophenotype using asymmetrically ascertained sib pairs. Computational Statistics and Data Analysis. 2009;53:1829–1842. doi: 10.1016/j.csda.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nature Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavano A, Sponda S, Fabbro F, Perlini C, Rambaldelli G, Ferro A, et al. Specific linguistic and pragmatic deficits in Italian patients with schizophrenia. Schiz Res. 2008;102:53–62. doi: 10.1016/j.schres.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Taylor MA, Reed R, Berenbaum S. Patterns of speech disorders in schizophrenia and mania. J Nerv Ment Dis. 1994;182:319–326. doi: 10.1097/00005053-199406000-00002. [DOI] [PubMed] [Google Scholar]
- Terzic J, Saraga-Babic M. Expression pattern of PAX3 and PAX6 genes during human embryogenesis. Int’l J Dev Biol. 1999;43:501–508. [PubMed] [Google Scholar]
- Titone D, Holzman PS, Levy DL. Idiom processing in schizophrenia: Literal implausibility saves the day for idiom priming. J Abnorm Psychol. 2002;111:313–320. doi: 10.1037//0021-843x.111.2.313. [DOI] [PubMed] [Google Scholar]
- Titone D, Levy DL. Lexical competition and spoke word identification in schizophrenia. Schiz Res. 2004;68:75–85. doi: 10.1016/S0920-9964(03)00212-3. [DOI] [PubMed] [Google Scholar]
- Titone D, Levy DL, Holzman PS. Contextual insensitivity in schizophrenic language processing: Evidence from lexical ambiguity. J Abnorm Psychol. 2000;109:761–767. doi: 10.1037//0021-843x.109.4.761. [DOI] [PubMed] [Google Scholar]
- Titone D, Libben M, Niman M, Ranbom L, Levy DL. Conceptual combination, in schizophrenia: Contrasting property and relational interpretations. Journal of Neurolinguistics. 2007;20:92–110. [Google Scholar]
- Vargha-Khadem F, Watkins KE, Alcock KJ, Fletcher P, Passingham R. Praxic and nonverbal cognitive deficits in a large family with a genetically transmitted speech and language disorder. Proc Nat’l Acad Sci. 1995;92:930–933. doi: 10.1073/pnas.92.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernes SC, Newbury DF, Abrahams BS, Winchester L, Nicod J, Groszer M, et al. A functional genetic link between distinct developmental language disorders. New England J Med. 2008;359:2337–2345. doi: 10.1056/NEJMoa0802828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernes SC, Spiteri E, Nicod J, Groszer M, Taylor JM, Davies KE, et al. High-throughput analysis of promoter occupancy reveals direct neural targets of FOXP2, a gene mutated in speech and language disorders. Am J Hum Genet. 2007;81:1232–1250. doi: 10.1086/522238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg K-E, Wynne LC, Oja H, Keskitalo P, Anais-Tanner H, Koistinen P, et al. Thought Disorder Index of Finnish adoptees and communication deviance of their adoptive parents. Psychol Med. 2000;30:127–136. doi: 10.1017/s0033291799001415. [DOI] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Watt NF, Anthony EJ. Children at Risk for Schizophrenia. Cambridge University press; New York: 1984. [Google Scholar]
- Whitehouse AJ, Barry JG, Bishop DVM. The broader language phenotype of autism: A comparison with specific language impairment. J Child Psychol Psychiatry. 2007;48:822–830. doi: 10.1111/j.1469-7610.2007.01765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse AJ, Barry JG, Bishop DVM. Further defining the language impairment of autism: Is there a specific language impairment subtype? J Commun Disord. 2008;41:319–336. doi: 10.1016/j.jcomdis.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Wynne LC. Family transactions and schizophrenia. II: Conceptual considerations for a research strategy. In: Romano J, editor. The Origins of Schizophrenia; Excerpta Medica International Congress Series; Amsterdam. 1967.pp. 165–178. [Google Scholar]
- Wynne LC, Singer MT. Thought disorder and family relations of schizophrenics. Arch Gen Psychiatry. 1965;12:187–200. doi: 10.1001/archpsyc.1965.01720320075009. [DOI] [PubMed] [Google Scholar]
- Wynne LC, Singer MT, Bartko J, Toohey ML. Schizophrenics and their families: Recent research on parental communication. In: Tanner JM, editor. Developments in Psychiatric Research. Hodder & Stroughton; Seven Oaks, Kent, England: 1977. pp. 254–286. [Google Scholar]
- Zahn TP. Word associations in adoptive and biological parents of schizophrenics. Arch Gen Psychiatry. 1968;19:501–503. doi: 10.1001/archpsyc.1968.01740100117017. [DOI] [PubMed] [Google Scholar]
- Zeesman S, Nowaczyk MJ, Teshima I, Roberts W, Cardy JO, Brian J, et al. Speech and language impairment and oromotor dyspraxia due to deletion of 7q31 that involves FOXP2. Am J Med Genet A. 2006;140:509–514. doi: 10.1002/ajmg.a.31110. [DOI] [PubMed] [Google Scholar]


